Abstract
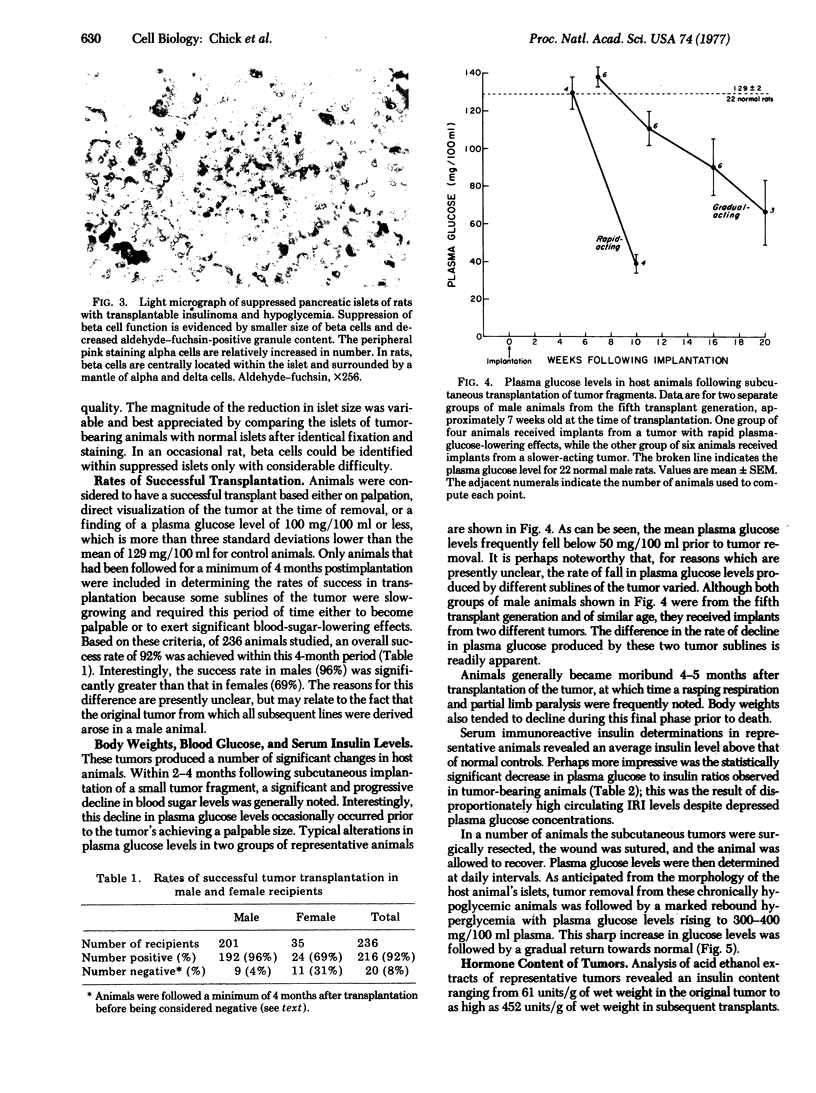
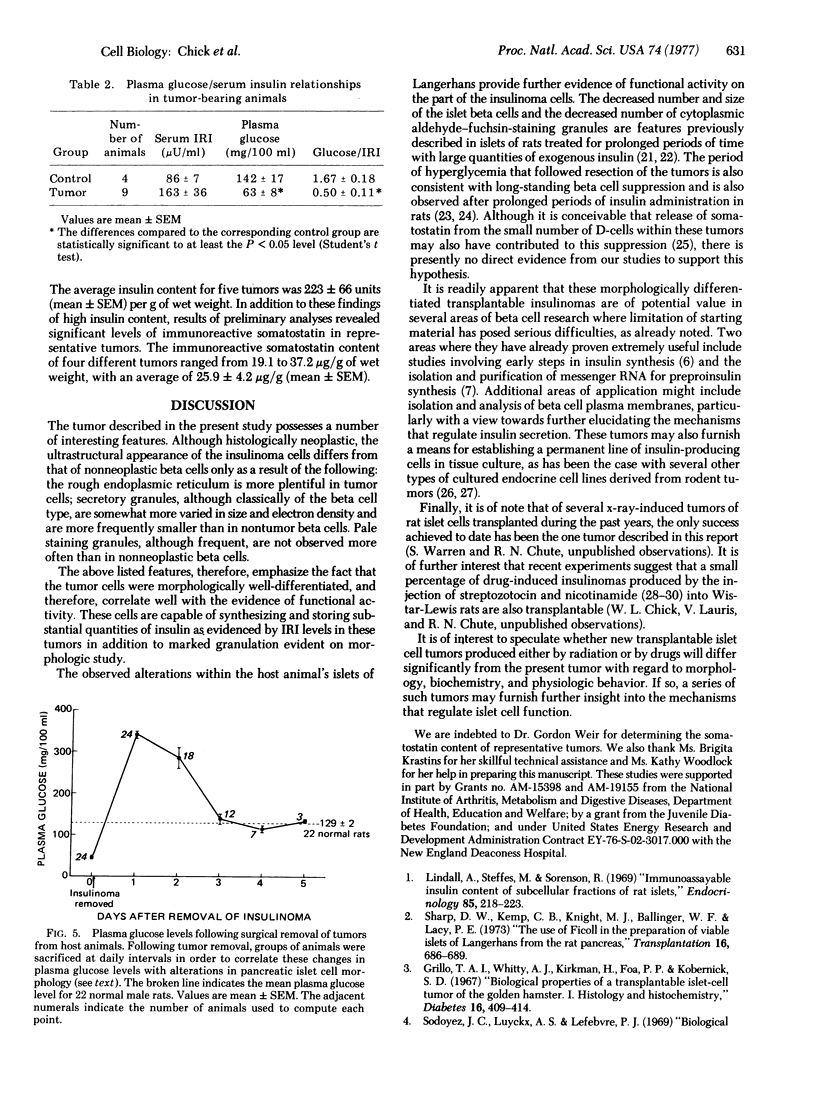
A transplantable insulinoma was developed in inbred albino rats of the NEDH strain. The original tumor, 1 cm in diameter, was removed from the pancreas of a male parabiont 566 days folowing 1000 rads (10J/kg) of total body x-irradiation. The time required for implanted fragments to grow to 0.5-1.5 cm in diameter decreased from 5-8 months in the first generation to 2-5 months in the seventh generation. Successful transplantation in male animals followed for 4 or more months after transplantation was significantly greater than in female animals followed for a similar period of time (96% versus 69%). Light and electron microscopy revealed that the tumors consisted predominantly of well-granulated beta cells. Ultrastructural studies also showed small numbers of D-cells. Tumor extracts contained an average of 223 units of immunoreactive insulin and 25.9 mug of immunoreactive somato-statin per gram wet weight of tissue. Tumors generally produced increasingly profound hypoglycemia within 2-4 months following transplantation, with plasma glucose levels frequently falling to 40 mg/100 ml or lower prior to death. Removal of tumors from chronically hypoglycemic animals resulted in transient rebound hyperglycemia with plasma glucose levels above 300mg/100 ml within the first 24 hr and a gradual decline to normal levels of 129 mg/100ml in 2-4 days. These observations correlated with findings of marked atropy and degranulation of the beta cells in the pancreata of tumor-bearing animals, and with gradual return of normal light microscopic morphology following tumor removal.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti K. G., Christensen N. J., Christensen S. E., Hansen A. P., Iversen J., Lundbaek K., Seyer-Hansen K., Orskov H. Inhibition of insulin secretion by somatostatin. Lancet. 1973 Dec 8;2(7841):1299–1301. doi: 10.1016/s0140-6736(73)92873-0. [DOI] [PubMed] [Google Scholar]
- Boschetti A. E., Moloney W. C. Observations on pancreatic islet cell and other radiation-induced tumors in the rat. Lab Invest. 1966 Mar;15(3):565–575. [PubMed] [Google Scholar]
- Chick W. L., Lauris V., Flewelling J. H., Andrews K. A., Woodruff J. M. Effects of glucose on beta cells in pancreatic monolayer cultures. Endocrinology. 1973 Jan;92(1):212–218. doi: 10.1210/endo-92-1-212. [DOI] [PubMed] [Google Scholar]
- Dixit P. K., Bauer G. E. Studies on rats with islet beta cell tumors induced by nicotinamide and streptozotocin. Proc Soc Exp Biol Med. 1976 Jun;152(2):232–236. doi: 10.3181/00379727-152-39368. [DOI] [PubMed] [Google Scholar]
- Duguid J. R., Steiner D. F., Chick W. L. Partial purification and characterization of the mRNA for rat preproinsulin. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3539–3543. doi: 10.1073/pnas.73.10.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunt J. A., Pallotta J. A., Soeldner J. S. Blood sugar, serum insulin and free fatty acid interrelationships during intravenous tolbutamide testing in normal young adults and in patients with insulinoma. Diabetes. 1970 Feb;19(2):122–126. doi: 10.2337/diab.19.2.122. [DOI] [PubMed] [Google Scholar]
- Horwitz D. L., Rubenstein A. H., Reynolds C., Molnar G. D., Yanaihara N. Prolonged suppression of insulin release by insulin-induced hypoglycemia: demonstration by C-peptide assay. Horm Metab Res. 1975 Nov;7(6):449–452. doi: 10.1055/s-0028-1093701. [DOI] [PubMed] [Google Scholar]
- Like A. A., Orci L. Embryogenesis of the human pancreatic islets: a light and electron microscopic study. Diabetes. 1972;21(2 Suppl):511–534. doi: 10.2337/diab.21.2.s511. [DOI] [PubMed] [Google Scholar]
- Lindall A., Steffes M., Sorenson R. Immunoassayable insulin content of subcellular fractions of rat islets. Endocrinology. 1969 Aug;85(2):218–223. doi: 10.1210/endo-85-2-218. [DOI] [PubMed] [Google Scholar]
- Melani F., Ryan W. G., Rubenstein A. H., Steiner D. F. Proinsulin secretion by a pancreatic beta-cell adenoma. Proinsulin and C-peptide secretion. N Engl J Med. 1970 Oct 1;283(14):713–719. doi: 10.1056/NEJM197010012831401. [DOI] [PubMed] [Google Scholar]
- ROSEN V. J., Jr, CASTANERA T. J., KIMELDORF D. J., JONESDC Pancreatic islet cell tumors and renal tumors in the male rat following neutron exposure. Lab Invest. 1962 Mar;11:204–210. [PubMed] [Google Scholar]
- Rakieten N., Gordon B. S., Beaty A., Cooney D. A., Davis R. D., Schein P. S. Pancreatic islet cell tumors produced by the combined action of streptozotocin and nicotinamide. Proc Soc Exp Biol Med. 1971 May;137(1):280–283. doi: 10.3181/00379727-137-35561. [DOI] [PubMed] [Google Scholar]
- SATO G. H., BUONASSISI V. HORMONE-SECRETING CULTURES OF ENDOCRINE TUMOR ORIGIN. Natl Cancer Inst Monogr. 1964 Apr;13:81–90. [PubMed] [Google Scholar]
- Sato G. H., Yasumura Y. Retention of differentiated function in dispersed cell culture. Trans N Y Acad Sci. 1966 Jun;28(8):1063–1079. doi: 10.1111/j.2164-0947.1966.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Scharp D. W., Kemp C. B., Knight M. J., Ballinger W. F., Lacy P. E. The use of ficoll in the preparation of viable islets of langerhans from the rat pancreas. Transplantation. 1973 Dec;16(6):686–689. doi: 10.1097/00007890-197312000-00028. [DOI] [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Turner R. C., Johnson P. C. Suppression of insulin release by fish-insulin-induced hypoglycaemia: with reference to the diagnosis of insulinomas. Lancet. 1973 Jun 30;1(7818):1483–1485. doi: 10.1016/s0140-6736(73)91817-5. [DOI] [PubMed] [Google Scholar]
- Turner R. C., Oakley N. W., Nabarro J. D. Control of basal insulin secretion, with special reference to the diagnosis of insulinomas. Br Med J. 1971 Apr 17;2(5754):132–135. doi: 10.1136/bmj.2.5754.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk B. W., Wellmann K. F., Brancato P. Fine structure of rat islet cell tumors induced by streptozotocin and nicotinamide. Diabetologia. 1974 Feb;10(1):37–44. doi: 10.1007/BF00421412. [DOI] [PubMed] [Google Scholar]
- WARREN S., CARLSTEIN R. G., STEINKE J., CHUTE R. N. ISLAND-CELL TUMORS IN IRRADIATED RATS IN PARABIOSIS. Proc Soc Exp Biol Med. 1964 Apr;115:910–912. doi: 10.3181/00379727-115-29075. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Goltsos P. C., Steinberg E. P., Patel Y. C. High concentration of somatostatin immunoreactivity in chicken pancreas. Diabetologia. 1976 May;12(2):129–132. doi: 10.1007/BF00428977. [DOI] [PubMed] [Google Scholar]






