Abstract
In addition to its maladaptive effects on psychiatric function, psychosocial deprivation impairs recovery from physical illness. Previously, we found that psychosocial deprivation, modeled by isolation rearing, depressed immediate early gene (IEG) expression in the medial prefrontal cortex (mPFC) and increased locomotion in the open field test (Levine, Youngs et al. 2007). In the present study, we examined whether similar changes in behavior and gene expression are associated with the maladaptive effects of psychosocial deprivation on physical injury healing. After weaning, anesthetized rats were subjected to a 20% total body surface area third degree burn injury and were subsequently either group or isolation reared. After four weeks of either isolation or group rearing (a period that encompasses rodent childhood and early adolescence), rats were sacrificed, and their healing and gene expression in the mPFC were assessed. Locomotion in the open field test was examined at 3 weeks post burn injury. We found that: 1) gross wound healing was significantly impaired in isolation reared rats compared to group reared rats, 2) locomotion was increased and IEG expression was suppressed for isolation reared rats during burn injury healing, 3) the decreased activity in the open field and increased IEG expression was greater for burn injury healing group reared rats than for uninjured group reared rats, 4) the degree of hyperactivity and IEG suppression was relatively similar between isolation reared rats during burn injury compared to uninjured isolation reared rats, 5) behavioral hyperactivity to novelty (the open field test) along with IEG suppression may constitute a detectable biomarker of isolation rearing during traumatic physical injury. Implications of the findings for understanding, assessing, and treating the maladaptive effects of psychosocial deprivation on physical healing during childhood are discussed.
Introduction
In addition to its detrimental impact on mental health (Kaufman, Plotsky et al. 2000; Heim and Nemeroff 2001; Rutter 2002; Schilling, Aseltine et al. 2007), psychosocial adversity also detrimentally impacts physical health (Flaherty, Thompson et al. 2006; Heim, Wagner et al. 2006; Sareen, Jacobi et al. 2006). In addition, we and others have shown that patients with psychiatric difficulties have worse medical outcomes (Levine, Covino et al. 1996; Katon 2003; Tarrier, Gregg et al. 2005) and are at heightened risk for sustaining subsequent physical injuries (Rockwell, Dimsdale et al. 1988; Swenson, Dimsdale et al. 1991). Finally, physical illness occuring in the context of psychiatric illness impairs functional outcomes and increases the financial cost of medical illness (Stein, Cox et al. 2006). Thus, in order to optimize treatment approaches for patients with concomitant physical illness and psychosocial adversity, it is important to understand the mechanism by which physical outcomes are mediated by psychosocial adversity.
In an effort to increase our understanding of how psychosocial adversity affects physical health, we applied an animal model of psychosocial deprivation (isolation rearing) that we had previously used (Levine, Youngs et al. 2007) to rats that were healing from a burn injury between PN days 20–40. This period corresponds to childhood and early adolescence in the rodent, so that findings using this model may provide some insights into the effect of deprivation states on physical healing during this developmental period.
Isolation rearing is a well established rodent model to study psychosocial deprivation (Lapiz, Fulford et al. 2003). In addition, isolation rearing has been found to impair wound healing (Detillion, Craft et al. 2004; Glasper and Devries 2005). Other animal models of psychological stress, particularly restraint stress, impair wound healing (Padgett, Marucha et al. 1998; Sheridan, Padgett et al. 2004; Horan, Quan et al. 2005; Eijkelkamp, Engeland et al. 2007), although interestingly, the social intruder model of stress did not affect wound healing (Sheridan, Padgett et al. 2004).
After determining that our model of isolation rearing (Levine, Youngs et al. 2007) led to impaired burn injury healing, we sought to determine whether behavioral and medial prefrontal cortex (mPFC) gene expression changes that we had observed with isolation rearing in healthy animals were associated with isolation rearing in the burn injury healing rats. Specifically, we had previously observed that isolation rearing in physically healthy rats downregulated immediate early gene (IEG) expression in the mPFC and increased locomotion in the open field test (Levine, Youngs et al. 2007). We reasoned that if similar changes were associated with the isolation reared rats during burn injury healing, such behavioral and molecular changes would provide useful biomarkers of the brain and behavioral changes associated psychosocial adversity during injury healing.
We focused on the mPFC because of its central role in modulating areas of the brain associated with stress responses such as the amygdala and hippocampus (Rauch, Shin et al. 2006). Furthermore, at the cellular level, chronic stress (restraint sress) changes dendritic arborization in the mPFC (Radley, Rocher et al. 2005; Radley, Rocher et al. 2006). Finally, cells in the mPFC have a high density of glucocorticoid receptors which receive input from cortisol released in response to physical and psychosocial stressors, and which modulate stress responses through connections with the hippoampus and hypothalamic-pituitary-adrenal (HPA) axis (Diorio, Viau et al. 1993).
The findings of the current study revealed that, as expected from the clinical literature, psychosocial adversity, as modeled by isolation rearing significantly worsened wound healing. Furthermore, isolation rearing after burn injury, significantly altered IEG expression in the mPFC and behavior in the open field test. For group reared rats (but not for isolation reared rats) burn injury healing resulted in significantly altered open field locomotion and IEG expression relative to uninjured control rats. The overall effect was that during burn injury healing the behavioral and IEG expression changes in the mPFC detected previously for uninjured isolation reared rats, continued to serve as useful biomarkers of isolation rearing. We discuss the implications of these findings for assessing and treating psychosocial adversity in children undergoing healing from physical injuries.
Methods
Animals
The animals were maintained in accordance with National Research Council guidelines and the experimental protocols were approved by the Subcommittee on Research Animal Care, Committee on Research, Massachusetts General Hospital. Male Sprague-Dawley rats (Taconic Farms, Germantown, New York; Charles River Laboratories, Wilmington) were obtained at postnatal day (PN) 17 with lactating dams. On PN 20, the pups were weaned and separated into 4 conditions: a) no injury, followed by 4 weeks of group rearing (n=3 per cage), b) no injury, followed by 4 weeks of isolation rearing, c) a 20% dorsal surface burn under ketamine/zylaxine anesthesia followed by 4 weeks of group-housing (n=3 per cage), d) the same burn injury followed by 4 weeks of isolation rearing. Uninjured rats received ketamine/zylaxine anesthesia when placed into group or isolation rearing conditions to control for the effect of anesthesia. Rats from both experimental conditions were housed in the same animal room. Rats weighed 40–50 g at the time of weaning. Rats were sacrificed by rapid decapitation on PN 46.
Wound Healing Analysis
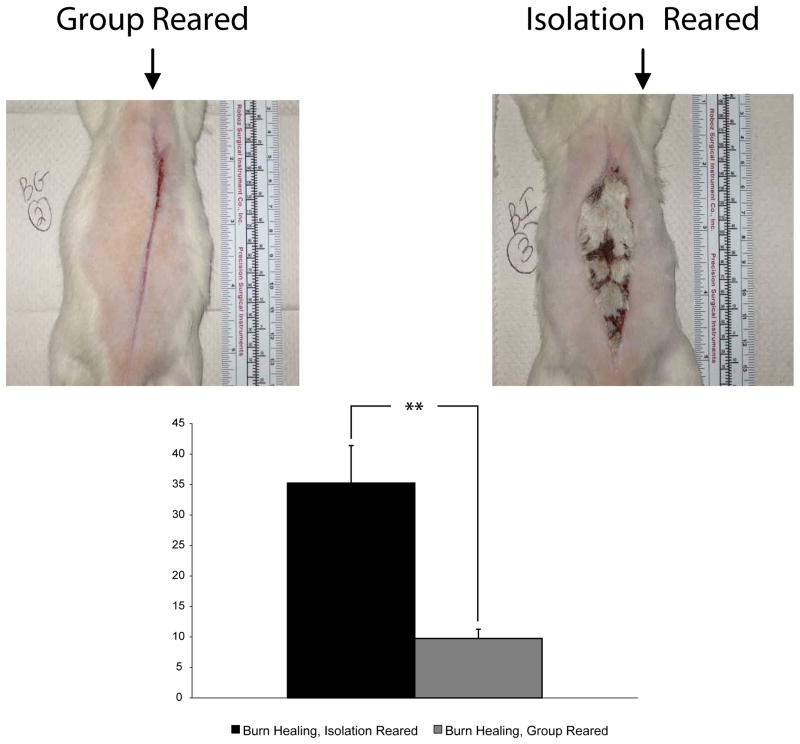
The approach to measure the wounds was as follows: The margins of the unhealed region of the burn injury were outlined with metamorph. The area inside of the outlined region was quantified by the number of pixels inside that region. This region was normalized to the number of pixels that comprised a one inch square based on the ruler pictured with each animal (see images in Figure 1A and 1B below). The healing was compared for 9 group reared and 9 isolation reared rats from 3 different experiments that compared the healing between these two conditions.
Figure 1.
Impaired burn healing for isolation reared rats. Gross morphology of burn injury healing was significantly impaired for isolation reared rats (B) than for group reared rats (A). The number of pixels of unhealed tissue normalized to the area comprising 1 square inch of the ruler shown in the pictures in A and B was determined for each group reared and each isolation reared rat. The average normalized pixels of unhealed tissue was significantly greater for the isolation reared compared to the group reared rats (C). Average ± S.E.M., **P<.01 (two tailed, unpaired T-Test, for sample with unequal variance). Group Reared (n=9), Isolation Reared (n=9).
Behavioral Assays
On PN 38 (after 18 days of group or isolation-rearing), rats were tested in locomotor boxes for behavior in the open field (Med Associates, St. Albans, Vermont). All testing was carried out over 5 consecutive hours on a single day. Animals were habituated to the room in which the test was done for 15 minutes prior to testing. Two rats were tested at a time, based on the availability of locomotor boxes. All rats were tested for at least 35 minutes in the open field, and locomotor activity was analyzed at 5-minute intervals over the first 30 minutes at which point inactivity tended to dominate in group-reared rats. Cages were wiped down with alcohol and water after each test. Behavioral data was obtained on four sets of rats a) control group reared rats that were uninjured (n=20), b) group reared rats undergoing burn injury healing (n=8), c) control isolation reared rats that were uninjured (n=18), and d) isolation reared rats undergoing burn injury healing (n=17).
Sample Processing
Animals were rapidly decapitated, whole brains extracted, immediately frozen in isopentane and stored at −80°C. The area of the brain corresponding to the medial prefrontal cortex (mPFC; cingulate cortex 1, prelimbic cortex, infralimbic cortex, medial orbital and ventral orbital cortex; from bregma, AP: +4.2 to +2.2) (Paxinos G 1998) was dissected on a freezing microtome. All samples were stored at −80°C until RNA extraction. RNA was extracted from approximately 20–30 mg tissue using the Qiagen kit (Valencia, CA). 1 – 7 μg of total RNA was used for cDNA synthesis with the SuperScript double-stranded cDNA synthesis kit (Invitrogen, Carlsbad, CA).
i) Microarray Processing
In vitro transcription was performed with the Affymetrix IVT labeling kit (Santa Clara, CA). Biotinylated RNA was hybridized to the RAE 230 array (Affymetrix, Santa Clara, CA) and washing and staining was carried out according to company protocol. Samples from individual rats were hybridized to individual arrays. The Affymetrix RAE230_2.0 array contains 31,100 probe sets. Each probe set is represented by 11 perfectly matched 25-mer oligonucleotides, and the same number of one-mismatch oligonucleotides to provide values for nonspecific binding. Gene arrays were completed for 4 rats in each of the burn injury conditions and for 4 uninjured, group reared rats.
ii) qPCR Processing
The gene expression patterns were assessed, using qPCR, on different rats from those used for the gene array analyses comparing the control group reared rats that were uninjured condition (n=5) to the same two experimental conditions used in the gene array analyses: group reared rats undergoing burn injury healing (n=5) and isolation reared undergoing burn injury healing (n=6). Tissue was dissected from the mPFC and the mRNA extracted in the same manner described for the gene array analyses above. Approximately 100ng-1 μg of total mRNA was reverse transcribed to cDNA using the TwoStep RT-PCR Kit (Qiagen, Valencia, CA) per manufacturer’s instructions and amplified in a Perkin Etus Thermal Cycler 480. Cycling conditions were: 1) 25 °C for 10 minutes; 2) 42 °C for 60 minutes; 3) 94 °C for 10 minutes, and then stored at 4 °C. cDNA was analyzed by kinetic PCR using the same cycling conditions for amplification: 1) 94°C for 10 minutes; 2) 30–40 cycles of 94°C for 30 seconds, 55°C for 30 seconds and 72 °C for 1 minute; and 3) a final extension step at 72 °C for 10 minutes.
Quantitative RT-PCR was performed in the Advanced Tissue Resource Center (ATRC) within the Harvard Center for Neurodegenerative Disease. Primers used for amplification were designed using the public software algorithm Primer3 (www-genome.wi.mit.edu/cgi-bin/primer/primer3.cgi.) for amplicons between 100 to 200 base pairs. Genes chosen for qPCR verification were selected from the IEGs that were regulated in the gene array between group and isolation reared rats in this (Figure 4) and the prior study (Levine, Youngs et al. 2007). Biorad Software (www.bio-rad.com) was used to analyze the data. Data from all experiments was combined for computation of gene expression differences. Reported values were normalized to the internal standard GAPDH. The base sequence of the forward and reverse primers used for each gene analyzed by qPCR is shown in Table 1.
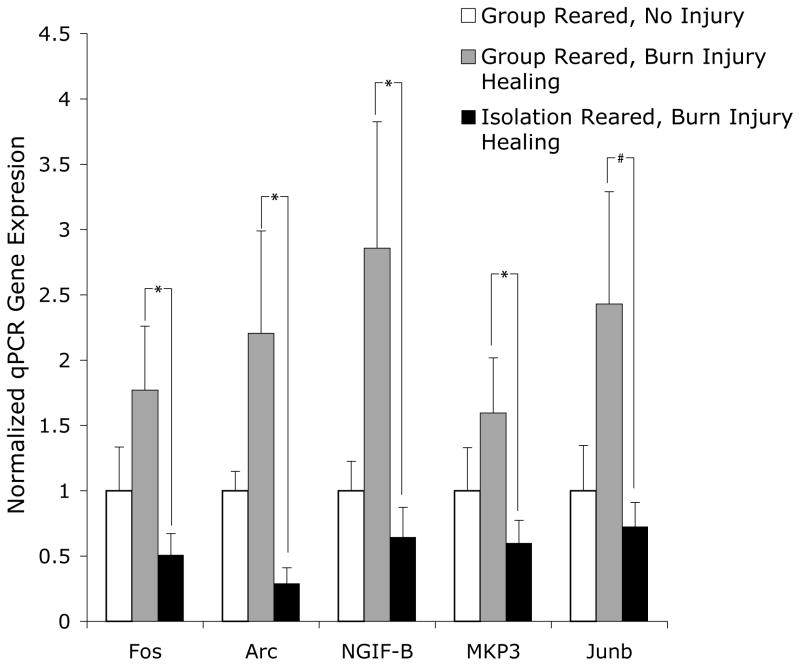
Figure 4.
Effect of isolation rearing condition on IEG expression during burn injury healing. The graph shows the IEG fold changes for the uninjured group reared rats (column 1, n=9), burn injury healing group reared rats (column 2, n=4), uninjured isolation reared rats (column 3, n=6) and burn injury healing isolation reared rats (column 4, n=4). The table below the graph shows the parametric p value and false discovery rates for the gene expression changes between the group versus isolation reared rats undergoing burn injury healing (columns 4 and 5) and the uninjured group versus isolation reared rats (columns 7 and 8 in table below graph) from the previous study (Levine, Youngs et al. 2007). The significance values in the table are for the log2 transformed values of the gene expression fold changes shown in the graph. Average ± SEM, *p<.05, **p<.01, ***p<.001.
Table 1.
Entrez GeneID Numbers and Primer Sequences of Genes Chosen for Quantitative Polymerase Chain Reaction Experiments
| Gene of Interest | Entrez Gene No. | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| Junb | 3726 | TAT GGA GCA AGG GAG GCT CT | CCT GGA GGA CAA GGT GAA GA |
| MKP1 | 1843 | AAT ACT CCG CCT CTG CTT CA | AGG ACA ACC ACA AGG CAG AC |
| Fos | 2353 | GAA GGA ACC AGA CAG GTC CA | TCA CCC TGC CTC TTC TCA AT |
| NGFI-B | 3164 | TCC AGC TTG AGG CAA AAG AT | TGC TCT GGT CCT CAT CAC TG |
| Arc/Arg3.1 | 23237 | GGT GTC ATT CAC CTG GCT CT | AGT CTT GGG CAG CAT AGC TC |
See Figure 4 for more complete description of gene names
Quality Control Criteria
RNA quality and quantity was assessed by spectroscopy.
i) Microarray Data
All quality control criteria defined by Affymetrix were met by the samples and no differences between the experimental groups were observed. The average percent “present” call across all arrays was 62.8%. The 3′/5′ ratio of glyceraldehyde 3-phophate dehydrogenase (GAPDH) was 1.8. Background was comparable between all groups.
ii) qPCR Data
Blanks were run with each primer to control for primer-dimer formation. A melt curve was used to confirm the specificity of each primer pair. Blanks and samples were run two-three times for each animal in separate qPCR experiments.
Data Analysis
i) Gene Array Analyses
The BRB Array program from NCBI was used (http://linus.nci.nih.gov/BRB-ArryTools.html; http://linus.nci.hin.gov/~brb/TechReport.htm) for the gene array analyses. Using this program, global normalization was used to median center the log-ratios on each array in order to adjust for differences in labeling intensities. Probe sets not passing a filter of less than 50% absent calls were removed from the analyses. Genes showing minimal variation across the set of arrays were excluded from the analysis. For the class comparison analyses, only genes whose expression differed by at least 1.5 fold from the median in at least 20% of the arrays were retained.
To determine if IEG expression was differentially expressed between the isolation and group reared rats undergoing burn injury healing, compared gene expression between these two conditions (class comparison) using the random-variance t-test. The random-variance t-test is an improvement over the standard separate t-test as it permits sharing information among genes about within-class variation without assuming that all genes have the same variance (Wright and Simon 2003). Genes were considered statistically significant if their p value was less than 0.001. A stringent significance threshold was used to limit the number of false positive findings.
To determine the false discovery rate for identified genes, we used a multivariate permutation test (Korn, Li et al. 2007). The false discovery rate is the proportion of the list of genes claimed to be differentially expressed that are false positives.
To determine if the genes that discriminated the uninjured isolation reared from group reared rats in the prior study could form prediction equation for distinguishing isolation reared from group rats during burn injury healing, we entered the genes that distinguished these two conditions in uninjured rats into the following models that predict the class of future samples: a) Compound Covariate Predictor (Radmacher, McShane et al. 2002), b) Diagonal Linear Discriminant Analysis (Dudoit, F et al. 2002), c) Nearest Neighbor Classification (Dudoit, F et al. 2002), and d) Support Vector Machines with linear kernel (Ramaswamy, Tamayo et al. 2001). The models incorporated genes that were differentially expressed among genes at the 0.001 significance level as assessed by the random variance t-test described above. We estimated the prediction error of each model using leave-one-out cross-validation (LOOCV) (Simon, Radmacher et al. 2003).
To compare the gene expression changes of interest from this study to those of uninjured group and isolation reared rats, we normalized the raw expression values of each gene of interest to that of uninjured, group reared and isolation reared rats. This allowed a comparison of gene expression of uninjured isolation reared rats to isolation reared rats undergoing burn injury healing since gene expression data for burn healing isolation reared rats was collected on affymetrix chip RAE230_2.0 and the gene expression data of uninjured isolation reared rats was collected on the Affymetrix RAE230_A chip in the prior study (Levine, Youngs et al. 2007). To do this, we first determined the fold changes for group and isolation reared rats undergoing burn injury healing relative to uninjured group reared rats from the current study. Next, we normalized the raw expression values of each gene of interest for uninjured isolation reared rats from the prior study (Levine, Youngs et al. 2007) to group reared rats from that study.
To determine biologically meaningful categories of genes that were differentially expressed between the group and isolation reared rats during burn injury healing, we identified gene ontology (GO) groups of genes whose expression was differentially regulated between these two groups of rats. By analyzing GO groups, rather than individual genes, we were able to reduce the number of tests conducted, and to enable findings among biologically related genes to reinforce each other. For each GO group we computed the number of genes represented on the microarray in that group, and the statistical significance value for each gene in the group. These p values reflect differential expression among classes and were computed based on random variance t-tests (Wright and Simon 2003). For each GO category, two significance levels are computed, corresponding to the two summary statistics: the Fisher (LS) statistic and the Kolmogorov-Smirnov (KS) statistic (as described in Simon, R. and Lam, A. BRB-ArrayTools User Guide, version 3.2. Biometric Research Branch, National Cancer Institute. http://linus.nci.nih.gov/brb). A GO category is considered significantly differentially regulated if either significance level was less than 0.01. We considered all GO categories with between 5 and 100 genes represented on the array.
ii) qPCR analysis
The PCR data was analyzed using the 2−ΔΔCT method. The relative expression of each gene examined was computed for each experimental condition by subtracting the internal control gene (GAPDH) from the gene of interest for the experimental group and then subtracting this from the difference between GAPDH expression and the gene of interest for the control rats (group reared rats that were uninjured). Thus the following equation was calculated for each rat in each condition: 2− ((GOIe-GAPDHe) − (GOIc-GAPDHc)) where GOI is the gene of interest, e is the experimental condition, and c is the control condition. After this calculation and elimination of outliers (mean + or − 2SDs), the average gene expression was determined for each experimental condition relative to the control condition. The SD and SEM for each experimental condition and the control condition was determined from the combination of all animals in each of these conditions.
iii) Behavioral (Open Field Test) analyses
The student’s t-test, for unpaired data with assumed unequal variance, was used to analyze the difference between rats in each set of conditions for ambulatory time, distance traveled, and resting time.
Results
Burn Injury Healing is improved in Group versus Isolation Reared Rats
Figures 1A and 1B illustrate the difference in burn injury healing between the rats reared in the two conditions. For the group reared rats (1A), wound margins closely apposed each other after 28 days of healing and healing appeared to occur mostly by intention. Healing by intention was significantly decreased for the isolation reared rats (1B) where healing by granulation predominated. As shown in 1C, the normalized (as described in methods) number of pixels of unhealed tissue for the isolation reared rats was significantly greater for the isolation reared compared to the group reared rats. To ensure reliability of the results, we repeated the experiment three times. The results shown combine the data for the animals in each condition for the 3 experiments (resulting in 9 animals obtained in total for each condition).
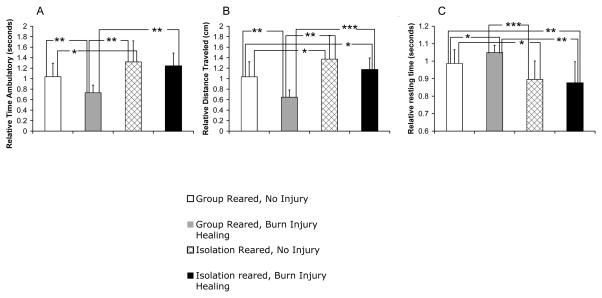
During Burn Injury Healing, Isolation Reared Rats evidenced Increased Locomotion in the Open Field Test Relative to Group Reared Rats, but not Relative to Control Isolation Reared Rats
During burn injury healing, rats reared in isolation had significantly greater distance traveled and significantly less resting time compared to both control uninjured group reared rats and group reared rats undergoing burn injury healing (compare column 4 to columns 1 and 2 in Figures 2B and 2C). For distance traveled, the burn injury healing isolation reared rats also had increased activity relative to the burn healing group reared rats, but not to the control uninjured group reared rats (compare columns 4 to columns 1 and 2, Figure 2A).
Figure 2.
Effect of Isolation Rearing on Open Field Test Behavior. Shown are ambulatory time (A), distance traveled (B), and resting time (C) for uninjured group reared rats (column 1, n=20), burn injury healing group reared rats (column 2, n=8), uninjured isolation reared rats (column 3, n=16), and burn injury healing isolation reared rats (column 4, n=17). Average ± SD,*p<.05, **p<.01, ***p<.001
Of note, the isolation reared rats undergoing burn injury healing were similarly hyperactive to the control uninjured isolation reared rats (compare columns 3 and 4, Figure 2A–C). On the other hand, for the group reared rats, there was a difference in activity level between control uninjured and burn healing rats on all activity measures in the open field (compare columns 1 and 2, Figures 2). As a result of this decrease in activity of burn injury healing group reared rats compared to control uninjured group reared rats, there was a greater difference in activity level between group and isolation reared rats undergoing burn injury healing compared to uninjured isolation and group reared rats (compare the difference between columns 1 and 3 to 2 and 4 in Figures 2A–2B).
Thus, overall, the results from the open field findings suggest that isolation rearing elicits an opposite open field test response to group reared rats during burn injury healing. Furthermore, this opposing pattern of activity is of a greater magnitude during burn injury healing compared to control uninjured rats, primarily due to the greater open field activity decrease in group reared burn healing rats compared to control group reared rats. On the other hand, isolation rearing seems to elicit a similar hyperactive response, regardless of whether the rats were undergoing burn injury healing or not.
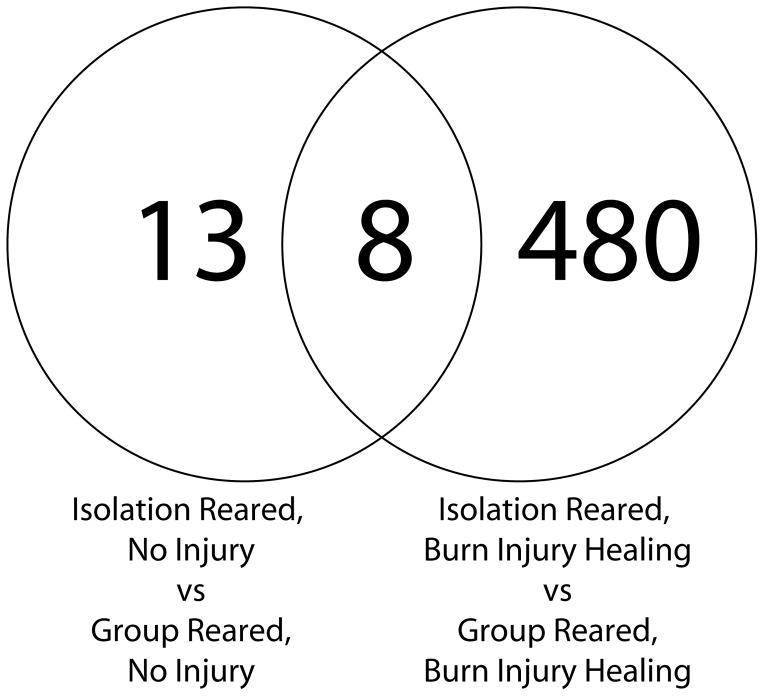
Although Many More Genes were Altered by Isolation Rearing During Burn Injury Healing, the Changes in IEG expression were Similar to Uninjured Isolation Reared Rats
To determine whether the changes in IEG expression in the mPFC that occurred in uninjured rats (Levine, Youngs et al. 2007) were also altered by isolation rearing during burn injury healing, we compared gene expression changes associated with isolation rearing during burn injury to gene expression changes that were altered by isolation rearing in the prior study with uninjured rats To control for false positives in this analysis we only included gene expression differences that met a very stringent p-value (.001) and that had an overall false discovery rate (FDR) of less than 0.1. These criteria identified 488 genes that were differentially expressed by by isolation rearing relative to group rearing during burn injury healing. The FDR for all 488 of these genes was less than .007. As shown in Figure 3, these genes included 8 of the 21 genes that were previously identified as altered by isolation rearing in uninjured rats. All 8 of these genes were IEGs (these are further discussed in the next section and are shown in Figure 4). Thus, although there were many previously unidentified genes that were differentially expressed between group and isolation reared rats during burn injury healing compared to when there was no injury, the key genes we previously identified as markers of isolation rearing in the mPFC (Levine, Youngs et al. 2007) continue to differentiate isolation from group reared rats during burn injury healing.
Figure 3.
Overlap of gene expression changes due to isolation rearing in uninjured and burn injury healing rats. All genes in the overlap were immediate early genes (discussed in the text).
Isolation Reared Rats Show Suppressed Immediate Early Gene Expression Relative to Group Reared Rats During Burn Injury Healing
All 8 of the IEGs that were altered by isolation rearing for both burn healing and uninjured rats (overlap of Figure 3) were suppressed to a statistically significant degree as measured by the parametric p value which compared the log base 2 transformed values of the raw expression data of these genes (note fold change direction and parametric p values for isolation compared to group reared rats for burn healing rats and uninjured rats on left and right sides, respectively, of the table below the graph in Figure 4). Using the students T test, these comparisons were also significant for the relative fold changes (compare columns 1 and 3 to columns 2 and 4 in graph in Figure 4).
The IEG expression fold changes were largely not significantly different between isolation reared rats with and without burn injury (compare columns 3 and 4 in graph in Figure 4), although it will be noted that Arc and Egr2 were altered. On the other hand, for group reared rats, the degree of IEG expression was significantly greater for every IEG examined compared to control, uninjured, group reared rats (compare columns 1 and 2 in graph in Figure 4). This indicates that the greater magnitude of IEG expression differences between group and isolation reared rats was largely due to the effect of burn injury on group reared rats.
Prediction of Group versus Isolation Rearing During Burn Injury Healing
Taken together with our previous findings that IEG expression is reduced in uninjured and surgically stressed rats (Levine, Youngs et al. 2007), the present observation that IEG expression is also downregulated in burn injury healing rats (Figure 4), suggests that decreased IEG expression is a predominant (“main effect”) of isolation rearing irrespective of the presence of other stressors. To examine this, we determined that the genes that differentiated isolation from group reared rats in the prior study (37 genes that included 13 IEGs) could predict the rearing status of the burn injury healing rats in the present study. We found that a compound predictor comprised of nine of these genes, all of which are IEGs, as shown in Table 2, predicted the rearing status of the rats in the present study with 100% accuracy. The positive predictive power, negative predictive power, sensitivity and specificity of these 9 genes for predicting the rearing status of the rats in the current study was 1 (values for these parameters range from 0.00–1.00). Eight of these nine genes overlapped with the genes that the class comparison analysis identified as differentiating isolation from group reared rats during burn injury healing (compare genes in Table 2 to genes in Figure 4). This suggests that these genes are main effect genes for isolation rearing and that an equation based upon these genes can predict isolation versus group rearing, both in the absence and presence of physical injury healing.
Table 2.
Class Prediction of Rearing Status During Burn Injury Healing from Genes Differentiating Rearing Status in Uninjured Rats
| A. Performance of classifiers during cross-validation. | |||||||
|---|---|---|---|---|---|---|---|
| Compound Covariate Predictor Correct? | Diagonal Linear Discriminant Analysis Correct? | 1-Nearest Neighbor Correct | 3-Nearest Neighbor Correct? | Nearest Centroid Correct? | Support Vector Machines Correct? | Bayesian Compound Predictor Correct? | |
| BurnGroup3 | YES | YES | YES | YES | YES | YES | YES |
| BurnGroup4 | YES | YES | YES | YES | YES | YES | YES |
| BurnGroup5 | YES | YES | YES | YES | YES | YES | YES |
| BurnGroup6 | YES | YES | YES | YES | YES | YES | YES |
| BurnIsolation1 | YES | YES | YES | YES | YES | YES | YES |
| BurnIsolation3 | YES | YES | YES | YES | YES | YES | YES |
| BurnIsolation5 | YES | YES | YES | YES | YES | YES | YES |
| BurnIsolation6 | YES | YES | YES | YES | YES | YES | YES |
| Mean percent of correct classification | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| B. Compostion of Classifier for Prediction of Rearing Status during Burn Injury Healing | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parametric p-value | t-value | % CV support | Geom mean of intensities of Group Reared Rats | Geom mean of intensities of Isolation Reared Rats | Ratio of geom means | Probe set | Description | Gene symbol |
| 0.000 | 5.29 | 25 | 124.6 | 59.8 | 2.084 | 1388792_at | growth arrest and DNA-damage-inducible 45 gamma (predicted) | Gadd45g_predicted |
| 0.000 | 5.88 | 100 | 177.8 | 66.3 | 2.682 | 1386994_at | B-cell translocation gene 2, anti-proliferative | Btg2 |
| 0.000 | 6.15 | 100 | 238 | 75.6 | 3.148 | 1376569_at | Kruppel-like factor | KLF |
| 0.000 | 7.13 | 100 | 336.8 | 54.5 | 6.18 | 1387306_a_at | early growth response 2 | Egr2 |
| 0.000 | 7.21 | 100 | 565.3 | 190.1 | 2.974 | 1368146_at | MAP Kinase Phosphatase 3 | MKP3 |
| 0.000 | 7.5 | 100 | 199.7 | 60.8 | 3.285 | 1387788_at | Jun-B oncogene | Junb |
| 0.000 | 9.28 | 100 | 622.7 | 191.3 | 3.255 | 1386935_at | nuclear receptor subfamily 4, group A, member 1 | NBFI-B |
| 0.000 | 10.05 | 100 | 674.5 | 117.7 | 5.731 | 1375043_at | FBJ murine osteosarcoma viral oncogene homolog | Fos |
| 0.000 | 10.2 | 100 | 575.8 | 103.5 | 5.563 | 1387068_at | activity regulated cytoskeletal-associated protein | Arc |
PCR Confirmation of Gene Array Findings for IEG Expression
To confirm the gene array findings for the IEG expression differences between group and isolation reared rats during burn injury healing, we used qPCR to analyze the expression of five of the IEGs that were regulated by rearing according to the array findings (Figure 4). For these analyses, we used different animals subjected to the two experimental conditions examined in the gene arrays: (group reared during burn healing; n=5 and isolation reared during burn healing; n=6) and one of the control conditions examined above (group reared, uninjured animals; n=5). The use of a separate set of animals for the qPRC analyses allowed these experiments to both validate the array findings with a different technique and to replicate the experimental findings. As expected, the qPCR results showed a pattern of gene expression for IEG expression that was the same as that found for the array data (Figure 5). Namely, relative to group reared rats undergoing burn healing, isolation reared rats undergoing burn injury healing had lower levels of IEG expression. Furthermore, as with the array data, the qPCR data indicated greater expression for each IEG examined for group reared rats undergoing burn injury healing compared to group reared, uninjured rats. The differences between group reared and isolation reared rats undergoing burn injury healing were significant for four of the five genes assessed with qPCR and marginally significant for the fifth (Figure 5).
Figure 5.
Quantitative PCR (qPCR) Verification of Isolation Rearing Effect on IEG Expression During Burn injury Healing. Using a separate group of rats, qPCR was computed in 2–3 different experiments for 5 of the 9 IEGs that differentiated the experimental and control conditions in Figure 3. Data were normalized to both control gene Gapdh and the control set of uninjured group reared rats using the ΔΔCT method as described in the Methods. As shown, the differences between group and isolation reared rats undergoing burn injury healing were significant for 4 of these 5 genes, and marginally significant for the 5th gene. Average ± SEM, *p<.05, #p=.08. Group reared, uninjured rats (n=5); group reared, burn injury healing rats (n=5), isolation reared, burn injury healing rats (n=6).
Discussion
The present study yielded several findings: i) burn injury healing is significantly worse for isolation reared rats than for group reared rats, ii), during their healing, isolation reared rats were significantly hyperactive in the open field test, compared to the group reared rats, iii) IEG expression in the mPFC was significantly suppressed for the isolation reared rats, relative to the group reared rats during burn injury healing, iv) although group reared rats showed a difference in behavior and IEG expression during injury healing compared to uninjured rats, isolation reared rats showed a similar pattern on these markers relative to group reared rats, regardless of whether they were undergoing burn injury healing or not. Taken together with our prior finding that isolation rearing produced these results in unperturbed and in surgically stressed rats (Levine, Youngs et al. 2007), the current study provides strong evidence that open field hyperactivity and IEG suppression in the mPFC can serve as a bio-behavioral signature of isolation rearing, even in the presence of other significant stressors.
Relation between isolation rearing and impaired wound healing
The present study clearly indicates a causal role between isolation rearing and impaired wound healing (as the rearing conditions were altered prior to changes in wound healing). This converges with other findings suggesting that psychosocial stress has a causal link to the impaired wound healing associated with it (Padgett, Marucha et al. 1998; Detillion, Craft et al. 2004; Sheridan, Padgett et al. 2004; Glasper and Devries 2005; Horan, Quan et al. 2005; Eijkelkamp, Engeland et al. 2007).
On the other hand, we can only definitely draw an association between the impaired wound healing and the neurobiological and behavioral findings of this study (as the behavioral, gene expression, and wound healing findings were gathered at approximately the same time). The large body of clinical literature suggesting a causal relationship between psychosocial distress and physical illness (Levine, Covino et al. 1996; Katon 2003; Tarrier, Gregg et al. 2005; Flaherty, Thompson et al. 2006; Heim, Wagner et al. 2006; Sareen, Jacobi et al. 2006) supports the view that the isolation rearing impairment of wound healing resulted from the associated brain and behavior changes we found. Nonetheless, to firmly establish whether these changes are cause or consequence of this relationship, further investigation is needed.
Regardless of whether the behavioral and gene expression findings we found associated with isolation rearing and poor wound healing are cause or consequence, the association has an interesting parallel with the research on sickness behavior (Dantzer and Kelley 2007). Studies of illness behavior have found that adaptive illness behavior and maladaptive illness behavior both involve a specific set of IEG expression patterns and behavioral activity patterns (Kozak, Conn et al. 1994; Yirmiya, Rosen et al. 1994; Konsman, Luheshi et al. 2000; Dantzer 2001; Engeland, Nielsen et al. 2001; Engeland, Kavaliers et al. 2003; Huang, Cheng et al. 2004; Konsman and Blomqvist 2005; Stone, Lehmann et al. 2006; Dantzer and Kelley 2007; Lawrence, Stroman et al. 2007). Most of these studies have found increased IEG expression and decreased locomotion during adaptive healing and the opposite pattern with maladaptive healing. It is tempting to analogize our IEG and behavioral findings for isolation rearing and group rearing to these patterns. This would suggest that the group reared rats evidenced more adaptive illness behavior than the isolation reared rats, as increased IEG expression and decreased locomotion was seen for the group reared rats during burn injury healing. However, almost all of the studies on adaptive and maladaptive healing studies involved data collected immediately after an illness induced perturbation such as lipopolysaccharide or formalin injection, whereas we measured behavior and gene expression several weeks into the burn injury healing. Further examination of the whether the open field and IEG changes were cause or consequence of the isolation rearing induced healing impairment will allow more definitive conclusions about whether these changes reflect maladaptive sickness behavior.
Behavioral Differences between Isolation and Group Reared Rats During Burn Injury Healing
The changes in the open field test (hyperactivity) found as an effect of isolation rearing in this study and our previous one (Levine, Youngs et al. 2007) are completely consistent with the vast body of literature on open field behavior, which finds increased locomotion during isolation rearing (Lapiz, Fulford et al. 2003). Most commonly, an increased response to novelty is posited as the basis for the hyperactive response of isolation reared rats to the open field (Hall, Humby et al. 1997; Hall, Huang et al. 2000), which may in turn may relate to deficient inhibitory controls (Morgan and Einon 1975) and decreased habituation (Einon, Morgan et al. 1975), as well as hyperactivity that is a response to anxiety of a novel situation (Holson 1986).
The present study, though, adds to our understanding of this effect as it occurred in the context of burn injury healing. The results of the present study are somewhat counterintuitive because they show that the addition of burn injury healing, a rather powerful peripheral stressor, had almost no effect on the hyperactivity in the open field test attained by control, isolation reared rats. In contrast burn injury did have a significant effect on activity in the open field for group reared rats. Since the healing was far worse for isolation reared rats, we might have expected that it would be during isolation rearing, rather than group rearing, that an effect of burn injury healing on behavior would be detected. The implication of this set of findings is that the effect of isolation rearing on behavior, in this model, prevails over the impact of burn injury healing on behavior. This is somewhat consistent with the results of the prior study (Levine, Youngs et al. 2007) where we found that isolation reared rats with or without surgical stress showed similar levels of hyperactivity in the open field test. Thus, hyperactivity in the open field test may serve as a signature of psychosocial deprivation (isolation rearing) that can demarcate it from a more psychosocially supportive situation (group rearing), both in the presence and absence of other stressors.
The Immediate Early Gene Expression Changes Parallel the Behavioral Findings
As with the behavioral findings just discussed, mPFC IEG expression was significantly different for isolation reared rats compared to group reared rats during burn injury healing. Also, similar to the behavioral pattern, while IEG expression was affected by burn injury healing for group reared rats, IEG expression was relatively impervious to the effect of burn injury healing for isolation reared rats. An exception to this pattern was that the expression of the IEGs Egr2 and Arc was regulated by burn injury for isolation reared rats, though in opposite directions. Egr genes do alter the expression of Arc (Li, Carter et al. 2005) so it is possible that a signaling cascade specific to Egr2 and Arc is affected by burn injury healing during isolation rearing. Nonetheless, during burn injury healing, these genes, as with the other IEGs, were suppressed relative to both injured and uninjured group reared rats.
Of note, as with the behavioral findings, in the prior study we also found that IEG suppression occurred in uninjured animals and in surgically stressed rats (Levine, Youngs et al. 2007). Thus, IEG suppression, along with hyperactivity in the open field test, demarcate isolation reared from group reared rats, both during injury healing and in uninjured control animals. We conclude that these behavioral and gene expression changes appear to be candidate biomarkers (a bio-behavioral “signature”) of isolation rearing and psychosocial deprivation with or without concomitant stressors.
Link between between Behavioral and IEG changes during Isolation Rearing
The present study provides some support to our prior hypothesis that locomotion in the open field has a direct relationship to IEG expression (Levine, Youngs et al. 2007). Namely, as just discussed, during burn healing, group and isolation reared rats displayed an activity pattern that followed the mPFC IEG expression pattern. A recent study in this Journal (Gallitano-Mendel, Izumi et al. 2007) found that when the transcriptional IEG Egr3 is knocked out, mice show a hyperactive response in the open field. These authors suggest that the absence of this IEG, which transcribes the effector IEG Arc (Li, Carter et al. 2005) and regulates long term potentiation (LTP) and long term depression (LTD) of neuronal synapses (Plath, Ohana et al. 2006), results in open field hyperactivity due to decreased habituation to its novelty. Consistent with this, impaired expression of the IEG Arc is associated with deficient long term memory and increased exploration of a novel object (Plath, Ohana et al. 2006). LTD (which is associated with altered IEG expression) is also associated with impaired spatial memory (Nakao, Ikegaya et al. 2002) and brief exposure to stressful situations (Xu, Anwyl et al. 1997). Together with our current findings, these findings support our earlier hypothesis (Levine, Youngs et al. 2007) that IEG suppression may serve as a molecular marker of hyperactivity in the open field, particularly if one considers the hyperactivity in the open field as impaired habituation to novelty stress.
Developmental and Anatomical Considerations
The current study was done on juvenile rats between ages PN20 and PN46. This period roughly corresponds to that of childhood and early adolescence (rodents become fully sexually mature around PN65). Therefore isolation rearing and physical injury stress studied here have to be considered in light of the developmental changes that occur in this time period. During this developmental period the prefrontal cortex normally undergoes retraction of some dendrite spiny processes (pruning) that follows rapid growth of spiny processes during infancy (Rakic, Bourgeois et al. 1986). This pruning, though, occurs at a specific rate allowing optimization of synaptic connectivity and continuing development of important new connections (Katz and Shatz 1996; Zhang and Poo 2001; Huang, Chou et al. 2005; Sur and Rubenstein 2005). If isolation rearing occurs during this developmental period, the dendrite spiny processes in the medial prefrontal cortex are reduced even beyond that of control rats (Silva-Gomez, Rojas et al. 2003) suggesting abnormal neuronal plasticity due to isolation rearing. Our findings of decreased IEG expression in the mPFC in isolation reared rats during this developmental period are consistent with this finding in that both IEG expression (Lanahan and Worley 1998; Guzowski 2002) and dendrite spiny process development (Yuste and Bonhoeffer 2001) are measures of neuronal plasticity. Furthermore IEG expression has been linked to both long term potentiation (LTP) and long term depression (LTD) (Abraham, Christie et al. 1994) and these processes (LTP and LTD) are associated with growth (Engert and Bonhoeffer 1999; Maletic-Savatic, Malinow et al. 1999) and retraction (Zhou, Homma et al. 2004) of spiny processes, respectively.
In this study we focused on the mPFC because of its key role in regulating the stress response, particularly in terms of the learning required to extinguish a conditioned fear both in animal studies (Quirk, Garcia et al. 2006; Burgos-Robles, Vidal-Gonzalez et al. 2007) and human studies (Milad, Wright et al. 2007). Although other key brain regions are involved in the stress response (amygdala, hippocampus, hypothalamus), the mPFC plays a central role in modulating these responses (Rauch, Shin et al. 2006). Furthermore the mPFC shows neuroplasticity in response to the kind of stressors we examined in this study (Wellman 2001; Kolb, Pellis et al. 2004; Radley, Rocher et al. 2005; Radley, Rocher et al. 2006; Ferdman, Murmu et al. 2007). Finally, cells in the mPFC have a high density of both mineralocorticoid and glucocorticoid receptors (Diorio, Viau et al. 1993). Studies relating the findings of this study to other brain regions are currently underway.
Gene Ontology (GO) Findings
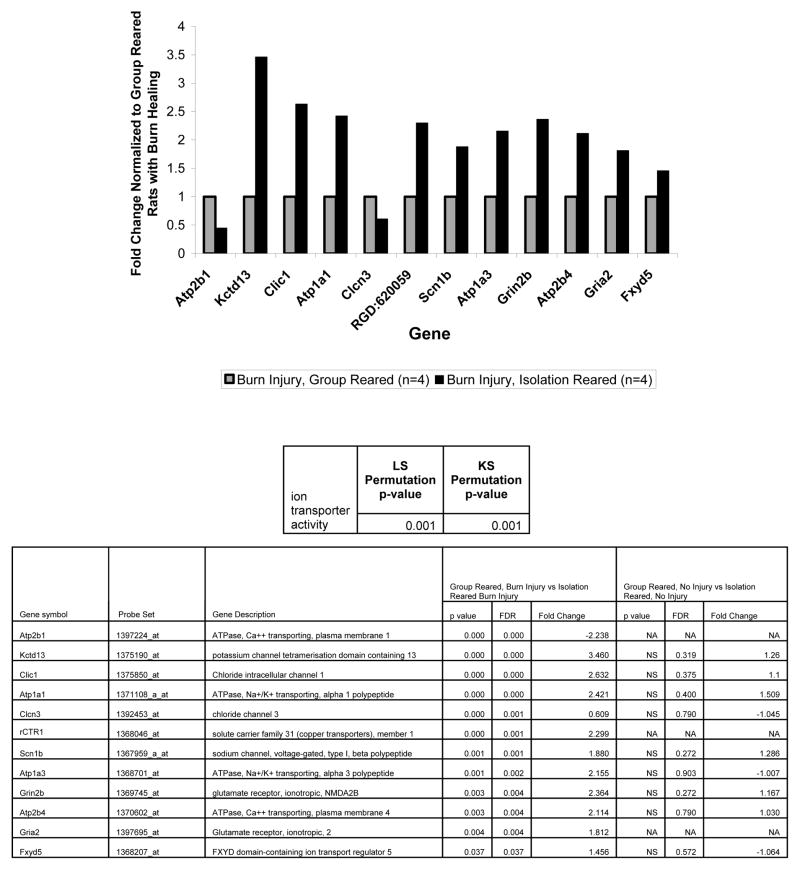
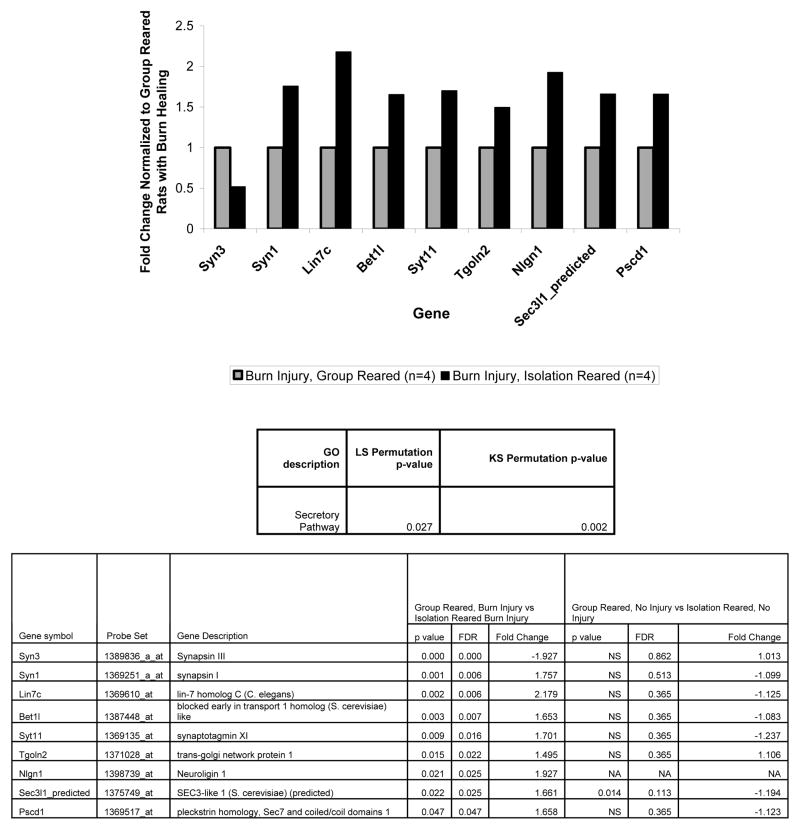
Although, in this study we largely focused on the IEG expression changes in the mPFC that resulted from isolation rearing during burn injury healing compared to group rearing during burn injury healing, we also found that many more genes were significantly altered by isolation rearing during burn injury healing compared to isolation rearing without injury. This suggests that a much more complicated set of cellular changes occur during isolation rearing in burn injury healing rats compared to in uninjured rats. This is consistent with the our findings and the literature discussed above suggesting that isolation rearing and physical illness (when adaptive) have opposing effects on locomotion and IEG expression. Also of note, the gene ontology categories associated with isolation rearing during burn injury healing all are affected by neuroplasticity and thus have some relation to the IEG changes we found. Specifically, the new categories identified related to a) genes regulating ion transporters (genes in the “ion transporter” GO category), b) genes affecting the recycling and endocytosis of vesicles containing ion channels (genes in the “cytoplasmic membrane bound vesicle” and “vesicle-mediated transport” GO categories), and c) genes affecting the exocytosis of vesicles containing neurotransmittors (genes in the “secretory pathway” GO category).
Homeostatic synaptic plasticity and synaptic scaling (Davis 2006) suggest that altered neuronal plasticity, such as that reflected by increased or decreased IEG expression, is optimized by compensatory changes that affect neuronal activity. For example, decreased Arc expression due to TTX application (which blocks the voltage sensitive sodium channel) results in increased AMPA receptor expression (Shepherd, Rumbaugh et al. 2006). In addition, an alternative form of synaptic scaling, increased presynaptic secretion, has been associated with decreased neuronal activity (Murthy, Schikorski et al. 2001). Thus, the increased expression of genes regulating ion transport, cytoplasmic vesicle transport, and secretory pathways in the isolation reared burn injury healing rats could reflect a compensatory response to decreased neuronal activity as indicated by their decreased IEG expression.
Therapeutic Implications
If IEG expression is consistently suppressed during poor wound healing, it might serve as a potential biomarker of patients at risk for poor wound healing due to psychosocial adversity, modeled here by isolation rearing. Studies of IEG expression in the human brain are unfeasible, but fMRI findings have been shown to reflect IEG expression following whisker stimulation in rats (Lu, Patel et al. 2004) and, particularly relevant for this study, following thermal injury (Lawrence, Stroman et al. 2007). This suggests that IEG expression, possibly measured in patients by fMRI, may represent a way to disentangle symptoms of psychiatric stress from those largely due to physical injury healing. Related to this, regional IEG expression may differ depending on whether the activator is a physical or mental stressor (Konsman, Luheshi et al. 2000; Huang, Cheng et al. 2004; Borsody and Weiss 2005; Stone, Lehmann et al. 2006). Differentiating stress symptoms that are part of normal illness behavior from those that indicate psychosocial distress is a particularly important goal as symptoms associated with psychiatric stress are particularly hard to discern in patients who have both psychiatric and physical illness (De La Garza 2005).
Therapeutic strategies aimed at improving physical healing in socially isolated patients may be informed by these findings. If further studies can identify a mechanistic link between IEG suppression and impaired healing, psychosocial and pharmacological strategies could be directed at activating these IEGs in individuals who have limited psychosocial supports. Furthermore, if a mechanism does underlie the association we have found between suppressed IEG expression and impaired healing, it may involve a circuit that includes suppressed neuronal activity in the mPFC, mPFC dysregulation of the HPA axis, and peripheral wound healing. The multiple levels in this circuit suggest potentially novel targets for improving both psychiatric and physical healing in socially isolated individuals.
Conclusion
Considered together with the prior study (Levine, Youngs et al. 2007), the present findings indicate that hyperactivity in the open field and IEG downregulation in the mPFC occurs in response to isolation rearing in healthy rats, surgically stressed rats, and burn injury healing rats. This suggests that this behavioral and gene expression pattern may be a bio-behavioral signature of psychosocial deprivation (as modeled by isolation rearing) irrespective of the presence or absence of other stressors. Furthermore, given that patients with low psychosocial support (Bunker, Colquhoun et al. 2003; Everson-Rose and Lewis 2005) cope poorly with medical illness, IEG expression could potentially demarcate patients with resilient and adaptive responses to physical illness from those with more maladaptive responses.
Figure 6.
Effect of rearing condition, during burn injury healing, on expression of genes in the ion transporter GO category. The table below the graph shows that both measures of this GO category (the LS and KS statistic) showed it to significantly differentiate isolation reared rats undergoing burn injury healing. The graph shows the fold changes for the significant genes in this GO category for the isolation reared rats undergoing burn injury healing normalized to the burn injury healing group reared rats. The bottom table shows the parametric p value and false discovery rates for these fold changes (columns 4–6) and contrasts them on the same parameters, where available, for the uninjured rats (columns 7–9) from the prior study (Levine, Youngs et al. 2007). The significance values in the table are for the log2 transformed values of the gene expression fold changes shown in the graph. Group reared, burn injury healing (n=4), isolation reared, burn injury healing (n=4).
Figure 7.
Effect of rearing condition, during burn injury healing, on expression of genes in the cytoplasmic membrane bound vesicle and vesicle-mediated transport GO categories. The table below the graph shows that the KS statistic was significant for both of these GO categories and the LS statistic was significant for one of them. The graph shows the fold changes for the significant genes in these GO categories for the isolation reared rats undergoing burn injury healing normalized to the burn injury healing group reared rats. The bottom table shows the parametric p value and false discovery rates for these fold changes (columns 4–6) and contrasts them on the same parameters, where available, for the uninjured rats (columns 7–9) from the prior study (Levine, Youngs et al. 2007). The significance values in the table are for the log2 transformed values of the gene expression fold changes shown in the graph. Group reared, burn injury healing (n=4), isolation reared, burn injury healing (n=4).
Figure 8.
Effect of rearing condition, during burn injury healing, on expression of genes in the secretory pathway GO category. The table below the graph shows that the KS and LS statistics both distinguished isolation from group reared rats during burn injury healing. The graph shows the fold changes for the significant genes in this GO category for the isolation reared rats undergoing burn injury healing normalized to the burn injury healing group reared rats. The bottom table shows the parametric p value and false discovery rates for these fold changes (columns 4–6) and contrasts them on the same parameters, where available, for the uninjured rats (columns 7–9) from the prior study (Levine, Youngs et al. 2007). The significance values in the table are for the log2 transformed values of the gene expression fold changes shown in the graph. Group reared, burn injury healing (n=4), isolation reared, burn injury healing (n=4).
Acknowledgments
Supported by National Institute of Mental Health 5T32MH016259: Clinical Research Training Program in Biological and Development/Psychosocial Research (JBL) and The Benson Henry Institute, Massachusetts General Hospital (JBL). We acknowledge Don Polsen, Medical Illustrator, Shriners Burns Hospital, Boston, for his help in making the Figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Christie BR, et al. Immediate early gene expression associated with the persistence of heterosynaptic long-term depression in the hippocampus. Proc Natl Acad Sci U S A. 1994;91(21):10049–53. doi: 10.1073/pnas.91.21.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsody MK, Weiss JM. The subdiaphragmatic vagus nerves mediate activation of locus coeruleus neurons by peripherally administered microbial substances. Neuroscience. 2005;131(1):235–45. doi: 10.1016/j.neuroscience.2004.09.061. [DOI] [PubMed] [Google Scholar]
- Bunker SJ, Colquhoun DM, et al. “Stress” and coronary heart disease: psychosocial risk factors. Med J Aust. 2003;178(6):272–6. doi: 10.5694/j.1326-5377.2003.tb05193.x. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, et al. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53(6):871–80. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15(1):7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21(2):153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21(2):153–60. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–23. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- De La Garza R., 2nd Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neurosci Biobehav Rev. 2005;29(4–5):761–70. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Detillion CE, Craft TK, et al. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29(8):1004–11. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, et al. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13(9):3839–47. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudoit SFF, et al. Comparison of discrimination methods for classification of tumors using DNA microarrays. Journal of the American Statistical Association. 2002;97:77–87. [Google Scholar]
- Eijkelkamp N, Engeland CG, et al. Restraint stress impairs early wound healing in mice via alpha-adrenergic but not beta-adrenergic receptors. Brain Behav Immun. 2007;21(4):409–12. doi: 10.1016/j.bbi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Einon D, Morgan MJ, et al. The development of intersession habituation and emergence in socially reared and isolated rats. Dev Psychobiol. 1975;8(6):553–9. doi: 10.1002/dev.420080613. [DOI] [PubMed] [Google Scholar]
- Engeland CG, Kavaliers M, et al. Sex differences in the effects of muramyl dipeptide and lipopolysaccharide on locomotor activity and the development of behavioral tolerance in rats. Pharmacol Biochem Behav. 2003;74(2):433–47. doi: 10.1016/s0091-3057(02)01024-9. [DOI] [PubMed] [Google Scholar]
- Engeland CG, Nielsen DV, et al. Locomotor activity changes following lipopolysaccharide treatment in mice: a multivariate assessment of behavioral tolerance. Physiol Behav. 2001;72(4):481–91. doi: 10.1016/s0031-9384(00)00436-4. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399(6731):66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Ferdman N, Murmu RP, et al. Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behav Brain Res. 2007;180(2):174–82. doi: 10.1016/j.bbr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Flaherty EG, Thompson R, et al. Effect of early childhood adversity on child health. Arch Pediatr Adolesc Med. 2006;160(12):1232–8. doi: 10.1001/archpedi.160.12.1232. [DOI] [PubMed] [Google Scholar]
- Gallitano-Mendel A, Izumi Y, et al. The immediate early gene early growth response gene 3 mediates adaptation to stress and novelty. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2007.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasper ER, Devries AC. Social structure influences effects of pair-housing on wound healing. Brain Behav Immun. 2005;19(1):61–8. doi: 10.1016/j.bbi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Guzowski JF. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 2002;12(1):86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, et al. Differential basis of strain and rearing effects on open-field behavior in Fawn Hooded and Wistar rats. Physiol Behav. 2000;71(5):525–32. doi: 10.1016/s0031-9384(00)00372-3. [DOI] [PubMed] [Google Scholar]
- Hall FS, Humby T, et al. The effects of isolation-rearing on preference by rats for a novel environment. Physiol Behav. 1997;62(2):299–303. doi: 10.1016/s0031-9384(97)00117-0. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Wagner D, et al. Early adverse experience and risk for chronic fatigue syndrome: results from a population-based study. Arch Gen Psychiatry. 2006;63(11):1258–66. doi: 10.1001/archpsyc.63.11.1258. [DOI] [PubMed] [Google Scholar]
- Holson RR. Feeding neophobia: a possible explanation for the differential maze performance of rats reared in enriched or isolated environments. Physiol Behav. 1986;38(2):191–201. doi: 10.1016/0031-9384(86)90154-x. [DOI] [PubMed] [Google Scholar]
- Horan MP, Quan N, et al. Impaired wound contraction and delayed myofibroblast differentiation in restraint-stressed mice. Brain Behav Immun. 2005;19(3):207–16. doi: 10.1016/j.bbi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Huang CC, Chou PH, et al. Neonatal isolation accelerates the developmental switch in the signalling cascades for long-term potentiation induction. J Physiol. 2005;569(Pt 3):789–99. doi: 10.1113/jphysiol.2005.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Cheng CY, et al. Expression of c-Fos-like immunoreactivity in the brain of mice with learned helplessness. Neurosci Lett. 2004;363(3):280–3. doi: 10.1016/j.neulet.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54(3):216–26. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–8. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, et al. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48(8):778–90. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kolb B, Pellis S, et al. Plasticity and functions of the orbital frontal cortex. Brain Cogn. 2004;55(1):104–15. doi: 10.1016/S0278-2626(03)00278-1. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Blomqvist A. Forebrain patterns of c-Fos and FosB induction during cancer-associated anorexia-cachexia in rat. Eur J Neurosci. 2005;21(10):2752–66. doi: 10.1111/j.1460-9568.2005.04102.x. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Luheshi GN, et al. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur J Neurosci. 2000;12(12):4434–46. doi: 10.1046/j.0953-816x.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- Korn EL, Li MC, et al. An investigation of two multivariate permutation methods for controlling the false discovery proportion. Stat Med. 2007 doi: 10.1002/sim.2865. [DOI] [PubMed] [Google Scholar]
- Kozak W, Conn CA, et al. Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice. Am J Physiol. 1994;266(1 Pt 2):R125–35. doi: 10.1152/ajpregu.1994.266.1.R125. [DOI] [PubMed] [Google Scholar]
- Lanahan A, Worley P. Immediate-early genes and synaptic function. Neurobiol Learn Mem. 1998;70(1–2):37–43. doi: 10.1006/nlme.1998.3836. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Fulford A, et al. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol. 2003;33(1):13–29. doi: 10.1023/a:1021171129766. [DOI] [PubMed] [Google Scholar]
- Lawrence J, Stroman PW, et al. Functional MRI of the cervical spinal cord during noxious and innocuous thermal stimulation in the alpha-chloralose- and halothane-anesthetized rat. Magn Reson Imaging. 2007 doi: 10.1016/j.mri.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Levine JB, Covino NA, et al. Psychological predictors of subsequent medical care among patients hospitalized with cardiac disease. J Cardiopulm Rehabil. 1996;16(2):109–16. doi: 10.1097/00008483-199603000-00005. [DOI] [PubMed] [Google Scholar]
- Levine JB, Youngs RM, et al. Isolation rearing and hyperlocomotion are associated with reduced immediate early gene expression levels in the medial prefrontal cortex. Neuroscience. 2007;145(1):42–55. doi: 10.1016/j.neuroscience.2006.11.063. [DOI] [PubMed] [Google Scholar]
- Li L, Carter J, et al. The neuroplasticity-associated arc gene is a direct transcriptional target of early growth response (Egr) transcription factors. Mol Cell Biol. 2005;25(23):10286–300. doi: 10.1128/MCB.25.23.10286-10300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Patel S, et al. Spatial correlations of laminar BOLD and CBV responses to rat whisker stimulation with neuronal activity localized by Fos expression. Magn Reson Med. 2004;52(5):1060–8. doi: 10.1002/mrm.20265. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, et al. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283(5409):1923–7. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Morgan M, Einon D. Incentive motivation and behavioral inhibition in socially-isolated rats. Physiol Behav. 1975;15(5):405–9. doi: 10.1016/0031-9384(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, et al. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32(4):673–82. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Nakao K, Ikegaya Y, et al. Hippocampal long-term depression as an index of spatial working memory. Eur J Neurosci. 2002;16(5):970–4. doi: 10.1046/j.1460-9568.2002.02159.x. [DOI] [PubMed] [Google Scholar]
- Padgett DA, Marucha PT, et al. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12(1):64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- Paxinos G, WC . The rat brain in stereotaxic coordinates. 4. San Diego CA: Academic Press; 1998. [Google Scholar]
- Plath N, Ohana O, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52(3):437–44. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, et al. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60(4):337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, et al. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196(1):199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16(3):313–20. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radmacher MD, McShane LM, et al. A paradigm for class prediction using gene expression profiles. J Comput Biol. 2002;9(3):505–11. doi: 10.1089/106652702760138592. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, et al. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232(4747):232–5. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Tamayo P, et al. Multiclass cancer diagnosis using tumor gene expression signatures. Proc Natl Acad Sci U S A. 2001;98(26):15149–54. doi: 10.1073/pnas.211566398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, et al. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60(4):376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rockwell E, Dimsdale JE, et al. Preexisting psychiatric disorders in burn patients. J Burn Care Rehabil. 1988;9(1):83–6. doi: 10.1097/00004630-198801000-00021. [DOI] [PubMed] [Google Scholar]
- Rutter M. The interplay of nature, nurture, and developmental influences: the challenge ahead for mental health. Arch Gen Psychiatry. 2002;59(11):996–1000. doi: 10.1001/archpsyc.59.11.996. [DOI] [PubMed] [Google Scholar]
- Sareen J, Jacobi F, et al. Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Arch Intern Med. 2006;166(19):2109–16. doi: 10.1001/archinte.166.19.2109. [DOI] [PubMed] [Google Scholar]
- Schilling EA, Aseltine RH, Jr, et al. Adverse childhood experiences and mental health in young adults: a longitudinal survey. BMC Public Health. 2007;7(1):30. doi: 10.1186/1471-2458-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, et al. Arc/Arg3.1 Mediates Homeostatic Synaptic Scaling of AMPA Receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan JF, Padgett DA, et al. Experimental models of stress and wound healing. World J Surg. 2004;28(3):327–30. doi: 10.1007/s00268-003-7404-y. [DOI] [PubMed] [Google Scholar]
- Silva-Gomez AB, Rojas D, et al. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983(1–2):128–36. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Simon R, Radmacher MD, et al. Pitfalls in the use of DNA microarray data for diagnostic and prognostic classification. J Natl Cancer Inst. 2003;95(1):14–8. doi: 10.1093/jnci/95.1.14. [DOI] [PubMed] [Google Scholar]
- Stein MB, Cox BJ, et al. Does co-morbid depressive illness magnify the impact of chronic physical illness? A population-based perspective. Psychol Med. 2006;36(5):587–96. doi: 10.1017/S0033291706007239. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lehmann ML, et al. Depressive behavior in mice due to immune stimulation is accompanied by reduced neural activity in brain regions involved in positively motivated behavior. Biol Psychiatry. 2006;60(8):803–11. doi: 10.1016/j.biopsych.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310(5749):805–10. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Swenson JR, Dimsdale JE, et al. Drug and alcohol abuse in patients with acute burn injuries. Psychosomatics. 1991;32(3):287–93. doi: 10.1016/S0033-3182(91)72067-7. [DOI] [PubMed] [Google Scholar]
- Tarrier N, Gregg L, et al. The influence of pre-existing psychiatric illness on recovery in burn injury patients: the impact of psychosis and depression. Burns. 2005;31(1):45–9. doi: 10.1016/j.burns.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49(3):245–53. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wright GW, Simon RM. A random variance model for detection of differential gene expression in small microarray experiments. Bioinformatics. 2003;19(18):2448–55. doi: 10.1093/bioinformatics/btg345. [DOI] [PubMed] [Google Scholar]
- Xu L, Anwyl R, et al. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387(6632):497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Rosen H, et al. Behavioral effects of lipopolysaccharide in rats: involvement of endogenous opioids. Brain Res. 1994;648(1):80–6. doi: 10.1016/0006-8993(94)91908-9. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–89. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;4(Suppl):1207–14. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, et al. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44(5):749–57. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]