Abstract
Marijuana discontinuation has been recently reported to be anxiogenic in humans, which may predict relapse. Limited animal research has been carried out to model this withdrawal-associated negative affect. The current study sought to investigate the potential anxiety-like effects of cannabinoid withdrawal in mice. Male ICR mice were injected s.c. with delta9-tetrahydrocannabinol (THC) at a dose of 10 mg/kg or vehicle once daily for 10 days. To precipitate withdrawal, the cannabinoid CB1 antagonist SR141716 (0.3, 1.0, or 3.0 mg/kg) or vehicle was administrated i.p. 4 h following the last THC or vehicle treatment. Thirty min later, mice were tested on the elevated plus-maze (EPM) for 5 min. SR141716 did not significantly change EPM behaviors in vehicle-treated mice. In contrast, SR141716 precipitated a reduction in exploration of the open arms of EPM in mice repeatedly treated with THC vs vehicle, with % open arm entries of the total arm entries, % open arm time of total time in arms, and the absolute time spent in open arms significantly less when SR141716 dose was at 3.0 mg/kg. No significant differences in the number of closed or total arm entries were observed, indicating that the behavioral changes were not due to altered motor activity. Collectively, the present results constitute the first evidence that cannabinoid withdrawal produces anxiety-like effects in mice. This animal model may help to identify the mechanisms that contribute to adaptations in the neuronal circuitry of the brain that are expressed as emotional symptoms of cannabinoid withdrawal.
Keywords: cannabinoid, THC, withdrawal, abstinence, SR141716, rimonabant, anxiety, elevated plus-maze, mice
Introduction
Recent data suggest that abstinence from marijuana smoking and oral delta9-tetrahydrocannabinol (THC, the main psychoactive component therein) in humans produces a consistent withdrawal pattern, including symptoms such as anxiety, aggression, hyperirritability, weight loss (decreased appetite), restlessness and sleep problems, which may be relevant to the motivation for the maintenance of cannabis addiction (see [5] for review; [2]). New strains of cannabis contain higher contents of THC. Accordingly, the likelihood of human users experiencing withdrawal is greater than ever [The United Nations Office on Drugs and Crime 2008 report (UNODC 2008)].
In contrast to the challenge of observing and quantifying spontaneous/abrupt cannabinoid withdrawal, SR141716, a selective cannabinoid CB1 receptor antagonist, elicits immediate and quantifiable withdrawal responses with a focus on somatic signs in a variety of animal species, including mouse, rat and dog (see [5,13,15] for reviews). For example, SR141716-precipitated THC withdrawal syndromes have been studied in mice by several groups (e.g., [4,11,12]). Depending on the mouse strain and dosing regimen used, a range of withdrawal behaviors have been documented, such as forepaw tremors, head shakes, wet dog shakes, increased or decreased locomotion, ataxia, hunched posture, mastication and piloerection. Paw tremor was the most reliable sign of THC withdrawal so far (e.g., [4,11,12,14]), and was also observed consistently during SR 141716-precipitated withdrawal in mice that had been repeatedly exposed to marijuana smoke [20]. Differences in the ability to demonstrate THC-precipitated withdrawal signs among mice strains might be due to strain differences in endocannabinoid tone during the withdrawal state. As a cannabinoid withdrawal syndrome has been characterized mainly by the presence of a number of somatic signs in mice, in this study we attempt to characterize the emotional aspects of SR141716-precipitated THC withdrawal in a mouse model of anxiety using the elevated plus-maze (EPM).
Materials and Methods
Animals
Male ICR mice were purchased from Ace Animals, Inc. (Boyertown, PA). Animals were allowed to acclimate to the animal facility for one week. They weighed 24–29 g at the start of the study. The mice were housed five animals per cage on a 12:12-h light/dark cycle with lights on at 7 am and with ad libitum access to food and water. Experimental protocols were approved by the Institutional Animal Care and Use Committee of Temple University School of Medicine. Animal care and experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Compounds
THC (in ethanol, 100 mg/ml) and SR141716 (freebase) were provided by the National Institute on Drug Abuse (Bethesda, MD). SR141716 was first dissolved in ethanol. Both drugs in ethanol were mixed with cremophor EL (Sigma) thoroughly by vortex, and then diluted with saline to form a vehicle mixture of ethanol/cremophor/saline in a ratio of 1:1:18. Injections were carried out in a final volume of 0.1 ml per 10 g of body weight.
SR141716-precipitated THC withdrawal
Mice were injected s.c. once daily with either THC at a dose of 10 mg/kg or vehicle for ten consecutive days, every day between 8:00–11:00 am, in the holding room. On the 10th day, 4 hrs after the last injection of THC or vehicle and after a 2~3-h habituation period in the testing room, each animal was challenged i.p. with vehicle or SR141716 at different doses (0.3, 1 and 3 mg/kg) and then remained in its home cage for 30 min. At the end of the 30-min period, each animal was subjected to the EPM test as follows.
Evaluation of behaviors of mice in EPM
Mice were tested for anxiety-like behaviors on a 50-cm high black Plexiglas EPM between 1:00–3:00 pm in the testing room, which was illuminated by a dimmer light (7 lux) than that in the holding room (260 lux). The EPM consisted of two open arms (28 × 7 cm), with a 0.5 cm lip on each open arm, and two enclosed arms (30 × 7 × 13.5 cm) that extended from a central platform (7 × 7 cm). To begin a test session, mice were placed in the center of the maze facing an open arm. The free exploration of the mouse in the maze was videotaped, and the entries into open and closed EPM arms and EPM center were recorded for the first 5 min of EPM exposure. The animal placing all four paws onto the arm was considered to be in arm, otherwise the animal was in center (see [10] for review). Between each trial, the maze was wiped clean with a damp sponge and dried with paper towels. The measures of anxiety are the percentage (%) of open arm entries and the percentage (%) of time spent on the open arms, both expressed as a % of the total entries onto, or time spent on, the open and closed arms, whereas the numbers of total and closed arm entries are considered locomotor measures (see [7,10] for reviews). In addition, the absolute time spent on the open arms, which has been used as an indicator of anxiety by some researchers (e.g., [17]), was also recorded in seconds (sec).
Data analysis
Data are expressed as the mean ± SEM, and examined for statistical significance by two-way Analysis of Variance (ANOVA) with the treatment (vehicle vs THC) and SR141716 dose [0 (vehicle), 0.3, 1.0 vs 3.0 mg/kg] as two variables followed by Bonferroni post-hoc tests (SigmaStat v 3.1).
Results
As can be seen in Fig. 1, a significant decrease in both open arm entries and open arm time was observed in mice repeatedly treated with THC vs vehicle for 10 days following the SR141716 challenge at a dose of 3.0 mg/kg rather than the challenge at a dose of 0.3 or 1.0 mg/kg or the vehicle challenge. Two-way ANOVA revealed a main effect of SR141716 dose (F(3, 87)=3.974, p=0.011; Fig. 1A) and a significant interaction between treatment (THC vs vehicle) and SR141716 dose (F(3, 87)=4.607, p=0.005; Fig. 1A) on % open arm entries. Post hoc comparisons indicated a significant 62.3% of decrease in % open arm entries (34.5 ± 4.4% vs 13.0 ± 3.8%, vehicle vs THC; ***, p < 0.001; Fig. 1A) with the SR141716 challenge at a dose of 3.0 mg/kg rather than a lower dose (0.3 or 1.0 mg/kg) or vehicle challenge. Similarly, two-way ANOVA demonstrated a significant interaction between treatment and SR141716 dose on % open arm time (F(3, 87)=3.011, p=0.034; Fig. 1B). Post hoc comparisons identified a significant 60.3% of decrease in % open arm time (30.5 ± 5.7% vs 12.1 ± 3.9%, vehicle vs THC; **, p < 0.01; Fig. 1B) only when the SR141716 challenge dose was at 3.0 mg/kg. Moreover, Two-way ANOVA revealed a main effect of SR141716 dose (F(3, 87)=4.607, p=0.005; Fig. 1C) and a significant interaction between repeated treatment (THC vs vehicle) and SR141716 dose (F(3, 87)=4.807, p=0.004; Fig. 1C) on time (sec) spent in open arms. Post hoc comparisons indicated a significant 54.0% of decrease in the open arm time (48.3 ± 10.0 sec vs 22.2 ± 9.7 sec, vehicle vs THC; **, p < 0.01; Fig. 1C) with the SR141716 challenge at a dose of 3.0 mg/kg rather than a lower dose (0.3 or 1.0 mg/kg) or vehicle challenge. In addition, in mice treated repeatedly with THC, SR141716 dose-dependently decreased % open arm entries (see ˆˆ p < 0.01, ### p <0.001 in Fig. 1A), % open arm time (see # p < 0.05 in Fig. 1B), and time (sec) spent in open arms (see ˆˆˆ p < 0.001, ### p <0.001 in Fig. 1C), whereas in mice treated repeatedly with vehicle, SR141716 challenge at any dose tested did not change the % open arm entries (Fig. 1A), % open arm time (Fig. 1B) and time (sec) spent in open arms (Fig. 1C) significantly in comparison to vehicle challenge.
Fig. 1. The anxiety measures (A. % open arm entries; B. % open arm time; C. open arm time (sec)) for the effects of SR141716 or vehicle challenge on EPM behaviors of mice repeatedly treated with vehicle or THC.
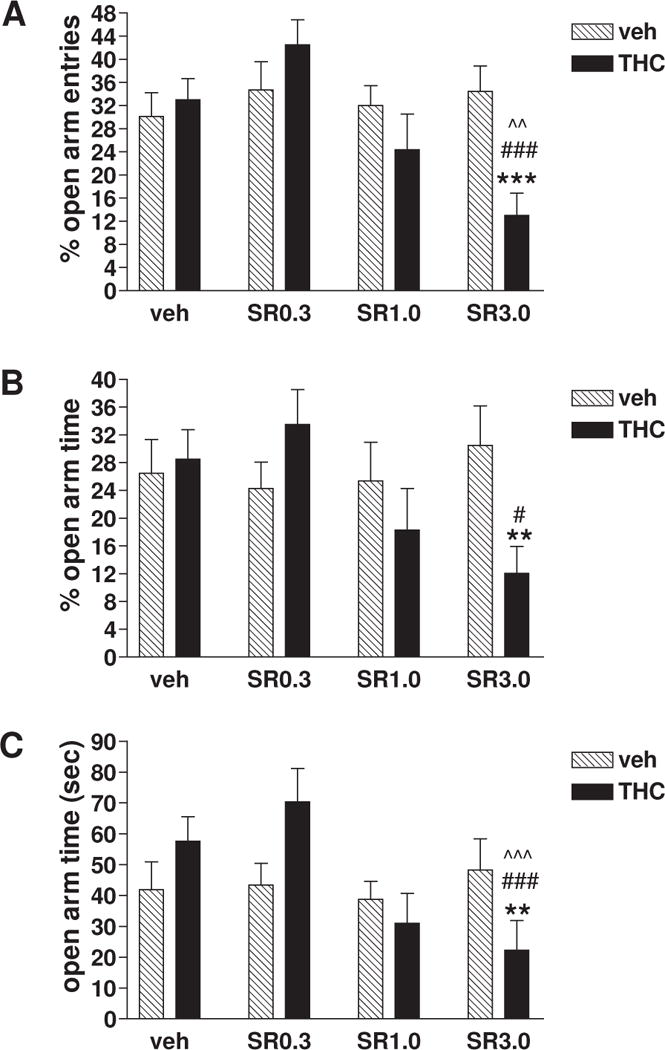
Mice were placed in the center of the maze facing an open arm and their behaviors videotaped and recorded for 5 min. SR141716 (SR) dose (0.3, 1.0 or 3.0 mg/kg, i.p.)-dependently decreased % open arm entries (A), % open arm time (B) and open arm time (sec) (C), and thereby suppressed the open arm exploration of the EPM, in mice repeatedly treated with THC (10 mg/kg, s.c.) rather than vehicle. Veh/veh n=15, THC/veh n=10, veh/SR0.3 n=10, THC/SR0.3 n=10, veh/SR1.0 n=10, THC/SR1.0 n=10, veh/SR3.0 n=15, THC/SR3.0 n=15. Data represent mean ± SEM, which were analyzed by two-way ANOVA with Bonferroni post-hoc tests. The significance is denoted by ** p < 0.01 and *** p < 0.001 vs mice repeatedly treated with vehicle and then challenged with SR141716 at a dose of 3.0 mg/kg, by # p < 0.05 and ### p < 0.001 vs mice repeatedly treated with THC and then challenged with SR141716 at a dose of 0.3 mg/kg, and by ˆˆ p < 0.01 and ˆˆˆ p < 0.001 vs mice repeatedly treated THC and then challenged with vehicle.
It appears from Figure 1B and 1C that the 0.3 mg/kg dose of SR 141716 might have an anxiolytic effect in THC-treated mice compared to the same dose in vehicle-treated mice, although this effect does not reach statistical significance by two-way ANOVA analyses followed by Bonferroni post-hoc tests. The statistical results for comparison between vehicle- and THC-treated mice at the 0.3 mg dose of SR141716 are P=0.24 (Fig. 1B) and P=0.066 (Fig. 1C), and the results for comparison between THC-vehicle and THC-SR0.3 groups are P=0.14 (Fig. 1B) and P=0.052 (Fig. 1C). In addition, another noteworthy fact is that the statistical result for comparison between THC-vehicle and THC-SR3.0 groups in Figure 1B is P=0.051.
As shown in Fig. 2, the decrease in both open arm entries and open arm time did not result from decreased motor activity, as no significant effects were detected by two-way ANOVA in treatment [F(1, 87)=0.23, p=0.63 (Fig. 2A) and F(1, 87)=0.63, p=0.43 (Fig. 2B)], SR141716 dose [F(3, 87)=0.61, p=0.61 (Fig. 2A) and F(3, 87)=2.09, p=0.11 (Fig. 2B)] and their interaction [F(3, 87)=1.49, p=0.22 (Fig. 2A) and F(3, 87)=0.80, p=0.50 (Fig. 2B)] on numbers of closed (Fig. 2A) and total (Fig. 2B) arm entries.
Fig. 2. The activity measures (A. number of closed arm entries; B. number of total arm entries) for the effects of SR141716 or vehicle challenge on EPM behaviors of mice repeatedly treated with vehicle or THC.
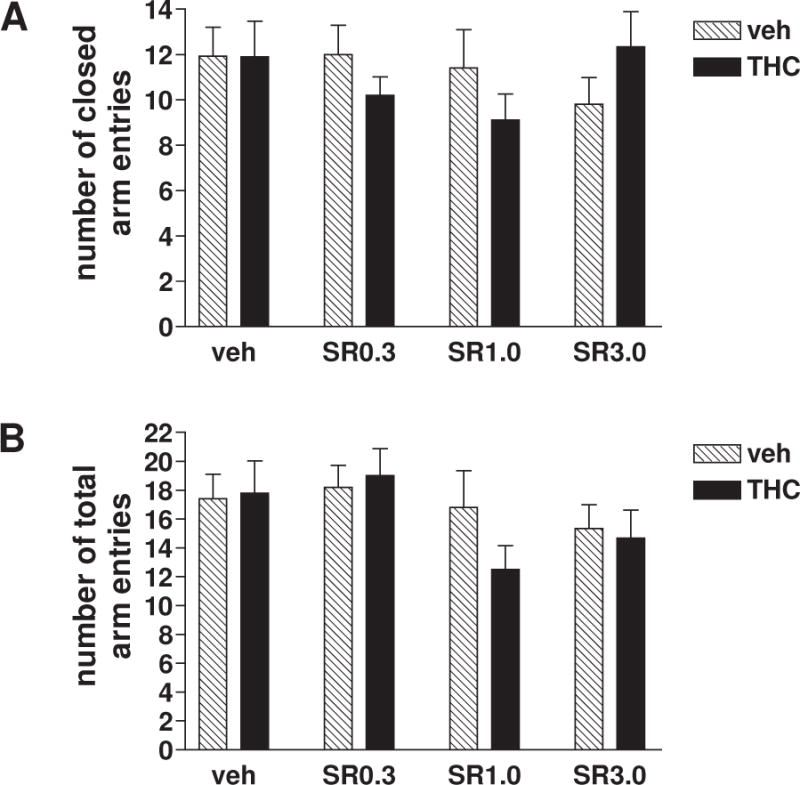
No significant change in motor activity, regarding the number of closed arm entries (A) and the number of total arm entries (B), was produced among the same eight cohorts of mice as those in Fig. 1. Veh/veh n=15, THC/veh n=10, veh/SR0.3 n=10, THC/SR0.3 n=10, veh/SR1.0 n=10, THC/SR1.0 n=10, veh/SR3.0 n=15, THC/SR3.0 n=15. Data were shown as mean ± SEM, and were analyzed by two-way ANOVA with Bonferroni post-hoc tests.
Discussion
The current study showed that a mouse EPM model can be used to demonstrate anxiety-like behaviors associated with THC withdrawal precipitated by SR141716. Because SR141716 failed to produce similar behaviors in mice repeatedly treated with vehicle, the SR141716-induced anxiety-like effects in EPM were deemed “precipitated withdrawal” rather than an intrinsic effect of the antagonist itself. To the best of our knowledge, this is the first report to study the anxiety-like responses of cannabinoid withdrawal in mice.
Our results are reminiscent of the findings in rats that the behavioral changes after administration of SR141716 at a dose of 3.0 mg/kg following repeated treatment with the potent synthetic cannabinoid HU-210 for 14 days were reflected in anxiety-like responses in the defensive withdrawal test, while administration of vehicle with the same cannabinoid treatment did not produce such responses [19]. The precipitated withdrawal was accompanied by a marked elevation in extracellular levels of the stress neurohormone corticotropin releasing factor and a distinct pattern of Fos activation in the central nucleus of the amygdala [19]. It is noteworthy that a single dose of SR141716 at 3.0 mg/kg was administrated to drug-naive rats, and produced anxiety-like responses in those rats to a similar level as that in rats pretreated with HU-210 for 14 days [19].
Acute SR141716-induced behaviors in drug-naive animals have been controversial in rodent models of anxiety, in which EPM has been most commonly used. Our findings that SR141716 at doses up to 3.0 mg/kg did not significantly change EPM behaviors of mice repeatedly treated with vehicle are consistent with the results from EPM trial 1 in a mouse study of Rodgers et al. [18]. Results from EPM trial 2 in the same report revealed an anti-anxiety profile of SR141716 in maze-experienced animals [18]. In addition, anxiolytic effects of SR141716 were observed in other studies [6,8,9]. SR141716 was also found to produce an anxiolytic effect in CB1 knockout mice, indicating that this effect was not mediated by CB1 receptors [9]. In contrast, SR141716 was shown to exhibit anxiogenic-like effects in rats and mice by several groups [1,16,17,19]. Among them, Patel et al. (2006) reported that SR141716 at a dose of 3.0 mg/kg produced significant decreases in % open arm time in EPM in ICR mice, the same mouse strain as used in our studies [17]. The experimental conditions were different (ours vs Patel’s) at least as follows: (1) repeated vehicle injection for 10 days before the EPM test vs no repeated injection; (2) dimmer vs normal light during testing; and (3) 1:00–3:00 pm vs 9:00 am–12:00 noon, for testing time. In addition, the % open arm time and % open arm entries appeared to be higher in control animals in our studies vs Patel’s, indicating varied basal level of anxiety. Thus, the discrepancies of anxiety-related effects of acute SR141716 in drug-naïve animals may be due to variations in multiple factors including species, strains and anxiety models used, and in particular the basal level of anxiety of the animal, which may be affected by repeated handling, the time of testing, light condition in the testing room and previous exposure to the maze (see [7,10] for reviews). In addition, the fact that the mice receiving the 3.0 mg/kg dose of SR141716 showed some aversive motor signs as mentioned below may complicate the interpretation of the anxiety-like effects.
In our studies, the minimum dose of SR141716 to produce reliable anxiety-like effects in THC-treated mice in EPM (i.e., decrease in open arm exploration) was 3.0 mg/kg, which was consistent with the SR141716 dose used in the aforementioned rat studies to reveal anxiety-like behaviors in the defensive withdrawal test [19]. This antagonist dose produced a nearly complete (95%) inhibition of THC-induced hypothermia and an almost complete (89%) inhibition of the antinociceptive effect of THC in the tail-flick procedure in mice [3]. Although SR141716 at a dose of 3.0 mg/kg was demonstrated to produce some somatic signs of withdrawal such as forepaw tremors and head shakes in ICR mice [4], during the 5-min period in EPM in current studies the somatic signs of withdrawal were barely detected. This supports the failure to see a significant change in the locomotor activity measure (number of total or closed arm entries). SR141716 at a dose of 10 mg/kg or higher was commonly used in mice to elicit high level of those somatic responses and/or produce other prominent somatic reactions such as wet dog shakes, ataxia, mastication and increased locomotor activity in different mouse strains (e.g., [4,11,12,14]). Nevertheless, the behavioral expression of THC withdrawal in mice precipitated by SR141716 at a dose of 10 mg/kg was reported to lack the aversive/dysphoric components in the conditioned place aversion test [12].
In summary, the anxiety-like responses associated with SR141716-precipitated THC withdrawal were revealed in a mouse EPM model. This model may allow further investigations of the underlying mechanisms of emotional aspects of cannabinoid withdrawal. Future studies are also planned to explore alternative behavioral measures of anxiety (e.g., defensive burying) and the potential anxiety-like behaviors induced by spontaneous/abrupt cannabinoid withdrawal in mice.
Acknowledgments
This work was supported by National Institute of Health Grants R01 DA17302 (LYLC), K01 MH63301 (LK) and P30DA013429 (LYLC). We thank Drs. Mary Abood, Alan Cowan and Ellen Unterwald for comments on an early version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Arevalo C, de Miguel R, Hernandez-Tristan R. Cannabinoid effects on anxiety-related behaviours and hypothalamic neurotransmitters. Pharmacol Biochem Behav. 2001;70:123–131. doi: 10.1016/s0091-3057(01)00578-0. [DOI] [PubMed] [Google Scholar]
- 2.Bonn-Miller MO, Moos RH. Marijuana discontinuation, anxiety symptoms, and relapse to marijuana. Addict Behav. 2009;34:782–785. doi: 10.1016/j.addbeh.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of delta 9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- 4.Cook SA, Lowe JA, Martin BR. CB1 receptor antagonist precipitates withdrawal in mice exposed to Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1998;285:1150–1156. [PubMed] [Google Scholar]
- 5.Cooper ZD, Haney M. Cannabis reinforcement and dependence: role of the cannabinoid CB1 receptor. Addict Biol. 2008;13:188–195. doi: 10.1111/j.1369-1600.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degroot A, Nomikos GG. Genetic deletion and pharmacological blockade of CB1 receptors modulates anxiety in the shock-probe burying test. Eur J Neurosci. 2004;20:1059–1064. doi: 10.1111/j.1460-9568.2004.03556.x. [DOI] [PubMed] [Google Scholar]
- 7.File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 8.Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry. 2005;57:261–267. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 9.Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- 10.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 11.Huang P, Liu-Chen LY, Unterwald EM, Cowan A. Hyperlocomotion and paw tremors are two highly quantifiable signs of SR141716-precipitated withdrawal from delta9-tetrahydrocannabinol in C57BL/6 mice. Neurosci Lett. 2009;465:66–70. doi: 10.1016/j.neulet.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutcheson DM, Tzavara ET, Smadja C, Valjent E, Roques BP, Hanoune J, Maldonado R. Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with delta-9-tetrahydrocannabinol. Br J Pharmacol. 1998;125:1567–1577. doi: 10.1038/sj.bjp.0702228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtman AH, Martin BR. Marijuana withdrawal syndrome in the animal model. J Clin Pharmacol. 2002;42:20S–27S. doi: 10.1002/j.1552-4604.2002.tb05999.x. [DOI] [PubMed] [Google Scholar]
- 14.Lichtman AH, Sheikh SM, Loh HH, Martin BR. Opioid and cannabinoid modulation of precipitated withdrawal in delta(9)-tetrahydrocannabinol and morphine-dependent mice. J Pharmacol Exp Ther. 2001;298:1007–1014. [PubMed] [Google Scholar]
- 15.Maldonado R. Study of cannabinoid dependence in animals. Pharmacol Ther. 2002;95:153–164. doi: 10.1016/s0163-7258(02)00254-1. [DOI] [PubMed] [Google Scholar]
- 16.Navarro M, Hernandez E, Munoz RM, del AI, Villanua MA, Carrera MR, Rodriguez dF. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport. 1997;8:491–496. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- 17.Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- 18.Rodgers RJ, Haller J, Halasz J, Mikics E. ‘One-trial sensitization’ to the anxiolytic-like effects of cannabinoid receptor antagonist SR141716A in the mouse elevated plus-maze. Eur J Neurosci. 2003;17:1279–1286. doi: 10.1046/j.1460-9568.2003.02548.x. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez dF, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- 20.Wilson DM, Varvel SA, Harloe JP, Martin BR, Lichtman AH. SR 141716 (Rimonabant) precipitates withdrawal in marijuana-dependent mice. Pharmacol Biochem Behav. 2006;85:105–113. doi: 10.1016/j.pbb.2006.07.018. [DOI] [PubMed] [Google Scholar]


