Abstract
Neurofibromatosis Type 1 (NF1) is characterized by the abnormal proliferation of neuroectodermal tissues and the development of certain malignancies, particularly neurofibromas, which may progress into malignant peripheral nerve sheath tumors (MPNSTs). Effective pharmacological therapy for the treatment of NF1 tumors is currently unavailable, and the prognosis for patients with MPNSTs is poor. Loss of neurofibromin correlates with increased expression of the epidermal growth factor receptor (EGFR) and ErbB2 tyrosine kinases, and these kinases have been shown to promote NF1 tumor-associated pathologies in vivo. We show here that while NF1 MPNST cells have higher EGFR expression levels and are more sensitive to EGF when compared to a non-NF1 MPNST cell line, the ability of the EGFR inhibitor gefitinib to selectively inhibit NF1 MPNST cell proliferation is marginal. We also show that NF1 MPNST proliferation correlates with activated ErbB2, and can be suppressed by nanomolar concentrations of the pan-ErbB inhibitor CI-1033 (canertinib). Consequently, targeting both EGFR and ErbB2 may prove an effective strategy for suppressing NF1 MPNST tumor growth in vivo.
Keywords: EGF receptor, ErbB2, NF1, tyrosine kinase inhibitor, MPNST
Introduction
Neurofibromatosis type 1 (NF1) is the most common inherited cancer predisposition syndrome and is associated with the aberrant proliferation of tissues derived from the neural crest.1, 2 The overall incidence of approximately 1 in 3,000 includes about 50% of cases that arise due to new mutations in the NF1 gene,3 which encodes a tumor suppressor protein called neurofibromin. Patients with NF1 present numerous clinical manifestations and are at an increased risk of developing certain tumors, most commonly neurofibromas.2 The majority of cells in neurofibromas are derived from Schwann cells, and these tumors can affect any peripheral nerve. In approximately 10% of cases, plexiform neurofibromas progress to malignant peripheral nerve sheath tumors (MPNSTs).4 There is currently no pharmacological treatment for MPNSTs. Prognosis for NF1 patients with MPNSTs is poor, with only 21% of patients surviving 5 years from time of diagnosis.5
While haploinsufficiency for functional neurofibromin protein drives some aspects of the disease, and provides a favorable microenvironment for tumor development,6–8 loss of heterozygosity is found in the transformed Schwann cell component of MPNSTs.9 Neurofibromin downregulates the activity of the small GTPase Ras.10 Ras has pivotal roles in cell survival, proliferation, and differentiation by transducing responses initiated at the cell surface to several intracellular signaling molecules, including those that constitute the Raf-MEK-ERK and PI3K-Akt axes.11, 12 We have previously demonstrated that basal N- and K-Ras and ERK1/2 activities are higher in NF1 MPNST cells, and that activation of the ERK1/2 signaling pathway is critical for their proliferation.13 A rational approach to NF1 MPNST therapy may derive from such characterization of the critical signal transduction pathways that drive MPNST proliferation.14, 15
In addition to loss of functional neurofibromin, neurofibromas and MPNSTs show significant alterations in the expression of one or more of the epidermal growth factor receptor (ErbB) family of receptor tyrosine kinases.16, 17 These changes significantly affect the responsiveness of Schwann cells to ErbB ligands.16 While quiescent Schwann cells do not express the EGFR, loss of neurofibromin has been shown to correlate with aberrant expression of this receptor in Schwann cells.16, 18 Increased EGFR abundance and activity are associated with the development and progression of multiple human solid tumors,19, 20 and play important roles in NF1-associated malignancy. For example, transgenic murine Schwann cells that have targeted overexpression of the EGFR exhibit features of neurofibromas, such as hyperplasia, collagen deposition, mast cell infiltration, and dissociation from axons.21 The EGFR confers a mechanism by which Schwann cells respond to mitogenic factors and activate Ras-mediated proliferative and pro-survival signaling pathways, which are greatly potentiated in the context of neurofibromin deficiency.22
Another ErbB family member that has been connected to NF1 tumor pathologies is the ErbB2 tyrosine kinase. ErbB2 is expressed in normal Schwann cells, and heterodimerization of ErbB2 with ErbB3 is believed to direct neuregulin-mediated signaling in Schwann cells.23 While there is no known ligand for ErbB2, this protein readily heterodimerizes with the other ErbB family members and greatly enhances their signaling.24 Overexpressed ErbB2 can also homodimerize, resulting in ligand-independent signaling and contributing to tumorigenesis.25, 26 Previous reports have found an inverse relationship between neurofibromin expression and ErbB2 levels,27 and constitutive activation of ErbB2 in Schwann cells results in formation of invasive Schwann cell tumors.27, 28
In this study, we found that two independent NF1 MPNST lines expressed higher levels of EGFR and have a prolonged and more potent ERK activation in response to EGF. We also found strong coupling between EGFR and ErbB2 in these NF1 MPNST cells. Despite the increase in EGFR signaling in NF1 MPNST cells, there was little inhibition of cell growth in response to the EGFR-selective inhibitor gefitinib unless exogenous ligand was present. In contrast, the pan-ErbB inhibitor CI-1033 (canertinib) strongly suppressed proliferation of the NF1 MPNST cells. Considering the roles of EGFR and ErbB2 in NF1-related pathology, inhibition of both of these ErbB kinases may prove an effective therapy for treatment of MPNSTs in vivo.
Materials and Methods
Cell Culture and Treatments
Human MPNST cell lines ST88-14 and NF90-8 (generous gifts from T. Glover, University of Michigan, Ann Arbor, MI, U.S.A.), and STS-26T (a generous gift from D. Scoles, Cedars-Sinai Medical Center, Los Angeles, CA, U.S.A.) were cultured in RPMI (Cellgro, Herndon, VA, U.S.A.) supplemented with 5% fetal bovine serum, L-glutamine, phenol red, 50 units/mL penicillin, and 50 μg/mL streptomycin in a humidified 37°C chamber, supplemented with 5% CO2. For EGF stimulation experiments, cells were grown to ~80% confluency, serum-starved for 1 h, and stimulated with human, recombinant EGF (Invitrogen, Carlsbad, CA, U.S.A.) as indicated in the figures. Gefitinib (AstraZeneca, Alderly Park, UK) and CI-1033 (Pfizer, Groton, CT) were prepared as stock solutions in dimethyl sulfoxide (DMSO) and double distilled H2O, respectively, and stored at −80°C.
Western Blotting
After the indicated treatments, cells were lysed with 2x SDS sample buffer and boiled immediately for 5 min.29 Proteins were separated using SDS-PAGE and transferred to nitrocellulose or PVDF membranes. The membranes were blocked with 5% milk in Tris-buffered saline with 0.5% Tween-20 (TBS-T) for 1 h to overnight. Primary antibodies were rabbit polyclonal IgG anti-EGFR at 1:1,000 (Cell Signaling Technology, Danvers, MA); rabbit immunoaffinity purified IgG anti-phospho-Tyr1068 of EGFR at 1:1000 (Cell Signaling Technology); anti-MAP Kinase at 1:2,000 (Upstate Cell Signaling Solutions, Charlottesville, VA); monoclonal IgG1 isotype anti-MAP Kinase activated, clone MAPK-YT at 1:2,000 (Sigma-Aldrich, St. Louis, MO); mouse monoclonal anti-ErbB2 (LabVision, Freemont, CA) at 1:1000; rabbit monoclonal and polyclonal anti-pTyr1221/1222 of ErbB2 (Cell Signaling Technology) at 1:500; mouse monoclonal anti-Cyclin D1 (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:1000; mouse monoclonal IgG1 anti-β-tubulin “E7” at 1:2,000 (developed by Michael Klymkowsky and obtained from the Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA). Secondary antibodies were HRP-conjugated goat anti-rabbit or goat anti-mouse IgG at 1:10,000 (Jackson Immunoresearch Laboratories, Inc., West Grove, PA). Immunoblots were incubated with Western Blotting Detection Reagents (Amersham Bioscience, Buckinghamshire, United Kingdom) or DuraSignal (Pierce Biotechnology, Inc., Rockford, IL) and recorded on X-ray film (KODAK Biomax Light; Fisher Scientific, Pittsburgh, PA). When necessary, brightness and contrast levels of scanned immunoblot images were uniformly adjusted using ADOBE Photoshop software (Adobe Systems, San Jose, CA). ImageQuant software (GE Healthcare, Piscataway, NJ) was used for densitometry and quantification of immunoblot signals.
Cell Proliferation Assays
Approximately equal numbers of cells (20,000 – 30,000) were plated onto either 30 mm or 60 mm dishes and allowed to attach overnight. For proliferation assays using gefitinib, cells were treated with the indicated concentration of gefitinib dissolved in DMSO or an equal volume of DMSO alone, and the cells harvested at the indicated time-points. For proliferation assays using CI-1033, cells were treated with the indicated concentration of CI-1033 dissolved in ddH2O, or ddH2O alone, and the cells harvested at the indicated time-points. Media and floating cells were collected and dishes rinsed with ice-cold PBS. Attached cells were incubated with 0.5 ml trypsin for 5 min to allow complete detachment. Trypsinized cells and floating cells were combined and centrifuged at 1000× g for 5 min to pellet. Media and trypsin were removed by aspiration, and the cells resuspended in an equal volume of serum-free media. Numbers of live and dead cells were determined by the Trypan blue exclusion assay.
Flow Cytometry
MPNST cell lines were harvested and processed for FACS analyses of DNA content as described previously. 30 DNA analyses were made with a FACScalibur instrument (BD Biosciences, San Jose, CA). Percentages of cells in the G0/G1, S, and G2/M stages of the cell cycle were determined with a DNA histogram-fitting program (MODFIT; Verity Software, Topsham, ME). A minimum of 104 events/sample was collected for subsequent analyses.
Receptor Turnover Assays
Cells were plated in 5% serum and grown to 80% confluency. Cells were serum-starved overnight, and then treated with either 50 μg/ml cycloheximide dissolved in ddH2O (Sigma-Aldrich) or ddH2O alone as indicated for 2 h. Cells were then treated with either 10 ng/mL of EGF in serum-free media, or serum-free media alone, and harvested at the indicated time-points. For assays involving a single time-point, EGF treatments were for 3 h. Cells were harvested in 2x SDS sample buffer and boiled immediately for 5 min. Proteins were separated using SDS-PAGE, transferred to nitrocellulose, and immunoblotted for EGFR, phosphorylated and total ErbB2, cyclin D1, and β-tubulin as described above.
Results
NF1 MPNSTs express higher levels of EGFR and ErbB2 and have a more potent response to EGF
Previous studies have found that MPNST cell lines express significantly different levels of the ErbB receptors.17 We analyzed a panel of MPNST cell lines: two (ST88-14 and NF90-8) were isolated from separate NF1 patients, and one (STS-26T) was isolated from a non-NF1 patient. STS-26T cells express neurofibromin and maintain lower levels of basal Ras activity.13 Both NF1 lines expressed substantially higher levels of EGFR and ErbB2 receptor than the STS-26T line (Fig. 1A). These results are consistent with previous reports that characterized the expression of ErbB family members in these lines using an alternate set of antibodies, and which also showed an absence of ErbB3 or ErbB4 receptors.16
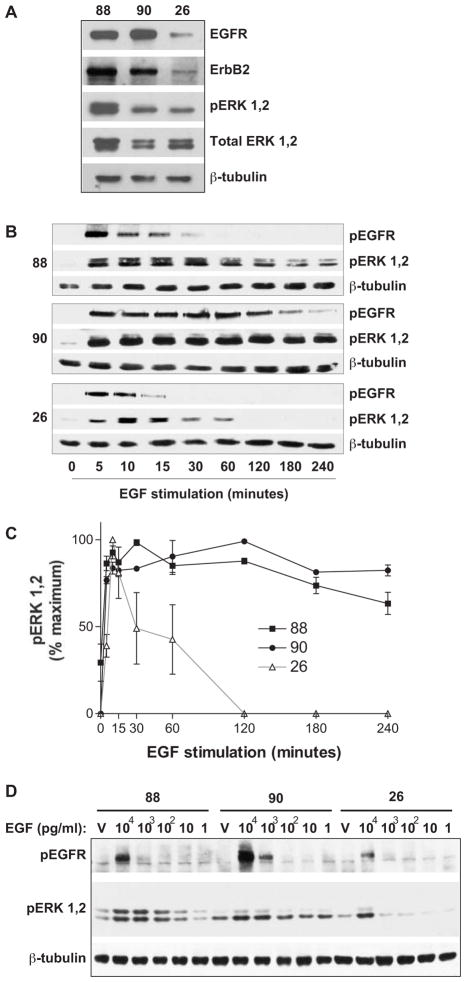
Figure 1.
Characterization of protein expression and MPNST cell line response to EGF. (A) Total cell lysates from the ST88-14 (“88”), NF90-8 (“90”), and STS-26T (“26”) MPNST lines were analyzed by immunoblotting for antibodies versus total protein levels of EGFR and ErbB2, and active and total ERK. Expression of β-tubulin is shown for a loading control. (B) The MPNST cell lines were stimulated as shown with 10 ng/ml EGF. EGFR and ERK activation were assessed by immunoblotting with phospho-specific antibodies against each. Expression of β-tubulin is shown for a loading control. (C) Densitometry of the amount of active ERK1,2 in each sample in panel (B). Background was calculated from equivalent areas in each lane and subtracted from the value of protein in that lane. Active ERK1,2 levels were recorded as percent maximum signal, and are shown as mean ± S.E.M. from three independent experiments. (D) MPNST cells were treated for 10 min with the indicated pg/ml concentrations of EGF or vehicle (RPMI media, “V”) and the activation of EGFR and ERK determined by immunoblotting. β-tubulin is included as a protein loading control. Panels A, B and D are representative of at least three independent experiments.
Loss of the NF1 tumor suppressor is believed to result in exaggerated Ras-mediated signaling.22 One of the primary Ras effector pathways is the ERK MAP kinase pathway, whose activation is required for proliferation of MPNST lines.13 To determine whether loss of neurofibromin correlated with prolonged EGF-mediated ERK signaling, we treated the MPNST lines with 10 ng/ml of EGF and harvested cells over a 4-h time period. Both of the NF1 MPNST lines show sustained ERK activation in response to EGF stimulation (Figs. 1B and 1C). The NF1 lines, particularly the NF90-8 cells, also show prolonged EGFR phosphorylation subsequent to EGF treatment, as determined by immunoblotting for phosphorylation of Tyr1068. Phosphorylation of the EGFR on Tyr1068 results in recruitment of the Grb2 adaptor protein and activation of the Ras-MEK-ERK pathway.31
To begin investigation of whether loss of neurofibromin in the ST88-14 and NF90-8 lines correlates with an increased sensitivity of the EGFR and the ERK pathway to EGF stimulation, we compared the ability of decreasing concentrations of EGF to stimulate EGFR and ERK activation in these NF1 lines versus the non-NF1 STS-26T line. Cells from each MPNST line were treated with 1 pg/ml-10 ng/ml EGF for 10 min. We detected phosphorylation of the EGFR on Tyr1068 at concentrations as low as 1 ng/ml in the NF1 lines, whereas Tyr1068 phosphorylation was detected in the non-NF1 STS-26T line only after treatment with 10 ng/ml EGF (Fig. 1D). In addition, both of the NF1 MPNST lines show ERK pathway activation in response to much lower concentrations of EGF than the STS-26T line (Fig. 1D).
MPNST cells show little turnover of the EGFR in the absence of exogenous EGF
Previous studies have identified the EGFR as partially driving the proliferation of NF1 MPNST cells.16 In addition, phosphorylation of the EGFR has been observed in histological sections of NF1 MPNSTs.32 However, we were not able to detect basal phosphorylation of the EGFR on Tyr1068 in non-stimulated MPNST cultures (Figs. 1B and 1D). As an alternative method to detect endogenous EGFR activity, we examined whether the EGFR showed detectable levels of receptor turnover. We treated these MPNST lines with either 10 ng/ml EGF, 50 μg/ml of the protein synthesis inhibitor cycloheximide, or both. As shown in Figs. 2A and 2B, treatment of cultures with EGF alone, without pretreatment with cycloheximide, resulted in negligible time-dependent decreases of EGFR levels over time. A much more pronounced EGF-induced decrease in total EGFR was detected in cycloheximide-pretreated cultures. These results are consistent with reports that EGF treatment triggers transport of the EGFR to lysosomes and subsequent receptor degradation.33 In addition, these data indicate that each of the MPNST lines was synthesizing EGFR protein to replace receptor lost due to EGF-induced receptor internalization and degradation. There was no detectable change in EGFR levels in cells treated with cycloheximide alone, indicating that protein synthesis was not required to maintain basal EGFR levels in the MPNST lines over the 3-h time frame of the experiment.
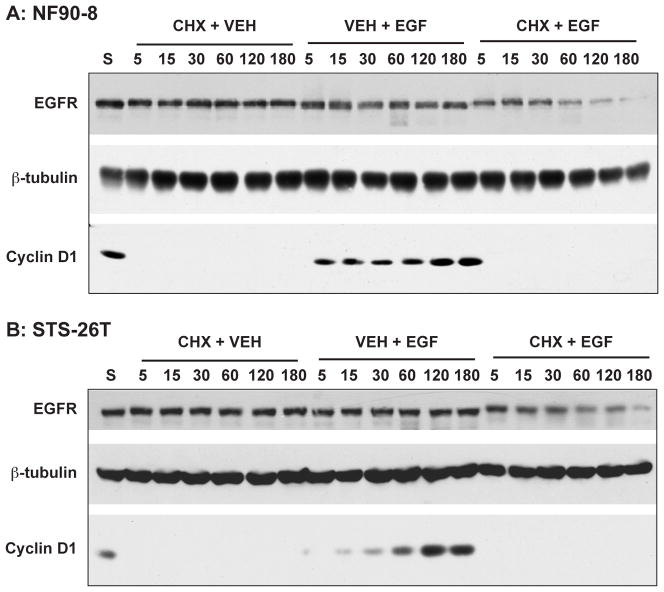
Figure 2.
Assessment of EGFR turnover in the MPNST cell lines. NF90-8 NF1 MPNST cells (A) and the non-NF1 MPNST line STS-26T (B) were pretreated with 50 μg/ml cycloheximide (or vehicle) for 2 h. Cells were then treated with 10 ng/ml EGF (or vehicle) for the indicated times. Lanes are indicated: “S”, untreated control; “CHX + VEH”, 2 h cycloheximide treatment followed by treatment with vehicle over the indicated time-course; “VEH + EGF”, pretreatment with ddH20 vehicle followed by treatment with 10 ng/ml EGF over the indicated time-course; “CHX + EGF”, 2 h treatment followed by treatment with 10 ng/ml EGF over the indicated time-course. Total EGFR protein levels were compared using immunoblotting. Cyclin-D1 is included to verify inhibition of EGF-stimulated protein synthesis by cycloheximide. β-tubulin is included as a protein loading control. These data are representative of at least three experiments per cell line.
EGF treatment has previously been shown to result in cyclin-D1 induction via Ras and ERK MAP kinase pathway activation.34 To confirm inhibition of protein synthesis by cycloheximide, we immunoblotted our samples for cyclin-D1. While cyclin D1 was detected in untreated cells, a 4-h pretreatment with cycloheximide resulted in a complete loss of immunoreactivity (Fig. 2, lanes “S” versus “CHX + VEH”). In addition, cycloheximide treatment prevented EGF-stimulated induction of cyclin D1 (Fig. 2, lanes “CHX + EGF”). The ST88-14 cell line showed similar results to the other two lines tested (data not shown). These results indicate that the concentration and duration of cycloheximide treatment were sufficient to inhibit protein synthesis in these MPNST cell lines.
Gefitinib suppresses EGF-mediated EGFR and ERK activation in MPNSTs
Gefitinib (Iressa or ZD1893) is an EGFR-selective inhibitor that competes for the ATP-binding site within the EGFR and inhibits ligand-induced tyrosine phosphorylation and receptor activation.19 Gefitinib has previously been shown to inhibit the EGFR-mediated proliferation of the A431 cell line at nanomolar concentrations.35 We thus hypothesized that gefitinib would inhibit EGFR-mediated proliferation of these cell lines. We first determined the concentrations of gefitinib required to inhibit EGF-mediated phosphorylation of the EGFR and the subsequent phosphorylation of ERK. We pretreated the three MPNST cell lines with increasing concentrations of gefitinib for 2 h and then treated them with 10 ng/ml of EGF for 10 min. We found that 1 μM of gefitinib was sufficient to inhibit phosphorylation of the EGFR at Tyr1068, as well as EGF-induced activation of ERK, in each of these MPNST cell lines (Figure 3A). Gefitinib was also able to inhibit EGF-stimulated proliferation of MPNST cells grown in very low (0.1%) serum (supplementary data).
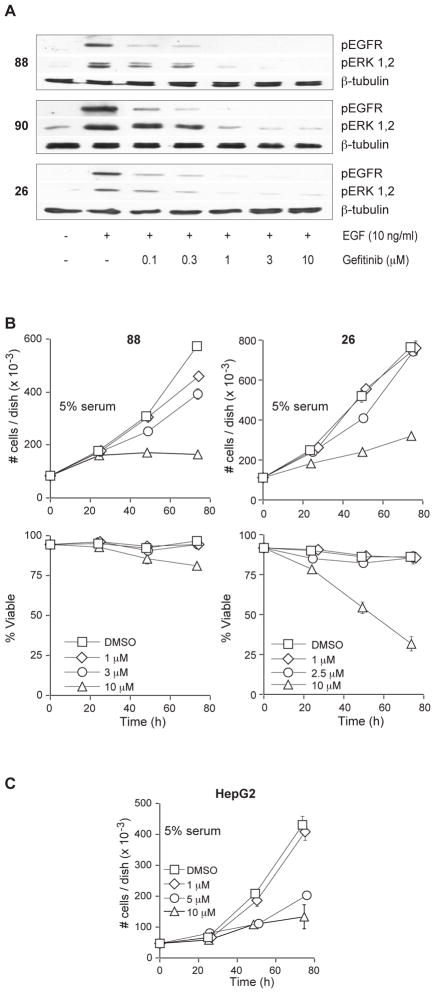
Figure 3.
Gefitinib inhibits EGF-stimulated activation of the EGFR-ERK pathway but does not inhibit MPNST proliferation. (A) Serum-starved cultures were treated with the indicated concentration of gefitinib (or vehicle) for 1 h, and subsequently treated with 10 ng/ml EGF for 10 min. EGFR and ERK activation were compared by immunoblotting with phospho-specific antibodies. β-tubulin was used as a total protein loading control. (B) Gefitinib does not inhibit the proliferation of MPNST lines at EGFR-selective concentrations. On day 0, plates were treated with either vehicle (DMSO) or the indicated concentration of gefitinib. Plates were harvested and cells counted at the indicated time-points and, the ability to exclude Trypan Blue was used to assess viability. These data show the effects of gefitinib on both total cell number (top panels) and percent viability (lower panels). Data represent the mean ± S.D. of three independent cultures for each line. (C) High concentrations of gefitinib have EGFR-independent effects. HepG2 cells were treated with either DMSO vehicle or the indicated concentration of gefitinib. Cells were harvested at the indicated time-points and counted using the Trypan Blue exclusion assay. Data represent the mean ± S.D. of three independent cultures.
Gefitinib does not significantly inhibit proliferation of MPNST cell lines in 5% serum at EGFR-selective concentrations
We next tested the ability of gefitinib to reduce the proliferation of cells grown in 5% serum. Neurofibromas and MPNSTs are highly vascular tumors and are likely to be exposed to numerous cytokines and growth factors.36, 37 In addition, a number of growth factors besides EGFR ligands are believed to drive MPNST proliferation.38, 39 Therefore, we believe that testing the ability of gefitinib to inhibit the proliferation of these MPNST lines in the presence of serum more accurately modeled the environment these tumor cells would encounter in vivo. We cultured the ST88-14 NF1 line and the STS-26T non-NF1 line in 5% serum supplemented with 1–10 μM of gefitinib and counted the number of cells. Gefitinib had an IC50 between 3 and 10 μM in the NF1 ST88-14 line, and did not effectively suppress proliferation of the non-NF1 STS-26T line at concentrations below 10 μM (Fig. 3B). Cell proliferation decreased significantly at 10 μM of gefitinib in both lines, and this was accompanied by a significant decrease of viable cells in the STS-26T MPNST line. The NF90-8 NF1 MPNST line responded similarly to gefitinib, with no inhibition of proliferation seen until treatment with 10 μM gefitinib (data not shown).
Because 1 μM of gefitinib is sufficient to inhibit EGFR-dependent proliferation in other cancer cell lines,40 and concentrations higher than 1 μM have been shown to have EGFR-independent effects,41 we hypothesized that the effects of high concentrations of gefitinib observed in our studies were due to anti-proliferative effects independent of this drug’s action at the EGFR. To test this hypothesis, we assessed whether gefitinib influences the proliferation of cells that do not rely on the EGFR for proliferation. We used two human cancer cell lines: the human hepatocellular carcinoma line HepG2, which expresses very low levels of EGFR,42 and a doxorubicin-resistant SKNSH neuroblastoma line, which does not express detectable levels of EGFR.43 Concentrations of 5 and 10 μM gefitinib significantly reduced HepG2 proliferation (Fig. 3C). In addition, 10 μM gefitinib also inhibited proliferation of the SKNSH line by approximately 25% (data not shown). These data support the conclusion that high concentrations of gefitinib result in anti-proliferative effects that are not EGFR-dependent. Thus, our data indicate that the EGFR does not drive proliferation of NF1 MPNST cells in vitro in the absence of exogenous EGF.
NF1 MPNST lines show a strong transactivation of ErbB2 in response to activation of EGFR by EGF
Differential expression of the various ErbB receptors has been demonstrated in both MPNST cell lines and primary tumor specimens.17 We have determined that the ST88-14 and NF90-8 NF1 lines expressed significantly higher levels of the EGFR and ErbB2 receptors than the non-NF1 STS-26T MPNST line (Fig. 1A). EGFR:ErbB2 heterodimers are significantly more active than EGFR homodimers24 and could further amplify EGFR-mediated NF1 tumor pathologies. To determine whether ErbB2 was involved in EGFR-mediated signaling, we treated the MPNST lines with 10 ng/ml EGF for 10 min. Addition of EGF resulted in a pronounced phosphorylation of ErbB2 at the Tyr1221/1222 autophosphorylation sites in the NF1 MPNST lines (Fig. 4A). Phosphorylation of these residues couples ErbB2 activation with the Ras-ERK pathway and Schwann cell mitotic activity.24, 44 Basal and EGF-induced Tyr1221/1222 phosphorylation were detected with both a rabbit monoclonal and polyclonal antibody against these phosphorylation sites of ErbB2. The rabbit monoclonal antibody does not immunoreact with several other phosphorylated receptor tyrosine kinases, including the EGFR and ErbB4. In addition, the phospho-ErbB2 immunoreactive band overlaid with ErbB2 when blots were reprobed with a mouse monoclonal antibody detecting total ErbB2 protein (Fig. 4B). We are therefore confident that this band represents basal and EGF-stimulated phosphorylation of ErbB2 on Tyr1221/1222.
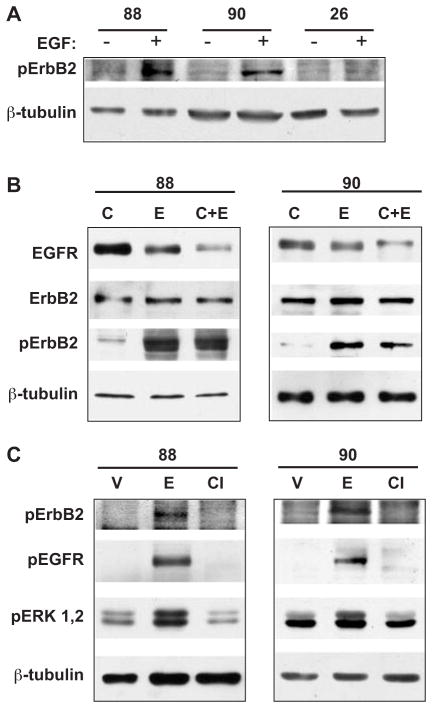
Figure 4.
Coupling of EGFR and ErbB2 in NF1 MPNST cell lines. (A) EGF induces phosphorylation of ErbB2 in NF1 MPNST cell lines. Serum-starved cultures of ST88-14 (“88”); NF90-8 (“90”); and STS-26T (“26”) cells were treated with vehicle or 10 ng/ml EGF for 10 min. Phosphorylation of Tyr1221/1222 of ErbB2 was assessed using a phospho-specific antibody raised against these sites (indicated as “pErbB2”). These data are representative of four independent experiments. (B) ErbB2 protein levels and activation remain elevated following EGF stimulation. ST88-14 (left panel) and NF90-8 (right panel) cells were pretreated with cycloheximide or vehicle for 2 h and subsequently treated with EGF or vehicle for 3 h. Lanes are indicated with “C”, cycloheximide treated only; “E”, EGF treated only; “C + E”, treated first with cycloheximide and then with EGF. (C) CI-1033 inhibits EGF-mediated phosphorylation of EGFR and ErbB2 in NF1 MPNSTs. ST88-14 (left panel) and NF90-8 (right panel) cells were treated with vehicle or 1 μM of CI-1033 for 1 h and subsequently treated with 10 ng/ml EGF for 10 min. Lanes are “V”, vehicle treated only; “E”, treated with EGF alone; “CI”, treated first with CI-1033 for 1 h and then with EGF. Results are representative of three separate experiments in both lines. In all panels, β-tubulin is included as a protein loading control.
The studies presented in Fig. 2 indicated that the MPNST lines utilize protein synthesis to maintain EGFR levels after EGF stimulation. Previous studies have determined that EGFR/ErbB2 heterodimers are resistant to ligand-induced endocytosis as well as ubiquitination of the EGFR through c-cbl activation.24 To determine whether ErbB2 was downregulated similarly to the EGFR after EGF stimulation, we examined ErbB2 phosphorylation status and expression levels in the NF1 lines following a 3-h treatment with 50 μg/ml cycloheximide, 10 ng/ml EGF, or both. We found that ErbB2 expression levels did not decline in response to treatment with EGF alone, or in combination with cycloheximide in either the ST88-14 (Fig. 4A) or NF90-8 (Fig. 4B) cells. In addition, EGF-induced phosphorylation of Tyr1221/1222 of ErbB2 was detected 3 h after addition of 10 ng/ml of EGF to the media (Fig. 4B). In contrast, both EGFR phosphorylation and total protein levels were decreased significantly at this time (Figs. 4B, and 2A and 2B, respectively). These results indicate that activation of the EGFR is associated with prolonged transactivation of ErbB2, and that EGFR downregulation does not correlate with downregulation of ErbB2 in the NF1 MPNST cell lines.
CI-1033 inhibits phosphorylation of Tyr1221/1222 of ErbB2 and Tyr1068 of EGFR
Our data indicated that NF1 MPNST lines express higher levels of both the EGFR and ErbB2 versus a non-NF1 line, and that prolonged transactivation of ErbB2 occurs after activation of the EGFR with exogenous EGF. CI-1033 is an irreversible inhibitor of all ErbB family member kinase activities.45 Treatment of both NF1 MPNST cell lines with 1 μM CI-1033 effectively suppressed EGF-induced phosphorylation of EGFR at Tyr1068, and ErbB2 at Tyr1221/1222 (Fig. 4C). In addition, CI-1033 also inhibited the EGF-stimulated activation of ERK in these lines (Fig. 4C).
CI-1033 inhibits the proliferation of NF1 but not the non-NF1 MPNST line in 5% serum
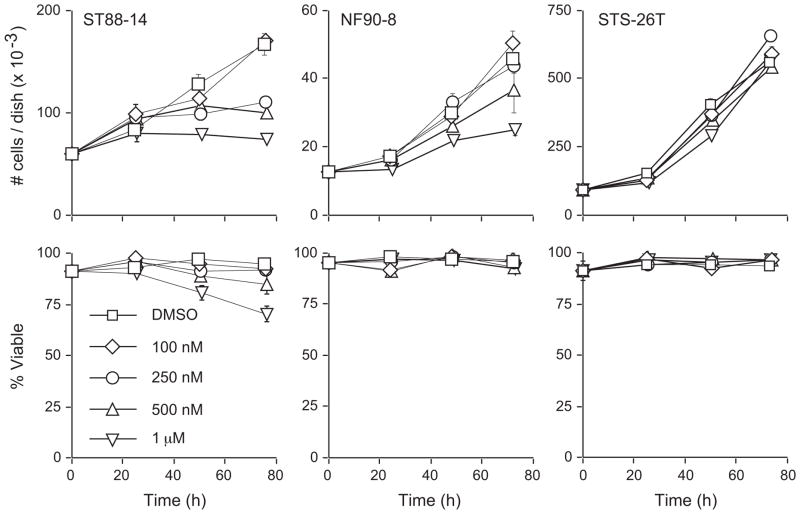
The in vivo proliferation of MPNSTs can be driven through ErbB-mediated neuregulin signaling as well as EGFR-mediated signaling.16, 17 We hypothesized that inhibition of ErbB signaling through a selective pan-ErbB inhibitor would be effective at suppressing multiple ErbB-mediated proliferation pathways in MPNST cells. Concentrations of CI-1033 as low as 250 nM and 500 nM suppressed the proliferation of ST88-14 and NF90-8 cultures, respectively (Fig. 5). In contrast, concentrations of CI-1033 up to 1 μM did not markedly affect the proliferation of the non-NF1 STS-26T line (Fig. 5). At the concentrations examined, CI-1033 had no effect on NF90-8 or STS-26T viability. A modest loss of viability in the ST88-14 cell line was only seen at the highest concentration tested (1 μM) (Fig. 5).
Figure 5.
CI-1033 selectively inhibits the proliferation of NF1 MPNST cell lines. On day 0, plates were treated with either vehicle or the indicated concentration of CI-1033. Cells were harvested and counted using the Trypan Blue exclusion assay. These data show the effects of CI-1033 on both total cell number (top panel) and percent viability (lower panel). Data are mean ± S.D. and are representative of three separate experiments for each line.
We next determined the effects of CI-1033 on MPNST cell cycle distribution (Table 1). Concentrations of CI-1033 having graduated effects on NF1 ST88-14 proliferation caused parallel gains in the percentage of cells in G1, coupled with parallel losses of S-phase cells. In contrast, but in agreement with proliferation analyses, CI-1033 treatment did not alter cell cycle phase distributions in STS-26T cells (Table 1).
Table 1. CI-1033 selectively decreases the proportion of ST88-14 NF1 MPNST cells in the S-phase of the cell cycle.
ST88-14 and STS-26T MPNST cells were treated as indicated with a range of CI-1033 concentrations that dose-dependently inhibit proliferation of NF1 MPNST cells (Fig. 5). The cells were processed for analysis of cell cycle distribution by flow cytometry.
| Treatment: [CI-1033] for 48 h | ST88-14 | STS-26T | ||||
|---|---|---|---|---|---|---|
| % of cells in: | % of cells in: | |||||
| G1 | S | G2/M | G1 | S | G2/M | |
| 0 | 63 | 28 | 9 | 70 | 21 | 9 |
| 100 nM | 66 | 25 | 9 | 70 | 21 | 9 |
| 250 nM | 70 | 22 | 8 | 70 | 21 | 9 |
| 1000 nM | 80 | 16 | 4 | 66 | 24 | 10 |
Discussion
Targeted expression of human EGFR in Schwann cells of newborn mice results in characteristics of the initial stages of NF1 tumor formation, including mast cell accumulation, collagen deposition, and disruption of axon-glial interactions.32 These EGFR-dependent developmental pathologies are inhibited by cetuximab administration, but only if administered for 2 weeks beginning immediately after birth. Treatments started later during development are much less effective, even if administered over a greater period of time.32 These results suggest that there may be a critical period during which aberrantly expressed EGFR mediates remodeling of the normal Schwann cell environment into a tumor-promoting environment. In the present study, we examined the effectiveness of EGFR inhibition in suppressing the proliferation of MPNST cell lines. While our data suggest that inhibition of the EGFR with gefitinib does not effectively suppress proliferation of MPNSTs in vitro, inhibition of aberrantly expressed EGFR may suppress development of the tumor-supporting microenvironment in vivo.
In addition to evaluating the EGFR as an inhibitor of MPNST proliferation, we examined the ErbB2 receptor as a potential pharmacological target. While there is no known ligand for ErbB2, this protein heterodimerizes with other members of the ErbB family and potentiates their ligand-mediated signaling.24 Overexpressed ErbB2 can undergo ligand-independent activation and contribute to tumorigenesis.25, 26 In normal Schwann cells, the only available heterodimerization partner for ErbB2 is thought to be ErbB3,46 and inhibition of ErbB3/ErbB2 signaling by pan-ErbB inhibitors may be significant to the effectiveness of this treatment approach in MPNSTs.17 Aberrant expression of the EGFR in NF1 MPNSTs introduces an additional dimerization partner for ErbB2.47 These EGFR:ErbB2 heterodimers are significantly more active than EGFR homodimers.24
The two NF1 MPNST lines used in our studies express greater levels of ErbB2 than the non-NF1 MPNST line (Figure 1A). Others have demonstrated that the NF1 MPNST cell lines used here do not express detectable ErbB3 and ErbB4 receptors.16 Thus, these lines are an excellent model to test the relationship between the EGFR and ErbB2 receptors in the absence of the other ErbB family members. Activation of EGFR with exogenous EGF resulted in phosphorylation of the Tyr1221/1222 phosphorylation sites and transactivation of ErbB2 in the NF1 MPNST lines (Figure 4A). A previous study did not find heterodimerization between EGFR and ErbB2 in normal Schwann cells.21 In that case, however, the result could have been affected by the system in which human EGFR was transgenically expressed and tested for interaction with endogenous murine ErbB2 in mouse Schwann cells.21 EGFR:ErbB2 heterodimers have previously been shown to avoid degradation through ubiquitination, as well as endocytosis-mediated transport to the lysosome, two mechanisms that efficiently downregulate EGFR homodimer activity.33 Our results indicate that ErbB2 protein levels remain relatively unchanged and that ErbB2 remains phosphorylated on Tyr1221/1222 despite EGFR deactivation and downregulation at the same time-points. Thus, in addition to prolonged EGFR-Ras mediated signaling due to loss of functional neurofibromin, our results suggest that coupling between EGFR and ErbB2 may result in further amplification of aberrant in vivo EGFR signaling in MPNSTs. We demonstrate here that the pan-ErbB kinase inhibitor CI-1033 was effective at inhibiting EGF-mediated phosphorylation of Tyr1221 of ErbB2, Tyr1068 of EGFR, and EGF-mediated activation of ERK. While efforts to selectively inhibit EGFR-mediated NF1 pathologies have met with mixed success,16, 17, 48, 49 our results suggest that inhibition of both EGFR and ErbB2 may provide an effective alternative.
The potency of CI-1033 correlates with suppression of ErbB2 transactivation in the NF1 MPNST cell lines. In agreement with a previous report that showed that micromolar levels of CI-1033 (PD158780) were required to block DNA synthesis in a chemically-induced MPNST cell line,50 we found that the non-NF1 STS-26T MPNST cell line was significantly less sensitive to inhibition of proliferation by CI-1033. Notably, CI-1033 effectively suppressed the proliferation of NF1 MPNST lines in the presence of 5% serum. Thus, we predict that ErbB inhibition may be effective in vivo, despite the presence of growth factors and cytokines that promote NF1 MPNST proliferation. This idea is supported by reports in which inhibition of ErbB2 in benign vestibular schwannomas significantly suppressed their proliferation.51, 52 Fortunately, inhibition of ErbB-mediated neuregulin signaling does not reduce the survival of mature Schwann cells in culture.53 Hence, ErbB inhibition may have the advantage of inhibiting NF1 tumor-promoting ErbB signaling without affecting normal Schwann cell survival.
In summary, we show that two independent NF1 MPNST cell lines demonstrate enhanced sensitivity and prolonged ERK activation in response to EGF stimulation. While EGFR expression has been shown to correlate with NF1 tumor pathologies, the EGFR per se does not appear to play a primary role in the proliferation of MPNST cells in culture. We show that EGFR activation by exogenously added EGF results in prolonged transactivation of ErbB2 in these NF1 MPNST lines, and that ErbB2 transactivation provides an additional potential mechanism for prolonged EGFR-Ras-ERK signaling in MPNSTs in vivo. Further, inhibition of both EGFR and ErbB2 with the inhibitor CI-1033 produces a profound and selective inhibition of NF1 MPNST cell proliferation. CI-1033 has reached Phase II clinical trials and so its ultimate clinical status is not yet clear.54 Our results suggest that targeted inhibition of the EGFR and ErbB2 may be an effective approach for inhibiting ErbB-mediated NF1 tumor pathologies and MPNST growth. The recent success of another combined EGFR/ErbB2 inhibitor, lapatinib, in advanced breast cancer demonstrates that such an approach can be effective in the treatment of human cancer.55
Supplementary Material
Acknowledgments
We thank AstraZeneca for providing us with gefitinib, and Pfizer for providing us with CI-1033.
This study was supported by grants DAMD17-03-1-0182 and W81XWH-05-1-0193 from the Department of the Army and a Strategic Research Initiative award from the Karmanos Cancer Institute. J.W.W. was supported by the National Institutes of Health grant T32 ES012163. This project was aided by Imaging and Cytometry Core facilities that were supported by National Institutes of Health grants P30 ES06639 and P30 CA22453.
Abbreviations
- NF1
Neurofibromatosis Type 1
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- MAP
mitogen-activated protein
- ERK
extracellular-signal regulated protein kinase
- MPNST
malignant peripheral nerve sheath tumor
- CI-1033
N-[4-(3-chloro-4-fluoro-phenylamino)-7-(3-morpholin4-yl-propoxy)-quinazol-in-6-yl]-acrylamide
Footnotes
The content of the information does not necessarily reflect the position or policy of the U.S. government, and no official endorsements should be inferred
References
- 1.Arun D, Gutmann DH. Recent advances in neurofibromatosis type 1. Curr Opin Neurol. 2004;17:101–5. doi: 10.1097/00019052-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Lynch TM, Gutmann DH. Neurofibromatosis 1. Neurol Clin. 2002;20:841–65. doi: 10.1016/s0733-8619(01)00019-6. [DOI] [PubMed] [Google Scholar]
- 3.Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet. 1999;89:1–6. [PubMed] [Google Scholar]
- 4.Friedrich RE, Kluwe L, Funsterer C, Mautner VF. Malignant peripheral nerve sheath tumors (MPNST) in neurofibromatosis type 1 (NF1): diagnostic findings on magnetic resonance images and mutation analysis of the NF1 gene. Anticancer Res. 2005;25:1699–702. [PubMed] [Google Scholar]
- 5.Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–4. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang FC, Chen S, Clegg T, Li X, Morgan T, Estwick SA, et al. Nf1+/− mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Hum Mol Genet. 2006;15:2421–37. doi: 10.1093/hmg/ddl165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang FC, Ingram DA, Chen S, Hingtgen CM, Ratner N, Monk KR, et al. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for Nf1+/− mast cells. J Clin Invest. 2003;112:1851–61. doi: 10.1172/JCI19195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HA, Ling B, Ratner N. Nf1-deficient mouse Schwann cells are angiogenic and invasive and can be induced to hyperproliferate: reversion of some phenotypes by an inhibitor of farnesyl protein transferase. Mol Cell Biol. 1997;17:862–72. doi: 10.1128/mcb.17.2.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Raedt T, Maertens O, Chmara M, Brems H, Heyns I, Sciot R, et al. Somatic loss of wild type NF1 allele in neurofibromas: Comparison of NF1 microdeletion and non-microdeletion patients. Genes Chromosomes Cancer. 2006;45:893–904. doi: 10.1002/gcc.20353. [DOI] [PubMed] [Google Scholar]
- 10.Feldkamp MM, Angelov L, Guha A. Neurofibromatosis type 1 peripheral nerve tumors: aberrant activation of the Ras pathway. Surg Neurol. 1999;51:211–8. doi: 10.1016/s0090-3019(97)00356-x. [DOI] [PubMed] [Google Scholar]
- 11.Parada LF. Neurofibromatosis type 1. Biochim Biophys Acta. 2000;1471:M13–9. doi: 10.1016/s0304-419x(00)00014-7. [DOI] [PubMed] [Google Scholar]
- 12.Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356:713–5. doi: 10.1038/356713a0. [DOI] [PubMed] [Google Scholar]
- 13.Mattingly RR, Kraniak JM, Dilworth JT, Mathieu P, Bealmear B, Nowak JE, et al. The mitogen-activated protein kinase/extracellular signal-regulated kinase kinase inhibitor PD184352 (CI-1040) selectively induces apoptosis in malignant schwannoma cell lines. J Pharmacol Exp Ther. 2006;316:456–65. doi: 10.1124/jpet.105.091454. [DOI] [PubMed] [Google Scholar]
- 14.Dilworth JT, Kraniak JM, Wojtkowiak JW, Gibbs RA, Borch RF, Tainsky MA, et al. Molecular targets for emerging anti-tumor therapies for neurofibromatosis type 1. Biochem Pharmacol. 2006;72:1485–92. doi: 10.1016/j.bcp.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Wojtkowiak JW, Fouad F, LaLonde DT, Kleinman MD, Gibbs RA, Reiners JJ, Jr, et al. Induction of apoptosis in neurofibromatosis type 1 malignant peripheral nerve sheath tumor cell lines by a combination of novel farnesyl transferase inhibitors and lovastatin. J Pharmacol Exp Ther. 2008;326:1–11. doi: 10.1124/jpet.107.135830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeClue JE, Heffelfinger S, Benvenuto G, Ling B, Li S, Rui W, et al. Epidermal growth factor receptor expression in neurofibromatosis type 1-related tumors and NF1 animal models. J Clin Invest. 2000;105:1233–41. doi: 10.1172/JCI7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stonecypher MS, Byer SJ, Grizzle WE, Carroll SL. Activation of the neuregulin-1/ErbB signaling pathway promotes the proliferation of neoplastic Schwann cells in human malignant peripheral nerve sheath tumors. Oncogene. 2005;24:5589–605. doi: 10.1038/sj.onc.1208730. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Velasco-Miguel S, Vass WC, Parada LF, DeClue JE. Epidermal growth factor receptor signaling pathways are associated with tumorigenesis in the Nf1:p53 mouse tumor model. Cancer Res. 2002;62:4507–13. [PubMed] [Google Scholar]
- 19.Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol. 2002;20:1S–13S. [PubMed] [Google Scholar]
- 20.Penne K, Bohlin C, Schneider S, Allen D. Gefitinib (Iressa, ZD1839) and tyrosine kinase inhibitors: the wave of the future in cancer therapy. Cancer Nurs. 2005;28:481–6. doi: 10.1097/00002820-200511000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Ling BC, Wu J, Miller SJ, Monk KR, Shamekh R, Rizvi TA, et al. Role for the epidermal growth factor receptor in neurofibromatosis-related peripheral nerve tumorigenesis. Cancer Cell. 2005;7:65–75. doi: 10.1016/j.ccr.2004.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cichowski K, Santiago S, Jardim M, Johnson BW, Jacks T. Dynamic regulation of the Ras pathway via proteolysis of the NF1 tumor suppressor. Genes Dev. 2003;17:449–54. doi: 10.1101/gad.1054703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garratt AN, Britsch S, Birchmeier C. Neuregulin, a factor with many functions in the life of a schwann cell. Bioessays. 2000;22:987–96. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Muthuswamy SK, Gilman M, Brugge JS. Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol Cell Biol. 1999;19:6845–57. doi: 10.1128/mcb.19.10.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burke CL, Stern DF. Activation of Neu (ErbB-2) mediated by disulfide bond-induced dimerization reveals a receptor tyrosine kinase dimer interface. Mol Cell Biol. 1998;18:5371–9. doi: 10.1128/mcb.18.9.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Fiore PP, Pierce JH, Kraus MH, Segatto O, King CR, Aaronson SA. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987;237:178–82. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- 27.Schlegel J, Muenkel K, Trenkle T, Fauser G, Ruschoff J. Expression of the ERBB2/neu and neurofibromatosis type 1 gene products in reactive and neoplastic schwann cell proliferation. Int J Oncol. 1998;13:1281–4. doi: 10.3892/ijo.13.6.1281. [DOI] [PubMed] [Google Scholar]
- 28.Huijbregts RP, Roth KA, Schmidt RE, Carroll SL. Hypertrophic neuropathies and malignant peripheral nerve sheath tumors in transgenic mice overexpressing glial growth factor beta3 in myelinating Schwann cells. J Neurosci. 2003;23:7269–80. doi: 10.1523/JNEUROSCI.23-19-07269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattingly RR, Felczak A, Chen CC, McCabe MJ, Rosenspire AJ. Low concentrations of inorganic mercury inhibit Ras activation during T cell receptor-mediated signal transduction. Toxicol Appl Pharm. 2001;176:162–8. doi: 10.1006/taap.2001.9272. [DOI] [PubMed] [Google Scholar]
- 30.Mattingly RR, Gibbs RA, Menard RE, Reiners JJ., Jr Potent suppression of proliferation of a10 vascular smooth muscle cells by combined treatment with lovastatin and 3-allylfarnesol, an inhibitor of protein farnesyltransferase. J Pharmacol Exp Ther. 2002;303:74–81. doi: 10.1124/jpet.102.036061. [DOI] [PubMed] [Google Scholar]
- 31.Rojas M, Yao S, Lin YZ. Controlling epidermal growth factor (EGF)-stimulated Ras activation in intact cells by a cell-permeable peptide mimicking phosphorylated EGF receptor. J Biol Chem. 1996;271:27456–61. doi: 10.1074/jbc.271.44.27456. [DOI] [PubMed] [Google Scholar]
- 32.Wu J, Crimmins JT, Monk KR, Williams JP, Fitzgerald ME, Tedesco S, et al. Perinatal epidermal growth factor receptor blockade prevents peripheral nerve disruption in a mouse model reminiscent of benign world health organization grade I neurofibroma. Am J Pathol. 2006;168:1686–96. doi: 10.2353/ajpath.2006.050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiley HS, Burke PM. Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic. 2001;2:12–8. doi: 10.1034/j.1600-0854.2001.020103.x. [DOI] [PubMed] [Google Scholar]
- 34.Lavoie JN, L’Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–16. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 35.Anderson NG, Ahmad T, Chan K, Dobson R, Bundred NJ. ZD1839 (Iressa), a novel epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, potently inhibits the growth of EGFR-positive cancer cell lines with or without erbB2 overexpression. Int J Cancer. 2001;94:774–82. doi: 10.1002/ijc.1557. [DOI] [PubMed] [Google Scholar]
- 36.Arbiser JL, Flynn E, Barnhill RL. Analysis of vascularity of human neurofibromas. J Am Acad Dermatol. 1998;38:950–4. doi: 10.1016/s0190-9622(98)70158-6. [DOI] [PubMed] [Google Scholar]
- 37.Wu M, Wallace MR, Muir D. Nf1 haploinsufficiency augments angiogenesis. Oncogene. 2006;25:2297–303. doi: 10.1038/sj.onc.1209264. [DOI] [PubMed] [Google Scholar]
- 38.Thomas SL, De Vries GH. Angiogenic expression profile of normal and neurofibromin-deficient human Schwann cells. Neurochem Res. 2007;32:1129–41. doi: 10.1007/s11064-007-9279-z. [DOI] [PubMed] [Google Scholar]
- 39.Mashour GA, Ratner N, Khan GA, Wang HL, Martuza RL, Kurtz A. The angiogenic factor midkine is aberrantly expressed in NF1-deficient Schwann cells and is a mitogen for neurofibroma-derived cells. Oncogene. 2001;20:97–105. doi: 10.1038/sj.onc.1204026. [DOI] [PubMed] [Google Scholar]
- 40.Weakling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–54. [PubMed] [Google Scholar]
- 41.Voigt W, Pickan V, Pfeiffer C, Mueller T, Simon H, Arnold D. Preclinical evaluation of ZD1839 alone or in combination with oxaliplatin in a panel of human tumor cell lines -- implications for clinical use. Onkologie. 2005;28:482–8. doi: 10.1159/000087344. [DOI] [PubMed] [Google Scholar]
- 42.Dalton SR, Jirtle RL, Meyer SA. EGF receptors of hepatocytes from rats treated with phenobarbital are sensitized to down-regulation by phenobarbital in culture. Toxicol Appl Pharmacol. 2000;165:115–26. doi: 10.1006/taap.2000.8935. [DOI] [PubMed] [Google Scholar]
- 43.Mattingly RR, Milstein ML, Mirkin BL. Down-regulation of growth factor-stimulated MAP kinase signaling in cytotoxic drug-resistant human neuroblastoma cells. Cell Signal. 2001;13:499–505. doi: 10.1016/s0898-6568(01)00173-5. [DOI] [PubMed] [Google Scholar]
- 44.Kwon YK, Bhattacharyya A, Alberta JA, Giannobile WV, Cheon K, Stiles CD, et al. Activation of ErbB2 during wallerian degeneration of sciatic nerve. J Neurosci. 1997;17:8293–9. doi: 10.1523/JNEUROSCI.17-21-08293.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen LF, Lenehan PF, Eiseman IA, Elliott WL, Fry DW. Potential benefits of the irreversible pan-erbB inhibitor, CI-1033, in the treatment of breast cancer. Semin Oncol. 2002;29:11–21. doi: 10.1053/sonc.2002.34049. [DOI] [PubMed] [Google Scholar]
- 46.Burden S, Yarden Y. Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron. 1997;18:847–55. doi: 10.1016/s0896-6273(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 47.Pomerantz RG, Grandis JR. The epidermal growth factor receptor signaling network in head and neck carcinogenesis and implications for targeted therapy. Semin Oncol. 2004;31:734–43. doi: 10.1053/j.seminoncol.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Mahller YY, Vaikunth SS, Currier MA, Miller SJ, Ripberger MC, Hsu YH, et al. Oncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model. Mol Ther. 2007;15:279–86. doi: 10.1038/sj.mt.6300038. [DOI] [PubMed] [Google Scholar]
- 49.Johansson G, Mahller YY, Collins MH, Kim MO, Nobukuni T, Perentesis J, et al. Effective in vivo targeting of the mammalian target of rapamycin pathway in malignant peripheral nerve sheath tumors. Mol Cancer Ther. 2008;7:1237–45. doi: 10.1158/1535-7163.MCT-07-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frohnert PW, Stonecypher MS, Carroll SL. Constitutive activation of the neuregulin-1/ErbB receptor signaling pathway is essential for the proliferation of a neoplastic Schwann cell line. Glia. 2003;43:104–18. doi: 10.1002/glia.10232. [DOI] [PubMed] [Google Scholar]
- 51.Hansen MR, Linthicum FH., Jr Expression of neuregulin and activation of erbB receptors in vestibular schwannomas: possible autocrine loop stimulation. Otol Neurotol. 2004;25:155–9. doi: 10.1097/00129492-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Hansen MR, Roehm PC, Chatterjee P, Green SH. Constitutive neuregulin-1/ErbB signaling contributes to human vestibular schwannoma proliferation. Glia. 2006;53:593–600. doi: 10.1002/glia.20316. [DOI] [PubMed] [Google Scholar]
- 53.Meier C, Parmantier E, Brennan A, Mirsky R, Jessen KR. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J Neurosci. 1999;19:3847–59. doi: 10.1523/JNEUROSCI.19-10-03847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steeghs N, Nortier JW, Gelderblom H. Small molecule tyrosine kinase inhibitors in the treatment of solid tumors: an update of recent developments. Ann Surg Oncol. 2007;14:942–53. doi: 10.1245/s10434-006-9227-1. [DOI] [PubMed] [Google Scholar]
- 55.Cameron DA, Stein S. Drug Insight: intracellular inhibitors of HER2-clinical development of lapatinib in breast cancer. Nat Clin Pract Oncol. 2008 doi: 10.1038/ncponc1156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.