Abstract
The frequency of claw regrowth, bony remnants, and complications in cats that underwent forelimb onychectomy using laser, scalpel, or guillotine techniques were evaluated. Eighty-seven client-owned cats were recruited from 27 veterinary clinics in the Canadian Atlantic provinces. At least 1 year after onychectomy the cats underwent a physical examination, gait analysis, and radiographic evaluation by 1 of 2 authors. There was no significant difference in the frequency of claw regrowth among the 3 methods (P = 0.283). Significantly more cats had bony remnants following guillotine onychectomy (P < 0.001). Bony remnants were significantly associated with claw regrowth (P = 0.001). Cats that underwent laser onychectomy had significantly less post-operative complications (P = 0.023). The long-term outcome was not significantly different among the 3 methods. We conclude that leaving remnants of the third phalanx in situ is associated with an increased frequency of claw regrowth. Laser onychectomy may be preferred to reduce the risk of post-operative complications, bony remnants, and claw regrowth.
Résumé
Comparaison de 3 méthodes d’onychectomie. La fréquence de la repousse des griffes, des reliquats osseux et des complications chez les chats qui avaient subi une onychectomie des pattes avant en utilisant le laser, le scalpel, ou les techniques de guillotine a été évaluée. Quatre-vingt-sept chats appartenant à des clients ont été recrutés dans 27 cliniques vétérinaires des provinces de l’Atlantique du Canada. Au moins 1 an après l’onychectomie, les chats ont subi une évaluation physique, une analyse de la démarche et une évaluation radiographique par 1 des 2 auteurs. Il n’y avait pas de différence significative dans la fréquence de la repousse des griffes entre les 3 méthodes (P = 0,283). Un nombre significativement supérieur de chats avaient des reliquats osseux après l’onychectomie à la guillotine (P < 0,001). Les reliquats osseux étaient significativement associés à la repousse de griffes (P = 0,001). Les chats qui ont subi une onychectomie au laser présentaient un nombre significativement inférieur de complications postopératoires (P = 0,023). Les résultats à long terme n’étaient pas significativement différents entre les 3 méthodes. Nous avons conclu que la présence de reliquats in situ dans la troisième phalange est associée à une fréquence accrue de repousse des griffes. L’onychectomie au laser peut être préférable pour réduire le risque de complications postopératoires, des reliquats et de la repousse des griffes.
(Traduit par Isabelle Vallières)
Introduction
Onychectomy (declaw) is an elective surgery that requires removal of the third phalanx that renders cats unable to scratch. The most common method of onychectomy is disarticulation and removal of the third phalanx (P3) by laser, scalpel, or nail trimmer. The use of a nail trimmer is referred to as the guillotine method and is used to disarticulate the third phalanx or amputate the third phalanx below the ungual process (1), leaving the flexor process in situ (Figure 1A).
Figure 1.
Schematic drawing of the third phalanx of the feline digit demonstrating relevant anatomy and the proposed amputation site for the bony amputation guillotine method of onychectomy (A), and the second (P2) and third phalanges (P3) demonstrating the transection site at the distal interphalangeal joint for the disarticulation method of onychectomy (B).
Onychectomy is a controversial procedure that is prohibited as an elective procedure in Australia (2), New Zealand (3), Brazil, and many European countries (4–8). Ethical concerns about elective onychectomies center around the high short-term complication rate and the possibility for long-term complications and behavioral changes (4,6–9). Short-term complications following onychectomy occur in as many as 50% of cases, and include pain, lameness, hemorrhage, decreased appetite, personality change, infection, lethargy, and cystitis (1,10–15). Long-term complications following onychectomy are infrequently reported but include claw regrowth, lameness, chronic pain, sore paws, flexor tendon contraction, and increased biting (4,6–9,13–17).
Although claw regrowth is a well-recognized complication of onychectomy, only 4 publications have reported regrowth of claws in cats following onychectomy, with a reported regrowth rate ranging from 1.8% to 10% (1,9,15,17). All reported cases of claw regrowth have occurred followed guillotine onychectomy. To the authors’ knowledge, no cases of claw regrowth have been documented following scalpel or laser onychectomy.
Previous studies investigating onychectomy performed by experienced veterinarians have had very short follow-up times, ranging from 7 to 21 days (14,18–21). Studies with longer follow-up times were conducted at veterinary teaching hospitals in which onychectomies were performed by senior veterinary students (1,15,17). No study has evaluated long-term follow-up after onychectomy by experienced veterinarians; long-term follow-up after laser onychectomy is not reported.
The first aim of this study was to report the frequency of claw regrowth in cats undergoing bilateral forelimb onychectomy, performed by experienced veterinary practitioners. A comparison of the frequency of claw regrowth after onychectomy by 3 methods would be made. We hypothesized that onychectomy performed by the guillotine (G) method would be associated with the highest frequency of claw regrowth compared to onychectomy performed with the laser (L) or scalpel (S). We also hypothesized that leaving bony remnants of P3 would be associated with claw regrowth. The second aim was to evaluate the short- and long-term outcome of cats following laser onychectomy. We hypothesized that cats undergoing laser onychectomy would have the same frequency of complications and behaviors as cats undergoing scalpel or guillotine onychectomy. The third aim was to assess the inter-observer agreement for the radiographic detection of claw regrowth and bony remnants of P3. We hypothesized that the agreement would be substantial for the detection of claw regrowth and almost perfect for the detection of bony remnants. We also hypothesized that the use of radiographs would increase the odds of detecting claw regrowth compared with physical examination.
Materials and methods
Cats
Cats that had undergone bilateral forelimb onychectomy by laser (n = 30), scalpel (n = 31), or guillotine (n = 26) methods with a minimum of 1-year follow-up were eligible for inclusion in the study. Cats were recruited from employees and students of the University of Prince Edward Island and veterinary practices in the Canadian Atlantic provinces between June 2009 and November 2011. Owner-consent was obtained, and the protocol for this study was approved by the University of Prince Edward Island Animal Care Committee.
Onychectomy surgery
All onychectomies were performed by experienced veterinarians who had been performing onychectomies for at least 1 y. The number of onychectomies performed by a single veterinarian was limited to 7 in order to reduce bias. All laser onychectomies were performed with a carbon dioxide laser using the disarticulation method (21) (Figure 1B). All scalpel onychectomies were performed with a scalpel blade using the disarticulation method (1). All guillotine onychectomies were performed with a sterile guillotine-type nail clipper using the disarticulation or bony amputation method. The disarticulation guillotine method was defined as removal of the entire third phalanx by either disarticulation of the distal interphalangeal joint space with the nail clippers or amputation of the third phalanx at the level of the flexor process with subsequent removal of the flexor process with a scalpel (1). The bony amputation guillotine method was defined as amputation below the ungual process leaving the flexor process of the third phalanx in situ (1) (Figure 1A).
Client questionnaire (Appendix)
Information on the age of the cat at the time of onychectomy, the method of onychectomy, the clinic in which the onychectomy was performed, any post-operative complications (including infection, draining tracts, fever, loss of appetite, reluctance to jump, reluctance to walk, reluctance to scratch, swelling of paws, chewing at paws, and increased aggression), current behaviors of the cat (including scratching with front paws, scratching with hind paws, biting, jumping, climbing trees, playing with toys, chewing at paws, lameness, and indoor/outdoor hunting), current medications, and current health problems was obtained from the owner by a questionnaire.
Clinical evaluation
The cats underwent visual gait evaluation in an examination room by 1 of 2 observers, a surgery resident, and board-certified veterinary surgeon (KC, TB). Lameness was graded as sound, mild weight-bearing lameness, moderate weight-bearing lameness, severe weight-bearing lameness, or non weight-bearing lameness. Reluctance to jump off the examination table and ease of jumping were noted. Each cat then underwent a general physical examination, an orthopedic examination, and a neurologic examination. All digits and digital pads were examined for signs of claw regrowth, previous pad laceration, and calluses. The front and hind paws were palpated for signs of discomfort. Discomfort was graded as none, mild, moderate, or severe.
Radiographic evaluation
A single dorsomedial-palmerolateral view was obtained for each forepaw. Radiographs were repeated if the distal aspect of the second phalanx could not be visualized for all 5 digits. All radiographs were reviewed by 2 board-certified veterinary radiologists (PR, AM), who were unaware of the onychectomy method. Radiographs were evaluated for signs of claw regrowth and remnants of P3. Claw regrowth was defined as evidence of an angular structure of soft tissue opacity seen extending distally from the digit or a P3 fragment that appeared to have an ungual crest and ungual process. Bony remnants of P3 were defined as small, often irregular mineral foci of variable size seen distal to the second phalanx.
Surgical evaluation
Three cats had claw regrowth surgically removed from the forepaws. At this time, surgical exploration was conducted in all 5 digits of each paw, regardless of radiographic evidence of claw regrowth. All excised tissue from 2 cats was submitted for histologic examination.
Cats were excluded from the study if there had been previous surgery to remove claw regrowth, the clinical evaluation could not be performed, or radiographs could not be taken without sedation.
Statistical analyses
Summary statistics were calculated for all 3 treatment groups. The Kruskal-Wallis and Mann-Whitney U-tests were used to compare non-parametric continuous outcomes. The combined findings of the physical examination, radiographs, and surgical evaluation were used to estimate the frequency of claw regrowth for each method of onychectomy, which was tested using Fisher’s exact test. The frequency of bony remnants of P3 was compared for the 3 methods using Chi-squared analysis. The association between claw regrowth and bony remnants was tested using Fisher’s exact test. The odds of detecting claw regrowth on radiographs compared with detecting claw regrowth on physical examination were calculated with 95% confidence intervals (CI).
The frequency of cats that had 1 or more post-operative complications was compared for the 3 methods using Chi-squared analysis. The frequency of specific post-operative complications, current behaviors, and physical examination findings for each method was compared using Chi-squared analysis or Fisher’s exact test. In all cases, Fisher’s exact test was used when the standard guidelines for the use of Chi-squared analysis were violated. Pairwise comparisons were used to determine which of the groups differed significantly from each other. Significance was set at P < 0.05 for a 2-sided hypothesis, and P-values were adjusted for multiple comparisons using the Bonferroni correction. A post hoc power analysis was conducted to evaluate variables that lacked statistical significance.
The kappa statistic was used to assess inter-observer agreement in the evaluation of the radiographs. A value of 1 indicates perfect agreement (Table 1). Continuous data were presented as the median and interquartile range, and binary outcomes were expressed as proportions with 95% binomial exact confidence intervals. Computer software (Minitab 16.1 Statistical Software, Minitab, State College, Pennsylvania, USA; Stata 12.0, StataCorp LP, College Station, Texas, USA) was used for all analyses.
Table 1.
Kappa agreement
| < 0 Less than chance agreement |
| 0.01–0.20 Slight agreement |
| 0.21–0.40 Fair agreement |
| 0.41–0.60 Moderate agreement |
| 0.61–0.80 Substantial agreement |
| 0.81–0.99 Almost perfect agreement |
Results
A total of 105 cats were considered for enrollment in the study. Cats were not enrolled if the onychectomy was performed by a veterinary student or if the veterinarian who had performed the onychectomy had performed onychectomies on 7 cats already enrolled in the study. A total of 88 cats were enrolled in the study (Table 2). One cat in the guillotine group was excluded from the study due to previous surgery to remove claw regrowth. The median age of cats was 4 y (3.0 to 5.5 y) in the laser group, 6 y (3.0 to 8.0 y) in the scalpel group, and 8 y (5.5 to 11 y) in the guillotine group. There was no significant difference in age at onychectomy, gender, and neuter status among the 3 groups. The median follow-up time was 4.9 y (2.3 to 4.7 y) for the scalpel group, 2.5 y (1.3 to 4.3 y) for the laser group, and 7.5 y (3.0 to 9.5 y) for the guillotine group. Cats in the laser group had a significantly shorter follow-up time compared with cats in the scalpel and guillotine groups (Kruskal-Wallis P = 0.000).
Table 2.
Signalment and frequency of immediate post-operative complications, current behaviors, physical examination findings, and radiographic findings in 87 cats that underwent scalpel (S), laser (L), or guillotine (G) onychectomy at least 1 year previously. Continuous data are expressed as a median and interquartile range and binary outcomes are expressed as the number of positive responses over the total
| Variable | Scalpel (S) | Laser (L) | Guillotine (G) | P-value |
|---|---|---|---|---|
| Age at declaw (months) | 7 (5–14) | 8 (5.0–15.5) | 7 (5–19) | 0.595 |
| Female spayed | 14 (45%) | 20 (67%) | 11 (42%) | 0.126 |
| Male castrated | 17 (55%) | 10 (33%) | 15 (58%) | 0.126 |
| Surgeons (n) | 21 | 7 | 14 | 0.499 |
| Number of cats with postoperative complications | 18/30 (60%)a,b | 10/30 (33%)a | 14/20 (70%)b | 0.023 |
| Reluctance to jump postoperatively | 14/30 (47%)a | 5/30 (17%)a | 8/19 (42%)a | 0.035 |
| Postoperative infection | 5/30 (16.7%)a | 0/30 (0%)a | 1/19 (5.3%)a | 0.038 |
| Current behaviors | ||||
| Climbing trees | 10/31 (32.2%)a | 5/30 (16.7%)a,b | 1/26 (3.8%)b | 0.021 |
| Hunting behavior | 11/31 (35.5%)a | 15/30 (50%)a | 1/26 (3.8%)b | 0.001 |
| Aggressive at home | 3/31 (9.7%) | 1/30 (3.3%) | 0/26 (0%) | 0.321 |
| Lameness in examination room | 3/31 (9.7%) | 2/30 (6.7%) | 6/26 (23.1%) | 0.197 |
| Pain on forepaw palpation | 12/31 (38.7%) | 5/29 (17.2%) | 11/26 (42.3%) | 0.093 |
| Reluctance to jump in examination room | 3/31 (9.7%) | 1/30 (3.3%) | 3/26 (11.5%) | 0.554 |
| Shaking paws | 2/31 (6.5%) | 3/30 (10%) | 2/26 (7.7%) | 0.891 |
| Claw regrowth on physical examination | 1/31 (3.2%) | 0/30 (0%) | 1/26 (3.8%) | 0.292 |
| Calluses | 9/31 (29%) | 18/30 (60%) | 12/26 (46.2%) | 0.051 |
| Pad lacerations | 10/31 (32.2%)a | 2/30 (6.7%)a | 6/26 (23.1%)a | 0.045 |
| Lifting paw when jumping | 0/31 (0%)a | 1/30 (3.3%)a,b | 5/26 (19.2%)b | 0.007 |
| Aggressive in room | 3/31 (9.7%) | 4/30 (13.3%) | 2/26 (7.7%) | 0.827 |
| Radiographic claw regrowth | 2/31 (6.5%) | 1/29 (3.4%) | 3/26 (11.5%) | 0.506 |
| Radiographic bony remnant | 10/31 (32%)a,b | 4/29 (14%)a | 17/26 (65.4%)b | 0.000 |
| Overall claw regrowth | 2/31 (6.5%) | 1/29 (3.4%) | 4/26 (15.4%) | 0.285 |
Values in a row with different superscript letters are significantly different (P < 0.05). Absence of superscript letters or same superscript letters indicates no significant differences.
Laser group
No cats had claw regrowth identified on physical examination. Radiographs from 1 cat were lost, and this cat was excluded from the analysis of radiographic findings. One cat had claw regrowth and bony remnants of P3 identified on radiographic evaluation (Table 2). The overall frequency of claw regrowth in cats undergoing laser onychectomy was 3.5% (n = 1/29; 95% CI 3.3% to 32.8%). Ten owners (33%) reported complications in the immediate post-operative period. The most common owner-reported complications were chewing at the paws, and reluctance to jump or walk (Table 2). Cats that underwent laser onychectomy had a significantly lower frequency of complications in the immediate post-operative period compared with cats that underwent scalpel or guillotine onychectomy (χ2 = 7.6, df = 2, P = 0.023).
Scalpel group
One cat had claw regrowth identified on physical examination and evidence of bony remnants and claw regrowth on radiographic evaluation (Figures 2 and 3). A second cat had evidence of claw regrowth with bony remnants on radiographic evaluation, but no evidence of claw regrowth on physical examination. The overall frequency of claw regrowth in cats undergoing scalpel onychectomy was 6.5% (n = 2/31; 95% CI: 6.5% to 44.1%). The scalpel onychectomy group had a significantly higher frequency of cats that climbed trees compared with the laser and guillotine onychectomy groups (χ2 = 7.7, df = 2, P = 0.021). Eighteen owners (60%) reported complications in the immediate post-operative period. Reluctance to jump, chewing at paws, swelling of paws, and reluctance to scratch were the most common owner-reported complications.
Figure 2.
Photograph of the left manus of a cat that had undergone scalpel onychectomy 6 years previously, demonstrating claw regrowth seen on physical examination.
Figure 3.
A dorsomedial-palmerolateral radiograph of the cat in Figure 2 demonstrating claw regrowth (arrow) and bony remnants (arrowhead).
Guillotine group
One cat had claw regrowth identified on physical examination, 3 cats had evidence of claw regrowth and bony remnants of P3 on radiographic evaluation, and 3 cats had claw regrowth identified during surgical exploration of the digits (Table 2). The overall frequency of claw regrowth in cats undergoing guillotine onychectomy was 15.4% (n = 4/26; 95% CI: 7.2% to 42.4%), which was not significantly higher than the frequency in cats undergoing laser or scalpel onychectomy (Fisher’s exact test, P = 0.283). Fourteen owners (70%) reported complications in the immediate post-operative period. Seven owners had not owned the cat at the time of the onychectomy and were unable to report complications; these cats were not included in the statistical analysis of post-operative complications. Reluctance to jump, reluctance to scratch, chewing at the paws, and swelling of the paws were the most common owner-reported complications. The guillotine onychectomy group had a significantly lower frequency of cats that displayed hunting behaviors (χ2 = 14.3, df = 2, P = 0.001) and a significantly higher frequency of cats that lifted a forepaw before or after jumping (Fisher’s exact test, P = 0.007) compared with the laser and scalpel onychectomy groups.
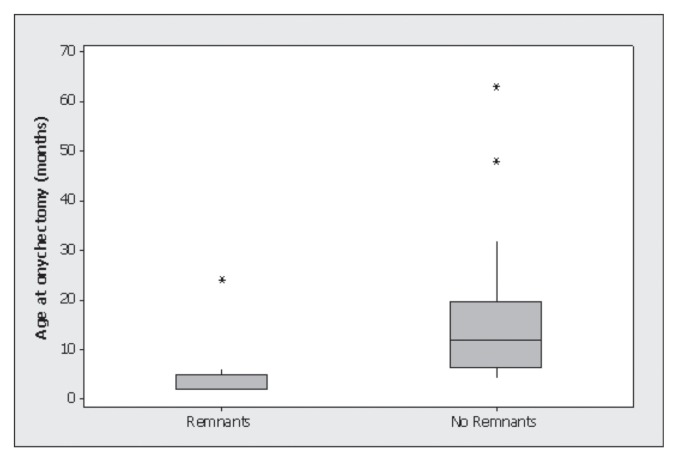
Cats that underwent guillotine onychectomy had a significantly higher frequency of bony remnants of P3 compared with the cats in the laser and scalpel groups (n = 17/26; Fisher’s exact test, P < 0.001). Cats that underwent guillotine onychectomy were more than 10 times as likely to have a bony remnant compared with cats that underwent laser onychectomy [odds ratio (OR) = 10.6, 95% CI: 2.95 to 37.8, P = 0.0001]. Cats that underwent scalpel onychectomy and had bony remnants at the time of follow-up had a median age of 5 months at the time of onychectomy (Figure 4). This was significantly younger than cats that underwent scalpel onychectomy and did not have bony remnants (median age 12 mo; Mann-Whitney U-test, P = 0.0012). Cats with bony remnants in the laser and onychectomy groups were not significantly younger at the time of onychectomy than cats without bony remnants.
Figure 4.
Box-and-whisker plot of the age of cats at the time of scalpel onychectomy. Cats that had bony remnants left in situ were significantly younger than cats without bony remnants (P = 0.0012).
The specific technique for the guillotine onychectomy was known for 23 cats. Eight cats underwent disarticulation onychectomy and 15 cats underwent bony amputation onychectomy. Cats that underwent bony amputation guillotine onychectomy had a significantly higher (Fisher’s exact test, P = 0.001) frequency of bony remnants (n = 13/15) compared with cats that had the disarticulation guillotine technique (n = 1/7). All cats with evidence of claw regrowth had bony remnants identified on radiographs (n = 7). Cats that had a bony remnant of P3 had a significantly higher frequency of claw regrowth (Fisher’s exact test, P = 0.001). Radiographs did not significantly increase the odds of detecting claw regrowth compared with physical examination (OR = 2.07, 95% CI: 0.55 to 7.81, P = 0.3).
The interobserver agreement for the radiographic detection of claw regrowth had an observed agreement of 0.94, an expected agreement of 0.92 and a calculated Kappa statistic of 0.27, indicating fair agreement (22). The interobserver agreement for the radiographic detection of bony remnants of P3 had an observed agreement of 0.86, an expected agreement of 0.58 and a calculated Kappa statistic of 0.67, indicating substantial agreement (22).
Gross findings during surgical exploration of the 3 cats with evidence of claw regrowth included seroma-like fluid pockets and inflammatory tissue on every digit. Discolored bone fragments and sharp keratin-like tissue were found in all digits. On histopathologic evaluation, the excised tissue contained bone fragments, segments of claw bed epithelium and fragments of keratin surrounded by macrophages and giant cells, indicating granulomatous pododermatitis secondary to claw regrowth. Radiographs from all 3 cats were evaluated and only 1 cat was identified as having claw regrowth. This cat had radiographic evidence of bony remnants of P3 on all digits, and histopathologic evaluation described claw regrowth in all digits.
Discussion
Claw regrowth after onychectomy occurs when germinal cells of the ungual process are left in situ (1). Intuitively, this is most likely to occur following the bony amputation technique of onychectomy, whereby the flexor process of the third phalanx is left in situ (Figure 1A). Inappropriate placement of the nail-clippers during this technique results in amputation through the ungual process leaving germinal cells behind. This may explain why the highest reported frequency of claw regrowth in the current study was seen following guillotine onychectomy and when bony remnants were detected on radiographs.
In this study, the frequency of claw regrowth following the guillotine method was 15.4%, which is higher than previous reports of 1.8% to 10% (1,9,15,17). This result was unexpected, given that the surgeries were performed by experienced veterinarians, unlike previous reports in which senior veterinary students performed the onychectomies (1,15,17). The detection of a higher frequency of claw regrowth may be explained by the fact that radiographs were used in addition to physical examination for the detection of claw regrowth. Claw regrowth was only detected in 2 of 87 cats on physical examination compared with 6 cats that were detected using radiographs; however, this finding was not statistically significant and may have been a result of the small sample size.
The interobserver agreement for the radiographic detection of claw regrowth in this study was fair. The Kappa statistic is affected by the prevalence of the event, and as claw regrowth was a rare finding this likely influenced the statistical evaluation (22). Radiograph evaluation is also highly influenced by the experience of the evaluator, exposure of the radiograph, and positioning, which likely contributed to the low level of agreement. Histopathologic evidence of claw regrowth was found in cats when no radiographic evidence of claw regrowth was seen. Based on this, it is the authors’ opinion that radiographs likely underestimate the true frequency of claw regrowth. The interobserver agreement for the radiographic detection of bony remnants of P3 was substantial (22). In comparison to the detection of claw regrowth, bony remnants are well-mineralized discreet fragments of bone that are easy to detect radiographically, which may explain why the agreement between observers was higher.
Bony remnants of P3 were detected in 4/29 (13.8%) cats in the laser group and 10/31 (32.3%) cats in the scalpel group, suggesting that it is not uncommon to leave bone fragments behind. Cats in the scalpel group with bony remnants were significantly younger at the time of onychectomy compared with cats in the scalpel group without bony remnants. Young cats with small incompletely mineralized bones may be more at risk of having bone fragments left in situ because the soft bone can be easily shaved off during dissection. Based on this finding, the authors conclude that careful dissection of P3 with the intent to remove the entire third phalanx is especially important in cats less than 6 mo old.
Claw regrowth has never been reported following laser or scalpel onychectomy. The current study identified claw regrowth in 1/29 (3.5%) cats in the laser group and 2/31 (6.5%) cats in the scalpel group. This demonstrates that claw regrowth can occur if attention is not paid to careful dissection and removal of the third phalanx, regardless of the technique. A significant association between bony remnants and claw regrowth was found. All cats with evidence of claw regrowth had bony remnants detected on radiographs, and cats had a significantly higher frequency of claw regrowth if bony remnants were left in situ. This finding strengthens the recommendation that the entire third phalanx should be removed in every onychectomy.
Although more cats that underwent guillotine onychectomy had claw regrowth compared to laser and scalpel onychectomy, this finding was not statistically significant. The calculated power for this statistic was 0.32; therefore, the lack of statistical significance may be a result of the small sample size. Given the frequency of claw regrowth in this study, the calculated sample size required for a statistical power of 0.8 is 100 cats per group.
Cats that underwent laser onychectomy had significantly less owner-reported post-operative complications compared to the scalpel and guillotine groups. This finding is similar to previous reports which found that laser onychectomy resulted in less discomfort, lameness, and complications compared with the scalpel technique (19–21). Cats from all 3 groups experienced similar types of post-operative complications, with reluctance to jump, reluctance to scratch, and chewing at paws being the most frequently reported complications. Cats in the scalpel group had a significantly higher percentage of post-operative infections. The lower infection rate in the guillotine group could be attributed to shorter surgical times; however, surgical times were not evaluated. The lower infection rate in the laser group could be attributed to the proposed bactericidal effect of the laser (23).
Twelve cats (13.6%) were classified as mildly lame at the long-term recheck, with pain localizing to the forepaws. While the majority of lame cats had been onychectomized with the guillotine method, this finding was not significant. Lameness in the onychectomized cats was difficult to observe, as most cats were symmetrically lame in both forelimbs. This may have resulted in an underestimation of the degree of lameness in this study. Long-term lameness is cited as an infrequent complication following onychectomy (9,13,15). Lameness in onychectomized cats is commonly thought to be secondary to chronic pain (5–8); however, it may also be a functional change in the gait secondary to the loss of the third phalanges, which are important for the normal digitigrade stance of the cat, or pain originating from osteoarthritis of other joints.
Significantly more cats in the guillotine group, compared with the scalpel group, lifted their paws before or after jumping. The guillotine group also had a significantly lower frequency of cats that displayed hunting behaviors compared with the laser and scalpel groups, which may reflect the older median age. Reluctance to jump, shaking/lifting of forepaws, and lack of hunting behaviors may be subtle signs of neuropathic pain following onychectomy (7). Neuropathic pain may develop as a consequence of inadequate pre- and post-operative analgesia (7). An objective evaluation of post-operative analgesia and long-term outcome was not performed in this study due to incomplete medical records; however, this should be objectively assessed in future studies.
A limitation of the current study was the reliance on physical examination and radiographs to detect cases of claw regrowth. Typically, physical examination is the sole method used in general practice for detecting claw regrowth. Surgical exploration of the digits of all cats would have been the definitive method to determine if claw regrowth had occurred; however, this was not considered ethical. In the cats that underwent surgical exploration of the digits, histological evidence of claw regrowth was found in all digits, despite the fact that radiographic evidence was only seen on a few of the digits. Radiographs may increase the index of suspicion that claw regrowth has occurred, especially if remnants of P3 are identified. The claw bed epithelium identified on histopathology of the excised remnants provides evidence that claw regrowth occurs when germinal cells of the ungual process are left in situ.
Another limitation of this study was the lack of objective measurements with respect to lameness evaluation. Lameness in cats could be objectively measured by the use of a pressure-sensitive mat. One previous study objectively measured the weight-bearing of cats after bilateral onychectomy (24). The technique of onychectomy was not specified. This study found no significant difference in the weight-bearing of the onychectomized limb compared with the control cats more than 6 months following surgery. Using the previously defined period of 3 to 6 months after surgery as “short term” (25), this study suggests there is no short-term lameness following onychectomy; however, it must be considered that cats with bilateral procedures may be obliged to use both limbs equally. Another study conducted kinetic gait analysis on cats before, and up to, 12 days after unilateral onychectomy by either scalpel or laser disarticulation methods (21). This study found significantly higher peak vertical forces on days 1, 2, and 12 following laser onychectomy compared with scalpel onychectomy. Future research utilizing pressure-sensitive mats could assess the long-term outcome of cats after onychectomy at the walk and during jumping. A final but considerable limitation of the study is that the postoperative complications were owner-reported. This may have resulted in recall-bias in the reporting of the complications.
This study found no significant difference in the frequency of claw regrowth in cats onychectomized by scalpel, laser, and guillotine methods. Leaving bony remnants of P3 in situ was significantly associated with a higher frequency of claw regrowth. Radiographs showed fair interobserver agreement for detection of claw regrowth, and are likely to underestimate the true frequency of claw regrowth. Cats that underwent laser onychectomy had significantly less owner-reported postoperative complications. There was no detectable difference in the long-term outcome of cats regardless of the method used. Laser onychectomy may be preferred to reduce the risk of postoperative complications, bony remnants, and claw regrowth. CVJ
Appendix: Client questionnaire
Name: _________________________________________________________
Breed: _________________________________________________________
Sex: _________________________________________________________
Neutered: _________________________________________________________
DOB: _________________________________________________________
When was your cat declawed? _________________________________________________________
How old was your cat when he/she was declawed? _________________________________________________________
-
Which technique was used to declaw your cat?
□ Scalpel □ Laser □ Nail Clippers
-
Where was the declaw performed?
Clinic: _________________________________________________________
Location: _________________________________________________________
-
Did you notice any of the following post-operative complications?
Infection □ Yes □ No Draining tracts □ Yes □ No Fever □ Yes □ No Loss of appetite □ Yes □ No Reluctance to walk □ Yes □ No Reluctance to jump □ Yes □ No Reluctance to scratch □ Yes □ No Swelling of paws □ Yes □ No Chewing at paws □ Yes □ No Increased aggression □ Yes □ No -
Does your cat currently engage in any of the following behaviours?
Scratching with front paws □ Yes □ No Biting □ Yes □ No Jumping □ Yes □ No Climbing trees □ Yes □ No Scratching with hind paws □ Yes □ No Playing with toys/animals □ Yes □ No Chewing at paws □ Yes □ No Limping on front limbs □ Yes □ No Hunting (indoor or outdoor) □ Yes □ No Is your cat currently on any medications? If yes, what are they currently taking, what is being treated and what is the dose of that medication? _________________________________________________________
Does your cat have any other health problems? Please explain _________________________________________________________
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
Supported by a grant from the Atlantic Veterinary College Companion Animal Trust Fund.
References
- 1.Martinez SA, Hauptman J, Walshaw R. Comparing two techniques for onychectomy in cats and two adhesives for wound closure. Vet Med. 1993;88:516–525. [Google Scholar]
- 2.Australian Veterinary Association [homepage on the Internet] Policy 3.1. [Last accessed October 22, 2013]. Available from: http://www.ava.com.au/policy/31-surgical-alteration-natural-state-animals.
- 3.New Zealand Veterinary Association [homepage on the Internet] Policy 3b. [Last accessed October 22, 2013]. Available from: http://www.nzva.org.nz/policies/surgical-alteration-natural-state-animals.
- 4.Bennett M, Houpt KA, Erb HN. Effects of declawing on feline behavior. Comp Anim Pract. 1988;2:7–12. [Google Scholar]
- 5.Patronek GJ. Assessment of claims of short- and long-term complications associated with onychectomy in cats. J Am Vet Med Assoc. 2001;219:932–937. doi: 10.2460/javma.2001.219.932. [DOI] [PubMed] [Google Scholar]
- 6.Hellyer P, Rodan I, Brunt J, Downing R, Hagedorn JE, Robertson SA. AAHA/AAFP pain management guidelines for dogs and cats. J Am Anim Hosp Assoc. 2007;43:235–248. doi: 10.5326/0430235. [DOI] [PubMed] [Google Scholar]
- 7.Robertson SA, Lascelles BD. Long-term pain in cats: How much do we know about this important welfare issue? J Feline Med Surg. 2010;12:188–199. doi: 10.1016/j.jfms.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor PM, Robertson SA. Pain management in cats — Past, present and future. Part 1. The cat is unique. J Feline Med Surg. 2004;6:313–320. doi: 10.1016/j.jfms.2003.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landsberg GM. Cat owners’ attitudes toward declawing. Anthrozoos. 1991;4:192–197. [Google Scholar]
- 10.Cambridge AJ, Tobias KM, Newberry RC, Sarkar DK. Subjective and objective measurements of postoperative pain in cats. J Am Vet Med Assoc. 2000;217:685–690. doi: 10.2460/javma.2000.217.685. [DOI] [PubMed] [Google Scholar]
- 11.Carroll GL, Howe LB, Slater MR, et al. Evaluation of analgesia provided by postoperative administration of butorphanol to cats undergoing onychectomy. J Am Vet Med Assoc. 1998;213:246–250. [PubMed] [Google Scholar]
- 12.Franks JN, Boothe HW, Taylor L, et al. Evaluation of transdermal fentanyl patches for analgesia in cats undergoing onychectomy. J Am Vet Med Assoc. 2000;217:1013–1020. doi: 10.2460/javma.2000.217.1013. [DOI] [PubMed] [Google Scholar]
- 13.Jankowski AJ, Brown DC, Duval J, et al. Comparison of effects of elective tenectomy or onychectomy in cats. J Am Vet Med Assoc. 1998;213:370–373. [PubMed] [Google Scholar]
- 14.Pollari FL, Bonnett BN. Evaluation of postoperative complications following elective surgeries of dogs and cats at private practices using computer records. Can Vet J. 1996;37:672–678. [PMC free article] [PubMed] [Google Scholar]
- 15.Tobias KS. Feline onychectomy at a teaching institution: A retrospective study of 163 cases. Vet Surg. 1994;23:274–280. doi: 10.1111/j.1532-950x.1994.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 16.Cooper MA, Laverty PH, Soiderer EE. Bilateral flexor tendon contracture following onychectomy in 2 cats. Can Vet J. 2005;46:244–246. [PMC free article] [PubMed] [Google Scholar]
- 17.Yeon SC, Flanders JA, Scarlett JM, Houpt KA. Attitudes of owners regarding tendonectomy and onychectomy in cats. J Am Vet Med Assoc. 2001;218:43–47. doi: 10.2460/javma.2001.218.43. [DOI] [PubMed] [Google Scholar]
- 18.Burns SM, Howerth EW, Rawlings CA, Cornell KK, Radlinsky MG, Mauck JW. Comparison of the carbon dioxide laser and the radiofrequency unit for feline onychectomies. J Am Anim Hosp Assoc. 2010;46:375–384. doi: 10.5326/0460375. [DOI] [PubMed] [Google Scholar]
- 19.Holmberg DL, Brisson BA. A prospective comparison of postoperative morbidity associated with the use of scalpel blades and lasers for onychectomy in cats. Can Vet J. 2006;47:162–163. [PMC free article] [PubMed] [Google Scholar]
- 20.Mison MB, Bohart GH, Walshaw R, Winters CA, Hauptman JG. Use of carbon dioxide laser for onychectomy in cats. J Am Vet Med Assoc. 2002;221:651–653. doi: 10.2460/javma.2002.221.651. [DOI] [PubMed] [Google Scholar]
- 21.Robinson DA, Romans CW, Gordon-Evans WJ, Evans RB, Conzemius MG. Evaluation of short-term function following unilateral carbon dioxide laser or scalpel onychectomy in cats. J Am Vet Med Assoc. 2007;230:353–358. doi: 10.2460/javma.230.3.353. [DOI] [PubMed] [Google Scholar]
- 22.Viera AJ, Garrett JM. Understanding interobserver agreement: The Kappa statistic. Fam Med. 2005;37:360–363. [PubMed] [Google Scholar]
- 23.Schoop U, Kluger W, Moritz A, et al. Bactericidal effect of different laser systems in the deep layers of dentin. Lasers Surg Med. 2004;35:111–116. doi: 10.1002/lsm.20026. [DOI] [PubMed] [Google Scholar]
- 24.Romans CW, Conzemius MG, Horstman CL, Gordon WJ, Evans RB. Use of a pressure platform gait analysis in cats with and without bilateral onychectomy. Am J Vet Res. 2004;65:1276–1278. doi: 10.2460/ajvr.2004.65.1276. [DOI] [PubMed] [Google Scholar]
- 25.Cook JL, Evans R, Conzemius MG, et al. Proposed definitions and criteria for reporting time frame, outcome, and complications for clinical orthopedic studies in veterinary medicine. Vet Surg. 2010;39:905–908. doi: 10.1111/j.1532-950X.2010.00763.x. [DOI] [PubMed] [Google Scholar]