Abstract
Helper-dependent adenoviral vectors are devoid of all viral coding sequences, possess a large cloning capacity, and can efficiently transduce a wide variety of cell types from various species independent of the cell cycle to mediate long-term transgene expression without chronic toxicity. These non-integrating vectors hold tremendous potential for a variety of gene transfer and gene therapy applications. Here, we review the production technologies, applications, obstacles to clinical translation and their potential resolutions, and the future challenges and unanswered questions regarding this promising gene transfer technology.
Introduction
Helper-dependent adenoviral vectors (HDAd) are deleted of all viral coding sequences, can efficiently transduce a wide variety of cell types from various species independent of the cell cycle, and can result in long-term transgene expression. Several small and large animal models of genetic disorders can be corrected effectively and long-term by HDAd without chronic toxicity [1]. An important advantage of HDAd vectors is their large cloning capacity of up to ~37 kb which allows for the delivery of large therapeutic genes and even whole genomic loci, multiple transgenes, and large cis-acting elements to enhance, prolong, and regulate transgene expression. The HDAd genome remains episomal in the nuclei of transduced cells where it associates with cellular histones and, depending on the nature of the stuffer sequence, may undergo repression or can be maintained transcriptionally active [2]. Because of its non-integrating nature, HDAd are not associated with an increased risk of germ-line transmission and insertional carcinogenesis [3]. Additionally, large amounts of high quality HDAd can now be quickly produced for evaluation in large animals and make potential human clinical trials possible [4]. Here, we review the current state of HDAd with regards to production technologies, applications, obstacles to clinical translation and their potential resolution, and the future challenges and unanswered questions still facing this promising gene transfer technology.
The adenovirus
The adenovirus (Ad) has a non-enveloped icosahedral capsid of ~100 nm containing a linear double-stranded DNA genome of ~ 36 kb [5]. Of the ~50 serotypes of human Ad, the most extensively characterized and thus vectorized are serotypes 2 (Ad2) and 5 (Ad5) of subgroup C. The 36 kb genome of Ad2 and Ad5 is flanked by cis-acting inverted terminal repeats (ITRs) which are required for viral DNA replication. A cis-acting packaging signal (Ψ), required for the encapsidation of the Ad genome is located near the left ITR (Figure 1). The Ad genome can be roughly divided into two sets of genes: the early region genes, E1A, E1B, E2, E3 and E4, are expressed before DNA replication and the late region genes, L1 to L5 are expressed to high levels after initiation of DNA replication. The E1A transcription unit is the first early region to be expressed during viral infection and it encodes two major E1A proteins that are involved in transcriptional regulation of the virus and stimulation of the host cell to enter an S phase-like state. The two major E1B proteins are necessary for blocking host mRNA transport, stimulating viral mRNA transport and blocking E1A-induced apoptosis. The E2 region encodes proteins required for viral DNA replication and can be divided into two subregions; E2a encodes the 72-kD DNA-binding protein and E2b encodes the viral DNA polymerase and terminal protein precursor (pTP). The E3 region, which is dispensable for virus growth in cell culture, encodes at least seven proteins most of which are involved in host immune evasion. The E4 region encodes at least six proteins, some functioning to facilitate DNA replication, enhance late gene expression and decrease host protein synthesis. The late region genes are expressed from a common major late promoter (MLP) and are generated by alternative splicing of a single transcript. Most of the late mRNAs encode virion structural proteins. In addition to the early and late region genes, four other small transcripts are also produced. The gene encoding protein IX (pIX) is colinear with E1B but uses a different promoter and is expressed at an intermediate time, as is the pIVa2 gene. Other late transcripts include the RNA polymerase III transcribed VA RNA I and II.
Figure 1.
Transcription map of human adenovirus serotype 5. The ~36 kb genome is divided into four early region transcription units, E1–E4, and five families of late mRNA, L1–L5, which are alternative splice products of a common late transcript expressed from the major late promoter (MLP). Four smaller transcripts, pIX, IVa, and VA RNA’s I and II, are also produced. The 103 bp inverted terminal repeats (ITRs) are located at the termini of the genome and are involved in viral DNA replication, and the packaging signal (ψ) located at the left end is involved in packaging of the genome into virion capsids.
Infection of the host cell is a two stage process involving an initial binding of the Ad fiber protein with CAR (coxsackie-adenovirus receptors) on the cell surface [6–8]. Furthermore, a secondary interaction occurs between the virion penton base and αvβ3 and αvβ5 integrins permitting internalization via-receptor-mediated endocytosis [9]. The efficiency of the infection, which is dependent on Ad binding and entry, is directly related to the level of primary and secondary receptors on the cell surface [10,11]. After Ad internalization, the virion escapes from the early endosome into the cytosol prior to lysosome formation [12,13]. During the translocation along the microtubule network toward the nucleus, the virion is disassembled and the DNA is released into the nucleus [14]. Once in the nucleus, viral DNA replication (beginning at 6–8h postinfection) and assembly of progeny virion occur. The entire life cycle takes about 24–36 h and generates about 104 virions per infected cell.
Until recently, it was believed that binding to cell surface CAR by the fiber of Ad was responsible for initiating infection of all cell types. However, this was recently demonstrated not to be the case for hepatocytes. Instead, vitamin K-dependent coagulation factors were found bound to the Ad capsid, acting as a bridge between hepatocytes and the virus [15–18]. The mechanism by which the vitamin K-dependent coagulation factor X (FX) interacts with Ad5 to promote infection of hepatocytes has recently been unraveled [19]. Electron cryomicroscopy studies determined that FX binds within cavities formed by trimeric hexon proteins and involves interaction with the Ad5 hexon hypervariable regions [19]. This interaction promotes the binding of the Ad5–FX complexes to cellular heparan sulfate proteoglycans (HSPGs) which are used as receptors on the hepatocytes. This seems to be important for liver transduction by Ad5 after systemic administration in vivo. Notably, in contrast with the species C serotypes Ad5 and Ad2, which have been shown to transduce hepatocytes after systemic injection, species B Ad35 and species D Ad26 have a weak-to-no binding to FX and do not transduce the liver [20].
Early generation ad vectors
First generation Ad vectors (FGAd) simply have foreign DNA inserted to replace all of the Ad early region 1 (E1) genes and are produced in an E1-complementing cell line [21]. FGAd can very efficiently infect a wide variety of cell types from many different species in a cell cycle independent manner to direct very high levels of transgene expression. Although deletion of E1 rendered FGAds replication deficient, all other viral genes remained in the vector backbone resulting in low level viral gene expression in the transduced cells. This targeted the transduced cells for elimination by the adaptive cellular immune response which results in transient transgene expression and chronic toxicity [20–26]. Processing of proteins derived from the viral capsid shell has also been implicated in this phenomenon [27]. Additional deletions of viral genes (E2 or E4) were implemented in so-called second generation Ad vectors in an attempt to overcome this problem. The advantages of second generation Ad over FGAd remain controversial as some studies show them to be superior in terms of toxicity and longevity of transgene expression [28–36] while others do not [22,37–41].
Helper-dependent adenoviral vectors
The easiest way to eliminate the cellular adaptive immune response against Ad-based vectors is to completely delete all of the viral genes from the Ad vector leaving only those cis-acting elements needed for vector genome replication (ITRs) and encapsidation (ψ). An important additional benefit to this large deletion of Ad sequences is the tremendous cloning capacity permitted; up to 37 kb of foreign sequences can be inserted to permit delivery of multiple transgenes, entire genomic loci and large cis-acting elements to enhance or regulate tissue-specific transgene expression. However, deletion of all viral genes meant that such a vector could not be produced on its own. It would be dependent on a helper virus (HV) for propagation, thus the term helper-dependent Ad (HDAd). The HV must replicate normally and express all of the viral proteins needed to replicate and package the HDAd genome. However, a strategy must be developed to inhibit propagation of infectious HV virions in order to obtain a relatively pure HDAd.
One of the first successful strategies to specifically limit propagation of infectious HV during HDAd production was described by Kochanek et al. [42]. In this strategy, a 91 bp deletion was introduced into the HV packaging signal which severely limited its packaging efficiency. This deletion, however, did not affect the ability of the HV to replicate and thus to trans-complement the HDAd genome. Since the HDAd contained the wild type packaging signal, it was preferentially packaged over the HV genome. Furthermore, because the HDAd and HV genomes differed in size, CsCl gradient purification allowed for physical separation of the two species resulting in ~1% helper virus contamination in the purified HDAd. While low, even lower levels of HV contamination would be desirable for clinical application. Moreover, the 91 bp deletion in packaging signal made producing HV difficult since it reduced HV yields 90-fold [43], making the production of large amounts of HV stocks problematic which would be needed to produce the considerable quantities of HDAd needed for clinical application. Clearly, further improvement in producing HDAd was required.
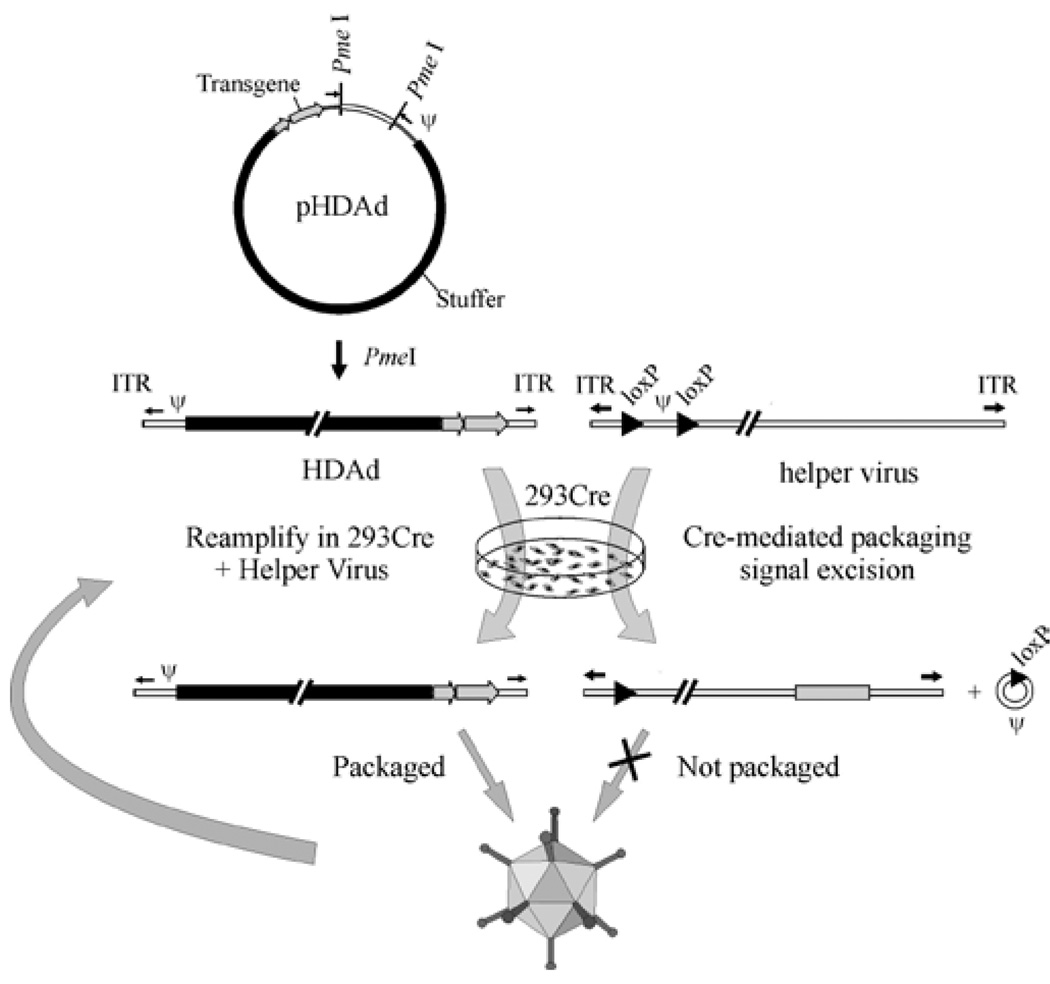
The most efficient method for producing HDAd is the Cre/loxP system [44] (Figure 2). In this system the HDAd genome, constructed in a bacterial plasmid, contains the following critical elements. First, ~500 bp of noncoding adenoviral DNA is needed. These include the adenoviral ITRs which are required for vector genome replication, and ψ which is the packaging signal required for encapsidation of the vector genome into the capsid. Second, noncoding eukaryotic “stuffer” DNA is needed to bring the vector genome size within the size range (27.7 kb to 37 kb) for efficient packaging into virions [45,46]. And third, the expression cassette of interest. To convert the plasmid form of the HDAd genome into the viral form, 293 cells expressing the site-specific Cre recombinase are transfected with the linearized HDAd genome and subsequently infected with the HV. The HV is an E1-deleted, FGAd bearing a packaging signal flanked by loxP sites so that following infection of 293 cells expressing Cre recombinase, the packaging signal is excised from the HV genome by Cre-mediated site-specific recombination between the loxP sites. This renders the HV genome unpackageable but still able to undergo DNA replication and thus trans-complement the replication and encapsidation of the HDAd genome. As with all FGAds propagated in 293 or 293-derived cells, the potential exists for the generation of replication competent Ad (RCA; E1+) as a consequence of homologous recombination between the HV and the Ad sequences present in 293 cells. To prevent the formation of RCA, a “stuffer” sequence was inserted into the E3 region to render any E1+ recombinants too large to be packaged [44]. As of this writing, the emergence of RCA has yet to be reported using HVs with an E3 stuffer. In contrast, RCA is readily detected using helper viruses without an E3 stuffer when propagated in 293 [44]. While packaging signal excision was relatively efficient in this original Cre/loxP system, it was not complete, leading to HV contamination, albeit at low levels [47]. Subsequent studies showed significant reduction in HV contamination could be achieved using producer cells that expressed much higher levels of Cre [4]. Because the genome size of the HDAd (~30 kb) and the HV (~36 kb) are engineered to be different, they can be physically separated by CsCl ultracentrifugation which provided an additional method to purify the HDAd from residual HV.
Figure 2.
The Cre/loxP system for generating HDAds. The HDAd contains only ~500 bp of cis-acting Ad sequences required for DNA replication (ITRs) and packaging (ψ); the remainder of the genome consists of the desired transgene and non-Ad stuffer sequences. The HDAd genome is constructed as a bacterial plasmid (pHDAd) and is liberated by restriction enzyme digestion (e.g., PmeI). To rescue the HDAd, the liberated genome is transfected into 293 cells expressing Cre and infected with a helper virus bearing a packaging signal (ψ) flanked by loxP sites. Cre-mediated excision of ψ renders the helper virus genome unpackageable, but still able to provide all of the necessary trans-acting factors for propagation of the HDAd. The titer of the HDAd is increased by serial coinfections of 293Cre cells with the HDAd and the helper virus.
Similar HDAd production systems based on the yeast FLP/frt site-specific recombination system have also been described which utilize the identical strategy of FLP-mediated recombination between frt sites flanking the packaging signal of the HV to select against encapsidation of HV in 293 cells that express FLP [48,49]. Alternative strategies to select against the HV have been developed. Sargent et al. [50] used a size-restricted approach based on deletion of the Ad protein IX (pIX) gene from the HV genome. pIX is a minor component of the Ad capsid without which no more than 35 kb of DNA can be encapsidated into the pIX-deficient capsid. Sargent et al. [50] constructed a 37.3 kb HV that was deleted of pIX which was produced in 293 cells stably expressing pIX. However, upon infection of non-pIX complementing 293 cells, the 37.3 kb pIX-deleted HV genome could not be efficiently packaged but was able to provide replicative and packaging functions for the propagation of a 30 kb HDAd. Sargent et al. [50] showed that using this strategy in combination with the Cre/loxP strategy of packaging signal excision resulted in 1000-fold reduction in HV contamination compared to the Cre/loxP strategy alone. However, this system produces HDAd virions lacking pIX and because stability of the capsid is conferred, at least in part by pIX, potential instability of HDAd is a concern [51]. Another interesting strategy to reduce the levels of helper virus contamination consists in inserting a DNA sequence derived from φC31 phage, between the 5’ ITR and the ψ in order to delay helper virus life cycle relative to that of HDAd [52]. This modified HV reaches maximum titer at 56 hours post-infection (h.p.i.) in contrast the HDAd which reaches maximum titer at 36 h.p.i.. Thus, HDAd can be preferentially packaged and harvested at 36 h.p.i. with negligible HV contamination [52]. None of these methods can eliminate 100% of the HV. Therefore future strategies could benefit from the combination of two or more of these approaches. Ultimately, a stable packaging cell line able to expression all of the necessary Ad genes for the production of HDAd would be desirable as it would eliminate helper virus contamination. However, the construction of such a cell line has yet to be achieved, probably due to the toxicity associated with the expression of Ad proteins.
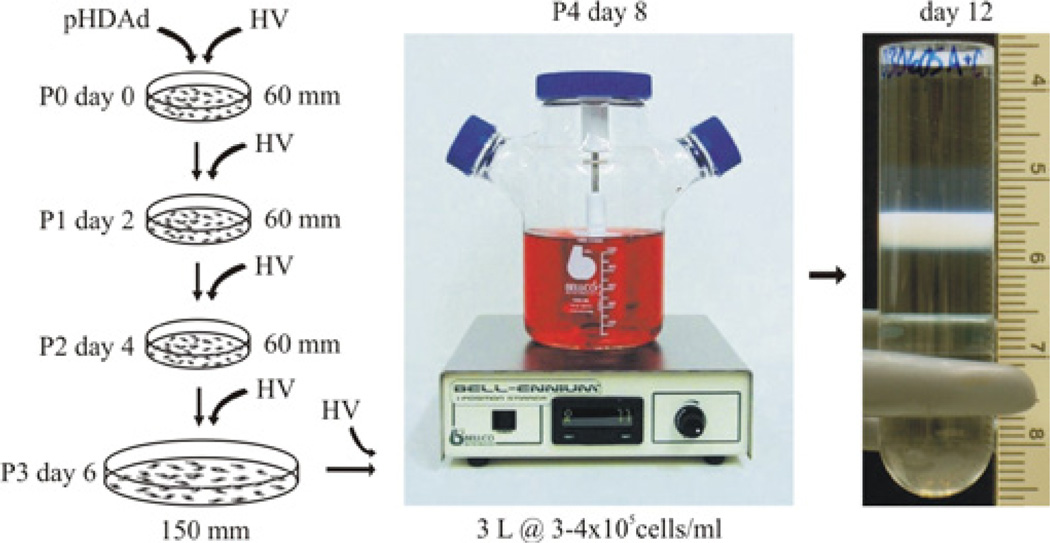
Based on the Cre/loxP system, a robust method for large-scale HDAd production has been developed comprised of a suspension adapted producer cell line that expressed very high levels of Cre to further reduce HV contamination, a HV resistant to mutation and refined protocols (Figure 3) [4]. With these improvements, >1×1013 viral particles (vp) could be easily produced from 3 liters of cells within 2 weeks of initial HDAd rescue, with specific yields of >10,000 vp/cell and with exceedingly low helper virus contamination of 0.4%-0.1% without relying on CsCl purification and 0.02%-0.01% following CsCl purification as determined by DNA-based assays. This advancement will significantly improve our ability to assess this promising gene therapy technology, especially in large animal models and for clinical applications. This improved system has recently been used to generate cGMP grade HDAd for human clinical trial with encouraging results [53]. Detailed methodologies for producing HDAd using this improved system is described elsewhere [54,55].
Figure 3.
Improved method of large scale production of HDAd. Overview of rescue (passage 0 or P0), amplification (P1 to P3) and large-scale (P4) production of HDAd. P0 involves transfecting producer cells with pHDAd followed by infection with the HV. Each subsequent serial passage involves coinfecting producer cells with the HDAd and HV. The entire process can take as little as two weeks with total yields of >1013 viral paricles (vp) per 3 liter culture and specific yields of >10,000 vp/cell.
Liver-directed gene therapy
The liver is a very attractive target for gene therapy because it is the affected organ in many genetic and acquired diseases and it can be used as a factory organ for systemic delivery through the vascular circulation of vector-encoded therapeutic proteins. Numerous examples of in vivo liver-directed gene therapy for disease models using HDAd have been reported [1]. In general, all of these studies have demonstrated that HDAd can mediate long-term transgene expression in the absence of chronic toxicity supporting the potential of HDAd for clinical applications. The purpose of this article is not to provide a comprehensive review of all of these studies. Instead, examples of particular significance or interest are highlighted.
The correction of hypercholesterolemia in apolipoprotein E (apoE)-deficient mice is a paradigmatic example of this great potential [56]. In this study, the efficacy of FGAd encoding mouse apoE cDNA (FG-Ad5-cE), was compared to a HDAd bearing the mouse genomic apoE locus (HD-Ad5-gE). Intravenous injection of ApoE deficient mice with FG-Ad5-cE resulted in an immediate fall in plasma cholesterol levels to within normal range. However this effect was transient and plasma cholesterol levels increased after 28 days, returning to pre-treatment levels by 112 days. Correlative with the plasma cholesterol levels, the levels of plasma apoE immediately increased shortly after injection but rapidly declined to pre-treatment levels by day 28. In contrast, intravenous injection of HD-Ad5-gE resulted in a complete and immediate lowering of plasma cholesterol to subnormal levels for about 9 months, with levels subsequently staying within the normal range for the rest of the natural lifespan of the animal (2.5 years). In this case, plasma apoE reached ~200% of wild-type levels within 4 weeks and remained at supraphysiological levels for >4 months at which time it slowly declined to about wildtype levels at 1 year and remained at 60%–90% physiological concentrations for the lifetime of the animals (2.5 years). Aortas from mice, examined at 2.3 yrs after treatment with HD-Ad5-gE, were essentially free of atherosclerotic lesions as determined by quantitative morphometry demonstrating that a single injection of HDAd encoding ApoE could confer lifetime protection against aortic atherosclerosis. Kim et al. [56] also investigated the associated toxicities and found that whereas injection of FG-Ad5-cE resulted in significant hepatotoxicity as indicated by significant elevation of AST and ALT (>10 to 20-fold), no such evidence of damage was observed following injection of HDAd. This study demonstrated that a single intravenous injection of HDAd resulted in lifelong expression of the therapeutic transgene and permanent phenotypic correction of a genetic disease. Additionally, negligible hepatotoxicity was associated with HDAd administrations.
Encouraging results have been also reported in the Crigler-Najjar syndrome animal model. Crigler-Najjar syndrome type I is a recessively inherited disorder caused by a deficiency of uridine diphosphoglucuronosyl transferase 1 A1 (UTG1A1) and characterized by severe unconjugated hyperbilirubinemia resulting in jaundice and increased risk of kernicterus. Current therapy consists of cumbersome and inconvenient life-long phototherapy to prevent kernicterus or liver transplantation [57]. Toietta et al. [58] showed that a single systemic injection of a HDAd expressing UTG1A1 at doses of 1×1013 vp/kg or 3×1012 vp/kg resulted in life-long expression of UTG1A1 and permanent phenotypic correction of hyperbilirubinemia in the Gunn rats, the Crigler-Najjar syndrome animal model. However, at a lower dose of 6×1011 vp/kg, only partial, life-long correction was observed.
HDAd liver-mediated gene transfer can be considered an important tool for numerous diseases beyond monogenic disorders. An interesting application of HDAd for the treatment of diabetes mellitus has also been reported. In this study, two HDAds, one expressing Neurod1 (a transcription factor expressed in developing and adult β-cells of the pancreas), and the other expressing betacellulin (a β-cell growth factor), were co-injected into diabetic mice at the dose of 3×1011 vp and 1×1011 vp respectively [59]. Following systemic administration of these two vectors, clusters of cells with immunohistochemical and ultrastructural properties similar to pancreatic islet were surprisingly noted in the liver of the treated mice. Remarkably, the diabetic mice also showed a normalization of glucose levels for the duration of the experiment of at least 120 days. These results showed the presence of endocrine pancreatic precursors in the liver and the possibility to manipulate their development to induce pancreatic islet formation by HDAd-mediated gene transfer to reverse the diabetic phenotype.
HDAds expressing short hairpin RNA (shRNA) to silence specific target genes in the liver have also been demonstrated. For example, HDAd-mediated expression of shRNA against specific mouse genes resulted in approximately 75–90% silencing [60,61] and in a mouse model of obesity and type 2 diabetes (db/db mice) silencing of the transcription factor sterol regulatory element-binding protein-1c (SREBP1), which is up-regulated in obese mice, resulted in a reduction in their body weight [61]. These initial studies could pave the way to a multitude of applications directed at silencing of specific genes for the treatment of a variety of genetic and acquired disorders. Interestingly, in contrast with previous reports showing severe toxicity and lethality following administration of AAV encoding shRNA [62], HDAd expressing shRNA was clinically well tolerated in mice with only mild pathological and biochemical signs of hepatotoxicity [60,61]. Moreover, saturation of the exportin-5 pathway, which shuttles cellular micro-RNA (miRNA) from the nucleus to the cytoplasm, was found in the case of AAV [62] and was thought to be involved in the observed toxicity. In contrast, saturation of the exportin-5 pathway was not seen with HDAd expressing shRNA [61]. These encouraging results with HDAd-shRNA may pave the way to a variety of applications involving the silencing of dominant mutations causing genetic and acquired diseases.
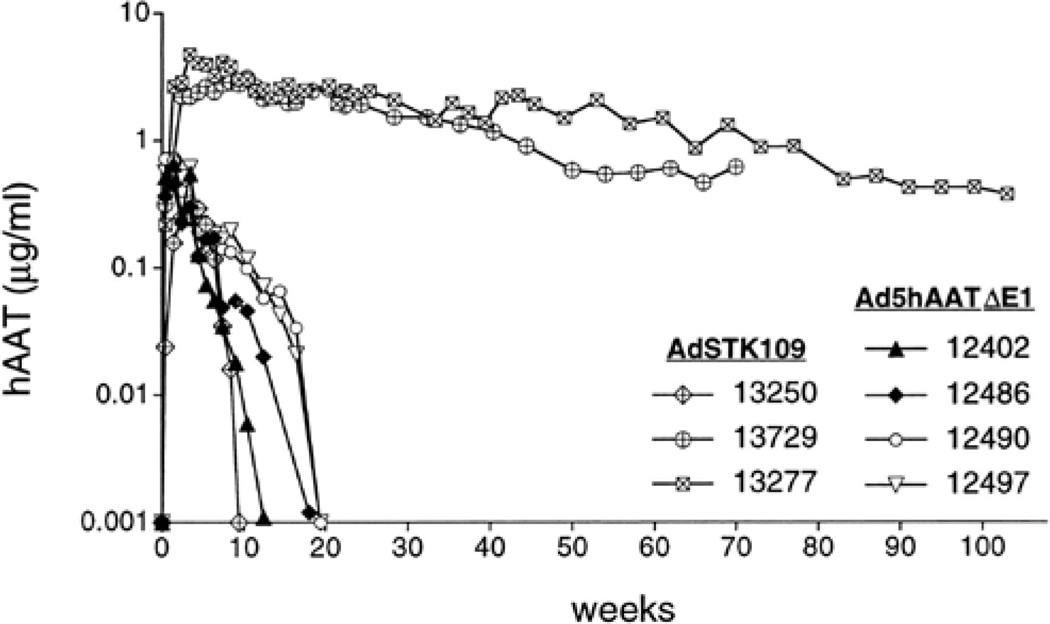
Importantly, long-term expression by HDAd has also been recapitulated in a clinically relevant large animal model [63–67]. In one study, three baboons were intravenously injected with 3.3 to 3.9×1011 vp/kg of HDAd expressing hAAT [63]. hAAT expression persisted for more than one year in two of the three animals (Figure 4). The third baboon injected with HDAd had significantly lower levels of serum hAAT which rapidly declined to undetectable levels after 2 months due to antibodies formation against the human AAT protein. It was significant that no abnormalities in blood cell counts and liver enzymes were observed in these three baboons at any time, starting 3 days post-injection. These results were in sharp contrast with the findings observed in the baboons injected with the FGAd expressing hAAT, in which the transgene levels were measurable for only 3 to 5 months after the injection (Figure 4). In the case of FGAd, this was shown not to be due to the generation of anti-hAAT antibodies but was attributed to the generation of a cellular immune response against viral proteins expressed from the vector backbone resulting in the elimination of vector transduced hepatocytes which was not seen in the HDAd injected baboons. In another large animal study, intravenous injection of 3×1012 vp/kg of a HDAd expressing the canine coagulation factor IX in two hemophilia B dogs resulted in sustained phenotypic improvement of the bleeding diathesis for the duration of the experiment of at least 604 and 446 days [64]. Other nonhuman primate examples illustrating long-term transgene expression following hepatocyte transduction by HDAd will be described below.
Figure 4.
Serum levels of hAAT in baboons following intravenous administration of the HDAd AdSTK109 or the FGAd AdhAATΔE1. Baboons 12402 and 12486 were injected with 6.2×1011particles/kg of AdhAATΔE1. Baboons 12490 and 12497 were injected with 1.4×1012 particles/kg of AdhAATΔE1. Baboons 13250, 13250 and 13277 were injected with 3.3×1011 particles/kg, 3.9×1011 particles/kg and 3.6×1011particles/kg, respectively, of AdSTK109.
One potential problem for gene therapy is that the therapeutic protein encoded by the vector may be immunogenic in patient. In this regard, Cerullo et al. [68] demonstrated that irreversible tolerization to an immunogenic protein could be achieved if that protein was expressed from HDAd using either a liver-specific or a ubiquitous cellular promoter following systemic delivery into immunocompetent C3H/HeJ mice. However, whether this important property will also be observed in humans is unknown.
Non-linear dose response to hepatocyte transduction
It is important to note that relatively high HDAd doses were used in the encouraging animal studies cited above. This is because high doses are required to achieve efficient hepatocyte transduction following systemic intravascular delivery of HDAd. It is now clear that there is a nonlinear dose response to hepatic transduction, with low doses yielding very low to undetectable levels of transgene expression, but with higher doses resulting in disproportionately high levels of transgene expression. Kupffer cells (KC) of the liver appear to play a significant role in the nonlinear dose response by sequestering intravenous Ad [69,70]. Other barriers may also exist in the liver. For example, trapping of the virus by liver sinusoidal cells [71], by endothelial cells and retention of the viral particle in the space of Disse [72]. Antibodies, both specific and nonspecific, for Ad appear to also play an important role in the nonlinear response [69,70], perhaps by opsonizing the virion to enhance the efficiency of Fc-receptor mediated vector uptake by macrophages such as KC. Furthermore, systemic administration of Ad vectors likely results in widespread transduction of a large number of various extrahepatic cell types (e.g. blood cells, endothelium, spleen, lung, etc.) which may also be important components of the barrier to efficient hepatocyte transduction. Another barrier to hepatocyte transduction by Ad vectors appears to be erythrocytes, at least in humans. Over 90% of a typical dose of Ad vectors binds to and is neutralized by human erythrocytes ex vivo [73]. Detailed studies showed erythrocytes from humans, but not from mice or rhesus macaques, bear the Ad receptor CAR responsible for sequestration of the vector [74]. Furthermore, human erythrocytes, but not mice, bear the complement receptor 1 (CR1) which binds Ad5 in the presence of antibodies and complement. These results have important implications for human application and highlight important potential limitations of animal models. A strategy to overcome sequestration by human erythrocytes is to coat the Ad vector with polymers containing quaternary amines [75]. It has also become clear that the liver microarchitecture plays a critical role in the efficiency of hepatocyte transduction by Ad vectors. The liver fenestrations normally form a structural barrier preventing large circulating macromolecules in the hepatic blood from accessing the hepatocytes. Several studies suggest that the diameter of the endothelial fenestrations of the liver (for example, ~141 nm in C57BL/6 mice and ~103 nm in NZW rabbits) plays a key role in the efficiency of Ad-mediated hepatocyte transduction [76,77]. Specifically, it was demonstrated that there is a positive correlation between the diameter of the fenestrations and the efficiency of hepatic transduction following systemic administration of Ad [76,77, 78]. Snoeys et al. [77] have determined that the Ad5 particle has a diameter of 93 nm with protruding fibers of 30 nm. However, the true functional diameter of the entire virus is difficult to determine because the Ad5 fiber is characterized by two flexible domains [79]. Importantly, Wisse et al. [78] demonstrated that the diameter of the human fenestration to be ~107 nm and thus may represent an obstacle for hepatocyte transduction by Ad based vectors in humans. In this regard, the balloon occlusion catheter methods for delivering HDAd into the liver of nonhuman primates described below may be useful because they cause a transient increase in intrahepatic pressure which appears to enlarge the fenestrations [66,67]. In summary, these studies demonstrate that there exists a barrier to efficient hepatocyte transduction following systemic administration of Ad-based vectors such that only at high vector doses is efficient hepatic transduction achieved. These high vector doses unfortunately lead to an acute toxic response.
HDAd-mediated acute toxicity
As discussed above, expression of viral genes from FGAd results in an adaptive cellular immune response against the transduced cells leading ultimately to their destruction and chronic toxicity beginning around 7 days after vector administration. This chronic toxicity is not observed with HDAd because of they do not contain viral genes. However, in addition to this chronic toxicity, administration of FGAd also leads to dose-dependent acute toxicity [80–84]. Unlike chronic toxicity, this acute toxic response manifests very shortly (within minutes to hours) after vector administration and is not triggered by expression of viral genes. Instead, the vector capsid activates the innate inflammatory response resulting in acute toxicity. Indeed the death of a patient injected with a second generation Ad vector was attributed to this acute toxicity [84]. HDAd can also provoke acute toxicity because its capsid is identical to FGAd [85,86]. It is important to emphasize that the severity of this acute toxic response is dose-dependent. At low vector doses, no toxic response is observed but unfortunately little to no hepatocyte transduction is also obtained. At the high vector doses required for efficient hepatocyte transduction, acute toxicity is observed, the severity of which increases as the dose increases. This acute toxicity is characterized by elevations in serum proinflammatory cytokines as a consequence of activation of the innate inflammatory response.
The mechanism(s) responsible for Ad-mediated activation of the acute inflammatory response is not completely understood. Vector uptake and activation to secrete pro-inflammatory cytokines by macrophages and dendritic cells (DCs) in the spleen have been implicated [80,81]. Indeed, the spleen appears to be a major contributor to interleukin (IL)-12 elevation following systemic Ad in mice [80]. Vector transduction of endothelial cells [87,88], peripheral mononuclear cells [89] and Ad-mediated complement activation [90–92] have all been implicated to play a role in acute toxicity. Ad has been shown to bind and activate the complement components including C3 and C4BP in the classical and alternative complement pathways. Complement and antibody interactions with Ad vectors result in an acute response with secretion of cytokines and chemokines [90]. These latter interactions promote the adhesion and migration of infiltrating leukocytes and platelet aggregation. Thrombocytopenia is caused by interaction between adenoviral particles and the coagulation system, resulting in formation of platelet-leukocyte aggregates [93]. Several studies also suggest that antibodies may play a role. Both neutralizing anti-Ad antibodies [90,94] and non-neutralizing or naturally occurring (nonspecific cross-reacting) antibodies [69,70,90] may contribute to acute toxicity, perhaps by opsonizing the viral particles and rendering them more susceptible to Fc-mediated uptake by macrophages which in turn may become activated to secrete proinflammatory cytokines. For example, systemic vector administration into nonhuman primates resulted in significantly higher IL-6 levels in animals with neutralizing anti-Ad antibodies compared to naïve animals [94]. Toll-like receptors (TLRs), crucial components in pathogen recognition processes, have recently emerging as important players in Ad-mediated acute toxicity. The important role of TLR-9 and TLR-2 in triggering the innate immune response has recently emerged [95]. TLR-2 is associated with the cellular membrane and is probably involved in the recognition of the capsid proteins [96,97]. However, the adenoviral ligand to TLR-2 is yet to be identified. TLR-9 is an endosomal receptor and recognizes the DNA component of the Ad vectors [98–100]. Ad-vector interaction with these two sensor-receptors engages a complex intracellular pathways through the activation of myeloid differentiation primary response gene 88 (MyD88) that culminate in the massive production of cytokine and chemokines, interferon (IF)-α,β and triggers DC maturation and development of T-cell and B-cell responses against the Ad vector components [101]. Type I-IFN (α and β) activate natural killer (NK) cells and have a predominant role in the subsequent regulation of the innate immune response machinery against the vector [102]. Subsequent activation of chemoattractant protein macrophage inflammatory protein-2 (MIP-2), IL-1 and tumor necrosis factor (TNF) contribute to leukocyte infiltration in the target tissue. NK cell activation releases several cytokines and promotes an adaptive immune response to the vector [103]. Importantly, Ad vectors elicit the innate immune response either through MyD88/TLR or in an independent pathway depending on the cell type [104]. For example, DCs use both MyD88 and TLR-9 for cytokine production, whereas activation of peritoneal macrophages and subsequent release of cytokines is independent of MyD88/TLRs system. Studies using MyD88-knockout mice showed that the immune response elicited by HDAd is greatly reduced in the absence of MyD88 when compared to wild-type mice or mice lacking TLR-2 [105]. Additionally, the loss of NOD2 in conjunction with MyD88 further decreased the innate immune response to HDAd in MyD88/NOD2 deficient mice and was shown to also function independently of MyD88 [106] suggesting that NOD2 is another important aspect of the innate immune response to HDAd. In the MyD88/TLR independent activation of innate immune response, double stranded viral DNA is recognized by a cytosolic molecular complex known as inflammasome [107]. The inflammasome consists of NALP3 and apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC) adaptor protein complex which induces maturation of pro-IL-1β in macrophages after the interaction with the viral DNA. This mechanism seems to be a key event in the innate immune response to Ad vectors and other DNA viruses. Another recent study identified IL-1α-IL-1receptor-I (IL-1R-1) as a key pathway to the activation of pro-inflammatory responses to the virus in macrophages, independently of recognition of the virus-associated nucleic acid by intracellular pattern recognition receptors [108]. The authors showed that the IL-1α-mediated response requires a selective interaction of virus RGD (peptide containing Arg-Gly-Asp) motifs with macrophage β3 integrins in response to Ad vector, leading to production of inflammatory cytokines and chemokines.
As discussed above, KCs of the liver are primarily responsible for the nonlinear dose response by preferentially sequestering systemic Ad, at least in mice and their activation following uptake of Ad has been implicated to play a role in the acute innate inflammatory response [109]. However, their precise role in acute toxicity remains to be fully elucidated and warrants further discussion because of their potential role in HDAd-mediated, liver-direct gene therapy. Most studies in mice utilizing selective depletion of KCs prior to Ad administration did not investigate markers of innate inflammatory response such as serum IL-6 [110–112]. Of the studies in which this was addressed, depletion of KCs in mice by gadolinium chloride nearly eliminated TNF but resulted in a more robust IL-6 increase and did not affect nuclear factor (NF)-κB following systemic Ad compared to mice bearing KCs [113]. In another study, depletion of KCs by chlodronate liposomes in mice prior to systemic Ad appeared to have no effect on the serum levels of Il-2, IL-4, IL-5, IL-6, TNF-α and INF-γ [70]. Likewise, Brunetti-Pierri et al. [114] showed that the amount of KC transduction did not correlate with serum IL-6 and IL-12 levels and Manickan et al. [115] showed that KC death as a consequence of Ad uptake did not result in elevated serum IL-6. It has been suggested that KCs may, in fact, play a protective role [116]. Clearly, a better understanding of the role of KCs in Ad-mediated acute toxicity is needed.
Another contributing factor may be the variations in responses to vector due to inter-individual variations, especially in an out-breed population like humans, or pre-existing pathological conditions. Smith et al. [117] for example, have shown that the biodistribution of adenoviral vectors is altered in cirrhotic rats due to the presence of pulmonary intravascular macrophages, which cause a shift in vector uptake from the liver to the lungs. Interestingly, the cirrhotic rats exhibited a potent activation of proinflammatory cytokines as compared to control non-cirrhotic rats.
Ultimately, the precise mechanism responsible for activation of the acute inflammatory response by systemic Ad is multifactorial and complex and remains to be fully elucidated. However, regardless of the precise mechanism, it appears that a threshold of innate immune activation must first be attained, as a consequence of high dose and systemic exposure of the vector to many cell types and blood borne components, before severe and lethal acute toxicity manifests. Evidence of robust activation of the acute inflammatory response is observed in both rodents and nonhuman primates given comparable systemic high dose Ad (on a per kg basis). However, it is important to emphasize that unlike primates, lethal systemic inflammatory response syndrome (SIRS) does not develop in rodents and in fact, such high doses are well tolerated. This may reflect species to species differences in the quality of the innate immune response or sensitivities of the end organs to pathologic sequelae [80,81] which likely accounts for the plethora of studies reporting negligible toxicity in mice given high dose HDAd and underscores the importance of safety and toxicity evaluations in larger animals.
Overcoming the threshold effect and acute toxicity
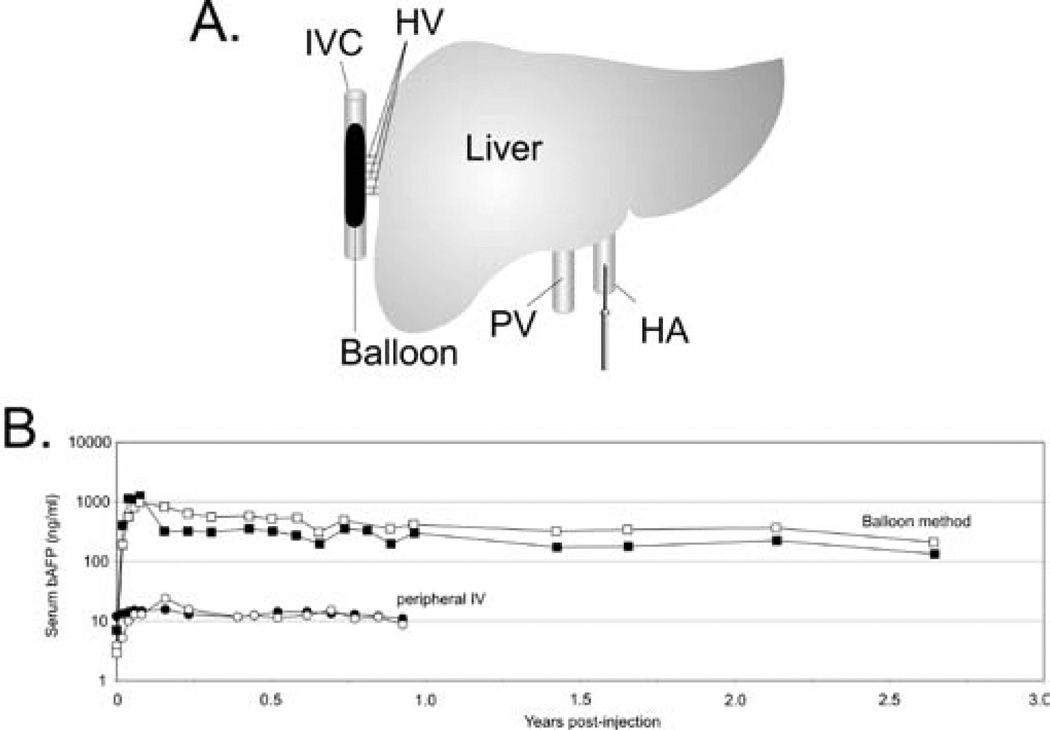
Given the tremendous potential of HDAd for liver-directed gene therapy, several groups have investigated different strategies to overcome the threshold to hepatocyte transduction and the obstacle of acute toxicity. Because the severity of the acute response is dose-dependent, one of these approaches aimed at preferential targeting of the vector to the liver thereby allowing for the use of lower vector doses. One strategy involved injection of HDAd directly into the surgically isolated liver of nonhuman primates and was shown to achieve higher efficiency hepatic transduction with reduced systemic vector dissemination compared to systemic injection resulting in stable, high level long term transgene expression for the duration of the observation period of at least 665 days in one animal and at least 560 days in two other animals with no chronic toxicity [65]. More recently, minimally invasive, percutaneous balloon occlusion catheter based methods were reported to achieve preferential hepatocyte transduction, resulting in high level, stable transgene expression in nonhuman primates using clinically relevant low doses of HDAd [66,67]. In one of these methods, a sausage-shaped balloon was inflated in the inferior vena cava to occlude the hepatic venous outflow and the HDAd was injected directly into the liver via the hepatic artery (Figure 5) [67]. This novel method of vector delivery resulted in up to 80-fold higher level of transgene expression compared to peripheral intravenous injection. Furthermore, this high level transgene expression persisted for several years in the absence of chronic toxicity. As discussed above, the size of the sinusoidal endothelial fenestrae (SEF) of the liver plays a critical role in hepatocyte transduction by Ad. Therefore, physical or chemical methods to enlarge SEF diameter could increase hepatocyte transduction with lower vector doses thereby reducing the acute toxicity. The size of the SEF can be enlarged by drugs such as Na-decanoate or N-acetylcysteine [118], combined with transient liver ischemia or pretreatment with the neuropeptide vasoactive intestinal peptide (VIP) [119] among others. As discussed above, KCs sequester intravenously administered Ad thereby reducing hepatocyte transduction. Recently, Prill et al. [120] showed that specific coupling of 5K PEG or transferrin to the hexon capsid protein of FGAd and HDAd can improve liver transduction by up to 11-fold and 18-fold, respectively. The mechanism for this improvement appears to be evasion of KCs. Another recent study has shown that avoiding Kupffer cell uptake may be possible by using a chimeric vector in which the hypervariable region of Ad5 is replaced with that of Ad6 and in so doing, increase liver specific transduction approximately 10-fold in BALB/c mice. Additionally, ALT levels were significantly lower in mice given the Ad5/6 chimeric vector than mice receiving the Ad5 vector [121]. One potential approach to reduce the visibility of the Ad vector to the immune system is to encapsulate the virion in a cationic liposome [122]. The encapsulated Ad resulted in 70–80% decrease in serum cytokines compared to unencapsulated Ad without compromising the hepatic transduction efficiency in mice. Another approach involved the administration of PEGylated Ad vectors which resulted in a 50% to 70% decrease in serum cytokine compared to un-PEGylated Ad [123,124]. In another study the combination of methylprednisolone, an anti-inflammatory glucocorticoid, and PEGylated Ad potently inhibited IL-6 elevation [125]. Similarly, a single administration of dexamethasone, another anti-inflammatory glucocorticoid, prior to Ad administration was able to significantly reduce both innate and adaptive immune response [126]. These approaches, individually or in combination, may improve the therapeutic index of HDAd.
Figure 5.
(A) A sausage-shaped balloon catheter is positioned in the inferior vena cava (IVC) under fluoroscopic guidance. Inflation of the balloon results in hepatic venous outflow occlusion from the hepatic veins (HV). The HDAd is administered by injection through a percutaneously positioned hepatic artery (HA) catheter. (B). Serum levels of the reporter baboon α-fetoprotein (bAFP) following administration of 3×1010 vp/kg of a HDAd expressing bAFP into baboons using the balloon method described above (squares) or by simple peripheral intravenous injection (circles). The balloon method of vector delivery yielded up to 80-fold higher level of transgene expression compared to peripheral intravenous injection of vector, and transgene expression persisted at high levels for at least 2.5 years.
Lung-directed gene therapy
The lung is an attractive target for gene transfer with the goal of treating cystic fibrosis (CF), one of the most common genetic disorders due to recessive mutations in the cystic fibrosis transmembrane conductance regulatory (CFTR) gene. Several CF gene therapy clinical trials have been conducted [127] but no single class of gene therapy vector or vector delivery strategy has yet emerged as obviously superior and the results to date have been disappointing.
FGAd, extensively studied for CF gene therapy, have a number of serious shortcomings. First, pulmonary delivery of FGAd is inefficient because the cellular receptor for Ad (and other viral vectors) resides on the basolateral surface of the airway epithelial cells and the tight junctions prevent vector-receptor interactions required for transduction [128]. Second, pulmonary delivery of FGAd results in dose-dependent inflammation and pneumonia [129–133] beginning about 3 to 4 days post-administration and becoming progressively more severe before eventually resolving. This latter problem has been attributed to the expression of the viral genes of the FGAd vector backbone which are cytotoxic and cause an adaptive cellular immune response against the transduced cells resulting in loss of transgene expression and chronic toxicity [24,25]. The first obstacle was addressed using compounds, such as EGTA, that disrupt the tight junctions prior to vector administration. This pre-treatement resulted in extensive Ad-mediated transduction of the proximal and distal airways (Figure 6) [134]. The second obstacle was solved with the use of HDAd: while administration of FGAd results in pulmonary inflammation with focal peribronchial lymphocytic infiltrates and focal alveolar macrophages, the lungs of mice given HDAd are free of inflammation and indistinguishable from saline treated animals presumably because of the absence of viral gene expression from HDAd [134]. Moreover, the duration of HDAd-mediated pulmonary transgene expression persisted for at least 15 weeks [134]. In these studies, transgene expression was driven by the human cytokeratin 18 (K18) promoter which is active in the epithelium of large airways and bronchioles and in submucosal glands with little expression in the alveoli, an expression pattern similar to CFTR [135]. Moreover, in contrast to commonly used viral promoters, the K18 promoter is less likely to suffer host shut-off and could reduce immune stimulation resulting in inappropriate expression in antigen presenting cells. The large cloning capacity of HDAd makes this vector ideal to accommodate the relatively large K18 control elements (4.1 kb) and the reporter or therapeutic cDNAs.
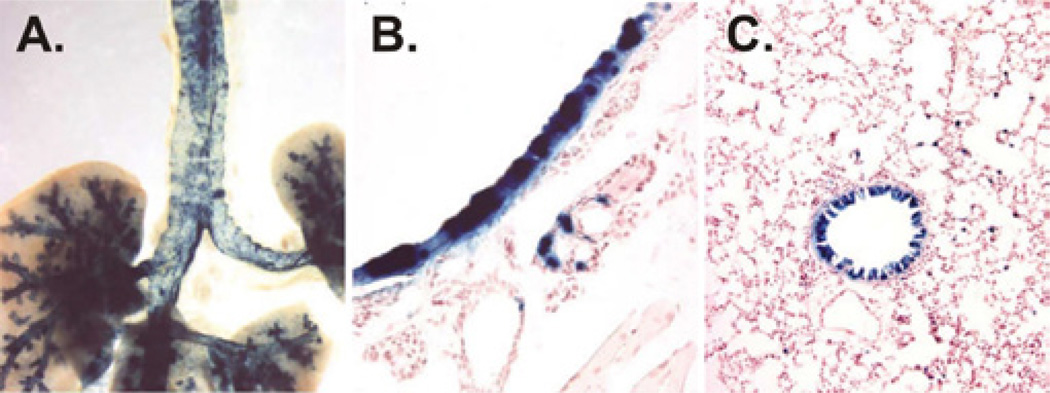
Figure 6.
Airway transduction by HDAd. Epithelia transduction of the proximal and distal airway (A), trachea (B), and bronchiole (C) of mice 3 days post-intranasal administration of HDAd-K18LacZ. Blue areas represent HDAd transduced cells.
An HDAd vector bearing the human CFTR cDNA under the control of the K18 was also found to express properly localized CFTR in cultured cells and in the apical airway epithelia of mice following intranasal administration [136]. Importantly, this vector was also found to improve resistance to acute lung infection in CFTR knockout mice [136]. High efficiency transduction of the airway epithelium has also been demonstrated in a large animal model (rabbit) using an HDAd, formulated in 0.1% L-lysophosphatidylcholine (LPC) to open the tight junctions, and delivered by an intracorporeal nebulizing catheter called the AeroProbe (Trudell Medical International) to aerosolize material directly into the trachea and lungs [137]. Although high interlobular variation was present, the delivery of HDAd revealed exceedingly high and unprecedented transduction from the trachea to terminal bronchioles (Figure 7). All rabbits, including those given only LPC as controls showed a transient decrease in dynamic lung compliance immediately following aerosol delivery. Fever and mild-to-moderate patchy pneumonia without edema were also observed. It is possible that LPC may have contributed to these effects which may be eliminated or minimized by optimizing the LPC and/or vector doses. Nevertheless, this study significantly demonstrated for the first time high efficiency transduction of the airway epithelium in a large animal which had previously been a major obstacle to CF gene therapy. This strategy has been applied to nonhuman primates which also yield high efficiency transduction of the airway epithelium but also high interlobular vector distribution [138]. More uniform vector distribution to all lung lobes was subsequently achieved in the nonhuman primate model by targeting HDAd aerosolization individually into each lung lobe. This strategy resulted in an exceedingly high transduction efficiency to all lung lobes with negligible toxicity [139]. It should be pointed out that the aforementioned studies were performed in animal models with generally intact airways and that transduction will likely be reduced in the lungs affected by multiple bacterial colonizations and thick mucus such as the human CF lungs. Up to now efficacy of gene therapy has been only addressed in animal models with unaffected airways such as the CFTR knock-out mice and the nonhuman primates. The recently developed pig model for CF could potentially provide a better model for assessing the efficacy of experimental treatments in the CF lung disease [140]. However, several strategies can be envisioned to address this obstacle in the clinical setting. For example, severely affected CF patients may undergo commonly employed regimens to clear their lungs before gene transfer. This could include inhaled antibiotics (such as Tobramycin) and systemic intravenous anti-pseudomonal antibotics (such as aminoglycosides, beta lactams, fluoroquionoes), pulmonary treatment with mucolytic agents (such as Pulmozyme), along with mechanical airway clearance to reduce the amount of mucus. Conducting gene transfer in CF patients with less affected lungs may be an alternative option, including the enrollment of younger CF patients with little or no lung disease. While somewhat controversial, this is not without precedence. Indeed, in a recent clinical trial using AAV, CF patients as young as 12 years of age were enrolled [141]. In summary, while the thickened mucus remains a barrier for all gene transfer vectors (viral or nonviral) as well as for small molecule therapeutics we do not believe it to be insurmountable, especially considering the low levels of gene transfer that may be required.
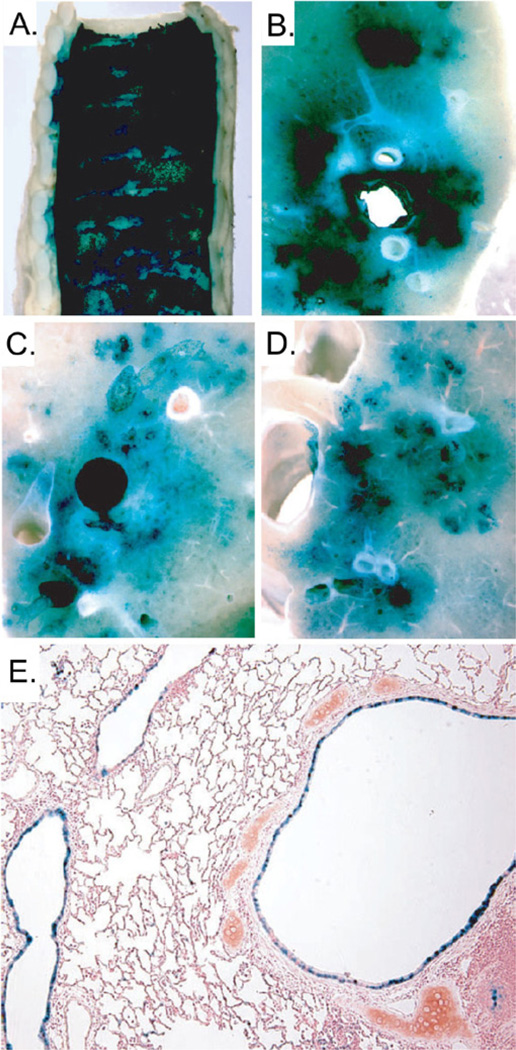
Figure 7.
Pulmonary transduction in rabbits following intratracheal aerosolization of L-α lysophosphatidylcholine and HDAd-K18LacZ. X-gal stained (A) trachea, (B) right upper lobe, (C) left lower lobe, (D) right lower lobe and (E) bronchus and bronchioles. Blue areas represent HDAd transduced cells.
Muscle-directed gene therapy
Duchenne Muscular Dystrophy (DMD) is an X-linked lethal disorder that affects 1 in 3500 male births and is caused by genetic mutation in the dystrophin gene. The protein dystrophin is an essential structural component of the skeletal muscle cell membrane and its deficiency results in instability of the muscle cell and fiber degeneration. Since there is a lack of effective treatments, gene therapy strategies aimed at transferring normal copies of the dystrophin gene into the muscle fibers of patients, appears one of the most desirable options to cure this disease. The cDNA for full-length dystrophin is approximately 14 kb, far above the size of most of gene therapy vectors. HDAd has opened the possibility to treat DMD animal models due to its large cloning capacity (up to 37 Kb) which can accommodate up to two copies of the dystrophin gene [142,143]. HDAds expressing full-length dystrophin gene have been shown to restore the full dystrophin-glycoprotein complex in the skeletal muscle. In these studies, neonate skeletal muscles of mdx mice (a model for DMD) injected with HDAd were able to express dystrophin for the duration of the experiment (up to one year) resulting in the amelioration of the pathogenesis of the disease and in a reduced level of muscle degeneration with functional correction of muscle contractility [142]. However a significant inflammatory response was observed which was accompanied by humoral response against the murine dystrophin protein expressed by transduced muscle. This immune response may also occur in humans, as many DMD patients have large dystrophin gene deletion. Therefore the dystrophin encoded by gene transfer vectors may be seen as a foreign antigen with the consequent development of an adaptive immune response and loss of long-term phenotypic correction. One potential strategy to bypass the immunity against the protein is the co-delivery of immunomodulatory molecules able to blunt the innate and adaptive immune response. Jiang et al. demonstrated that blockade of the costimulatory interaction between naïve T cells and antigen-presenting cells by co-delivering CTLA4Ig alone or in combination with CD40Ig, diminishes innate and adaptive immunity induced by HDAd-dystrophin and prolong the transgene expression [144,145]. HDAd have also been explored for in utero gene therapy for DMD. The immaturity of the fetal immune system accompanied by the survival advantage of the muscle cells expressing dystrophin over the dystrophin-deficient fiber, makes this approach very attractive. The application of this strategy in vivo, showed that HDAd is less toxic compared to FGAd and capable of driving stable transgene expression and restoration of the sarcoglycan complex [146]. In order to be an effective treatment for this disease, dystrophin needs to be expressed by multiple muscles, including the diaphragm because respiratory dysfunction is a main cause of death among DMD patients. Therefore, diaphragm-directed gene therapy has been investigated in mdx mice with HDAd which leads to reversal of functional abnormalities of dystrophic diaphragms for at least 30 days [147].
The muscle is a very attractive target for gene transfer because, like the liver, it can be used as a cell factory for production and secretion of therapeutic proteins. In fact, skeletal myofibers constitute about 40% of the total body mass, have a relatively long half-life and can be easily transduced in vivo because of easy access. High seroprevalence of pre-existing anti-Ad neutralizing antibody in the adult human population represents an obstacle for intravenous delivery, and it has been shown that this could be minimized by local delivery of the vector. Interestingly, when HDAd was injected intramuscularly (i.m) into previously immunized mice, stable transgene expression could be achieved; in contrast, the same mice injected i.m. with FGAd lost transgene expression after 3 weeks [148]. This study also showed that even though it is possible to administer HDAd into pre-immunized mice, a 30- to 100 fold higher dose (compared to naïve mice) was required to achieve 87% and 100% transduction of the muscles.
Finally, another hurdle that hampers muscle-directed gene therapy with Ad vectors is that mature muscule is not transduced efficiently because of low level of CAR receptor expression on the surface of adult muscle cells. Bramson L et al. [149] showed that the incorporation of polylysine into the H-I loop of the adenoviral fiber protein can improve HDAd transduction of mature muscle cells, giving up to 21- fold increase compared to the unmodified counterpart [149].
Brain and eye-directed gene therapy
Because of their intrinsic ability to infect post-mitotic cells and to mediate long-term transgene expression, Ad vectors constitute a very promising gene-delivery platform for central nervous system (CNS) disorders [150–153]. The above features are critical in order to successfully treat disorders ranging from simple monogenic disorders (such as Lesh-Nyhan syndrome, leukodistrophies, lysosomal storage diseases, amyotrophic lateral sclerosis among others) to multifactorial diseases including Parkinson’s disease and Alzeimer’s disease.
Following systemic administration of an FGAd vector, a rapid decline in transgene expression has been observed in peripheral organs whereas the same FGAd vector is able to stably transduce adult brain cells [150–152]. Indeed, intraparenchymal injection of FGAd vectors into the brain elicits a minimal, transient local inflammation which does not compromise the duration of transgene expression. One possible explanation for this phenomenon is the “immune-privileged” status of the brain, being relatively protected from the effect of the immune response. In fact, Ad injections into the brain result in an ineffective T cell response against brain-transduced cells in presence of viral protein expression from the backbone of FGAd vectors [154,155]. However, the immune system can respond to antigenic stimuli in the brain if the host organism has pre-existing immunity against that antigen, which would be the case for pre-immunization or re-administration with the same vector. In this case, loss of transgene expression and chronic inflammation are observed following FGAd injection into the brain [156]. In contrast, injection of HDAd into the brain of pre-immunized mice does not show these detrimental effects. Instead, HDAd was able to mediate significantly higher levels of transgene expression with substantially reduced immune response [156,157]. Recently, interesting results have been reported by delivering HDAd into the cerebrospinal fluid through a lumbar puncture in primates [158]. In this study, it was shown that injection of an HDAd vector by lumbar puncture into the cerebrospinal fluid (CSF) of non-human primates allows long-term (three months) transduction of neuroepithelial cells. This result was also observed in monkeys bearing a pre-existing anti-adenoviral immunity [158]. Another study from the same authors showed that by using the same route of administration in immune-competent mice, it was possible to deliver HDAd expressing anti-inflammatory cytokines and achieve long term transgene expression without any signs of toxicity [159]. This latter strategy makes HDAd-CSF gene delivery a very attractive therapeutic approach for brain inflammatory condition such as multiple sclerosis.
Encouraging results have been obtained in a study in which stereotactic injection of HDAd expressing a short hairpin RNA to silence the Huntington disease gene was able to inhibit Huntington protein aggregation [160,161]. However, the vector had limited brain distribution not extending beyond a few millimeters from the needle track, making this approach still far from optimal. The brain is a complex organ with intricate interconnections between various cell types and therefore it could be challenging to develop a targeting strategy with HDAd for the correction of diseases with diffuse involvement. Nevertheless, diseases requiring localized gene delivery to a discrete set of neurons such as Parkinson’s disease or brain tumors may be more suitable.
HDAd vectors have recently emerged as an important therapeutic strategy for brain tumor treatment. In a preclinical study for the treatment of glioblastoma multiforme, intratumoral injection with HDAd encoding the conditionally cytotoxic herpes simplex type 1 thymidine kinase (TK) and the immunostimulatory cytokine fms-like tyrosine kinase ligand 3 (Flt3L) was associated with increased survival and development of antiglioma immunological memory without signs of neuropathology or systemic toxicity [162]. Given the high risk that FGAd treatment of glioblastoma multiforme can be compromised by prior exposure to natural Ad infection, HDAd vectors could offer a safer and more effective treatment for patients with this type as well other types of brain cancer.
There have been a limited number of studies investigating HDAd vectors for ocular gene therapy. In one study, HDAd vector was able to transduce and rescue cells from the neurosensory retina in a mouse model of retinal degeneration [163]. Moreover, HDAd vectors showed a great potential in targeting the retinal pigment epithelium following subretinal injection, without evidence of adverse immune reactions [164].
Human gene therapy with HDAd
There has been a single case of intravascular administration of HDAd into a human patient. In this clinical trial, 4.3×1011 vp/kg of a HDAd expressing factor VIII (FVIII) was intravenously injected into a hemophilia A patient [165]. This subject developed grade 3 liver toxicity, marked increase in interleukin-6 (IL-6), thrombocytopenia and laboratory signs of disseminated intravascular coagulopathy, but all these values returned to baseline by day 19 post-infusion. Unfortunately, no evidence of FVIII expression was detected [164]. Also unfortunate is that this study has yet to be published in a peer-reviewed format so that much of the details remain unknown.
HDAd has recently been used in a clinical trial to treat anemic chronic kidney disease (CDK) patients [166]. In this Phase I–II study, a small number of autologous dermal fibroblasts were removed from under the skin of anemic CDK patients under local anesthesia and transduced ex vivo with an HDAd expressing erythropoietin (EPO). Following transduction, the amount of EPO produced by the transduced cells was measured so that the precise number of transduced cells could be reimplanted subcutaneously to achieve the requisite dose of EPO. No adverse events were reported in this trial and, importantly, elevated hemoglobin levels were sustained for up to one year after a single treatment with the HDAd transduced cells. Significantly, this study also clearly demonstrates that HDAd can be manufactured under cGMP.
HDAd as genetic vaccines
FGAds have been developed to express antigens and have proven to be valuable genetic vaccines. Recent studies have shown that HDAd may be superior than FGAd for this application. For example, Harui et al. [167] compared the ability of FGAd and HDAd expressing β-galactosidase to generate an immune response in mice and found that HDAd generated a stronger T cell and antibody response against β-galactosidase than FGAd. Weaver et al. [168] also found that HDAd-based vaccines generated stronger immune responses against the encoded antigen than FGAd-vaccines in mice. In addition, administration of HDAd–based vaccines resulted in lower tissue damage and anti-Ad T cell responses than FGAd. In a subsequent study, Weaver et al. [169], demonstrated that rhesus macaques vaccinated with HDAd expressing HIV-1 envelope protected the animals from subsequent mucosal SHIV challenge. Because most humans are seropositive for adenovirus serotype 5, HDAd vaccines based on serotype 5 may be minimally, if at all, effective. To overcome this, Weaver et al. used HDAd vaccines based on serotypes 1, 2 and 6 and showed that the presence of pre-existing immunity to adenovirus serotype 5 in both the mice and rhesus macaques did not prevent successful vaccination [168,169]. These studies demonstrate the potential utility of HDAd as a genetic vaccine.
Vascular gene therapy with HDAd
Expression of therapeutic atheroprotective genes in arterial endothelial cells is attractive for atherosclerosis gene therapy by preventing or reversing atherosclerosis. Wen et al. [170] demonstrated that HDAd can provide long-term transgene expression following transduction of vascular wall with minimal inflammation. This was in contrast to FGAd in which transgene expression was transient and accompanied by greater inflammation and neointimal formation. Moreover, in contrast to FGAd, transduction of arterial endothelial cells by HDAd did not alter critical physiological functions (proliferation, migration, apoptosis, metabolic activity, and nitric oxide production) and did not stimulate proinflammatory pathways [171]. Flynn et al. [172] showed that localized infusion of carotid arteries with HDAd expressing apoA-1 significantly retards althrogenesis in hyperlipidemic rabbits for at least 48 weeks with stable apoA-1 expression and a lack of systemic vector dissemination and toxicity.
HDAd and stem cells
Embryonic stem (ES) cells and induced pluripotent stem cells (iPSCs) have recently attracted much attention because of their self-renewal capacity and pluripotency render them promising for cell-based therapies. Targeted gene repair/editing of stem cells would offer the potential for autologous cell therapy for the treatment of multiple human diseases. As a gene targeting vector for stem cells, HDAd offer numerous advantages. HDAd can transduce stem cells with high efficiency and low cytotoxicity. The large cloning capacity of HDAd can accommodate very long regions of homology to the target chromosome locus to enhance homologous recombination. Introduction of artificial double strand breaks, which are potentially mutagenic, are not required. Indeed, HDAd-mediated gene targeting has been accomplished in mouse ES cells [173], monkey ES cells [174] and human iPS cells [175]. For example, Liu et al demonstrated that HDAd can efficiently correct mutations in the transcriptionally inactive lamin A locus, which is associated with various degenerative diseases, in patient-specific iPS cells without off-target effects and/or introduction of additional mutations [175]. Because of HDAd’s large cloning capacity, long homologous arms can be used which permit a wide range of choices for promoters and selectable markers and correction of multiple mutations at the target locus. Furthermore, high efficient transduction by HDAd contributes to high efficiency targeting thus allowing for smaller numbers of starting cells. Importantly, the disease phenotype was reversed in patient-derived cells after HDAd targeting.
iPS cells have gained prominence in recent years because of the eithical issues surrounding the use of human ES cells. A popular method to reprogram human adult somatic cells to iPS cells is by retroviral integration of c-Myc, Klf4, Oct4 and Sox2. However, the use of integrating viruses is a concern, especially for clinical application, because they may cause insertional mutagensis, interfere with endogenous gene transcription and induce malignant transformation. Since only transient expression of the four aforementioned genes are required for reprogramming, Zhou and Freed [176] reasoned that E1-deleted Ad would be safer because they do not integrate into the host genome but provided the necessary transient transgene expression required for reprogramming. Indeed, following transduction with four E1-deleted Ads, each expressing one of the aforementioned genes, human adult fibroblasts were reprogram to iPS cells. Unfortunately, the efficiency was low. The authors’ hypothesized that this was due to low efficiency of fibroblast being transduced by all four E1-deleted Ads. HDAd’s large cloning capacity could easily accommodate all four genes into a single vector, and thus may increase the efficiency of reprogramming.
Concluding remarks and future challenges
In all animal models studied to date, HDAd transduced hepatocytes (as well as all other target cell types examined) are not destroyed by an adaptive cellular immune response, thus leading to long-term transgene expression. However, this may not hold true in humans, especially considering the outcome of a recent liver-directed clinical trial for FIX-deficiency using rAAV2 [177]. rAAV2, like HDAd, do not contain any viral genes and directs long-term transgene expression following hepatocyte transduction in all animal models tested. However, in humans, rAAV2-mediated transgene expression from transduced hepatocytes is short-lived due to their killing by AAV-specific CTLs. The source of immunogen in this case has been attributed to AAV capsid peptides derived directly from the injected particle [177–179]. Similar findings suggesting immune rejection of transduced cells due to AAV capsid peptide presentation was recently observed in another liver-directed scAAV8 clinical trial [180] as well as an AAV1 muscle-directed clinical trial [181] Similar to AAV, the HDAd capsid proteins derived directly from the administered particle may be a source of immunogen, analogous to rAAV2 [182,183]. In this regard, Roth et al. [184] showed that HDAd transduction of DCs in vitro can stimulate activation of anti-Ad CD8+ T-cells. Indeed, Muruve et al. [86] showed that Ad-specific CTL were generated following intravascular administration of HDAd into mice. Similarly, Kushwah et al. [185] showed that intranasal administration of HDAd resulted in Ad-specific CD8+Tcells. These studies show that HDAd can indeed provoke the generation of a CTL response directed against the viral protein derived directly from the capsid independent of de novo viral protein synthesis following administration into mice. However, what has not been documented is whether these Ad-specific CTLs can kill HDAd transduced cells in vivo. It should be noted that the onset of transgene expression by AAV2 is delayed. This is due, in part, to the fact that AAV2 uncoats very slowly and virion proteins are metabolized slowly, remaining in the transduced cells for up to eight weeks [186]. This results in prolonged presentation of antigenic epitopes on the hepatocyte surface for continued recognition and targeting by AAV-specific CTLs for a long period of time. In contrast, onset of transgene expression by HDAd is very rapid and can be detected within hours post-transduction. This suggests that HDAd uncoats very rapidly and perhaps Ad peptides derived from the virion capsid are cleared quickly from the transduced cell rendering them invisible to Ad-specific CTLs even if they are generated. This is an important issue that remains to be answered. However, animal modeling may not be useful for addressing this issue, at least not for rAAV2 [187–189]. The ex vivo clinical trial results using HDAd showing at least one year of sustained EPO expression in patients with chronic kidney disease may be encouraging in this regard [166].
Acknowledgements
PN is supported by the National Institutes of Health (R01 DK069369 and R01 HL083047). AR is supported by a National Institute of General Medical Sciences training grant (T32 GM08307).
References
- 1.Brunetti-Pierri N, Ng P. Helper-dependent adenoviral vectors for liver-directed gene therapy. Hum Mol Genet. 2011;20:R7–R13. doi: 10.1093/hmg/ddr143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross PJ, Kennedy MA, Parks RJ. Host cell detection of noncoding stuffer DNA contained in helper-dependent adenovirus vectors leads to epigenetic repression of transgene expression. J Virol. 2009;83:8409–8417. doi: 10.1128/JVI.00796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephen SL, Montini E, Sivanandam VG, Al-Dhalimy M, Kestler HA, et al. Chromosomal integration of adenoviral vector DNA in vivo. J Virol. 2010;84:9987–9994. doi: 10.1128/JVI.00751-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer DJ, Ng P. Improved system for helper-dependent adenoviral vector production. Mol Ther. 2003;8:846–852. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Shenk T, Horwitz MS. In: Adenoviridae: the viruses and their replication. Fields BN, Knipe DM, Howley PM, editors. Philadelphia: Lipponcott-Raven Publishers; 1996. pp. 2265–2326. [Google Scholar]
- 6.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 7.Hong SS, Karayan L, Tournier J, Curiel DT, Boulanger PA. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. Embo J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 10.Wickham T, Segal DM, Roelvink PW, Carrion ME, Lizonova A, et al. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J Virol. 1996;70:6831–6838. doi: 10.1128/jvi.70.10.6831-6838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman M, Su Q, Wilson JM. Gradient of RGD-dependent entry of adenoviral vector in nasal and intrapulmonary epithelia: implications for gene therapy of cystic fibrosis. Gene Ther. 1996;3:811–818. [PubMed] [Google Scholar]
- 12.Mellman I. The importance of being acid: the role of acidification in intracellular membrane traffic. J Exp Biol. 1992;172:39–45. doi: 10.1242/jeb.172.1.39. [DOI] [PubMed] [Google Scholar]
- 13.Leopold PL, Ferris B, Grinberg I, Worgall S, Hackett NR, et al. Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells. Hum Gene Ther. 1998;9:367–378. doi: 10.1089/hum.1998.9.3-367. [DOI] [PubMed] [Google Scholar]
- 14.Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 15.Parker AL, Waddington SN, Nicol CG, Shayakhmetov DM, Buckley SM, et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- 16.Parker AL, McVey JH, Doctor JH, Lopez-Franco O, Waddington SN, et al. Influence of coagulation factor zymogens on the infectivity of adenoviruses pseudotyped with fibers from subgroup D. J Virol. 2007;81:3627–3631. doi: 10.1128/JVI.02786-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waddington SN, Parker AL, Havenga M, Nicklin SA, Buckley SM, et al. Targeting of adenovirus serotype 5 (Ad5) and 5/47 pseudotyped vectors in vivo: fundamental involvement of coagulation factors and redundancy of CAR binding by Ad5. J Virol. 2007;81:9568–9571. doi: 10.1128/JVI.00663-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Alba R, Bradshaw AC, Parker AL, Bhella D, Waddington SN, et al. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: effect of mutagenesis on FX interactions and gene transfer. Blood. 2009;114:965–971. doi: 10.1182/blood-2009-03-208835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1997;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 22.Morral N, O’Neal W, Zhou H, Langston C, Beaudet A. Immune responses to reporter proteins and high viral dose limit duration of expression with adenoviral vectors: comparison of E2a wild type and E2a deleted vectors. Hum Gene Ther. 1997;8:1275–1286. doi: 10.1089/hum.1997.8.10-1275. [DOI] [PubMed] [Google Scholar]
- 23.Dai Y, Schwarz EM, Gu D, Zhang WW, Sarvetnick N, et al. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, et al. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Xiang Z, Ertl HC, Wilson JM. Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kafri T, Morgan D, Krahl T, Sarvetnick N, Sherman L, et al. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc Natl Acad Sci USA. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelhardt JF, Litzky L, Wilson JM. Prolonged transgene expression in cotton rat lung with recombinant adenoviruses defective in E2a. Hum Gene Ther. 1994;5:1217–1229. doi: 10.1089/hum.1994.5.10-1217. [DOI] [PubMed] [Google Scholar]
- 29.Gao GP, Yang Y, Wilson JM. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Nunes FA, Berencsi K, Gonczol E, Engelhardt JF, et al. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- 31.Goldman MJ, Litzky LA, Engelhardt JF, Wilson JM. Transfer of the CFTR gene to the lung of nonhuman primates with E1-deleted, E2a-defective recombinant adenoviruses: a preclinical toxicology study. Hum Gene Ther. 1995;6:839–851. doi: 10.1089/hum.1995.6.7-839. [DOI] [PubMed] [Google Scholar]
- 32.Dedieu JF, Vigne E, Torrent C, Jullien C, Mahfouz I, et al. Long-term gene delivery into the livers of immunocompetent mice with E1/E4-defective adenoviruses. J Virol. 1997;71:4626–4637. doi: 10.1128/jvi.71.6.4626-4637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, Greenburg G, Bunch D, Farson D, Finer MH. Persistent transgene expression in mouse liver following in vivo gene transfer with a delta E1/delta E4 adenovirus vector. Gene Ther. 1997;4:393–400. doi: 10.1038/sj.gt.3300404. [DOI] [PubMed] [Google Scholar]
- 34.Van Linthout S, Lusky M, Collen D, De Geest B. Persistent hepatic expression of human apo A-I after transfer with a helper-virus independent adenoviral vector. Gene Ther. 2002;9:1520–1528. doi: 10.1038/sj.gt.3301824. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs F, Snoeys J, Feng Y, Van Craeyveld E, Lievens J, et al. Direct comparison of hepatocyte-specific expression cassettes following adenoviral and nonviral hydrodynamic gene transfer. Gene Therapy. 2008;15:594–603. doi: 10.1038/sj.gt.3303096. [DOI] [PubMed] [Google Scholar]
- 36.Van Craeyveld E, Gordts S, Nefyodova O, Jacobs F, De Geest B. Regression and stabilization of advanced murine atherosclerotic lesions: a comparison of LDL lowering and HDL raising gene transfer strategies. J Mol Med. 2011;89:555–567. doi: 10.1007/s00109-011-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang B, Wang H, Gordon G, Bellinger DA, Read MS, et al. Lack of persistence of E1- recombinant adenoviral vectors containing a temperature-sensitive E2A mutation in immunocompetent mice and hemophilia B dogs. Gene Ther. 1996;3:217–222. [PubMed] [Google Scholar]
- 38.O’Neal WK, Zhou H, Morral N, Aguilar-Cordova E, Pestaner J, et al. Toxicological comparison of E2a-deleted and first-generation adenoviral vectors expressing alpha1-antitrypsin after systemic delivery. Hum Gene Ther. 1998;9:1587–1598. doi: 10.1089/hum.1998.9.11-1587. [DOI] [PubMed] [Google Scholar]
- 39.Lusky M, Christ M, Rittner K, Dieterle A, Dreyer D, et al. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J Virol. 1998;72:2022–2032. doi: 10.1128/jvi.72.3.2022-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddy PS, Sakhuja K, Ganesh S, Yang L, Kayda D, et al. Sustained human factor VIII expression in hemophilia A mice following systemic delivery of a gutless adenoviral vector. Mol Ther. 2002;5:563–573. doi: 10.1006/mthe.2001.0510. [DOI] [PubMed] [Google Scholar]
- 41.Brough DE, Hsu C, Kulesa VA, Lee GM, Cantolupo LJ, et al. Activation of transgene expression by early region 4 is responsible for a high level of persistent transgene expression from adenovirus vectors in vivo. 1997;71:9206–9213. doi: 10.1128/jvi.71.12.9206-9213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kochanek S, Clemens PR, Mitani K, Chen HH, Chan S, et al. A new adenoviral vector: Replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc Natl Acad Sci USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gräble M, Hearing P. Adenovirus type 5 packaging domain is composed of a repeated element that is functionally redundant. J Virol. 1990;64:2047–2056. doi: 10.1128/jvi.64.5.2047-2056.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, et al. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bett AJ, Prevec L, Graham FL. Packaging capacity and stability of human adenovirus type 5 vectors. J Virol. 1993;67:5911–5921. doi: 10.1128/jvi.67.10.5911-5921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parks RJ, Graham FL. A helper-dependent system for adenovirus vector production helps define a lower limit for efficient DNA packaging. J Virol. 1997;71:3293–3298. doi: 10.1128/jvi.71.4.3293-3298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng P, Evelegh C, Cummings D, Graham FL. Cre levels limit packaging signal excision efficiency in the Cre/loxP helper-dependent adenoviral vector system. J Virol. 2002;76:4181–4189. doi: 10.1128/JVI.76.9.4181-4189.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng P, Beauchamp C, Evelegh C, Parks R, Graham FL. Development of a FLP/frt system for generating helper-dependent adenoviral vectors. Mol Ther. 2001;3:809–815. doi: 10.1006/mthe.2001.0323. [DOI] [PubMed] [Google Scholar]
- 49.Umana P, Gerdes CA, Stone D, Davis JR, Ward D, et al. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat Biotechnol. 2001;19:582–585. doi: 10.1038/89349. [DOI] [PubMed] [Google Scholar]
- 50.Sargent KL, Ng P, Evelegh C, Graham FL, Parks RJ. Development of a size-restricted pIX-deleted helper virus for amplification of helper-dependent adenovirus vectors. Gene Ther. 2004;11:504–511. doi: 10.1038/sj.gt.3302107. [DOI] [PubMed] [Google Scholar]
- 51.Parks RJ. Adenovirus protein IX: a new look at an old protein. Mol Ther. 2005;11:19–25. doi: 10.1016/j.ymthe.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 52.Alba R, Hearing P, Bosch A, Chillon M. Differential amplification of adenovirus vectors by flanking the packaging signal with attB/attP-PhiC31 sequences: implications for helper-dependent adenovirus production. Virology. 2007;367:51–58. doi: 10.1016/j.virol.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 53.Stern BS, Shoshani W, Pearlman AL, Ng P, Nissenson AR, et al. Erythropoeisis Sustained 1 Year by the EPODURE BioPump in Patients with Chronic Kideney Disease: Further Results of PhaseI/II Proof of Concept Trial. Mol Ther. 2010;18:S239. [Google Scholar]
- 54.Palmer DJ, Ng P. Methods for the production and characterization of helper-dependent adenoviral vectors. In: Friedmann T, Rossi J, editors. DNA Delivery/Gene Transfer Vector Lab Manual. Cold Spring Harbor Press; 2007. pp. 167–181. [Google Scholar]
- 55.Palmer DJ, Ng P. Methods for the production of helper-dependent adenoviral vectors. Methods Mol Biol. 2008;433:33–53. doi: 10.1007/978-1-59745-237-3_3. [DOI] [PubMed] [Google Scholar]
- 56.Kim IH, Jozkowicz A, Piedra PA, Oka K, Chan L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2001;98:13282–13287. doi: 10.1073/pnas.241506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strauss KA, Robinson DL, Vreman HJ, Puffenberger EG, Hart G, et al. Management of hyperbilirubinemia and prevention of kernicterus in 20 patients with Crigler-Najjar disease. Eur J Pediatr. 2006;165:306–319. doi: 10.1007/s00431-005-0055-2. [DOI] [PubMed] [Google Scholar]
- 58.Toietta G, Mane VP, Norona WS, Finegold MJ, Ng P, et al. Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2005;102:3930–3935. doi: 10.1073/pnas.0500930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9:596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 60.Ruiz R, Witting SR, Saxena R, Morral N. Robust hepatic gene silencing for functional studies using helper-dependent adenovirus vectors. Hum Gene Ther. 2009;20:87–94. doi: 10.1089/hum.2008.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Witting SR, Brown M, Saxena R, Nabinger S, Morral N. Helper-dependent adenovirus-mediated short hairpin RNA expression in the liver activates the interferon response. J Biol Chem. 2008;283:2120–2128. doi: 10.1074/jbc.M704178200. [DOI] [PubMed] [Google Scholar]
- 62.Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 63.Morral N, O’Neal W, Rice K, Leland MM, Piedra PA, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunetti-Pierri N, Nichols TC, McCorquodale S, Merricks E, Palmer DJ, et al. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum Gene Ther. 2005;16:811–820. doi: 10.1089/hum.2005.16.811. [DOI] [PubMed] [Google Scholar]
- 65.Brunetti-Pierri N, Ng T, Iannitti DA, Palmer DJ, Beaudet AL, et al. Improved hepatic transduction, reduced systemic vector dissemination, and long-term transgene expression by delivering helper-dependent adenoviral vectors into the surgically isolated liver of nonhuman primates. Hum Gene Ther. 2006;17:391–404. doi: 10.1089/hum.2006.17.391. [DOI] [PubMed] [Google Scholar]
- 66.Brunetti-Pierri N, Stapleton GE, Palmer DJ, Zuo Y, Mane VP, et al. Pseudo-hydrodynamic delivery of helper-dependent adenoviral vectors into non-human primates for liver-directed gene therapy. Mol Ther. 2007;15:732–740. doi: 10.1038/sj.mt.6300102. [DOI] [PubMed] [Google Scholar]
- 67.Brunetti-Pierri N, Stapleton GE, Law M, Breinholt J, Palmer DJ, et al. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol Ther. 2009;17:327–333. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cerullo V, McCormack W, Seiler M, Mane V, Cela R, et al. Antigen-specific tolerance of human alpha1-antitrypsin induced by helper-dependent adenovirus. Hum Gene Ther. 2007;18:1215–1224. doi: 10.1089/hum.2006.036. [DOI] [PubMed] [Google Scholar]
- 69.Tao N, Gao GP, Parr M, Johnston J, Baradet T, et al. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- 70.Schiedner G, Hertel S, Johnston M, Dries V, Van RN, et al. Selective depletion or blockade of Kupffer cells leads to enhanced and prolonged hepatic transgene expression using high-capacity adenoviral vectors. Mol Ther. 2003;7:35–43. doi: 10.1016/s1525-0016(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 71.Yu Q, Que LG, Rockey DC. Adenovirus-mediated gene transfer to nonparenchymal cells in normal and injured liver. Am J Physiol Gastrointest Liver Physiol. 2002;282:G565–G572. doi: 10.1152/ajpgi.00512.2000. [DOI] [PubMed] [Google Scholar]
- 72.Di Paolo NC, Van RN, Shayakhmetov DM. Redundant and synergistic mechanisms control the sequestration of blood-born adenovirus in the liver. Mol Ther. 2009;17:675–684. doi: 10.1038/mt.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lyons M, Onion D, Green NK, Aslan K, Rajaratnam R, et al. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol Ther. 2006;14:118–128. doi: 10.1016/j.ymthe.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Carlisle RC, Di Y, Cerny AM, Sonnen AF, Sim RB, et al. Human erythrocytes bind and inactivate type 5 adenovirus by presenting coxsackievirus-adenovirus receptor and complement receptor 1. Blood. 2009;113:1909–1918. doi: 10.1182/blood-2008-09-178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Subr V, Kostka L, Selby-Milic T, Fisher K, Ulbrich K, et al. Coating of adenovirus type 5 with polymers containing quaternary amines prevents binding to blood components. J Control Release. 2009;135:152–158. doi: 10.1016/j.jconrel.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 76.Lievens J, Snoeys J, Vekemans K, Van Linthout S, de Zanger R, et al. The size of sinusoidal fenestrae is a critical determinant of hepatocyte transduction after adenoviral gene transfer. Gene Ther. 2004;11:1523–1531. doi: 10.1038/sj.gt.3302326. [DOI] [PubMed] [Google Scholar]
- 77.Snoeys J, Lievens J, Wisse E, Snoeys J, Wisse E, et al. Species differences in transgene DNA uptake in hepatocytes after adenoviral transfer correlate with the size of endothelial fenestrae. Gene Ther. 2007;14:604–612. doi: 10.1038/sj.gt.3302899. [DOI] [PubMed] [Google Scholar]
- 78.Wisse E, Jacobs F, Topal B, Frederik P, De Geest B. The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther. 2008;15:1193–1199. doi: 10.1038/gt.2008.60. [DOI] [PubMed] [Google Scholar]
- 79.Wu E, Pache L, Von Seggern DJ, Mullen TM, Mikyas Y, et al. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J Virol. 2003;77:7225–7235. doi: 10.1128/JVI.77.13.7225-7235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, et al. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 81.Schnell MA, Zhang Y, Tazelaar J, Gao GP, Yu QC, et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- 82.Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 83.Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10:935–940. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- 84.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 85.Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, et al. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- 86.Muruve DA, Cotter MJ, Zaiss AK, White LR, Liu Q, et al. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramalingam R, Rafii S, Worgall S, Hackett NR, Crystal RG. Induction of endogenous genes following infection of human endothelial cells with an E1(−) E4(+) adenovirus gene transfer vector. J Virol. 1999;73:10183–10190. doi: 10.1128/jvi.73.12.10183-10190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morral N, O’Neal WK, Rice K, Leland MM, Piedra PA, et al. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum Gene Ther. 2002;13:143–154. doi: 10.1089/10430340152712692. [DOI] [PubMed] [Google Scholar]
- 89.Higginbotham JN, Seth P, Blaese RM, Ramsey WJ. The release of inflammatory cytokines from human peripheral blood mononuclear cells in vitro following exposure to adenovirus variants and capsid. Hum Gene Ther. 2002;13:129–141. doi: 10.1089/10430340152712683. [DOI] [PubMed] [Google Scholar]
- 90.Cichon G, Boeckh-Herwig S, Schmidt HH, Wehnes E, Müller T, et al. Complement activation by recombinant adenoviruses. Gene Ther. 2001;8:1794–1800. doi: 10.1038/sj.gt.3301611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang H, Wang Z, Serra D, Frank MM, Amalfitano A. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol Ther. 2004;10:1140–1142. doi: 10.1016/j.ymthe.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 92.Kiang A, Hartman ZC, Everett RS, Serra D, Jiang H, et al. Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol Ther. 2006;14:588–598. doi: 10.1016/j.ymthe.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 93.Othman M, Labelle A, Mazzetti I, Elbatarny HS, Lillicrap D. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. 2007;109:2832–2839. doi: 10.1182/blood-2006-06-032524. [DOI] [PubMed] [Google Scholar]
- 94.Varnavski AN, Zhang Y, Schnell M, Tazelaar J, Louboutin JP, et al. Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity. J Virol. 2002;76:5711–5719. doi: 10.1128/JVI.76.11.5711-5719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, et al. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. 2008;181:2134–2144. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- 97.Appledorn DM, Patial S, Godbehere S, Parameswaran N, Amalfitano A. TRIF, and TRIF-interacting TLRs differentially modulate several adenovirus vector-induced immune responses. J Innate Immun. 2009;1:376–388. doi: 10.1159/000207194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cerullo V, Seiler MP, Mane V, Brunetti-Pierri N, Clarke C, et al. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol Ther. 2007;15:378–385. doi: 10.1038/sj.mt.6300031. [DOI] [PubMed] [Google Scholar]
- 99.Hartman ZC, Black EP, Amalfitano A. Adenoviral infection induces a multi-faceted innate cellular immune response that is mediated by the toll-like receptor pathway in A549 cells. Virology. 2007;358:357–372. doi: 10.1016/j.virol.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 100.Hartman ZC, Kiang A, Everett RS, Serra D, Yang XY, et al. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J Virol. 2007;81:1796–1812. doi: 10.1128/JVI.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu J, Huang X, Yang Y. Type I IFN signaling on both B and CD4 T cells is required for protective antibody response to adenovirus. J Immunol. 2007;178:3505–3510. doi: 10.4049/jimmunol.178.6.3505. [DOI] [PubMed] [Google Scholar]
- 102.Zhu J, Huang X, Yang Y. A critical role for type I IFN-dependent NK cell activation in innate immune elimination of adenoviral vectors in vivo. Mol Ther. 2008;16:1300–1307. doi: 10.1038/mt.2008.88. [DOI] [PubMed] [Google Scholar]
- 103.Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nociari M, Ocheretina O, Schoggins JW, Falck-Pedersen E. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J Virol. 2007;81:4145–4157. doi: 10.1128/JVI.02685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suzuki M, Cerullo V, Bertin TK, Cela R, Clarke C, et al. MyD88-Dependent Silencing of Transgene Expression During the Innate and Adaptive Immune Response to Helper-Dependent Adenovirus. Hum Gene Ther. 2010;21:325–336. doi: 10.1089/hum.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Suzuki M, Cela R, Bertin TK, Sule G, Cerullo V, et al. NOD2 signaling contributes to the innate immune response against helper-dependent adenovirus vectors independently of MyD88 in vivo. Hum Gene Ther. 2011;22:1071–1082. doi: 10.1089/hum.2011.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 108.Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, et al. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity. 2009;31:110–121. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schiedner G, Bloch W, Hertel S, Johnston M, Molojavyi A, et al. A hemodynamic response to intravenous adenovirus vector particles is caused by systemic Kupffer cell-mediated activation of endothelial cells. Hum Gene Ther. 2003;14:1631–1641. doi: 10.1089/104303403322542275. [DOI] [PubMed] [Google Scholar]
- 110.Wolff G, Worgall S, van Rooijen N, Song WR, Harvey BG, et al. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J Virol. 1997;71:624–629. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuzmin AI, Finegold MJ, Eisensmith RC. Macrophage depletion increases the safety, efficacy and persistence of adenovirus-mediated gene transfer in vivo. Gene Ther. 1997;4:309–316. doi: 10.1038/sj.gt.3300377. [DOI] [PubMed] [Google Scholar]
- 112.Kuzmin AI, Galenko O, Eisensmith RC. An immunomodulatory procedure that stabilizes transgene expression and permits readministration of E1-deleted adenovirus vectors. Mol Ther. 2001;3:293–301. doi: 10.1006/mthe.2000.0258. [DOI] [PubMed] [Google Scholar]
- 113.Lieber A, He CY, Meuse L, Schowalter D, Schowalter D, et al. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brunetti-Pierri N, Palmer DJ, Mane V, Finegold M, Beaudet AL, et al. Increased hepatic transduction with reduced systemic dissemination and proinflammatory cytokines following hydrodynamic injection of helper-dependent adenoviral vectors. Mol Ther. 2005;12:99–106. doi: 10.1016/j.ymthe.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 115.Manickan E, Smith JS, Tian J, Eggerman TL, Lozier JN, et al. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol Ther. 2006;13:108–117. doi: 10.1016/j.ymthe.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 116.Jooss K, Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther. 2003;10:955–963. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- 117.Smith JS, Tian J, Lozier JN, Byrnes AP. Severe pulmonary pathology after intravenous administration of vectors in cirrhotic rats. Mol Ther. 2004;9:932–941. doi: 10.1016/j.ymthe.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 118.Jacobs F, Feng Y, Van CE, Lievens J, Snoeys J, et al. Species differences in hepatocyte-directed gene transfer: implications for clinical translation. Curr Gene Ther. 2009;9:83–90. doi: 10.2174/156652309787909562. [DOI] [PubMed] [Google Scholar]
- 119.Vetrini F, Brunetti-Pierri N, Palmer DJ, Bertin T, Grove NC, et al. Vasoactive intestinal peptide increases hepatic transduction and reduces innate immune response following administration of helper-dependent Ad. Mol Ther. 2010;18:1339–1345. doi: 10.1038/mt.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Prill JM, Espenlaub S, Samen U, Engler T, Schmidt E, et al. Modifications of adenovirus hexon allow for either hepatocyte detargeting or targeting with potential evasion from Kupffer cells. Mol Ther. 2011;19:83–92. doi: 10.1038/mt.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khare R, May SM, Vetrini F, Weaver EA, Palmer D, et al. Generation of a Kupffer Cell-evading Adenovirus for systemic and Liver-directed Gene Transfer. Mol Ther. 2011;19:1254–1262. doi: 10.1038/mt.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yotnda P, Chen DH, Chiu W, Piedra PA, Davis A, et al. Bilamellar cationic liposomes protect adenovectors from preexisting humoral immune responses. Mol Ther. 2002;5:233–241. doi: 10.1006/mthe.2002.0545. [DOI] [PubMed] [Google Scholar]
- 123.Croyle MA, Chirmule N, Zhang Y, Wilson JM. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum Gene Ther. 2002;13:1887–1900. doi: 10.1089/104303402760372972. [DOI] [PubMed] [Google Scholar]
- 124.Hofherr SE, Mok H, Gushiken FC, Lopez JA, Barry MA. Polyethylene glycol modification of adenovirus reduces platelet activation, endothelial cell activation, and thrombocytopenia. Hum Gene Ther. 2007;18:837–848. doi: 10.1089/hum.2007.0051. [DOI] [PubMed] [Google Scholar]
- 125.De Geest B, Snoeys J, Van LS, Lievens J, Collen D. Elimination of innate immune responses and liver inflammation by PEGylation of adenoviral vectors and methylprednisolone. Hum Gene Ther. 2005;16:1439–1451. doi: 10.1089/hum.2005.16.1439. [DOI] [PubMed] [Google Scholar]
- 126.Seregin SS, Appledorn DM, McBride AJ, Schuldt NJ, Aldhamen YA, et al. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol Ther. 2009;17:685–696. doi: 10.1038/mt.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gene Therapy Clinical Trials Worldwide. J Gene Med [Google Scholar]
- 128.Walters RW, Grunst T, Bergelson JM, Finberg RW, Welsh MJ, et al. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 129.Yei S, Mittereder N, Wert S, Whitsett JA, Wilmott RW, et al. In vivo evaluation of the safety of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA to the lung. Hum Gene Ther. 1994;5:731–744. doi: 10.1089/hum.1994.5.6-731. [DOI] [PubMed] [Google Scholar]
- 130.Wilmott RW, Amin RS, Perez CR, Wert SE, Keller G, et al. Safety of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA to the lungs of nonhuman primates. Hum Gene Ther. 1996;7:301–318. doi: 10.1089/hum.1996.7.3-301. [DOI] [PubMed] [Google Scholar]
- 131.Simon RH, Engelhardt JF, Yang Y, Zepeda M, Weber-Pendleton S, et al. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: toxicity study. Hum Gene Ther. 1993;4:771–780. doi: 10.1089/hum.1993.4.6-771. [DOI] [PubMed] [Google Scholar]
- 132.Joseph PM, O’Sullivan BP, Lapey A, Dorkin H, Oren J, et al. Aerosol and lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. I. Methods, safety, and clinical implications. Hum Gene Ther. 2001;12:1369–1382. doi: 10.1089/104303401750298535. [DOI] [PubMed] [Google Scholar]
- 133.Harvey BG, Maroni J, O’Donoghue KA, Chu KW, Muscat JC, et al. Safety of local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions. Hum Gene Ther. 2002;13:15–63. doi: 10.1089/10430340152712638. [DOI] [PubMed] [Google Scholar]
- 134.Toietta G, Koehler DR, Finegold MJ, Lee B, Hu J, et al. Reduced inflammation and improved airway expression using helper-dependent adenoviral vectors with a K18 promoter. Mol Ther. 2003;7:649–658. doi: 10.1016/s1525-0016(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 135.Koehler DR, Hannam V, Belcastro R, Steer B, Wen Y, et al. Targeting transgene expression for cystic fibrosis gene therapy. Mol Ther. 2001;4:58–65. doi: 10.1006/mthe.2001.0412. [DOI] [PubMed] [Google Scholar]
- 136.Koehler DR, Sajjan U, Chow YH, Martin B, Kent G, et al. Protection of Cftr knockout mice from acute lung infection by a helper-dependent adenoviral vector expressing Cftr in airway epithelia. Proc Natl Acad Sci USA. 2003;100:15364–15369. doi: 10.1073/pnas.2436478100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koehler DR, Frndova H, Leung K, Louca E, Palmer D, et al. Aerosol delivery of an enhanced helper-dependent adenovirus formulation to rabbit lung using an intratracheal catheter. J Gene Med. 2005;7:1409–1420. doi: 10.1002/jgm.797. [DOI] [PubMed] [Google Scholar]
- 138.Hiatt P, Brunetti-Pierri N, Koehler D, McConnell R, Katkin J, et al. Aerosol delivery of helper-dependent adenoviral vector into nonhuman primate lungs results in high efficiency pulmonary transduction with minimal toxicity. Mol Ther. 2005;11:S317. [Google Scholar]
- 139.Hiatt P, Brunetti-Pierri N, McConnell R, Palmer D, Zuo Y, et al. Bronchoscope-Guided, Targeted Lobar Aerosolization of HDAd into Nonhuman Primate Lungs Results in Uniform, High Level Pulmonary Transduction, Long Term Transgene Expression and Negligible Toxicity. Mol Ther. 2007;15:S161. [Google Scholar]
- 140.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moss RB, Rodman D, Spencer LT, Aitken ML, Zeitlin PL, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest. 2004;125:509–521. doi: 10.1378/chest.125.2.509. [DOI] [PubMed] [Google Scholar]
- 142.Dudley RW, Lu Y, Gilbert R, Matecki S, Nalbantoglu J, et al. Sustained improvement of muscle function one year after full-length dystrophin gene transfer into mdx mice by a gutted helper-dependent adenoviral vector. Hum Gene Ther. 2004;15:145–156. doi: 10.1089/104303404772679959. [DOI] [PubMed] [Google Scholar]
- 143.Gilbert R, Nalbantoglu J, Howell JM, Davies L, Fletcher S, et al. Dystrophin expression in muscle following gene transfer with a fully deleted (“gutted”) adenovirus is markedly improved by trans-acting adenoviral gene products. Hum. Gene Ther. 2001;12:1741–1755. doi: 10.1089/104303401750476249. [DOI] [PubMed] [Google Scholar]
- 144.Jiang Z, Feingold E, Kochanek S, Clemens PR. Systemic delivery of a high-capacity adenoviral vector expressing mouse CTLA4Ig improves skeletal muscle gene therapy. Mol Ther. 2002;6:369–376. doi: 10.1006/mthe.2002.0676. [DOI] [PubMed] [Google Scholar]
- 145.Jiang Z, Schiedner G, Van RN, Liu CC, Kochanek S. Sustained muscle expression of dystrophin from a high-capacity adenoviral vector with systemic gene transfer of T cell costimulatory blockade. Mol Ther. 2004;10:688–696. doi: 10.1016/j.ymthe.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 146.Bilbao R, Reay DP, Hughes T, Biermann V, Volpers C. Fetal muscle gene transfer is not enhanced by an RGD capsid modification to high-capacity adenoviral vectors. Gene Ther. 2003;10:1821–1829. doi: 10.1038/sj.gt.3302084. [DOI] [PubMed] [Google Scholar]
- 147.Matecki S, Dudley RW, Divangahi M, Gilbert R, Nalbantoglu J, et al. Therapeutic gene transfer to dystrophic diaphragm by an adenoviral vector deleted of all viral genes. Am J Physiol Lung Cell Mol Physiol. 2004;287:L569–L576. doi: 10.1152/ajplung.00117.2004. [DOI] [PubMed] [Google Scholar]
- 148.Maione D, Della RC, Giannetti P, D’Arrigo R, Liberatoscioli L, et al. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc Natl Acad Sci USA. 2001;98:5986–5991. doi: 10.1073/pnas.101122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bramson JL, Grinshtein N, Meulenbroek RA, Lunde J, Kottachchi D, et al. Helper-dependent adenoviral vectors containing modified fiber for improved transduction of developing and mature muscle cells. Hum Gene Ther. 2004;15:179–188. doi: 10.1089/104303404772679986. [DOI] [PubMed] [Google Scholar]
- 150.Persson A, Fan X, Widegren B, Englund E. Cell type- and region-dependent coxsackie adenovirus receptor expression in the central nervous system. J Neurooncol. 2006;78:1–6. doi: 10.1007/s11060-005-9055-3. [DOI] [PubMed] [Google Scholar]
- 151.Davidson BL, Allen ED, Kozarsky KF, Wilson JM, Roessler BJ. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993;3:219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- 152.Doran SE, Roessler BJ, Hartman JW, Hoff JT, Shewach DS, et al. Adenovirus-mediated in vivo gene transfer into the central nervous system of a nonhuman primate (resident award paper) Clin Neurosurg. 1994;41:242–257. [PubMed] [Google Scholar]
- 153.Doran SE, Ren XD, Betz AL, Pagel MA, Neuwelt EA. Gene expression from recombinant viral vectors in the central nervous system after blood-brain barrier disruption. Neurosurgery. 1995;36:965–970. doi: 10.1227/00006123-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 154.Byrnes AP, Wood MJ, Charlton HM. Role of T cells in inflammation caused by adenovirus vectors in the brain. Gene Ther. 1996;3:644–651. [PubMed] [Google Scholar]
- 155.Byrnes AP, MacLaren RE, Charlton HM. Immunological instability of persistent adenovirus vectors in the brain: peripheral exposure to vector leads to renewed inflammation, reduced gene expression, and demyelination. J Neurosci. 1996;16:3045–3055. doi: 10.1523/JNEUROSCI.16-09-03045.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci USA. 2009;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zou L, Yuan X, Zhou H, Lu H, Yang K. Helper-dependent adenoviral vector-mediated gene transfer in aged rat brain. Hum Gene Ther. 2001;12:181–191. doi: 10.1089/104303401750061249. [DOI] [PubMed] [Google Scholar]
- 158.Butti E, Bergami, Recchia A, Brambilla E, Franciotta D, et al. Absence of an intrathecal immune reaction to a helper-dependent adenoviral vector delivered into the cerebrospinal fluid of non-human primates. Gene Ther. 2008;15:233–238. doi: 10.1038/sj.gt.3303050. [DOI] [PubMed] [Google Scholar]
- 159.Butti E, Bergami A, Recchia A, Brambilla E, Del CU, et al. IL4 gene delivery to the CNS recruits regulatory T cells and induces clinical recovery in mouse models of multiple sclerosis. Gene Ther. 2008;15:504–515. doi: 10.1038/gt.2008.10. [DOI] [PubMed] [Google Scholar]
- 160.Huang B, Schiefer J, Sass C, Landwehrmeyer GB, Kosinski CM, et al. High-capacity adenoviral vector-mediated reduction of huntingtin aggregate load in vitro and in vivo. Hum Gene Ther. 2007;18:303–311. doi: 10.1089/hum.2006.160. [DOI] [PubMed] [Google Scholar]
- 161.Huang B, Schiefer J, Sass C, Kosinski CM, Kochanek S. Inducing huntingtin inclusion formation in primary neuronal cell culture and in vivo by high-capacity adenoviral vectors expressing truncated and full-length huntingtin with polyglutamine expansion. J Gene Med. 2008;10:269–279. doi: 10.1002/jgm.1150. [DOI] [PubMed] [Google Scholar]
- 162.Muhammad AK, Puntel M, Candolfi M, Salem A, Yagiz K, et al. Study of the Efficacy, Biodistribution, and Safety Profile of Therapeutic Gutless Adenovirus Vectors as a Prelude to a Phase I Clinical Trial for Glioblastoma. Clin Pharmacol Ther. 2010;88:204–213. doi: 10.1038/clpt.2009.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kumar-Singh R. Barriers for retinal gene therapy: separating fact from fiction. Vision Res. 2008;48:1671–1680. doi: 10.1016/j.visres.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kreppel F, Luther TT, Semkova I, Schraermeyer U, Kochanek S. Longterm transgene expression in the RPE after gene transfer with a high-capacity adenoviral vector. Invest Ophthalmol Vis Sci. 2002;43:1965–1970. [PubMed] [Google Scholar]
- 165.White GC, Monahan PE. In: Gene Therapy for Hemophilia A, in Textbook of Hemophilia. Lee CA, Berntorp EE, Hoots WK, Aledort LM, editors. Oxford, UK: Blackwell Publishing Ltd; 2007. [Google Scholar]
- 166.Stern BS, Shoshani W, Pearlman AL, Ng P, Nissenson AR. Erythropoeisis Sustained 1 Year by the EPODURE BioPump in Patients with Chronic Kideney Disease: Further Results of PhaseI/II Proof of Concept Trial. Mol Ther. 2010;18:S239. [Google Scholar]
- 167.Harui A, Roth MD, Kiertscher SM, Mitani K, Basak SK. Vaccination with helper-dependent adenovirus enhances the generation of transgene-specific CTL. Gene Ther. 2004;11:1617–1626. doi: 10.1038/sj.gt.3302332. [DOI] [PubMed] [Google Scholar]
- 168.Weaver EA, Nehete PN, Buchl SS, Senac JS, Palmer D, et al. Comparison of replication-competent, first generation, and helper-dependent adenoviral vaccines. PLoS One. 2009;4:5059. doi: 10.1371/journal.pone.0005059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Weaver EA, Nehete PN, Nehete BP, Buchl SJ, Palmer D, et al. Protection against mucosal SHIV challenge by peptide and helper-dependent adenovirus vaccines. Viruses. 2009;1:920–938. doi: 10.3390/v1030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wen S, Graf S, Massey PG, Dichek DA. Improved vascular gene transfer with a helper-dependent adenoviral vector. Circulation. 2004;110:1484–1491. doi: 10.1161/01.CIR.0000141574.78032.A9. [DOI] [PubMed] [Google Scholar]
- 171.Flynn R, Buckler JM, Tang C, Kim F, Dichek DA. Helper-dependent adenoviral vectors are superior in vitro to first-generation vectors for endothelial cell-targeted gene therapy. Mol Ther. 2010;18:2121–2129. doi: 10.1038/mt.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Flynn R, Qian K, Tang C, Dronadula N, Buckler JM, et al. Expression of Apolipoprotein A-I in Rabbit Carotid Endothelium Protects Against Atherosclerosis. Mol Ther. 2011;19:1833–1841. doi: 10.1038/mt.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ohbayashi F, Balamotis MA, Kishimoto A, Aizawa E, Diaz A, et al. Correction of chromosomal mutation and random integration in embryonic stem cells with helper-dependent adenoviral vectors. Proc Natl Acad Sci USA. 2005;102:13628–13633. doi: 10.1073/pnas.0506598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Suzuki K, Mitsui K, Aizawa E, Hasegawa K, Kawase E, et al. Highly efficient transient gene expression and gene targeting in primate embryonic stem cells with helper-dependent adenoviral vectors. Proc Natl Acad Sci USA. 2008;105:13781–13786. doi: 10.1073/pnas.0806976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu GH, Suzuki K, Qu J, Sancho-Martinez I, Yi F, et al. Targeted gene correction of laminopathy-associated LMNA mutations in patient-specific iPSCs. Cell Stem Cell. 2011;8:688–694. doi: 10.1016/j.stem.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:2667–2674. doi: 10.1002/stem.201. [DOI] [PubMed] [Google Scholar]
- 177.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 178.Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 179.Pien GC, Basner-Tschakarjan E, Hui DJ, Mentlik AN, Finn JD, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest. 2009;119:1688–1695. doi: 10.1172/JCI36891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Davidoff AM. PhaseI/II clinical trial of AAV8 FIX for hemophilia B. Scientific symposium presented at American Society of Gene and Cell Therapy 14th Annual Meeting; Seattle, WA. 2011. [Google Scholar]
- 181.Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Smith CA, Woodruff LS, Kitchingman GR, Rooney CM. Adenovirus-pulsed dendritic cells stimulate human virus-specific T-cell responses in vitro. J Virol. 1996;70:6733–6740. doi: 10.1128/jvi.70.10.6733-6740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Molinier-Frenkel V, Gahery-Segard H, Mehtali M, Le Boulaire C, Ribault S, et al. Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J Virol. 2000;74:7678–7682. doi: 10.1128/jvi.74.16.7678-7682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Roth MD, Cheng Q, Harui A, Basak SK, Mitani K, et al. Helper-dependent adenoviral vectors efficiently express transgenes in human dendritic cells but still stimulate antiviral immune responses. J Immunol. 2002;169:4651–4656. doi: 10.4049/jimmunol.169.8.4651. [DOI] [PubMed] [Google Scholar]
- 185.Kushwah R, Cao H, Hu J. Characterization of pulmonary T cell response to helper-dependent adenoviral vectors following intranasal delivery. J Immunol. 2008;180:4098–4108. doi: 10.4049/jimmunol.180.6.4098. [DOI] [PubMed] [Google Scholar]
- 186.Thomas CE, Storm TA, Huang Z, Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang L, Figueredo J, Calcedo R, Lin J, Wilson JM. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum Gene Ther. 2007;18:185–194. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- 188.Li H, Murphy SL, Giles-Davis W, Xiang Z, Li Y, et al. Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol Ther. 2007;15:792–800. doi: 10.1038/sj.mt.6300090. [DOI] [PubMed] [Google Scholar]
- 189.Li H, Lin SW, Giles-Davis W, Li Y, Zhou D, et al. A preclinical animal model to assess the effect of pre-existing immunity on AAV-mediated gene transfer. Mol Ther. 2009;17:1215–1224. doi: 10.1038/mt.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]