Abstract
1.1. Background
A large literature documents the detrimental effects of socioeconomic disparities on intelligence and neuropsychological development. Researchers typically measure environmental factors such as socioeconomic status (SES), using income, parent's occupation and education. However, SES is more complex, and this complexity may influence neuropsychological outcomes.
1.2. Methods
This studyused principal components analysis to reduce 14 SES and 28 family stress indicators into their core dimensions (e.g. community and educational capital, financial resources, marital conflict). Core dimensions were used in path analyses to examine their relationships with parent IQ and cerebral volume (white matter, grey matter and total brain volume), to predict child IQ in a sample of typically developing children.
1.3. Results
Parent IQ affected child IQ directly and indirectly through community and educational capital, demonstrating how environmental factors interact with familial factors in neuro-development. There were no intervening effects of cerebral white matter, grey matter, or total brain volume.
1.4. Conclusions
Findings may suggest that improving community resources can foster the intellectual development of children.
Keywords: Socioeconomic status (SES), IQ, Brain volume, Family stress
3. Introduction
Thirty years of research have established that family income and other measures of socioeconomic status (SES) are highly associated with cognitive, intellectual and achievement outcomes in childhood [1-6]. The correlational studies between SES and child outcomes in typically developing children suggest that higher SES is associated with higher IQ [7,8], higher vocabulary development [9], better school achievement [10,11], and a variety of domains indicated better child health and development than children of lower SES{[4].
The strong relationship between SES and measures of intellectual ability illustrate the relevance of SES to neuroscience. At birth, the brain is dependent upon experiences and environmental stimulation for healthy development [12,13]. In turn, there is now a large literature which documents this interdependency between familial factors (e.g. genes) and the environment [14-18]. Over the course of development, familial factors and the environment continue to influence and interact with each other.
It follows then, that unfavorable environmental experiences are harmful for both structural and functional brain development [16,19]. Based on decades of neuroscience research, it is clear that stress and environmental complexity are the two primary experiential influences on brain development [20,21]. Because SES is incontrovertibly correlated with differences in life stress and family resources, it is understandable that SES would influence experience-dependent patterns of neural activity and development [3,4,22-24]. Thus, the differences in families across the continuum of SES may create different experiences of stress and environmental complexity, potentially creating systematic changes to cognitive and brain development [19,20,25], and intellectual outcomes [17,26]. Lower language and executive function—the two main components of a two-factor IQ score, can be seen in lower SES children, as early as kindergarten [27].
In addition, there is an area of research in developmental psychology on the Family Stress model, designed to understand the processes by which SES and economic disadvantage affect children's well-being [3,4,28-31]. The thesis of the Family Stress model is that material hardship operates through parenting behavior, to affect children by weakening parents' mental health and marital relationship, creating systematic changes in the reciprocal dynamic between parent and child [3-5,28,29,32,33]. Understandably, families with low SES experience higher levels of family stress and material hardship, and are more likely to experience negative life events [6,32,34,35], have lower cognitive and intellectual function, and higher rates of mental disorders [36]. Hence, one cannot examine the effects of SES on child development, without considering the important role of family stress.
Research from several disciplines provides a motivation to examine how family stress covaries with different aspects of SES, to affect neuropsychological development and cognition in childhood. While prior research has examined the effects of SES on various child outcomes, few studies have incorporated complex measures of both SES and family stress, and no studies to date have incorporated both of these constructs with the study of brain volume. Because both family factors and environmental factors are key determinants of brain development and function, we examine both in the present study.
One inherent problem in researching the effects of SES is the operationalization and measurement of this diverse construct, encompassing anything from family structure to health status. Typically, researchers attempt to capture SES using income, parent's occupational status and years of education (i.e. Hollingshead Index). While empirical testing shows that these three components measure specific aspects of SES [37,38], the experience of material hardship or socioeconomic privilege is much more complex than these factors alone.
Many scholars have proposed utilizing a more comprehensive measure of poverty and SES to fully capture the experience of families. Because SES is a multi-dimensional construct, it is recommended that researchers use multiple components of SES in their operationalization [39]. Specifically, Blank proposed a construct consisting of six indicators: health status, health insurance access, food security, housing conditions, education and labor market access [40]. The present study used the Blank framework to operationalize SES by selecting items from participant surveys that best captured these six indicators. The SES variables used in the analyses were created using Principal Components Analysis—a mathematical technique that reduces an original set of variables into a smaller number of key, or ‘principal’, components [41].
Hackman et sl. [42] summarized the research across a number of disciplines to identify potential environmental mechanisms through which SES may affect cognitive development. They suggest that prenatal factors, parent-offspring interactions and cognitive stimulation partly underlie the effects of SES, corroborating the hypotheses of the Family Stress model, whereby economic disadvantage affects children's well-being through its effects on the parent [3,4,29,32,33,43-45]. This model nicely synthesizes how two mechanisms from different disciplinary perspectives—parent-offspring interactions and stress—can converge to shape cognitive and brain development, and provides a strong foundation for the interdisciplinary approach of the present study.
Brain volume has been shown to be positively correlated with IQ [46,47]. However, total brain volume does not mediate the relationship between parental education and IQ [46], indicating that SES variables may be contributory. Brain development during childhood and adolescence is characterized by progressive myelination of neural networks. Findings from cross-sectional studies suggest that cerebral white matter volume and the area of the corpus callosum, the main interhemispheric commissure, increase significantly from childhood through late adolescence [48]. Longitudinal MRI studies of healthy children and adolescents have confirmed these age-related linear increases in cerebral white matter and corpus callosum area [49,50]. These observations may reflect experience dependent in vivo evidence of age-related progressive events, such as axonal growth and myelination of neural networks. Thus these neurobiological events are considered strongly influenced by experience and SES, and both contribute to neuropsychological development.
In spite of the complimentary and interdisciplinary evidence, the neuropsychological and intellectual effects of SES differences are relatively unexplored. This is due in part to the complexity in measuring an SES construct, and because most neurological studies are conducted with individuals from restricted income ranges; primarily middle class [21]. The primary purpose of this investigation is to examine the relative contributions of both environmental factors—SES and family stress—and familial factors—biological parent's IQ—in affecting child IQ through their effects on structural brain development. We combine neuropsychological measurement with multiple item measures of social constructs, and a large, diverse sample to examine the neurological and cognitive effects of SES and family stress. We also modify and extend the Family Stress model by giving empirical bases to components of the constructs of family stress and SES.
2.1. Research questions
We explored three primary research hypothesizes: (1) Does brain volume mediate the effect of SES on child IQ? (2) Do any components of SES mediate the effect of a biological parent's IQ on child IQ? (3) Do any components of family stress mediate the effect of SES on child IQ? For all three questions, it is hypothesized that measures of cerebral volume (white, grey, and total brain volumes) will mediate the relationship between SES, family stress and children's intellectual ability.
3. Methods
3.1. Data collection
The study was conducted at the Duke Healthy Childhood Brain Development and Developmental Traumatology Research Program All participants were recruited from the surrounding communities via IRB approved advertisements. The clinical assessment portion included interviews of all subjects and their legal guardian, using the Kiddie Schedule for Affective Disorders and Schizophrenia for School Aged Children Present and Lifetime Version (KSADS-PL) [51]. This semi-structured interview was administered to 151 caregivers and youth. Interviewers were individually trained to obtain over 90% agreement for the presence of any lifetime major Axis I disorder, with a board certified child and adolescent psychiatrist and experienced child trauma interviewer. Discrepancies were resolved by reviewing archival information (e.g. school records, birth and pediatric medical records), or by re-interviewing the child or caregiver. If diagnostic disagreements were not resolved with this method, consensus diagnoses were reached between a child psychiatrist [M.D.D.B.] and child psychologist [S.R.H.]. Adolescents also received saliva and urine toxicology screens to confirm the absence of alcohol, tobacco or other drug use on the day of interview and neuropsychological data collection and MRI scan. Participants with an Axis I diagnosis, or a positive alcohol or drug toxicology screen, were excluded from this study.
The sample was invited for another research day to undergo an anatomical MRI brain scan. Of the total 151 children in the sample who were interviewed, 49 children did not have brain data due to poor resolution, movement artifact, equipment problems, or chose to not participate in this aspect of the study. Therefore, the outcome analyses only included children with brain imaging data (n=102). Tests for sample restriction bias indicated that this subsample was no different from the larger sample on age (p=.85), race (p=.84) and gender (p=.97). Because the n=151 subjects did not differ significantly from the sample with brain data, we included all the 151 interviewed children in the principal components analysis, to reduce targeted informational data on SES and family stress into their core dimensions. Using the full sample also helped to increase variation and statistical power.
Exclusion criteria for subjects were: (1) current or lifetime history of DSM-IV Axis I psychiatric disorders, including alcohol and substance use disorders, (2) significant medical, neurological or psychiatric disorder, (3) history of head injury or loss of consciousness, (4) pregnancy (5) history of prenatal or birth confounds that could have influenced brain maturation, such as significant prenatal exposure to substances that caused severe birth complications, birth weight under 5 lbs. or severe postnatal compromise with neonatal intensive care unit stay; (6) morbid obesity or growth failure (7) full scale IQ lower than 70 (8) contraindications to safe participation in MRI research. The IRB of the Duke University Medical Center approved this study.
3.2. Sample
The study sample included 102 healthy children and adolescents who participated in the Duke Healthy Childhood Brain Development and Developmental Traumatology Research Program studies. Sample demographics are summarized in table 1. The sample was 57.6% female, 58.3% Caucasian and 33% African-American. Approximately, 97.9% were living with their biological parents, with caregiver intellectual functioning falling within the average range (mean=113.1, SD=14.0). Subjects ranged in age from 4.2 to 18.6 years (mean=11.7, SD=3.7). While measures of IQ are conventionally known to be stable starting at age seven [52], a study of preschool children suggests that IQ is stable at younger ages for typically developing children, as well [53]. Although the sample mean of the Hollingshead scale (mean=45.15, SD=13.1) would indicate that the average family in our sample is considered to be middle-class, there was also a wide range of SES level in the sample based on this measure (range=14-66). Nearly 89% of the sample was right handed and intellectual functioning fell within the average range (mean=110.3, SD=15.6, range=74,132) (Table 1).
Table 1. Sample Demographics.
| Complete Sample | Male | Female | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean (sd) | Range | Mean (sd) | Range | Mean (sd) | Range | |
| Child's IQ | 107.67 (13.59) | 74-132 | 107.22 (14.67) | 85-132 | 108.28 (12.10) | 74-132 |
| Parent's IQ | 112.78 (14.27) | 62-137 | 112.85 (14.19) | 62-137 | 112.70 (14.56) | 82-133 |
| Subject's Age in years | 11.54 (3.62) | 4.17-17.58 | 11.02 (3.49) | 4.17-17.58 | 12.25 (3.71) | 4.42-17.58 |
| Hollingshead Score* | 45.15 (13.10) | 14-66 | 43.97 (14.55) | 14-66 | 46.77 (10.75) | 14-66 |
| % Male | 42.2 | -- | -- | |||
| % White | 53.9 | 43.1 | 56.9 | |||
| N | 102 | 43 | 59 | |||
Hollingshead Score is a continuous measure from 8 (lowest) to 66 (highest) of socioeconomic class.
3.3. Measures
3.3.1. Outcome measure
IQ was measured with either the 2-subtest version (Vocabulary, Matrix Reasoning) of the Wechsler Abbreviated Scale of Intelligence (WASI) [54], the 2-subtest short-form of the Wechsler Intelligence Scale for Intelligence-III (WISC-III) [55], or the Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R) [56]. All IQ scores were combined to create an overall measure for IQ.
3.3.2. Predictor measures
This study focused on several key predictors, including multidimensional measures of SES, family stress, biological parent IQ and cerebral brain structures based on brain and intellectual development literature from several disciplines.
3.3.3. Socioeconomic status
The SES components were constructed from 14 items selected from the parent self-report questionnaires and the parent and child interviews, listed as they appear in the parent questionnaires and interview schedules in table 2. The various instruments used in the questionnaires containing the items used in the principal components analysis are described. The items were selected using the six indicators from the Blank SES measurement framework: health status, health insurance access, food security, housing conditions, education and labor market access (2008).
Table 2. SES Component Loadings.
| Component | |||
|---|---|---|---|
|
|
|||
| Variable | Community and Educational Capital | Health Status | Financial Resouces |
| Median housing value of census tract1 | .883 | .126 | -.046 |
| Median real estate taxes of census block1 | .818 | .163 | .023 |
| Median income of census tract1 | .751 | .100 | -.077 |
| Father's Education2 | .673 | .269 | -.104 |
| Mother's Education2 | .612 | .191 | -.296 |
| Is parent health limited in exertive activities?3 | .137 | .783 | -.113 |
| Is parent health limited in moderate activities?3 | .067 | .770 | -.227 |
| Does parent have a lot of energy?3 | .176 | .694 | -.038 |
| Does pain interfere with parent's normal work?3 | .095 | .689 | .075 |
| Parent's self description of health3 | .212 | .557 | -.173 |
| Source of Income: Non- employment support2 | -.030 | -.217 | .883 |
| Source of Income: Child Support2 | -.097 | .008 | .819 |
| Source of Income: Employment2 | .156 | .154 | -.757 |
| Child's current health insurance coverage2 | -.470 | .006 | .412 |
Notes. Correlations above 0.4 bolded
Item Sources: 2000 Census;
Healthy Brain KSADS-PL;
Pittsburgh Medical Center SF-12 Health Survey.
To capture potential unobserved differences in SES, we used data at different levels of aggregation, as recommended by prior research [39,57,58]. We extracted neighborhood variables to use as indicators of relative income and assets from the 2000 Census1 (sample data collected between 2003 and 2008). Median housing value is at the census block level, median income is at the block-group level, and median real estate taxes are at the census tract level (Tables 2 and 3).
Table 3. Family Stress Component Loadings.
| Component | |||||
|---|---|---|---|---|---|
|
|
|||||
| Variable | Marital Conflict - Physical | Depressive Symptoms | Marital Conflict - Verbal | Marital Conflict Resolution | Intrafamily Dyadic Relationships |
| Respondent slapped or spanked him/her1 | .991 | -.028 | -.019 | -.022 | -.022 |
| Respondent hit or tried to hit with something1 | .991 | -.021 | -.058 | -.012 | -.018 |
| Respondent pushed, grabbed, or shoved him/her1 | .990 | -.026 | -.048 | -.017 | -.016 |
| Respondent beat him/her up1 | .988 | -.018 | -.066 | -.026 | -.019 |
| Respondent threw something at him/her1 | .988 | -.027 | -.005 | -.003 | -.007 |
| Respondent threw or smashed something1 | .968 | -.010 | -.007 | .038 | -.023 |
| Respondent threatened to hit or throw something at him/her1 | .866 | -.068 | .193 | .043 | .003 |
| Feeling blue2 | -.014 | .783 | .139 | .035 | .100 |
| Feeling lonely2 | -.021 | .777 | .093 | .121 | -.109 |
| Have you felt downhearted and blue?3 | .065 | -.762 | -.027 | .027 | -.007 |
| Feeling hopeless about the future2 | -.010 | .711 | -.017 | -.134 | -.025 |
| Thoughts of ending your life2 | .015 | .623 | -.055 | -.143 | -.055 |
| Feelings of worthlessness2 | -.002 | .617 | -.117 | .192 | .107 |
| Respondent called the person a name, insulted or swore at them1 | .025 | -.037 | .852 | .116 | -.088 |
| Respondent did or said something to spite him/her just to be mean1 | -.024 | .011 | .799 | -.123 | -.104 |
| Respondent yelled or screamed at him/her1 | .015 | .041 | .782 | .259 | .067 |
| Respondent sulked and/or refused to talk about it1 | .094 | -.005 | .630 | -.129 | -.057 |
| FES Conflict Scale Index (score/number answered)4 | -.086 | .054 | .489 | .303 | -.059 |
| Respondent stomped out of room or house (or yard)1 | .026 | -.044 | .440 | .373 | .338 |
| Respondent discussed the issue calmly together1 | .076 | .043 | -.059 | .610 | -.318 |
| Respondent got information to back up their side of things1 | .018 | -.123 | .217 | .582 | -.097 |
| Respondent tried to bring in someone to help settle things1 | .011 | .079 | .199 | .565 | .260 |
| Mother-Child Relationship5 | -.014 | -.040 | -.042 | .505 | .369 |
| Father-Child Relationship5 | -.015 | .047 | -.104 | .068 | .840 |
| Biological parents Relationship5 | -.041 | -.012 | -.083 | -.195 | .789 |
| Family History Elements- Total Count6 | -.053 | .208 | .028 | .382 | .032 |
| Have you felt calm and peaceful?3 | .148 | -.233 | -.250 | .027 | -.029 |
| Respondent got into a fight (hit or pushed second)1 | .002 | -.089 | -.033 | .189 | -.066 |
Note. Correlations above 0.4 bolded
Item Sources: Conflict Tactics Scale;
Brief Symptom Inventory;
Pittsburgh Medical Center SF-12 Health Survey;
Family Environment Scale;
Healthy Brain KSADS-PL;
Healthy Brain Family History Interview.
3.3.4. Family stress
Based on the previously cited literature, exploring family stress and parenting using the Family Stress model, we identified four subconstructs that capture family stress: Marital conflict, parenting stress, depressive symptoms and maternal history of psychiatric disorders. These sub-constructs were used to guide the selection of items into a separate (from SES) principal components analysis. The family stress components were constructed from 28 items listed, as they appear in the parent questionnaires in table 3. The various instruments used in the questionnaires are described below.
3.3.5. Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (KSADS-PL)
Because mental illness is associated with lower IQ, children and parents were interviewed using the KSADS-PL to screen for current and lifetime mental illness, defined as DSM-IV Axis I disorders. This is a semi-structured diagnostic interview to assess current and past episodes of psychopathology in children and adolescents according to DSM-IV criteria, and generate reliable and valid child psychiatric diagnoses [51]. Probes and objective criteria are provided to rate individual symptoms. The schedule was modified to include items to measure the following: 1) life events, including traumatic events from the Child and Adolescent Psychiatric Assessment [59]; 2) disorders not present in the instrument; 3) a structured scale to quantify symptom frequency with a minimum score of zero meaning no history of a symptom and maximum score of ten indicating symptoms present several times a day; 4) algorithms to determine Axis I psychiatric disorders based on DSM-IV criteria; and 5) eleven items about participants background information, which included questions regarding the primary sources of income in the household (non-employment support, employment, Social Security Income, child support), limited choice describing family relationships (e.g. friendly, conflictual, or no contact), and whether the child had health insurance coverage. Disorders were assigned a severity score of mild, moderate or severe. This modified version of the KSADS-PL is available upon request.
3.3.6. University of Pittsburgh Medical Center short form-12 health survey
The 12-Item Short Form Health Survey (SF-12) measures one's perception of general health status, and was developed as a shorter alternative to the 36-Item Short Form Health Survey (SF-36) [60]. The resulting short-form survey instrument was designed to reduce respondent burden and maintain precision across multiple health dimensions. The Short Form is comprised of the Physical Component Summary and Mental Component Summary scales [61]. Items from both scales were used to assess parent's overall health and ability to participate in the labor market, and their mental well-being, including depressive symptoms. Validation studies indicate that SF-12 scores are highly predictive of both Physical Component Summary-36 scores (r=.95) and Mental Component Summary-36 scores (r=.97) [62].
3.3.7. Revised Conflict Tactics Scale (CTS2
The original Conflict Tactics Scale was designed to capture intrafamily violence, conflict and marital conflict and relations [63]. The Revised Conflict Tactics Scale 2 was used in this study to measure these aspects of family stress, as well as environmental and emotional stimulation [64]. The revised version also captures psychological and physical attacks on a partner in a marital, cohabiting, or dating relationship, and also use of negotiation. Reliability ranges from .79-.96 [64].
3.3.8. Brief Symptom Inventory (BSI)
The Brief Symptom Inventory is a short form of the Symptom Checklist 90 (SCL-90), and includes nine scales of symptom dimensions: somatization, depression, obsessive compulsiveness, interpersonal sensitivity, anxiety, hostility, phobic anxiety, paranoid ideation and psychoticism [65]. We used the depressive symptom items in the family stress Principal Components Analysis because of the prior research indicating that maternal depression affects child outcomes. The BSI has strong stability and internal consistency in adolescent samples [65].
3.3.9. Family Environment Scale (FES)
Each parent filled out the Conflict Scale Index subscale of the Moos Family Environment Scale [66]. This index generates a score based on the number answered. Reliability of the Conflict Index subscale is satisfactory (.71) [67].
3.3.10. Structured Clinical Interview for DSM-IV Axis I Diagnosis (SCID-I)
During the parent interview, the SCID-I was used to assess parent's psychological functioning [68]. The maternal history of psychiatric diagnosis was based on the Family History-Research Diagnostic Criteria, for the clinical diagnostic evaluation of psychiatric disorders in parents using an interview checklist approach for all DSM- IV diagnoses of major mental disorders [69]. This approach is based on the Family Study Method [70], and was used to provide information on the subject's biological parent. We counted number of the parental lifetime disorders (0-7): mood (major depressive disorder, dysthymia, mania), psychotic (delusion, schizophrenia, affective disorder), anxiety (phobia, generalized anxiety, panic, obsessive-compulsive, post-traumatic stress disorder), Attention Deficit Hyperactivity, eating, substance abuse (alcohol abuse disorder, drug abuse), and antisocial (anti-social personality, conduct disorder).
3.3.11. Brain structures
All subjects underwent anatomical magnetic resonance imaging using a Siemens Trio 3.0 Tesla MRI system (Trio, Siemens Medical Systems) running version VA 24 software (3D GRE (IMPRSGE), axial, TR=1750 ms, T1=1100 ms, 25.6 cm FOV, 1.0 mm slice, flip=20o, Bandwidth: (220 Hz/pixel), 256 (phase)×256 (frequency), 1NEX). Cerebral white, grey, and total brain matter volumes were measured at the Duke Neuropsychiatric Imaging Research Laboratory, using the Katholieke Universiteit Leuven [71,72] procedure and the GRID program [73], that was modified for pediatric imaging. All subjects tolerated the procedure well. No sedation was used. Intraclass correlation of intra-rater reliability for independent designation of regions on segmented images obtained from 10 subjects were 0.99 for total brain volume, total white matter, total grey matter, and cerebral spinal volumes.
3.3.12. Other covariates
In addition to our targeted predictor measures, we include several other key covariates that have shown significant relationships to our predictor variables, and/or outcome variables. These included chronological age, race/ethnicity, gender and biological parental IQ. The latter variable was used as a proxy measure for familial (e.g. genetic) contribution, and was operationalized by the two-subtest (Vocabulary, Matrix Reasoning) version of the Wechsler Abbreviated Scale of Intelligence [54].
4. Data Analysis
4.1. Construction of SES and family stress components
Principal components analysis is a mathematical technique that reduces an original set of variables into a smaller number of uncorrelated variables, to explain part of the variation in a set of observed variables with a few underlying dimensions [41]. These dimensions or components are a linear weighted combination of the initial variables, where each consecutive component captures an additional dimension in the data, while explaining smaller and smaller proportions of the variation in the original variables [74]. Unlike factor analysis, there is no statistical model underlying principal components analysis, because it is merely a transformation of the data [75]. Another difference between the two approaches is that in principal components analysis, all of the observed variance is analyzed, while in factor analysis, it is only the shared variance that is analyzed. We chose to use principal components analysis over factor analysis because there are fewer assumptions about the relationships between the items.
We used PASW Statistics 18 software (SPSS 18.0), to conduct the principal components analysis on the full sample of children who were interviewed (n=151), using varimax rotation to construct orthogonal (independent) components. These factor loadings were then used in the outcome analyses. We used varimax rotation, so that the resulting factors would be unique variables for use in the outcome analyses. The number of components was determined by the Kaiser-Guttman criterion, keeping the number of eigenvalues larger than one. We also examined the components using a scree-plot, by displaying the eigenvalues against their rank, and determined the appropriate number of components using the ‘elbow’ of the curve [76]. These two sets of components were used to operationalize each construct and test the research questions in the outcome models.
Table 4 displays the rotated eigenvalues and percent variance explained by each component. The first component explains the most variance, with the next component explaining the second most, and so on. Specific component content, respective instrument and loadings are displayed in tables 2 and 3. Henceforth, we will refer to the components by their substantive names, instead of their component numbers (Table 4).
Table 4. Eigenvalues and Percent Variance Explained by Separate Factor Analyses of SES and Family Stress Components.
| Initial Eigenvalues | |||
|---|---|---|---|
|
|
|||
| Total | % of Variance | Cumulative % | |
| SES Components | |||
|
|
|||
| Community and Educational Capital | 4.365 | 29.103 | 29.103 |
| Health Status | 1.86 | 12.401 | 41.504 |
| Financial Resources | 1.369 | 9.127 | 50.632 |
|
| |||
| Family Stress Components | |||
|
|
|||
| Marital Conflict - Physical | 6.696 | 23.091 | 23.091 |
| Depressive Symptoms | 3.908 | 13.476 | 36.567 |
| Marital Conflict - Verbal | 3.431 | 11.830 | 48.398 |
| Marital Conflict Resolution | 2.011 | 6.935 | 55.333 |
| Intrafamily Dyadic Relationships | 1.647 | 5.679 | 61.012 |
Note. Specific component content is available from the corresponding author.
The principal components analysis indicated three unique dimensions of SES, in this order: Community and educational capital, health status, and financial resources. Food security was the only construct from the Blank (2008) framework that was dropped, because there was no variation in this item across subjects. Community and educational capital represents the parents' education level and the census block, and tract variables that measure the average level of income and housing costs of the family's neighborhood. These neighborhood variables may also capture investment in community resources like public schools, parks and other local private and public programs and amenities. Health status represents the parent's perception of their physical abilities and health, their energy level, pain experiences, and whether their health interferes with their everyday life. The financial resources variable represents the family's sources of income (e.g. employment, alimony, Social Security Income), and whether the focal child is covered by health insurance (Yes/No) (Table 2 for item wording and instrument source).
The principal components analysis for family stress generated five unique components: marital conflict-physical, mother's depressive symptoms, marital conflict-verbal, marital conflict resolution, and intrafamily dyadic relationships. Marital conflict-physical represents violent acts between the mother and the child's father, or mother's intimate partner. Depressive symptoms capture the mother's responses on all items related to depression (e.g. feeling blue, feeling hopeless about the future). Marital conflict-verbal includes the items that describe verbal discord, such as yelling, screaming, insulting or swearing and the Family Environment Scale Conflict Scale index score. Marital conflict resolution includes the items that describe positive coping and resolution skills for marital discord, such as discussing the issue calmly together. An intrafamily dyadic relationship includes the description of the mother-father and father-child relationship items (e.g. conflictual, loving and friendly). Note that the mother-child relationship item loaded on the marital conflict resolution component (Table 3 for item wording and instrument source).
4.2. Child IQ analyses
Our primary research questions were as follows: (1) Does brain volume mediate the effect of SES on child IQ? (2) Do any components of SES mediate the effect of one biological parent's IQ on child IQ? (3) Do any components of family stress mediate the effect of SES on child IQ? Each question was examined using path analysis, a type of structural equations modeling, in Mplus version 5.21. This allowed us to examine patterns of directional and nondirectional linear relationships among variables [77]. To test for the intervening effects of brain volume between SES, family stress and child outcomes, we included measures of grey matter, white matter, and total brain volume, as well as controls for age, sex and parent IQ as our genetic proxy.
The dependent variable in all models (white, grey and total brain volume) was child IQ. Each model included child age, an indicator for sex (male=1), parent IQ, child race (white, non-white), the three SES components (community and educational capital, health status and financial resources), and the five family stress components (physical and marital conflict, depressive symptoms, verbal marital conflict, marital conflict resolution, intrafamily dyadic relationships), as independent variables. All independent variables were allowed to predict the outcome variable, child IQ in each model. The SES and family stress components were allowed to freely correlate with one another. Directionality was fixed from parent IQ to the SES and family stress components, and from race to the SES and family stress components. Mplus enabled us to explore the all the possible channels through which the independent variables could affect child IQ. For all pathways, the models were unconstrained to explore any possible mediating effects of the component measures. Child outcomes and path results are in terms of standardized coefficients (i.e. standardized by the variance of both the independent and dependent variable).
In testing for both direct and indirect effects in path analysis, this model assumes that all unmeasured causes of the dependent variables (i.e. brain volume and child IQ) are uncorrelated with the SES and family stress measures, and the other included covariates after controlling for the covariation between all of the covariates [78]. This model also assumes that the omitted causes of the outcome variables are unrelated to each other. Because of the comprehensiveness of our constructed SES and family stress variables in capturing the numerous environmental factors which may influence brain volume and IQ, and the inclusion of biological parent IQ to capture familial factors, we found these assumptions plausible. Additionally, the effects in this study are presumed to be directional, in that the covariates are hypothesized to determine brain volume and child IQ, but that child IQ does not affect brain volume or the other included controls. In other words, this is a recursive model with no feedback loops.
Each model had good fit across all tests of overall model fit including; the chi-square test, root mean square error of approximation (RMSEA<.08) [79], the comparative fit index (CFI>0.95), the standardized root mean square residual (SRMR, <0.07), and the non-normed fit index. Root mean square error of approximation and standardized root mean square residual measures were given precedence in accordance with the SEM literature in psychology [77].
5. Results
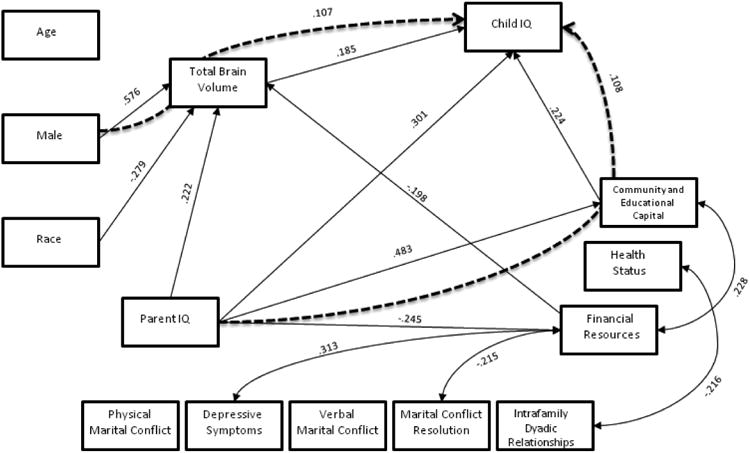
The white matter model had acceptable fit across all measures (RMSEA=0.074; SRMR=0.046; chi-square=29.687, p=0.056; CFI=0.947), and significant path model results are displayed in figure 1. The grey matter model had good fit across all measures (RMSEA=.056; SRMR=.045; chi-square= 27.649, p=.150; CFI=.971), and significant path model results are displayed in figure 2. The total brain volume also had good model fit across all measures (RMSEA=0.069; SRMR=0.045; chi-square=28.126, p=0.081; CFI=0.956), and significant results are displayed in figure 3. Each path coefficient (β) represents a standard deviation change in child IQ, as a result of a one standard deviation increase in the explanatory variable (e.g. age), operating through the meditational variable (e.g. total brain volume), holding other variables in the model constant. Child IQ has a standard deviation of 15 points (Figures 1-3).
Figure 1. White Matter.
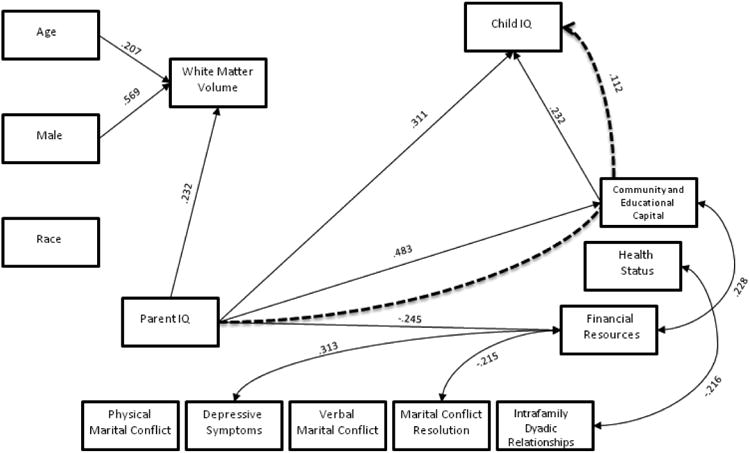
Figure 2. Grey Matter.
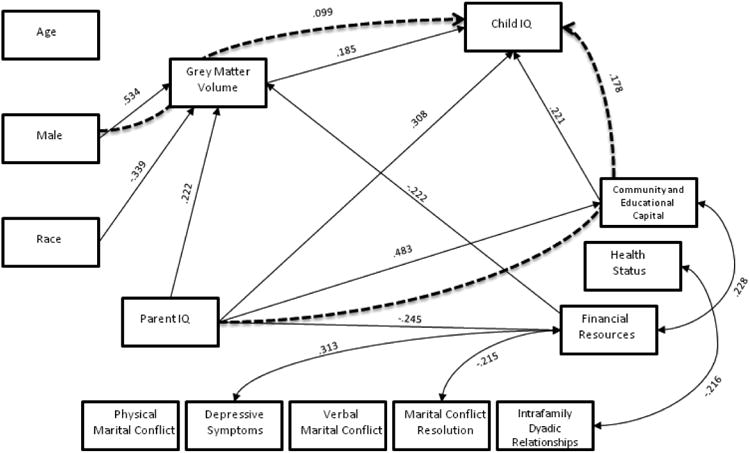
Figure 3. Total Brain Volume.
5.1.1. Does brain volume mediate the effect of SES on child IQ?
5.1.1.1. White matter mediation
White matter did not mediate any of the paths to child IQ, but sex, age and parent IQ predicted white matter volume directly (β=.569, SE=.061, p<.001; β=.207, SE=.070, p=.003 and β=.232, SE=.095, p=.015, respectively).
5.1.1.2. Grey matter mediation
The only significant grey matter mediation path was that grey matter mediated the effect of sex (male) on IQ (β=.099, SE=.050, p=.047). This means that male child IQ is 1.5-points higher than female IQ through its effects on males grey matter volume. Both race and the financial resources component predicted grey volume directly (β=.339, SE=.088, p<.001 and -β=.222, SE=.084, p=.008, respectively).
5.1.1.3. Total brain volume mediation
Total brain volume mediated the effect of sex (male) on IQ (β=.107, SE=.053, p=.044). This means that male child IQ is 1.5-points higher than female IQ, through its effects on male total brain volume. Sex, race and the financial resources component predicted total brain volume directly (β=.576, SE=.061, p<.001; β=.279, SE=.085, p=.001 and β=.198, SE=.078, p=.011). Parent IQ directly predicted child IQ (β=.301, SE=.109, p=.006), with the same magnitude as the grey and white matter models.
5.1.2. Do any components of SES mediate the effect of one biological parent's IQ (genetic proxy) on child IQ?
5.1.2.1. White matter mediation
The community and educational capital component of SES mediated the effects of parent IQ on child IQ (β=.112, SE=.054, p=.037), and was the only significant indirect path. This means that a 15-point increase in parent IQ (one standard deviation in IQ) predicts a 1.1-point increase in child IQ, by way of increasing the family's community and educational capital. Parent IQ directly predicted child IQ (β=.311, SE=.110, p=.005), meaning that a 15-point increase in parent's IQ predicts a 4.6-point increase in child IQ, holding all else constant. Additionally, parent IQ directly predicted community and educational capital (β=.483, SE=.091, p<.001), and financial resources (β=.245, SE=.112, p=.028) components of SES.
5.1.2.2. Grey matter mediation
Community and educational capital mediated the effects of parent IQ on child IQ (β=.107, SE=.053, p=.044). This means that a one standard deviation increase in parent's IQ produces a .1 standard deviation increase in child IQ via its effects on community and educational capital, which is approximately a 1.5-point increase in child IQ. Parent IQ directly predicted child IQ (β=.308, SE=.109, p=.005), with approximately the same magnitude as in the white matter model. Parent IQ also directly predicted the SES components of community and educational capital and financial resources in the same magnitude as in the white matter model.
5.1.2.3. Total cerebral brain volume mediation
As in the grey and white matter models, the SES component of community and educational capital mediated the effects of parent IQ (β=.108, SE=.053, p=.042) on child IQ. This means that a 15-point increase in parent IQ (one standard deviation in IQ) predicts a 1-point increase in child IQ, by way of increasing the family's community and educational capital. Parent IQ directly predicted community and educational capital and financial resources, with the same magnitude as the white and grey matter models.
5.1.3. Do any components of family stress mediate the effect of SES on child IQ?
None of the family stress components mediated the effect of the SES components on child IQ. There were non-directional correlations amongst the SES and family stress components. Health status and intrafamily dyadic relationships were related (β=-.216, SE=.094, p=.022), and financial resources was related to both depressive symptoms (β=.313, SE=.089, p<.001) and marital conflict resolution (β=-.215, SE=.094, p=.023). These relationships were the same in the white, grey and total cerebral volume models.
5.1.4. Summary of findings
Community and educational capital, the primary SES component, mediated the effects of parent IQ on child IQ, providing support for our second hypothesis. None of the brain volume measures or the family stress components mediated the effects of the SES components on child IQ, and therefore, our hypotheses for the first and last research questions were not supported.
6. Discussion
The primary purpose of this study was to investigate how well-defined environmental factors influence children's brain development and intellectual functioning, and makes several contributions to the literature. The unique interdisciplinary research approach combined the neuropsychological, sociological and developmental psychological literature, to explore the ways that SES affects child development, by conducting an empirical test of the Family Stress model. This analysis yielded more comprehensive measures of SES and family stress, using multiple items with principal components analysis. In particular, the operationalized SES components are stronger and more robust representations of this construct, than what is typically used in the literature. This paper also extends the neuroscience literature by using meditational models to explore potential mechanisms through which SES operates in child neuro-development.
This study explored numerous meditational relationships through which SES and family stress may affect child outcomes, by using these components in path analysis, which also included measures of cerebral volume and parent IQ. We proposed three major research questions: (1) Does brain volume mediate the effect of SES on child IQ? (2) Do any components of SES mediate the effect of one biological parent's IQ on child IQ? (3) Do any components of family stress mediate the effect of SES on child IQ? We hypothesized that cerebral white matter will have the strongest level of mediation between SES and family stress, and child cognitive outcomes because of the known environmental impact of myelination processes during development [80]. However, none of the brain volume measures or the family stress components mediated the effects of SES on child IQ; therefore, our hypotheses for the first and third research questions were not supported. This is concurrent with the findings from another recent neurological study, with a large sample of typically developing children that found a small association between total brain volume and IQ, and a larger association between parental education and IQ, using parental education as a proxy for SES [46]. As in our study, they found that total brain volume did not mediate the relationship between parental education and IQ.
The foremost finding was that in all three models, community and educational capital—the primary SES component—mediated the effect of parent's IQ on child IQ, providing supportive evidence for the second research question. Community and educational capital can be thought of as the average and median resources of families living in the child's community, and serves as a proxy measure for local investments in public goods. In this case, higher community and educational capital means higher housing costs, taxes, and perhaps, schools with better performance measures. Other aspects, such as reduced crime, higher quality child care, more extra-curricular opportunities or access to community health resources are likely correlated with better-resourced communities. Our results show that an increase in parent's IQ was associated with a small increase in child IQ that operated through a family's community and educational capital, in addition to the direct affect of parent IQ on child brain volume.
This is a particularly interesting pathway because it involves both a familial (e.g. genetic), and an environmental factor. This also corroborates numerous developmental theories, which hypothesize that the environment shapes child outcomes in concert with genetics through dynamic developmental processes, and the large literature that documents the interdependency between familial factors (e.g. genes) and the environment [14-18,81-84]. Over the course of development, familial factors and the environment continue to influence and interact with each other. This suggests that even if parents have a high IQ, if community supports are not available, the child's IQ would be negatively affected. Since community and educational capital also predicted IQ directly, this implies that increasing resources in the community could enhance child IQ. Together, the model results indicate that the effect of parent IQ on child IQ operates both indirectly through the environment via community and educational capital, and directly through familial effects.
Other neuropsychological research indicates that environmental factors related to SES disproportionately affect certain cognitive systems, suggesting further exploration of more specific neural pathways [21,27,85]. Farah [25] suggests that SES most profoundly affects language, memory and executive function systems. Another study using electrophysiological recording found that attention responses and measures of executive functioning were significantly better for children with high SES [86]. Our future work will continue to explore these more precise pathways. In fact, our lack of findings in the present study may be due to the broad measures of the brain and cognition we used to develop our SES measurement model. Nonetheless, few studies have brought together structural measures of the brain, with sophisticated measures of SES and family stress.
The correlations between the SES and family stress components were expected based on the hypotheses and intuition of the Family Stress model (e.g. financial resources is associated with marital conflict resolution and depressive symptoms) providing further support for this body of literature. The direct negative effect of financial resources on grey matter cerebral volume and total cerebral volume also likely reflects the family financial stress mechanisms posited in the Family Stress model; fewer financial resources increases parental stress, negatively affecting child development through its affects on parenting behavior. Lower SES is associated with higher cortisol levels in children [87], which may lead to negative effects on grey matter.
Males in the sample have larger brains on average, and thus more grey matter, corroborating other studies finding that males have about 8% larger brains than females [46,47,88,89]. The pathway going from sex (male) to child IQ through the brain in the grey matter model is consistent with the broad differences between males and females found in recent research [46], but may also be an artifact of the large effect size of for greater male versus female brain volume seen in our dataset, and confirmed in the literature. However, this does not imply that females have proportionally less grey matter or lower IQ on average. There were no significant differences between the IQ of girls and boys in this study.
7. Implications
These findings have several implications for child neuropsychological research, clinical practice, policy and intervention. First, these findings provide further evidence that both nature and nurture are important for healthy brain development. Studies have shown the positive influence of environmental stimulation, caregiving and experience for age-appropriate healthy development [12,13,90,91]. Therefore, cognitive stimulation interventions that relate to community and educational capital can use ‘nurture’ to influence brain development for children in low-income families. For example, if a child has a low IQ, and the parent is not as able to provide environmental stimulation for the child's neuropsychological development at home, then child placement in a well-resourced school district may help to improve child IQ. It is important to note that because of the wide age range of children in this study, the specific mechanisms along the pathway between community and educational capital and child IQ may be different for children at different ages. For example, communities with more resources likely have better funding for public schooling, which can directly and additively affect child IQ for older children. This may also have implications for children who are neurologically and intellectually disabled, and for children living in poverty, where schools are more likely to be under-performing due to lack of resources [92,93].
For clinical practice, it is important that assessments of children include detailed items about the family life and household and community resources, as we have used here, in order to more fully understand where intervention is needed. It is also unclear how these environmental interventions are mediated by brain structure and function. Can the environmental intervention change pathways in the brain and associated brain functions to affect neuropsychological outcomes? Future work can continue to examine these types of brain-by-environment interactions. Relatedly, it will be important that future studies test whether these brain-by-environment pathways differ between typical and impaired samples.
These findings may have policy implications for neurologically and intellectually disabled children, and for children living in poverty, where schools are more likely to be under-performing due to lack of resources [92,93]. In fact, early education for children living in poverty has larger and longer lasting positive effects than similar community and educational resources provided to the whole population [94]. These findings may also contribute to the literature about the effects of neighborhoods on child outcomes because communities with more institutional resources may be able to mitigate the effects of familial factors, and influence development by enabling children to participate in educational and recreational activities [95-97].
8. Limitations
There are several limitations to our study: 1) Though our sample size was large for a neuroscience study, it is fairly small for a modeling study, and thus, our analyses may have failed to uncover a host of other effects. Thus, our findings should be seen as exploratory, and not causal; 2) Parent self-selection into communities may have biased our findings because parent IQ predicts parent's educational attainment and family income, and this influences their choice and ability to live in better-resourced communities; 3) Our results may have been different if we had administered the full IQ test on subjects, instead of the two-subtest IQ measure; 4) We used a typical sample of children with no pathologies, and thus these findings cannot be generalized to atypical samples. This also limited the distribution of sample IQ to be above 70; 5) The use of standardized path coefficients imposes certain assumptions on the model, in that it equates the difference between scaling units in the path variables, which may not be plausible [98]. For example, one-unit changes in IQ and the SES and family stress components are not likely the same; 6) We did not incorporate a direct measure of family income in our SES components; 7) The range of SES in our sample was wide based on the Hollingshead Index, but a majority of the families were middle-class. The study site was located in a university setting, increasing the representation of middle-class families.
This study provides an innovative approach to the measurement and conceptualization of the elements, comprising SES and its relationship to child structural brain development and neuropsychological outcomes. Future research should replicate the use of this framework and test our model on different samples, such as children with developmental disabilities and other neurological impairments. Testing the relationships between SES components and other child neuropsychological outcomes, including memory and social behavioral outcomes, is also needed. If similar results and pathways are replicated in other studies, we can continue to build a unique neuropsychological literature on the key elements of the child's environment, and their relationships to neurological development. By using an interdisciplinary approach and incorporating new frameworks for measurement, we can advance the study of SES and child poverty, and apply these findings to clinical research, intervention, and policy.
Acknowledgments
The authors thank the staff the Duke Healthy Childhood Brain Development and Developmental Traumatology Research Program, and the participants and their families for making this work possible. This work was supported by funding from National Institutes of Health (K24 MH071434 and K24 DA028773 and RO1- MH61744, R01-AA12479, RO1-MH63407).
Footnotes
Census tracts and blocks were approximated for a small number of subjects whose household addresses did not exist at this time (n=9).
References
- 1.Brooks-Gunn J, Duncan GJ. The effects of poverty on children. Future of Children. 1997;7(2):55–71. [PubMed] [Google Scholar]
- 2.Cunha F, Heckman JJ. The Technology of Skill Formation. American Economic Review. 2007;97(2) [Google Scholar]
- 3.McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- 4.Bradley RH, Corwyn RF. Socioeconomic Status and Child Development. Annual Review of Psychology. 2002;53(1):371. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 5.Mistry RS, Biesanz JC, Taylor LC, Burchinal MR, Cox M. Family Income and Its Relation to Preschool Children's Adjustment for Families in the NICHD Study of Early Child Care. Developmental psychology. 2004;40(5):727–745. doi: 10.1037/0012-1649.40.5.727. [DOI] [PubMed] [Google Scholar]
- 6.McLoyd VC. The Impact of Economic Hardship on Black Families and Children: Psychological Distress, Parenting, and Socioemotional Development. Child development. 1990;61(2):311. doi: 10.1111/j.1467-8624.1990.tb02781.x. [DOI] [PubMed] [Google Scholar]
- 7.Gale CR, O'Callaghan FJ, Godfrey KM, Law CM, Martyn CN. Critical periods of brain growth and cognitive function in children. Brain. 2004 Feb 1;127(2):321–329. doi: 10.1093/brain/awh034. [DOI] [PubMed] [Google Scholar]
- 8.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 9.Hoff E. The Specificity of Environmental Influence: Socioeconomic Status Affects Early Vocabulary Development Via Maternal Speech. Child Development. 2003;74(5):1368–1378. doi: 10.1111/1467-8624.00612. [DOI] [PubMed] [Google Scholar]
- 10.Duncan GJ, Brooks-Gunn J, editors. Consequences of growing up poor. New York: Russell Sage Foundation; 1997. [Google Scholar]
- 11.Duncan GJ, Brooks-Gunn J. Economic Deprivation and Early Childhood Development. Child development. 1994;65(2):296–318. [PubMed] [Google Scholar]
- 12.Dawson G, Ashman SB, Carver LJ. The role of early experience in shaping behavioral and brain development and its implications for social policy. Development and psychopathology. 2000;12(04):695–712. doi: 10.1017/s0954579400004089. [DOI] [PubMed] [Google Scholar]
- 13.Als H, Duffy FH, McAnulty GB, et al. Early Experience Alters Brain Function and Structure. Pediatrics. 2004 Apr 1;113(4):846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 14.Meaney M. Epigenetics and the Biological Definition of Gene & Environment Interactions. Child Development. 2010;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 15.Rutter M. Gene-environment interdependence. Developmental Science. 2007;10(1):12–18. doi: 10.1111/j.1467-7687.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 16.Rutter M, Moffitt TE, Caspi A. Gene–environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47(3-4):226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 17.Turkheimer E, Haley A, Waldron M, D'Onofrio B, Gottesman II. Socioeconomic Status Modifies Heritability of IQ in Young Children. Psychological Science. 2003;14(6):623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- 18.Caspi A, McClay J, Moffitt TE, et al. Role of Genotype in the Cycle of Violence in Maltreated Children. Science. 2002 Aug 2;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 19.De Bellis MD, Keshavan M, Clark DB, et al. A.E. Bennett Research Award. Developmental Traumatology Part II: Brain Development. Biological Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 20.Farah MJ, Shera DM, Savage JH, et al. Childhood poverty: Specific associations with neurocognitive development. Brain research. 2006;1110(1):166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 21.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends in cognitive sciences. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conger RD, Donnellan MB. An Interactionist Perspective on the Socioeconomic Context of Human Development. Annual Review of Psychology. 2007;58(1):175–199. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- 23.Duncan GJ, Yeung WJ, Brooks-Gunn J, Smith JR. How Much Does Childhood Poverty Affect the Life Chances of Children? American Sociological Review. 1998;63(3):406–423. [Google Scholar]
- 24.Evans GW. The Environment of Childhood Poverty. The American Psychologist. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 25.Farah MJ. Mind, Brain and Education in Socioeconomic Context. In: Ferrari M, Vuletic L, editors. The Developmental Relations among Mind, Brain and Education. New York, NY: Springer; 2010. [Google Scholar]
- 26.Capron C, Duyme M. Assessment of effects of socio-economic status on IQ in a full cross-fostering study. Nature. 1989;340:552–554. [Google Scholar]
- 27.Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science. 2005;8(1):74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 28.Conger KJ, Elder GH. Families in troubled times: Adapting to change in rural America. Hillsdale, NJ: Aldine; 1994. [Google Scholar]
- 29.Linver MR, Brooks-Gunn J, Kohen DE. Family processes as pathways from income to young children's development. Developmental psychology. 2002;38:719–734. [PubMed] [Google Scholar]
- 30.Conger RD, McCarty JA, Yang RK, Lahey BB, Kropp JP. Perception of Child, Child-Rearing Values, and Emotional Distress as Mediating Links between Environmental Stressors and Observed Maternal Behavior. Child Development. 1984;55(6):2234–2247. [PubMed] [Google Scholar]
- 31.Bornstein MH, Bradley RH. Socioeconomic status, parenting and child development. Mahwah, NJ: Erlbaum; 2003. [Google Scholar]
- 32.Gershoff ET, Raver CC, Aber JL, Lennon MC. Income Is Not Enough: Incorporating Material Hardship Into Models of Income Associations With Parenting and Child Development. Child development. 2007;78(1):70–95. doi: 10.1111/j.1467-8624.2007.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayoub C, O'Connor E, Rappolt-Schlictmann G, Vallotton C, Raikes H, Chazan-Cohen R. Cognitive skill performance among young children living in poverty: Risk, change and the promotive effects of Early Head Start. Early Childhood Research Quarterly. 2009;24:289–305. [Google Scholar]
- 34.McLeod JD, Kessler RC. Socioeconomic Status Differences in Vulnerability to Undesirable Life Events. Journal of health and social behavior. 1990;31(2):162–172. [PubMed] [Google Scholar]
- 35.McLoyd VC, Jayaratne TE. Unemployment and Work Interruption among African American Single Mothers: Effects on Parenting and Adolescent Socioemotional Functioning. Child development. 1994;65(2):562–589. [PubMed] [Google Scholar]
- 36.Melzer D, Fryers T, Jenkins R, Brugha T, McWilliams B. Social position and the common mental disorders with disability Estimates from the National Psychiatric Survey of Great Britain. Soc Psychiatry Psychiatr Epidemiol. 2003;38:238–243. doi: 10.1007/s00127-003-0628-1. [DOI] [PubMed] [Google Scholar]
- 37.Bollen KA, Glanville JL, Stecklov G. Socioeconomic status and class in studies of fertility and health in developing countries. Chapel Hill, NC: MEASURE Evaluation, Carolina Population Center, University of North Carolina at Chapel Hill; 1999. [Google Scholar]
- 38.Hauser RM, Huang MH. Verbal ability and socioeconomic success: a trend analysis. Madison, WI: University of Wisconsin--Madison, Institute for Research on Poverty; 1998. [Google Scholar]
- 39.Sirin SR. Socioeconomic Status and Academic Achievement: A Meta-Analytic Review of Research. Review of Educational Research Fall. 2005;75(3):417–453. [Google Scholar]
- 40.Blank RM. Presidential address: How to improve poverty measurement in the United States. Journal of Policy Analysis and Management. 2008;27(2):233–254. [Google Scholar]
- 41.Dunteman GH. Principal components analysis. Newbury Park: Sage Publications; 1989. [Google Scholar]
- 42.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11(9):651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mistry RS, Vandewater EA, Huston AC, McLoyd VC. Economic Well-Being and Children's Social Adjustment: The Role of Family Process in an Ethnically Diverse Low-Income Sample. Child development. 2002;73(3):935–951. doi: 10.1111/1467-8624.00448. [DOI] [PubMed] [Google Scholar]
- 44.Lamb ME. Infant-Father attachments and their impact on child development. In: Tamis-LeMonda CS, Cabrera N, editors. Handbook of Father Involvement. Mahwah, N.J.: Lawrence Erlbaum; 2002. pp. 93–117. [Google Scholar]
- 45.Lamb ME. Nonparental child care: Context, Quality Correlates and Consequences. In: Sigel I, Renninger K, editors. Handbook of Child Psychology. Fifth. New York: Wiley; 1998. [Google Scholar]
- 46.Lange N, Froimowitz MP, Bigler ED, Lainhart JE Brain Development Cooperative Group. Associations Between IQ, Total and Regional Brain Volumes, and Demography in a Large Normative Sample of Healthy Children and Adolescents. Developmental Neuropsychology. 2010;35(3):296–317. doi: 10.1080/87565641003696833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. Brain. 1996 Oct 1;119(5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 48.De Bellis MD. Developmental traumatology: The psychobiological development of maltreated children and its implications for research, treatment and policy. Development and psychopathology. 2001;13:539–564. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- 49.Giedd JN, Blumenthal J, Jeffries NO, et al. Development of the human corpus callosum during childhood and adolescence: A longitudinal MRI study. Prog Neuro-Psychopharmacol & Biol Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 50.Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Research Bulletin. 2001;54(3):255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 51.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective Disorders and Schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 52.Moffitt TE, Caspi A, Harkness AR, Silva PA. The Natural History of Change to Intellectual Performance: Who Changes? How Much? Is it Meaningful? Journal of Child Psychology and Psychiatry. 1993;34(4):455–506. doi: 10.1111/j.1469-7610.1993.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 53.Dietz C, Swinkels SH, Buitelaar JK, van Daalen E, van Engeland H. Stability and change of IQ scores in preschool children diagnosed with autistic spectrum disorder. European Child & Adolescent Psychiatry. 2007;16(6):405–410. doi: 10.1007/s00787-007-0614-3. [DOI] [PubMed] [Google Scholar]
- 54.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 55.Wechsler D. Wechsler Intelligence Scale for Children. 3rd. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- 56.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-Revised. San Antonio, TX: The Psychological Corporation; 1989. [Google Scholar]
- 57.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic Status in Health Research. JAMA: The Journal of the American Medical Association. 2005 Dec 14;294(22):2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 58.Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Social Science & Medicine. 2002;55(1):125–139. doi: 10.1016/s0277-9536(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 59.Angold A, Prendergast M, Cox A, Harrington R, Simonoff E, Rutter M. The Child and Adolescent Psychiatric Assessment (CAPA) Psychological Medicine. 1995;25:739–753. doi: 10.1017/s003329170003498x. [DOI] [PubMed] [Google Scholar]
- 60.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form survey (SF-36): I. conceptual framework and items selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 61.Ware JE, Jr, Kosinski M, Turner-Bowker DM, Gandek B. How to score version 2 of the SF-12 health survey. QualityMetric Incorporated; 2002. [Google Scholar]
- 62.Riddle DL, Lee KT, Stratford PW. Use of SF-36 and SF-12 health status measures: A quantitative comparison for groups versus individual patients. Medical Care. 2001;39:867–878. doi: 10.1097/00005650-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 63.Straus MA. Measuring intrafamily conflict and violence: The Conflict Tactics Scales. Journal of Marriage and Family. 1979;41:75–88. [Google Scholar]
- 64.Straus MA, Hamby SL, Boney McCoy S, Sugarman DB. The revised Conflict Tactics Scales (CTS2): Development and preliminary psychometric data. Journal of Family Issues. 1996;17:283–316. [Google Scholar]
- 65.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological Medicine. 1983;13(03):595–605. [PubMed] [Google Scholar]
- 66.Moos RH, Insel PM, Humprehy B. Family work and group environment scales. Palo Alto, CA: Consulting Psychologists Press; 1974. [Google Scholar]
- 67.Boyd CP, Gullone E, Needleman G, Burt T. The Family Environment Scale: Reliability and Normative Data for an Adolescent Sample. Family Process. 2004;36(4):369–373. doi: 10.1111/j.1545-5300.1997.00369.x. [DOI] [PubMed] [Google Scholar]
- 68.Williams JB, M G, First MB, et al. The Structured Clinical Interview for DSM-III-R (SCID). II., Multisite test-retest reliability. Archives of General Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 69.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition Text Revision. Washington D.C: American Psychiatric Press; 2000. [Google Scholar]
- 70.Andreasen NC, Rice J, Endicott J, Reich T, Coryell W. The family history method approach to diagnosis: How useful is it? Archives of General Psychiatry. 1986;43:421–429. doi: 10.1001/archpsyc.1986.01800050019002. [DOI] [PubMed] [Google Scholar]
- 71.Payne ME, Fetzer DL, MacFall JR, Provenzale JM, Byrum CE, Krishnan KR. Development of a semi-automated method for quantification of MRI gray and white matter lesions in geriatric subjects. Psychiatry Research. 2002;15:63–77. doi: 10.1016/s0925-4927(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 72.Beyer JL, Kuchibhatla M, Payne ME, MacFall J, Cassidy F, Krishnan KRR. Gray and white matter brain volumes in older adults with bipolar disorder. Int J Geriatr Psychiatry. 2009;24:1445–1452. doi: 10.1002/gps.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MacFall J, Byrum C, Parashos I. Relative accuracy and reproducibility of regional MRI brain volumes for point-counting methods. Psychiatry Research. 1994;55:167–177. doi: 10.1016/0925-4927(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 74.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy and Planning. 2006 Nov 1;21(6):459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 75.Skrondal A, Rabe-Hesketh S. Generalized Latent Variable Modeling: Multilevel, Longitudinal, and Structural Equation Models. Boca Raton, FL: Chapman & Hall; 2004. [Google Scholar]
- 76.Cattell RB. The scree test for the number of factors. Multivariate Behavioural Research. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- 77.MacCallum RC, Austin JT. Applications of Structural Equation Modeling in Psychological Research. Annual Review of Psychology. 2000;51:201–226. doi: 10.1146/annurev.psych.51.1.201. [DOI] [PubMed] [Google Scholar]
- 78.Kline RB. Principles and Practice of Structural Equation Modeling. Third. New York: Guilford Press; 2011. [Google Scholar]
- 79.Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. [Google Scholar]
- 80.Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- 81.Sameroff A. A Unified Theory of Development: A Dialectic Integration of Nature and Nurture. Child Development. 2010;81(1):6–22. doi: 10.1111/j.1467-8624.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- 82.Gottlieb G. Individual development and evolution: The genesis of novel behavior. New York: Oxford University Press; 1992. [Google Scholar]
- 83.Cox M, Paley B. Understanding Families as Systems. Current Directions in Psychological Science. 2003;12(5):193–196. [Google Scholar]
- 84.Bronfenbrenner U. Developmental research, public policy, and the ecology of childhood. Child Development. 1974;45:1–5. [Google Scholar]
- 85.Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 86.Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, Knight RT. Socioeconomic Disparities Affect Prefrontal Function in Children. Journal of cognitive neuroscience. 2009;21(6):1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- 87.Lupien SJ, King S, Meaney MJ, McEwen BS. Child's stress hormone levels correlate with mother's socioeconomic status and depressive state. Biological Psychiatry. 2000;48(10):976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- 88.Giedd JN, Snell JW, Lange N, et al. Quantitative Magnetic Resonance Imaging of Human Brain Development: Ages 4-18. Cerebral Cortex. 1996 Jul 1;6(4):551–559. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 89.Schultz RT, Cho NK, Staib LH, et al. Brain morphology in normal and dyslexic children: The influence of sex and age. Annals of Neurology. 1994;35(6):732–742. doi: 10.1002/ana.410350615. [DOI] [PubMed] [Google Scholar]
- 90.Greenough WT, Black JE, Wallace CS. Experience and Brain Development. Child development. 1987;58(3):539. [PubMed] [Google Scholar]
- 91.Knudsen EI, Heckman JJ, Cameron JL, Shonkoff JP. Economic, Neurobiological, and Behavioral Perspectives on Building America's Future Workforce. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(27):10155–10162. doi: 10.1073/pnas.0600888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Locke A, Ginsborg J, Peers I. Development and disadvantage: implications for the early years and beyond. International Journal of Language & Communication Disorders. 2002;37(1):3–15. doi: 10.1080/13682820110089911. [DOI] [PubMed] [Google Scholar]
- 93.Battistich V, Solomon D, Kim D, Watson M, Schaps E. Schools as Communities, Poverty Levels of Student Populations, and Students' Attitudes, Motives, and Performance: A Multilevel Analysis. American Educational Research Journal. 1995;32(3):627–658. [Google Scholar]
- 94.Magnuson KA, Ruhm C, Waldfogel J. Does prekindergarten improve school preparation and performance? The Economics of Early Childhood Education. 2007;26(1):33–51. [Google Scholar]
- 95.Leventhal T, Brooks-Gunn J. The neighborhoods they live in: The effects of neighborhood residence on child and adolescent outcomes. Psychological Bulletin. 2000;126(2):309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- 96.Dearing E, Wimer C, Simpkins SD, et al. Do neighborhood and home contexts help explain why low-income children miss opportunities to participate in activities outside of school? Developmental psychology. 2009;45(6):1545–1562. doi: 10.1037/a0017359. [DOI] [PubMed] [Google Scholar]
- 97.Kohen DE, Leventhal T, Dahinten VS, McIntosh CN. Neighborhood Disadvantage: Pathways of Effects for Young Children. Child Development. 2008;79(1):156–169. doi: 10.1111/j.1467-8624.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 98.Hotchkiss L. A Technique for Comparing Path Models Between Subgroups. Sociological Methods & Research. 1976 Aug 1;5(1):53–76. [Google Scholar]


