Abstract
We examined the effect of a controlled diet and two probiotic preparations on urinary oxalate excretion, a risk factor for calcium oxalate kidney stone formation, in patients with mild hyperoxaluria. Patients were randomized to a placebo, a probiotic, or a synbiotic preparation. This tested whether these probiotic preparations can increase oxalate metabolism in the intestine and/or decrease oxalate absorption from the gut. Patients were maintained on a controlled diet to remove the confounding variable of differing oxalate intake from food. Urinary oxalate excretion and calcium oxalate supersaturation on the controlled diet were significantly lower compared with baseline on a free-choice diet. Neither study preparation reduced urinary oxalate excretion nor calcium oxalate supersaturation. Fecal lactobacilli colony counts increased on both preparations, whereas enterococcal and yeast colony counts were increased on the synbiotic. Total urine volume and the excretion of oxalate and calcium were all strong independent determinants of urinary calcium oxalate supersaturation. Hence, dietary oxalate restriction reduced urinary oxalate excretion, but the tested probiotics did not influence urinary oxalate levels in patients on a restricted oxalate diet. However, this study suggests that dietary oxalate restriction is useful for kidney stone prevention.
Keywords: diet therapy, lactobacilli, nephrolithiasis, probiotic, synbiotic, urolithiasis
The formation of calcium oxalate (CaOx) kidney stones is extremely common,1 and evidence suggests that minimal, perhaps transient, elevations in urinary oxalate concentration may be important factors in at least a subgroup of these patients with ‘idiopathic’ CaOx urolithiasis.2 In the case of enteric hyperoxaluria, the pathogenic role of oxalate is clear, and renal scarring can also be observed as a consequence of oxalate exposure and CaOx crystal deposition.3–6 Unfortunately, few satisfactory specific treatments for hyperoxaluria are available. Dietary restriction is commonly advised, but is difficult for patients to follow effectively without extensive education, in part because oxalate content is not listed on food labels.7 Typical treatment strategies for enteric hyperoxaluria also include low-fat diets to limit malabsorption and effects of fatty acids and bile acids on oxalate absorption in the distal colon,8,9 oral calcium to bind oxalate,10 and bile acid sequestrants such as cholestyramine.8,11 These more intensive strategies have not been widely employed for patients with CaOx stones and, even if effective, they do not seem likely to be accepted by the majority of patients with mild hyperoxaluria and idiopathic CaOx stone disease.
Previous studies have shown that components of the endogenous digestive microbiota can utilize oxalate, potentially limiting its absorption from the intestinal lumen.12 A recent preliminary study demonstrated that a preparation of lactic acid bacteria degraded oxalate in vitro and reduced urinary oxalate excretion when given by mouth.13 We previously demonstrated that the same preparation of lactic acid bacilli (Oxadrop, VSL Pharmaceuticals, Gaithersburg, MD, USA) can reduce urinary oxalate excretion in patients with enteric hyperoxaluria.14 Agri-King Synbiotic (AKSB) is a candidate synbiotic preparation extensively studied at Mayo Clinic that might have beneficial effects on gastrointestinal health, although there is no evidence it should influence oxalate metabolism directly. In this study, we tested the effect of these two preparations on oxalate excretion in subjects with mild hyperoxaluria. Our hypothesis was that such preparations can increase oxalate metabolism in the intestine, and/or decrease gastrointestinal oxalate absorption from the gut and reduce oxalate elimination in the urine. Importantly, subjects were maintained on a controlled diet while conducting the urinary oxalate measurements, to remove the potential confounding variable of differing amounts of dietary oxalate intake from food.
RESULTS
Baseline characteristics of the subjects are listed in Table 1. There were 36 men and 4 women of a mean age of 53±14 years who had passed or had 2.24±1.84 stones (median 2.0) removed. Demographic characteristics did not vary between groups.
Table 1.
Patient characteristics
| Placebo | AKSB | Oxadrop | Total | |
|---|---|---|---|---|
| Number | 14 | 12 | 14 | 40 |
| Male: female | 12:2 | 10:2 | 14:0 | 36:4 |
| Age (mean±s.d.) | 50±13 | 55±18 | 53±15 | 53±14 |
| Stones passed (mean; median) | 2.5; 2.0 | 2.36; 1.0 | 1.86; 2.0 | 2.24; 2.0 |
Abbreviation: AKSB, Agri-King Synbiotic.
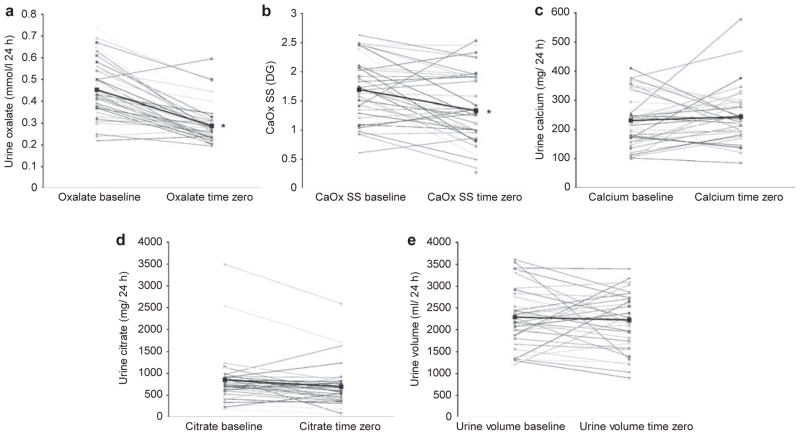
The controlled metabolic diet was well tolerated by the study group. Table 2 contains baseline 24-h urinary parameters for all subjects regardless of group on the free- choice diet (pre-study) and those on the controlled metabolic diet at time zero. Urinary sodium fell, as expected, to 123±38 mEq/day, thus confirming compliance with the controlled metabolic diet, whereas urinary creatinine, volume, and calcium did not change. Urinary oxalate fell significantly from 0.45±0.12 to 0.29±0.09 mmol/l/day, as did CaOx and brushite supersaturation (SS), whereas uric acid SS increased slightly. Figure 1 demonstrates the significant fall in urinary oxalate excretion and CaOx SS values for most subjects between baseline and time zero, as well as for the overall mean. Conversely, 24-h urinary calcium, citrate, and volume excretion did not change.
Table 2.
Summary of urinary values for 40 idiopathic CaOx stone patients at baseline on free-choice diet and at time zero after 1 week on a controlled low-oxalate diet
| Urine parameter | Baseline (free-choice diet) | Time zero (controlled diet) | P-valuea |
|---|---|---|---|
| Oxalate (mmol/l) | 0.45 (0.12) | 0.29 (0.09) | <0.000 |
| ss CaOx (DG) | 1.69 (0.52) | 1.34 (0.61) | 0.000 |
| Volume (ml) | 2288 (692) | 2223 (644) | 0.56 |
| Osmolality (mOsm/kg) | 521 (205) | 483 (191) | 0.15 |
| Calcium (mg) | 231 (92) | 244 (93) | 0.44 |
| Citrate (mg) | 849 (570) | 695 (460) | 0.005 |
| pH | 6.08 (0.83) | 5.92 (0.40) | 0.20 |
| Uric acid (mg) | 770 (265) | 725 (224) | 0.32 |
| Sodium (mEq) | 214 (69) | 121 (34) | <0.001 |
| Chloride (mEq) | 199 (70) | 109 (28) | <0.001 |
| Potassium (mEq) | 81 (25) | 76 (20) | 0.22 |
| Magnesium (mg) | 144 (60) | 133 (38) | 0.15 |
| Sulfate(mEq) | 26 (8) | 34 (7) | <0.001 |
| Phosphorous (mg) | 1203 (435) | 1079 (253) | 0.11 |
| ss BR (DG) | −0.06 (1.19) | −0.51 (1.05) | 0.04 |
| ss Uric acid | −0.37 (2.77) | 0.86 (2.26) | 0.02 |
| Creatinine (mg) | 1944 (503) | 1833 (446) | 0.15 |
Abbreviations: BR, brushite; CaOx, calcium oxalate; DG, delta Gibbs; SS, supersaturation.
Paired t-test comparing time zero with baseline. Values are given as mean (s.d.).
Values in bold were significantly different on controlled diet.
Figure 1.
Changes in urinary oxalate on a free-choice diet compared with a controlled metabolic low-oxalate diet. Urine oxalate (a) and CaOx SS (b) values fell on the controlled metabolic diet for the majority of subjects. Other key determinants of SS, including urinary calcium excretion (c), citrate excretion (d), and urine volume (e), did not change. *P<0.001 time zero versus baseline. CaOx, calcium oxalate; SS, supersaturation.
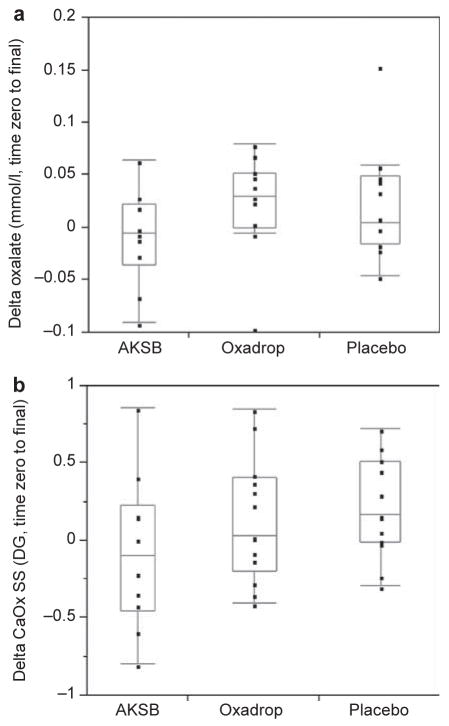
Table 3 lists time zero and final urinary chemistries for the three study groups by preparation. There was no further improvement in any urinary parameter on either of the study drug. In particular, neither urinary oxalate nor CaOx SS fell from the time zero values (Figure 2).
Table 3.
Summary of urinary values for 35 idiopathic CaOx stone patients randomized to placebo, AKSB, or Oxadrop
| Urine parameter | Placebo (n=12)
|
AKSB (n=10)
|
Oxadrop (n=13)
|
P-valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time zero | Final | P-valuea | Time zero | Final | P-valuea | Time zero | Final | P-valuea | ||
| Oxalate (mmol/l) | 0.28 (0.07) | 0.30 (0.10) | 0.18 | 0.29 (0.07) | 0.28 (0.07) | 0.56 | 0.28 (0.10) | 0.31 (0.09) | 0.07 | 0.14 |
| ss CaOx (DG) | 1.19 (0.56) | 1.40 (0.65) | 0.045 | 1.49 (0.69) | 1.42 (0.49) | 0.67 | 1.22 (0.57) | 1.36 (0.67) | 0.23 | 0.30 |
| Volume (ml) | 2437 (676) | 2256 (632) | 0.12 | 1987 (620) | 2001 (761) | 0.94 | 2305 (632) | 2265 (858) | 0.79 | 0.60 |
| Osmolality (mOsm/kg) | 419 (148) | 468 (156) | 0.11 | 543 (239) | 495 (202) | 0.42 | 478 (173) | 511 (205) | 0.28 | 0.20 |
| Calcium (mg) | 235 (55) | 256 (68) | 0.09 | 265 (153) | 238 (121) | 0.39 | 244 (77) | 228 (64) | 0.08 | 0.07 |
| Citrate (mg) | 738 (422) | 774 (583) | 0.62 | 737 (365) | 669 (239) | 0.49 | 768 (564) | 776 (638) | 0.83 | 0.96 |
| pH | 5.86 (0.30) | 5.90 (0.43) | 0.70 | 6.19 (0.44) | 6.32 (0.60) | 0.10 | 5.92 (0.36) | 5.90 (0.36) | 0.78 | 0.13 |
| Uric acid (mg) | 774 (225) | 745 (198) | 0.51 | 652 (199) | 642 (182) | 0.92 | 790 (157) | 771 (157) | 0.58 | 0.59 |
| Sodium (mEq) | 111 (39) | 122 (24) | 0.32 | 131 (43) | 112 (31) | 0.16 | 124 (34) | 120 (33) | 0.48 | 0.07 |
| Chloride (mEq) | 99 (30) | 107 (20) | 0.36 | 113 (39) | 96 (27) | 0.15 | 115 (18) | 118 (31) | 0.66 | 0.19 |
| Potassium (mEq) | 73 (18) | 75 (17) | 0.79 | 81 (28) | 72 (18) | 0.26 | 80 (17) | 89 (22) | 0.06 | 0.25 |
| Magnesium (mg) | 137 (53) | 140 (50) | 0.50 | 140 (34) | 133 (35) | 0.51 | 132 (28) | 128 (25) | 0.46 | 0.63 |
| Sulfate (mEq) | 34 (6) | 33 (5) | 0.29 | 32 (11) | 31 (10) | 0.49 | 35 (6) | 35 (8) | 0.82 | 0.77 |
| Phosphorous (mg) | 1106 (240) | 1088 (220) | 0.74 | 1023 (349) | 935 (265) | 0.38 | 1103 (172) | 1153 (258) | 0.40 | 0.89 |
| ss BR (DG) | −0.78 (1.18) | −0.48 (1.13) | 0.13 | 0.11 (0.70) | 0.18 (0.61) | 0.72 | −0.51 (1.05) | −0.55 (1.15) | 0.85 | 0.20 |
| ss Uric acid | 1.07 (1.95) | 1.23 (1.90) | 0.78 | −0.22 (3.01) | −0.95 (3.72) | 0.35 | 1.15 (2.11) | 1.36 (2.39) | 0.06 | 0.43 |
| Creatinine (mg) | 1815 (488) | 1756 (524) | 0.44 | 1788 (582) | 1545 (389) | 0.17 | 1909 (369) | 1929 (420) | 0.80 | 0.75 |
Abbreviations: AKSB, Agri-King Synbiotic; BR, brushite; CaOx, calcium oxalate; DG, delta Gibbs; SS, supersaturation.
P-value from signed-rank test that shows mean within group change is zero.
P-value from Kruskal–Wallis test of equal change across groups.
No improvements in key urinary parameters were observed in any group before (time zero) or after 4 weeks treatment (final). Values are given as mean (s.d.).
Figure 2.
Changes in urine composition while on AKSB and Oxadrop compared with placebo. Urine oxalate (a) and CaOx SS (b) did not change after 4 weeks on either study drug or placebo. CaOx, calcium oxalate; SS, supersaturation. AKSB, Agri-King Synbiotic.
Stool colony counts for lactobacilli increased among subjects on AKSB and Oxadrop (Table 4). Fecal enterococcal colony counts also increased in those on AKSB. No changes in fecal flora occurred in those on placebo, and baseline colony counts also did not differ between groups. Exact confirmation of the presence or numbers of specific microorganism strains by PCR was not attempted. Among 21 patients with both pre- and post-stool culture data, changes in urinary oxalate level did not significantly correlate with changes in fecal lactobacillus, enterococcal, or yeast colony number, although power to detect this association was limited.
Table 4.
Quantitative stool cultures before and after administration of study drug
| Stool organism (microbes per gram dry stool) | Placebo (n=10)
|
AKSB (n=8)
|
Oxadrop (n=11)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Time zero | Final | P-valuea | Time zero | Final | P-valuea | Time zero | Final | P-valuea | |
| MRS Lactobacilli (×106) | 9.08 (11.03) | 12.30 (13.42) | 0.58 | 2.75 (2.04) | 193.98 (306.94) | 0.02 | 24.09 (41.24) | 53.03 (48.93) | 0.04 |
| KF Enterococci (×106) | 0.29 (0.43) | 1.10 (2.11) | 0.68 | 0.13 (0.26) | 124.66 (287.10) | 0.002 | 13.32 (31.30) | 4.15 (8.22) | 0.28 |
| PDA Yeast (×103) | 0.32 (0.54) | 0.74 (1.46) | 0.89 | NA | 55.10 (91.84) | NA | 34.95 (66.18) | 37.73 (92.12) | 0.77 |
Abbreviations: AKSB, Agri-King Synbiotic; KF, streptococcus medium (supplemented with 2,3,5-triphenyl-2H-tetrazolium chloride for increased selectivity of enterococci); MRS, lactobacilli deMan–Rogosa–Sharpe medium (supplemented with amphotericin B for suppression of fungal contamination); NA, not applicable; PDA, potato dextrose agar (supplemented with tartaric acid for suppression of bacterial contamination).
Comparison between time zero and final.
Values are given as mean (s.d.).
Urinary oxalate (P<0.001, r = 0.38) and calcium (P<0.001, r = 0.36) showed significant positive correlations with CaOx SS, whereas urine volume was negatively correlated (P<0.0001, r = −0.56). Conversely, urinary citrate was not significantly correlated (P = 0.15, r = 0.16). In a multiple regression model (adjusting for diet and repeated readings), urinary CaOx SS was positively associated with oxalate (P<0.001) and calcium (P<0.001) levels, and negatively associated with urine volume (P<0.001) and log citrate levels (P = 0.008) (Table 5). Among these factors, urine volume and calcium had the most significant effects. On the basis of the model slopes (and assuming other predictors remain fixed), an increase from the 25 to 75th percentile in urine volume or citrate would be projected to decrease CaOx SS by 0.57 and 0.068 delta Gibbs (DG), respectively. Similarly, decreases in calcium and oxalate from the 75th to 25th percentile led to decreases of 0.50 and 0.30 DG in CaOx SS, respectively. A regression model predicting change in CaOx SS (from time zero to final) as a function of changes in oxalate, calcium, volume, and citrate resulted in similar significant associations for all four parameters (Table 6).
Table 5.
Multivariate predictors of CaOx SS (Y)a
| Predictor (X) | 25th, 75th Percentile of X | Slope (s.e.) | P-value | Predicted change in CaOx SS (Y) with independent increase in X from 25 to 75th percentile | Predicted change in Y relative to median (1.69), % |
|---|---|---|---|---|---|
| Intercept | — | 2.52 (0.40) | — | — | — |
| Oxalate (mmol/l) | 0.37, 0.52 | 1.99 (0.44) | <0.0001 | 0.30 | 18 |
| Volume (ml) | 1839, 2794 | −0.0006 (0.0001) | <0.0001 | −0.57 | −34 |
| Calcium (mg) | 165, 317 | 0.0033 (0.0004) | <0.0001 | 0.50 | 30 |
| Citrate (log −e, mg) | 6.43, 6.83 | −0.1690 (0.0634) | 0.0077 | −0.068 | −4.0 |
| Post-diet visit (1=yes, 0=no) | — | −0.15 (0.0558) | 0.0074 | — | — |
Abbreviations: CaOx, calcium oxalate; SS, supersaturation.
Multiple regression analysis (generalized estimating equations model) based on 80 readings in 40 patients.
Table 6.
Multivariate predictors of change in CaOx SS from baseline to time zeroa
| Predictor | Slope (s.e.) | P-value |
|---|---|---|
| Intercept | −0.15 (0.09) | — |
| Change in oxalate (mmol/l) | 2.01 (0.48) | 0.0002 |
| Change in urine volume (ml) | −0.00051 (0.00008) | <0.0001 |
| Change in calcium (mg) | 0.0024 (0.0005) | <0.0001 |
| Change in citrate (mg) | −0.00057 (0.00017) | 0.0018 |
Abbreviations: CaOx, calcium oxalate; SS, supersaturation.
Multiple regression analysis based on 40 patients. Positive changes imply an increase.
Two subjects (one on placebo, one on Oxadrop) dropped out within the first week because of demands of the study diet and protocol. The 24-h urine samples for the second visits were lost for one subject (placebo) and there was a protocol error in another (insufficient quantity of AKSB supplied). One patient on Oxadrop reported diarrhea and did not complete the protocol. As urine values for the final visit were not available for five subjects, they were not included in analyses regarding the effect of the study preparations on urine oxalate levels. One patient on placebo reported cramps and mild diarrhea, but did complete the study and was included.
DISCUSSION
Our current study provides evidence that a controlled metabolic diet, low in oxalate and moderate in content of calcium, protein, and sodium, can reduce urinary oxalate excretion and CaOx SS. Therefore, these data strongly support the key role of diet as a determinant of urinary oxalate excretion and CaOx SS. Addition of either of two probiotic combinations (AKSB, Oxadrop) in an effort to enhance intraintestinal oxalate metabolism or decrease oxalate absorption from the gut did not augment the effect of controlled metabolic diet alone. The relative significance of net intestinal oxalate absorption as a determinant of urinary oxalate excretion and the potential for use of agents to modify these processes, remain to be established.
The importance of the urinary composition in the pathogenesis of stone disease is suggested by the observation that urinary SS predicts stone composition 15 and that oxalate concentration is a critical determinant of SS.16 To date, all successful kidney stone treatment trials have employed strategies to directly reduce the urinary excretion of elements that might influence SS, such as thiazide diuretics to decrease urinary calcium,17 allopurinol to decrease urinary uric acid,18 and a diet with increased calcium and reduced protein, sodium, and oxalate content designed to have multiple complementary effects on urinary SS.19 However, no treatment trial has directly shown that reducing urinary oxalate will change stone formation rates, largely because no strategy other than diet is currently available to reduce urinary oxalate excretion.
Mild hyperoxaluria is commonly observed in CaOx stone patients.16 In normal urine, the ratio of calcium to oxalate is usually ≥5:1 (calcium to oxalate). As the stoichiometric relationship between calcium and oxalate in CaOx crystals is 1:1, and as the greatest crystalline mass is produced when they are closest to equimolar concentrations, increases in urinary oxalate should have a greater influence on crystalline mass than increases in calcium.2 Furthermore, in the normal urinary range small changes in oxalate concentration influence CaOx SS, with respect to CaOx far more than changes in calcium concentrations.16 These predictions are supported by in vitro studies performed in urine, as it is easy to initiate CaOx crystallization by addition of oxalate, but difficult to do so by adding excess calcium.20 Our analysis of urine chemistries from stone-forming patients suggested that changes in urinary calcium and oxalate had roughly similar effects on CaOx SS (Tables 5, 6). Therefore, all lines of evidence suggest that changes in urinary oxalate can have important effects on urinary CaOx SS, at least equal to that of the other key analytes, calcium and citrate.
It has been estimated that on a typical Western diet, only ~10% of ingested oxalate is absorbed, and this is thought to constitute ~1/3 of the total oxalate in the urine, the other 2/3 being synthesized by the liver.21 However, these estimates may not be accurate. In an intriguing study, two normal individuals were placed on a rigorously defined, essentially oxalate-free diet, and urinary oxalate excretions fell from normal levels (mean 19.5 mg/day) to very low levels within a week (7 mg/day).22 Urinary oxalate excretion promptly rose after a normal diet was resumed. Therefore, a larger percentage of urinary oxalate may arise from diet than is traditionally assumed. As a consequence, factors that mediate oxalate absorption from food might critically influence urinary oxalate levels, independent of dietary oxalate intake.
Recent studies support the hypothesis that increased gastrointestinal absorption of oxalate could mediate hyperoxaluria in a subgroup of CaOx stone formers. When a standard oxalate load was administered to 60 CaOx stone formers, those with mild hyperoxaluria at baseline (n = 21) responded with a significantly greater peak in urinary oxalate excretion 4 h later.23 Utilizing a standardized protocol to measure intestinal absorption and urinary excretion of [13C2]oxalate, Hesse et al.24 found that, as a group, both male and female CaOx stone formers absorbed a greater percentage of the oxalate load (9.45 versus 6.61% for males and 8.07 versus 6.86% for females; both P<0.01). Multiple older studies also support the hypothesis that CaOx stone formers, as a group, tend to overabsorb oxalate from their diet.25–27 Furthermore, two recent studies suggest that dietary instructions can effectively reduce urinary oxalate levels in a stone clinic setting.28,29
Factors that are known to influence availability of oxalate for absorption include the availability of free calcium and magnesium ions that can complex with oxalate and decrease its rate of absorption, and the presence of free fatty acids in the distal colon, which can form soaps with calcium and thereby increase concentration of free oxalate.30 The presence or absence of colonization with oxalate-degrading bacteria (Oxalobacter formigenes) may also be important.31 As a portion of an oxalate load appears in the urine within hours after ingestion, it is assumed that some can be absorbed in the proximal intestine.32 However, during disease states associated with fat malabsorption and enteric hyperoxaluria, it is clear that a majority of oxalate overabsorption occurs in the colon,33 and is thought to be passive and paracellular.30
In the absence of fat malabsorption, there is evidence that specific energy-dependent transporters might mediate both net oxalate absorption, as well as oxalate secretion.34
The latter might be particularly critical in eliminating excess oxalate when renal function is reduced.35 The relative importance of these mechanisms to intestinal oxalate transport in healthy individuals, compared with mildly hyperoxaluric stone formers, remains unclear.
Generally, as is the case for Oxadrop, probiotics are viable preparations of commensal microorganisms useful for the control or inhibition of pathogens in the digestive tract while promoting the establishment of the normal beneficial microbiota.36–38 The mixture of organisms in Oxadrop were also selected for their ability to degrade oxalate in vitro.13 A prebiotic is a food ingredient that is not digestible and can benefit the patient by aiding the growth of intestinal bacteria.37,38 Synbiotics, such as AKSB, are a combination of prebiotic and a probiotic. This product uniquely contains both bacteria and yeast, whereas most products contain one or the other; the bacteria and yeast are viable, and the fructooligosaccharide provides an active growth substrate that is indigestible by the subject and preferentially used by Enterococcus faecium in AKSB (as opposed to gut pathogens). Although there are no published data in humans supporting the efficacy of AKSB, the preparation SF68, which is widely used in Europe, contains a key component (E. faecium) and appears to decrease rates of antibiotic-associated diarrhea.39 There are no data to suggest that components in AKSB can degrade oxalate, and it was used in this study to determine whether a preparation that has been used to promote overall gastrointestinal health might nonspecifically alter oxalate metabolism or absorption. Oxadrop, on the other hand, also appears to directly metabolize oxalate, at least to a modest degree.13
Despite these potential beneficial effects of the probiotic and synbiotic preparation on oxalate absorption from the gut, we did not demonstrate any effect of either preparation in this study in subjects on a controlled metabolic low-oxalate diet. Although the number of subjects in each group was modest, there was no indication of positive effect in the conditions tested. Therefore, we do not think including more subjects in this exact protocol would have altered results. In a previous study of the same probiotic preparation in patients with enteric hyperoxaluria, we did find a modest reduction in net urinary oxalate excretion.14 However, unlike this study, the previous one was not randomized or placebo-controlled, and the participants had clear evidence for enteric hyperoxaluria. It is unknown whether AKSB or Oxadrop might have had an effect in the current population if the subjects had been studied on a higher oxalate diet, or even a free-choice diet. For example, it is possible that the low-oxalate diet prevented adequate gastrointestinal growth and activity of the oxalate-degrading bacteria in Oxadrop, as has been observed in animal studies with O. formigenes.40 However, a previous small crossover study of Oxadrop in patients with idiopathic CaOx stones and mild hyperoxaluria on a free- choice diet also failed to demonstrate an effect.41 It is also possible that the relatively short period of time on the controlled diet altered the equilibrium of the gastrointestinal tract flora, and that longer-term studies might demonstrate a more dramatic effect.
This study supplements evidence in the literature that diet is an effective tool to reduce urinary oxalate excretion Furthermore, analysis of the data confirms that urinary oxalate intake is an independent determinant of urinary CaOx excretion. The strength of this protocol was the use of exactly defined diets prepared in a Clinical Research Unit. Although one could question the practicality of advocating a low-oxalate diet for long-term management, two recent studies in an outpatient stone clinic setting confirmed that effective dietary counseling can reduce urinary oxalate excretion.28,29 A third study demonstrated successful compliance with a low-sodium diet that secondarily reduced urinary calcium.42 Therefore, amongst idiopathic CaOx stone formers, efforts should be directed toward improved strategies to educate patients in order to improve compliance with a stone prevention diet that includes low sodium, normal calcium, reduced oxalate, and increased fluid intake. Furthermore, a controlled trial to demonstrate the effect of such a diet on stone formation rates seems warranted.
METHODS
This double-blind, placebo-controlled study was approved by the Mayo Clinic Institutional Review Board and registered with the United States National Library of Medicine (NCT 00587041; www.clinicaltrials.gov).
Oxadrop
Each gram of the mix (Oxadrop) contains 2 × 1011 bacteria (Lactobacillus acidophilus, L. brevis, Streptococcus thermophilus, and Bifidobacterium infantis). The different strains are mixed in a 1:1:4:4 weight and prepared as a granulate. The organisms were chosen on the basis of their ability to degrade oxalate in vitro, and Oxadrop is different than the more widely studied VSL#3,43 which contains three of the above four bacterial species with the exception of L. brevis, as well as three other species of lactobacillus and two other species of bifidobacteria. In clinical trials performed with Oxadrop, there have been no adverse events noted, and no case of clinical infection has been traced to ingested probiotic lactic acid bacteria in a normal host.44
AKSB
AKSB ingredients were mixed into powder and provided to Mayo clinic, where it was encapsulated by the Mayo Research Pharmacy. Each AKSB capsule contains the following active ingredients (subjects were given two AKSB capsules per day for a total of 1010 organisms): (1) Fructooligosaccharide (115 mg), manufactured as Ultra-FOS ST by Encore Technologies, Minnetonka, MN, as food-grade quality and is a prebiotic component of AKSB; (2) E. faecium (E. faecium SF68; 4.5 billion) produced by Cerbios-Pharma SA (Barbengo, Switzerland); (3) Saccharomyces cerevisiae subsp. boulardi (300 million), a yeast produced as LEVUCELL SB by Lallemand Biochem International, Ontario, Canada, as ‘food-grade’ quality; and (4) S. cerevisiae (200 million), a food-grade yeast produced as active dry yeast by SAF (USA) Corporation in Milwaukee, WI. AKSB was developed by Agri-King (Fulton, IL, USA), with the primary aim of improving gut performance in animals so that the routine use of antibiotics in animal feeds could be reduced or eliminated. Studies by Agri-King. have confirmed that the preparation improves intestinal health and reduces the risk of illness when animals fed with the preparation are challenged with food- or water-borne pathogens, and improves overall animal growth rates.
Study population
Subjects were identified as those who had CaOx nephrolithiasis and mild hyperoxaluria of unknown etiology (>0.35 mmol/l/day) between May 2006 and May 2009. Stone disease was defined as the presence of radio-opaque stones on X-ray, or a history consistent with passage of a stone, or stone surgery, or extracorporeal shock wave lithotripsy in the last 5 years. Stone composition could be confirmed either by stone analysis demonstrating composition ≥50% CaOx or by radiographic demonstration of a calcific renal stone in the presence of hyperoxaluria. Inclusion criteria allowed subjects taking drugs for the prevention of stone disease, including pyridoxine, thiazides, citrate supplements, and allopurinol, as long as there was no change in these medications for at least three previous months and during the study, and hyperoxaluria was present. Study subjects were selected from among those who carried out 24-h collections for routine clinical purposes in the Mayo renal stone clinic. Subjects were excluded if they were on immunosuppressive medications (excluding small stable doses of prednisone of ≤10 mg), had human immunodeficiency virus infection, known enteric bacterial infection, or a history of splenectomy; had a current malignancy other than superficial skin cancers that had been excised or were felt to be in complete remission (>5 years); had previous colectomy; or had completed a course of oral or parenteral antibiotics <2 weeks before initiation of the study. Subjects who required a course of antibiotics during the period of preparation administration were withdrawn from the study and excluded from the final analysis. Subjects were also told to avoid probiotic preparations or dairy products containing live cultures.
Study design
Baseline urine collection was the most recent 24-h urine study available that was used to qualify subjects for the study, and was obtained on free-choice diet as per clinical routine (Figure 3). Eligible subjects were then placed on a constant metabolic diet to eliminate oxalate intake as a variable. The diet contained reduced oxalate (80–100 mg), normal calcium (1000 mg), adequate protein (15% of total calories), low fat (25% of total calories), vitamin C (100–150 mg), and moderate sodium (120–150 mEq). Calorie intake was calculated to avoid weight loss. Controlled metabolic diet contents were calculated using ProNutra nutritional analysis software (Viocare Technologies, Princeton, NJ, USA, Version 3.1.0.13., Copyright(C) 2002). Diets were individualized to allow for food allergies and intolerances, but remained constant for oxalate-containing foods, which were batched for each subject to minimize variation in oxalate content for the duration of study. All meals were prepared in the Mayo Clinic Clinical Research Unit. Subjects received the morning meal in the clinical research unit, and were given the noon and dinner meal to take home. All calcium supplements were held for this period. A 4-day run-in period on the controlled metabolic diet is required to achieve steady-state status in relation to calcium balance and sodium balance.45 After 4 days on the controlled metabolic diet, two initial 24-h urines were collected on consecutive days (days 5–6; time zero urine collections). Subjects then took of the following regimens: (1) the study preparation (Oxadrop one packet q.d. plus one placebo capsule b.i.d.; (2) AKSB one capsule b.i.d. plus one placebo packet q.d.; (3) one placebo packet q.d. and one placebo capsule b.i.d.). Subjects were instructed to avoid high-oxalate foods and live culture dairy products (for example, yogurt) between controlled metabolic diet phases. At the end of the fourth week, the subjects were placed on the identical controlled metabolic diet. After 4 days, two repeat 24-h urines were collected (days 5–6 back on the controlled metabolic diet; final urine collections). Final urine oxalate values were compared with the time zero numbers.
Figure 3.
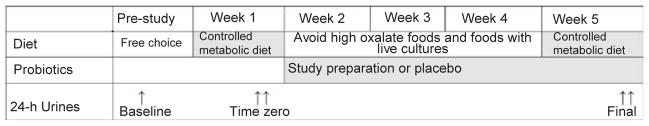
Study design. Baseline urine collections to qualify for the study were collected on a free-choice diet. Patients were placed on a controlled metabolic diet on the first and last week of the study. Two time zero 24-h urine samples were collected on days 5 and 6 of the first week on the controlled metabolic diet (time zero urines) before study preparation was initiated. The probiotic or placebo was then administered for 4 weeks (weeks 2–5). Patients were next placed back on the controlled metabolic diet for the final week of the study (week 5), with two more final urine sample collections on days 5 and 6 of that week (final urines).
Urine chemistries
Urinary concentrations (24 h) of oxalate, calcium, and other determinants of SS were measured in the Mayo Clinic Renal Testing Laboratory. Urine oxalate was measured by oxalate oxidase. SS was calculated using the EQUIL2 program.46
Stool cultures
When possible, stool samples were collected at the time of urine collections during weeks 1 and 5 of the study (successful in 33 of 44 subjects). Quantitative stool cultures for lactobacilli, enterococci, and yeast were performed by technicians at Agri-King, and were performed blinded to the study identifiers.
Statistics
Differences between baseline and time zero urine values were evaluated by the paired t-test and signed-rank tests. Treatment group differences (AKSB, Oxadrop, and Placebo) were compared using the Kruskal–Wallis test. Bivariate Spearman’s rank correlation was used to test the linear association between urinary CaOx SS and the urinary composition (urinary oxalate, volume, calcium, and citrate) after pooling all readings (baseline and time zero). Multiple linear regression analysis was used to identify independent predictors of CaOx SS, with generalized estimating equations models employed to adjust for potential lack of independence (using an exchangeable correlation structure). The primary outcomes were changes in urinary oxalate and CaOx SS between the time zero and final urine collections. All reported P-values were two-sided, and P<0.05 was considered statistically significant. With a minimum of 14 persons per group, we had 80% power to detect a change in urine oxalate of 0.11 mmol/l/day and in CaOx SS of 0.66 DG. Statistical analyses were performed using SAS software, version 9.1 (SAS Institute, www.sas.com).
Acknowledgments
This work was supported by AT R21AT2534, the Mayo Clinic O’Brien Urology Research Center P50 DK083007, the Rare Kidney Stone Consortium U54 DK082908, and the Mayo Clinic Center for Translational Science Activities, all funded by the National Institutes of Health. We are thankful to Dave Spangler (Agri-King) for completing quantitative stool cultures and to VSL and Agri-King for supplying the study preparations used in this protocol. We are also thankful to Thomas Witzig for his support during the design and interpretation of this study.
Footnotes
DISCLOSURE
Agri-King performed quantitative stool cultures for the study, and VSL and Agri-King supplied the study preparations used in this protocol. One of the authors (CDS) has a financial interest in VSL.
References
- 1.Unwin R, Wrong O, Cohen E, et al. Unraveling of the molecular mechanisms of kidney stones. Lancet. 1996;348:1561–1565. [PubMed] [Google Scholar]
- 2.Smith LH. Diet and hyperoxaluria in the syndrome of idiopathic calcium oxalate urolithiasis. Am J Kidney Dis. 1994;17:370–375. doi: 10.1016/s0272-6386(12)80625-1. [DOI] [PubMed] [Google Scholar]
- 3.Lieske JC, Spargo B, Toback FG. Endocytosis of calcium oxalate crystals and proliferation of renal tubular epithelial cells in a patient with type 1 primary hyperoxaluria. J Urol. 1992;148:1517–1519. doi: 10.1016/s0022-5347(17)36954-9. [DOI] [PubMed] [Google Scholar]
- 4.Mandell I, Krauss E, Millan JC. Oxalate-induced acute renal failure in Crohn’s disease. Am J Med. 1980;69:628–632. doi: 10.1016/0002-9343(80)90479-9. [DOI] [PubMed] [Google Scholar]
- 5.Gelbart DR, Brewer LL, Fajardo LF, et al. Oxalosis and chronic renal failure after intestinal bypass. Arch Intern Med. 1977;137:239–243. [PubMed] [Google Scholar]
- 6.Canos HJ, Hogg GA, Jeffery JR. Oxalate nephropathy due to gastrointestinal disorders. Can Med Assoc J. 1981;124:729–733. [PMC free article] [PubMed] [Google Scholar]
- 7.Parivar F, Low RK, Stoller ML. The influence of diet on urinary stone disease. J Urol. 1996;155:432–440. [PubMed] [Google Scholar]
- 8.Stauffer JQ. Hyperoxaluria and intestinal disease. The role of steatorrhea and dietary calcium in regulating intestinal oxalate absorption. Am J Dig Dis. 1977;22:921–928. doi: 10.1007/BF01076170. [DOI] [PubMed] [Google Scholar]
- 9.Andersson H, Bosaeus I. Hyperoxaluria in malabsorptive states. Urol Int. 1981;36:1–9. doi: 10.1159/000280387. [DOI] [PubMed] [Google Scholar]
- 10.Hylander E, Jarnum S, Nielsen K. Calcium treatment of enteric hyperoxaluria after jejunoileal bypass for morbid obesity. Scand J Gastroenterol. 1980;15:349–352. doi: 10.3109/00365528009181482. [DOI] [PubMed] [Google Scholar]
- 11.McLeod RS, Churchill DN. Urolithiasis complicating inflammatory bowel disease. J Urol. 1992;148:974–978. doi: 10.1016/s0022-5347(17)36794-0. [DOI] [PubMed] [Google Scholar]
- 12.Argenzio RA, Liacos JA, Allison MJ. Intestinal oxalate-degrading bacteria reduce oxalate absorption and toxicity in guinea pigs. J Nutr. 1988;118:787–792. doi: 10.1093/jn/118.6.787. [DOI] [PubMed] [Google Scholar]
- 13.Campieri C, Campieri M, Bertuzzi V, et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int. 2001;60:1097–1105. doi: 10.1046/j.1523-1755.2001.0600031097.x. [DOI] [PubMed] [Google Scholar]
- 14.Lieske JC, Goldfarb DS, De Simone C, et al. Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int. 2005;68:1244–1249. doi: 10.1111/j.1523-1755.2005.00520.x. [DOI] [PubMed] [Google Scholar]
- 15.Parks JH, Coward M, Coe FL. Correspondence between stone composition and urine supersaturation in nephrolithiasis. Kidney Int. 1997;51:894–900. doi: 10.1038/ki.1997.126. [DOI] [PubMed] [Google Scholar]
- 16.Robertson WG, Hughes H. Importance of mild hyperoxaluria in the pathogenesis of urolithiasis – new evidence from studies in the Arabian peninsula. Scanning Microsc. 1993;7:391–401. [PubMed] [Google Scholar]
- 17.Ettinger B, Citron JT, Livermore B, et al. Chlorthalidone reduces calcium oxalate calculous recurrence but magnesium hydroxide does not. J Urol. 1988;139:679–684. doi: 10.1016/s0022-5347(17)42599-7. [DOI] [PubMed] [Google Scholar]
- 18.Ettinger B, Tang A, Citron JT, et al. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med. 1986;315:1386–1389. doi: 10.1056/NEJM198611273152204. [DOI] [PubMed] [Google Scholar]
- 19.Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 20.Robertson WG, Peacock M, Nordin BEC. Activity products in stone- forming and non-stone-forming urine. Clin Sci. 1968;34:579–594. [PubMed] [Google Scholar]
- 21.Nemeh MN, Weinman EJ, Kayne LH, et al. Absorption and excretion of urate, oxalate, and amino acids. In: Coe FC, Favus MJ, Pak CYC, Parks JH, Preminger GM, editors. Kidney Stones: Medical and Surgical Management. Lippincott-Raven; Philadelphia: 1996. pp. 303–322. [Google Scholar]
- 22.Holmes RO, Goodman DG, Assimos DG. Dietary oxalate and its intestinal absorption. Scanning Microsc. 1995;9:1109–1120. [PubMed] [Google Scholar]
- 23.Krishnamurthy MS, Hruska KA, Chandhoke PS. The urinary response to an oral oxalate load in recurrent calcium stone formers. J Urol. 2003;169:2030–2033. doi: 10.1097/01.ju.0000062527.37579.49. [DOI] [PubMed] [Google Scholar]
- 24.Hesse A, Schneeberger W, Engfeld S, et al. Intestinal hyperabsorption in calcium oxlate stone formers: application of a new test with [13C2]oxalate. J Am Soc Nephrol. 1999;10(Suppl 14):S329–S333. [PubMed] [Google Scholar]
- 25.Marangella M, Frutero B, Bruno M, et al. Hyperoxaluria in idiopathic calcium stone disease: further evidence of intestinal hyperabsorption of oxalate. Clin Sci. 1982;63:381–385. doi: 10.1042/cs0630381. [DOI] [PubMed] [Google Scholar]
- 26.Lindsjo M, Bo G, Fellstrom B, et al. Intestinal oxalate and calcium absorption in recurrent renal stone formers and healthy subjects. Scan J Urol Nephrol. 1989;23:55–59. doi: 10.1080/00365599.1989.11690431. [DOI] [PubMed] [Google Scholar]
- 27.Schwille PO, Hanisch E, Scholz D. Postprandial hyperoxaluria and intestinal oxalate absorption in idiopathic renal stone disease. J Urol. 1984;132:650–655. doi: 10.1016/s0022-5347(17)49808-9. [DOI] [PubMed] [Google Scholar]
- 28.Penniston KL, Nakada SY. Effect of dietary changes on urinary oxalate excretion and calcium oxalate supersaturation in patients with hyperoxaluric stone formation. Urology. 2009;73:484–489. doi: 10.1016/j.urology.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 29.Nouvenne A, Meschi T, Guerra A, et al. Diet to reduce mild hyperoxaluria in patients with idiopathic calcium oxalate stone formation: a pilot study. Urology. 2009;73:725–730. 730 e1. doi: 10.1016/j.urology.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Williams A, Wilson DM. Dietary intake, absorption, metabolism, and excretion of oxalate. Semin Nephrol. 1990;10:2–8. [PubMed] [Google Scholar]
- 31.Sidhu H, Schmidt ME, Cornelius JG, et al. Direct correlation between hyperoxaluria/oxalate stone disease and the absence of the gastrointestinal tract-dwelling bacterium Oxalobacter formigenes: possible prevention by gut recolonization or enzyme replacement therapy. J Am Soc Nephrol. 1999;10:S334–S340. [PubMed] [Google Scholar]
- 32.Asplin JR. Hyperoxaluric calcium nephrolithiasis. Endocrinol Metab Clin N Am. 2002;31:927–949. doi: 10.1016/s0889-8529(02)00030-0. [DOI] [PubMed] [Google Scholar]
- 33.Modigliani R, Labayle D, Aymes C, et al. Evidence for excessive absorption of oxalate by the colon in enteric hyperoxaluria. Scand J Gastroent. 1978;13:187–192. doi: 10.3109/00365527809181746. [DOI] [PubMed] [Google Scholar]
- 34.Hatch M, Freel RW, Vaziri ND. Regulatory aspects of oxalate secretion in enteric oxalate elimination. J Am Soc Nephrol. 1999;10:S324–S328. [PubMed] [Google Scholar]
- 35.Hatch M, Freel RW, Vaziri ND. Intestinal excretion of oxalate in chronic renal failure. J Am Soc Nephrol. 1994;5:1339–1343. doi: 10.1681/ASN.V561339. [DOI] [PubMed] [Google Scholar]
- 36.Duffy LC, Leavens A, Griffiths E, et al. Perspectives on Bifidobacteria as biotherapeutic agents in gastrointestinal health. Dig Dis Sci. 1999;44:1499–1505. doi: 10.1023/a:1026632704628. [DOI] [PubMed] [Google Scholar]
- 37.Collins MD, Gibson GR. Probiotics, prebiotics, and symbiotics: approaches for modulating the microbial etiology of the gut. Am J Clin Nutr. 1999;69:1052S–1057S. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- 38.Gibson GR. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J Nutr. 1999;129:1438S–1441S. doi: 10.1093/jn/129.7.1438S. [DOI] [PubMed] [Google Scholar]
- 39.Wunderlich PF, Braun L, Fumagali I, et al. Double-blind report on the efficacy of lactic acid-producing Entercoccus SF68 in the prevention of antibiotic-associated diarrhea and in the treatment of acute diarrhea. J Int Med Res. 1989;17:333–338. doi: 10.1177/030006058901700405. [DOI] [PubMed] [Google Scholar]
- 40.Goldfarb DS. Microorganisms and calcium oxalate stone disease. Nephron Physiol. 2004;98:48–54. doi: 10.1159/000080264. [DOI] [PubMed] [Google Scholar]
- 41.Goldfarb DS, Modersitzki F, Asplin JR. A randomized, controlled trial of lactic acid bacteria for idiopathic hyperoxaluria. Clin J Am Soc Nephrol. 2007;2:745–749. doi: 10.2215/CJN.00600207. [DOI] [PubMed] [Google Scholar]
- 42.Nouvenne A, Meschi T, Prati B, et al. Effects of a low-salt diet on idiopathic hypercalciuria in calcium-oxalate stone formers: a 3-mo randomized controlled trial. Am J Clin Nutr. 2010;91:565–570. doi: 10.3945/ajcn.2009.28614. [DOI] [PubMed] [Google Scholar]
- 43.Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double- blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 44.Salminen S, von Wright A, Morelli L, et al. Demonstration of safety of probiotics – a review. Int J Food Microbiol. 1998;44:93–106. doi: 10.1016/s0168-1605(98)00128-7. [DOI] [PubMed] [Google Scholar]
- 45.Brinkley L, Pak CYC. The metabolic balance regimen and nutritional aspects of clinical research. In: Pak CYC, Adams PM, editors. Techniques of Patient-Oriented Research. Raven Press; New York: 1994. pp. 143–148. [Google Scholar]
- 46.Werness PJ, Brown CM, Smith LH, et al. EQUIL2: a BASIC computer program for the calculation of urinary saturation. J Urol. 1985;134:1242–1244. doi: 10.1016/s0022-5347(17)47703-2. [DOI] [PubMed] [Google Scholar]