Abstract
Evidence is accumulating that skin can act as an independent steroidogenic organ. It can respond to various stresses including UV light, trauma and oncogenesis by upregulating glucocorticoid production via elements of the local hypothalamic-pituitary-adrenal (HPA) axis. Recent data by Takei and collaborators provided in this issue of Experimental Dermatology included dryness to the list of stressors stimulating cutaneous cortisol synthesis with a possible involvement of IL-1β as a mediator of this regulation. Thus, the last decade of research has not only documented that skin can produce cortisol, but that levels of its production change in response to environmental stress. The role of this regulated steroidogenic system in physiological or pathological outcomes requires further studies with focus on cutaneous homeostasis, formation of epidermal barrier, antimicrobial activity and display of immune (both pro- and anti-inflammatory) properties.
Keywords: Cortisol, epidermal barrier, dryness, stress, cytokines
1. Dry environment and cortisol production
Recently Takei et al (1), using organotypic culture system, have demonstrated that human skin exposed to dry environment can increase the production and release of cortisol by as much as three folds in comparison to the control skin. Steroid 11β-hydroxylase (CYP11B1), the enzyme responsible for the conversion of 11-deoxycortisol to cortisol, and its corresponding mRNA levels were also shown to be dramatically increased in the same experiment. Gene expression of IL-1β, a cytokine involved in regulation of the epidermal barrier (2), also increased by six times. These changes have been partially (but significantly) reduced by isolating the skin via a water-impermeable plastic membrane. The authors further show that cortisol levels could increase to the same level as that of dry exposure when IL-1β was added to the culture media under a humid condition. They propose that IL-1β may be an important cytokine stimulating glucocorticosteroidogenesis in response to the stress of a dry environment.
These results have confirmed previous observations made by many investigators that skin can function as an independent steroidogenic organ with the ability to produce cortisol in response to environmental stress [reviewed in (3, 4)].
2. Steroidogenic activity of the skin
Studies over the last two decades have well established the notion that skin is a bona fide endocrine organ of steroidogenesis, capable of sensing and counteracting environmental stressors [reviewed in (5)]. Human skin cells under various conditions, are able to produce critical corticosteroids including deoxycorticosterone (DOC), 18(OH)DOC, corticosterone and cortisol as it has been shown for the first time by Slominski and Paus groups (6–10). Human skin is also geared with all the enzymes, receptors and transport proteins necessary for the production of these corticosteroids such as CYP11A1, 3βHSD, CYP11B1, CYP17, CYP21A2, MC2, StAR and StAR related proteins as it has been shown for the first time in (11) and further confirmed by many investigators [reviewed in (3, 12)]. This glucocorticoids synthesis, including cortisol, in the skin can proceed either from cholesterol (13, 14) or from progesterone of systemic origin, or through transformation of cortisone to cortisol [reviewed in (12)]. The expression of CYP11A1 have been shown not only in normal melanocytes, keratinocytes and fibroblasts but also in number of tumor cells of non-cutaneous and cutaneous origin including melanomas (3, 14–17). Although expression of CYP17A, CYP21A2, CYP11B1, and of HSD11B1 have already been well documented in normal human skin and skin cells [reviewed in (3, 12)], we tested their expression in an extended panel of melanomas and other cancer cells (Table 1). Surprisingly, these genes are expressed at considerable levels in all melanoma lines tested (3, 12, 13, 16) as well as in selected breast carcinomas, oral squamous cell carcinomas, osteosarcomas, gliomas and leukemia cells (Table 1). This suggests that melanoma and other tumors can utilize glucocorticosteroids to modify their local environment facilitating a tumor survival in the host. Interestingly, HSD11B1 has also been expressed in all tested human neonatal and adult epidermal keratinocytes, melanocytes and dermal fibroblasts, with expression significantly higher in intact skin and adipose tissue, with the latter expression level similar to the adrenal gland (see legend to the Table 1).
Table 1.
Expression of steroidogenic genes in cell lines of melanoma (MM), breast carcinoma (BC), oral squamous cell carcinoma (OSCC), leukemia, osteosarcoma, glioma and human embryonic kidney cell line (HEK293).
| Cell line | CYP11B1 | CYP17A1 | CYP21A2 | HSD11B1 |
|---|---|---|---|---|
| MM: YUWERA | 13.55± 0.28 | 17.34±0.0001 | 11.53±0.32 | 20.92±0.08 |
| MM: YUTICA | 16.1± 0.085 | 17.27± 0.32 | 14.69±0.395 | 23.16±0.075 |
| MM: YUKSI | 16.04± 0.09 | 18.65± 0.27 | 13.94±0.176 | 22.72±0.24 |
| MM: YULAC | 3.75±0.47 | 4.48±0.52 | 12.85±0.3629 | 23.21±0.06 |
| MM: YUSIV | 14.62± 0.236 | 16.86± 0.0001 | 12.46±0.1997 | 19.7±0.205 |
| MM: YUAME | 6.06± 0.1 | nd | 4.92±0.13 | 17.29±0.51 |
| MM: YUROB | 3.08±0.47 | 5.46±0.23 | 1.8±0.245 | 21.87.±0.48 |
| MM: YUCOT | 14.24± 0.1 | 9.36± 0.34 | 12.59±0.2569 | 15.17±0.226 |
| MM: YUKIM | 7.08± 0.09 | 9.3± 0.38 | 4.88±0.017 | 15.17±0.254 |
| MM: YUMUT | 2.81± 0.275 | 12.75± 0.266 | 0.067±0.2879 | 17.17±0.16 |
| MM: YUKOLI | 17.19± 0.208 | 12.72± 0.275 | 15.08±0.383 | 12.5±0.083 |
| MM: WM98D | 3.08±0.23 | 3.08±0.23 | 13.94±0.0001 | 1.00±0.25 |
| MM: WM164 | 17.57±0.20 | 15.45±0.15 | 5.24±0.48 | 6.48±0.11 |
| MM: WM1341 | 11.01±0.19 | 13.93±0.09 | 4.47±0.28 | 17.54±0.17 |
| Leukemia: K562 | 2.99±0.01 | 0.15±0.28 | −2.06±0.09 | 1.83±0.14 |
| Leukemia: U937 | 1.96±0.32 | −1.27±0.26 | nd | 3.25±0.49 |
| Leukemia: Jurkat | 3.08±0.23 | −0.74±0.18 | −7.59±0.31 | 4.98±0.43 |
| Leukemia: HL-60 | 14.28±0.46 | 8.2±0.29 | 16.75±0.29 | 16.75±0.29 |
| Osteosarcoma: MG63 | 12.03±0.45 | −0.97±0.36 | 14.25±0.25 | 2.06±0.25 |
| Glioma: U87 | 16.75±0.07 | 1.22±0.07 | 16.75±0.07 | 10.64±0.08 |
| BC: MCF7 | 15.23±0.39 | 8.82±0.39 | 15.23±0.39 | 5.65±11.77 |
| BC: MDA-MB 453 | 14.84±0.42 | 1.57±0.46 | 4.86±10.08 | 12.38±0.31 |
| BC: MDA-MB 231 | 16.26±0.30 | 16.64±0.40 | −1.46±0.48 | 14.81±0.13 |
| OSCC: SCC15 | 14.78±0.39 | 8.40±0.31 | 15.64±0.03 | 8.44±0.32 |
| OSCC: SCC25 | 16.70±0.37 | 17.52±0.36 | 4.98±0.24 | 12.43±0.26 |
| HEK293 | 15.83±0.15 | 11.72±0.15 | 17.81±0.13 | 17.81±0.13 |
nd: not done. The lower the number the higher is relative gene expression.
RNA isolation, reverse transcription and real-time PCR were performed as described previously (16). Reactions were performed at 95°C for 5min and then 50 cycles (95°C for 10sec, 60°C for 30 sec and 72°C for 30 sec). Data was collected on a Roche Light Cycler 480. The amounts were compared to reference gene (Cyclophilin B) using a comparative CT method. Relative gene expression data are calculated using ΔΔCt method. Changes in gene expression are presented as a relative quantities using mean ΔCt (normalized target) as a difference between target gene and reference gene in cycle of appearance in time (C). Sequences of the primers for CYP17A1, CYP21A2, CYP11B1 and 11HSDB1are are in (3), while sequences for cyclophilin are: left: TGTGGTGTTTGGCAAAGTTC and right: GTTTATCCCGGCTGTCTGTC. Data are presented as means ± SD (n=3).
In addition, expression of HSD11B1 in normal human skin cells in comparison to comparison to control tissues was as follows: neonatal epidermal melanocytes (11.71±0.17); adult epidermal melanocytes (14.51±0.11); neonatal epidermal keratinocytes (14.21±0.18); adult epidermal keratinocytes (13.01±0.05); neonatal dermal fibroblasts (9.82±0.12); HaCaT keratinocytes (22.51±0.24); skin from white patient (6.38±0.28); subcutaneous adipose tissue (8.36±0.3); adrenal gland (9±0.09).
3. Hypothalamic–pituitary–adrenal (HPA) axis in the skin
Since Takei et al (1) have mentioned cutaneous HPA axis without a proper context, it is important to note that skin is also subjected to HPA regulation through central and local mechanisms [reviewed in (18)]. Specifically, corticotropin releasing hormone (CRH) and related peptides as well as the proopiomelanocortin-derived peptides, including ACTH and their corresponding receptors are expressed by skin [reviewed in (18, 19)]. Functional studies further demonstrated the critical roles of these hormones in regulating skin homeostasis [reviewed in (5, 18, 19)]. For example, application of CRH and ACTH would stimulate the production of cortisol by hair follicle and follicular keratinocytes (10) and by cultured melanocytes (8). The production of cortisol is dependent on the CRH receptor type 1 (CRH-R1) (8, 10), which is similar to other skin compartments (5, 18, 19). It must be noted, that IL-1 is a recognized positive regulator of CRH and POMC expression [reviewed in (18)]. Thus, further studies on the communication between cutaneous cytokines and different elements of the HPA axis are warranted to understand the mechanisms regulating epidermal responses to stress including restoration of epidermal barrier functions.
4. Skin stress response system and cortisol
The current study by Takei et al (1) not only confirmed previous observations that skin could generate cortisol locally (6, 8–10) but also added dryness (Fig. 1) to the list of environmental stressors such as solar radiation (20–22), trauma (23), and skin cancer (12). The next step to be tested is cutaneous HPA-like response to pathogens. It is well known that the immune system of skin needs to be well balanced to defend against pathogens invasion but also to not overreact to them. Both hypersensitivity or insufficiency of skin immune function results in skin pathology such as acne vulgaris, psoriasis, atopic dermatitis, lupus erythematous and many other autoimmune diseases. Although previous investigations had centered on the roles of cytokines and immune cells, the contribution of local steroids to various skin diseases is also emerging. For instance, locally produced glucocorticoids can counteract the proinflammatory activity caused by cytokines, CRH and related peptides (4, 18, 24) and deficiency in local glucocorticoid production or its receptor function may at least partially account for the development of atopic dermatitis, psoriasis and alopecia areata (25–28). The present study shows that upregulation of local cortisol production is mediated by IL-1β (1), which is consistent with previous hypothesis that inflammatory cytokines are potentially important regulators of local production of cortisol [reviewed in (5)].
Figure 1.
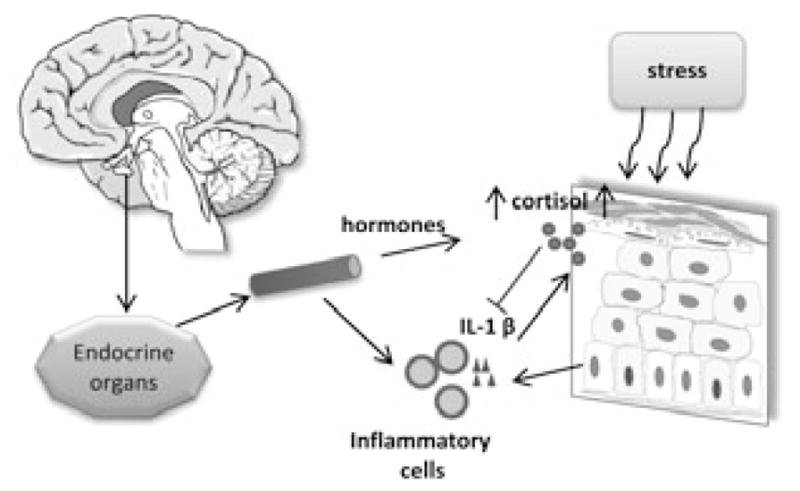
Skin senses the environmental changes (including dryness) and increases cortisol production possibly through IL-1β mediated inflammatory reaction. This response is under the regulation of HPA axis and might feedback to a systemic level.
5. Concluding remarks
The current study by Takei et al (1) added dryness to the list of stressors such as UV light, trauma and oncogenesis that may result in the upregulation of local cortisol production (Fig. 1). The possible mechanism of this regulation could include different elements of the HPA axis or IL-1β as previously proposed (4, 5). Thus, regulation of local steroidogeneic systems can play a role in skin physiology or in pathological outcomes.
Acknowledgments
This commentary was supported in part by grants from National Science Foundation (# IOS-0918934) and National Institutes of Health (#1R01AR056666-01A2; # 2R01AR052190-06A1) to AS, and dermatopathology fellowship directed by Dr. Slominski. We thank Dr. Diane Kovacic, dermatopathology fellow, for editing English. Drs. Zhu Guo, Zorica Janjetovic and Andrzej Slominski analyzed the data and wrote the paper.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Takei K, Denda S, Kumamoto J, Denda M. Low environmental humidity induces synthesis and release of cortisol in an epidermal organotypic culture system. Exp Dermatol. 2013;22:662–664. doi: 10.1111/exd.12224. [DOI] [PubMed] [Google Scholar]
- 2.Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slominski A, Zbytek B, Nikolakis G, et al. Steroidogenesis in the skin: Implications for local immune functions. J Steroid Biochem Mol Biol. 2013;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265–266:143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Metabolism of progesterone to DOC, corticosterone and 18OHDOC in cultured human melanoma cells. FEBS Lett. 1999;455:364–366. doi: 10.1016/s0014-5793(99)00889-3. [DOI] [PubMed] [Google Scholar]
- 8.Slominski A, Zbytek B, Szczesniewski A, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288:E701–706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 9.Slominski A, Zbytek B, Szczesniewski A, Wortsman J. Cultured human dermal fibroblasts do produce cortisol. J Invest Dermatol. 2006;126:1177–1178. doi: 10.1038/sj.jid.5700204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 11.Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81:2746–2749. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 12.Slominski AT, Zmijewski MA, Semak I, et al. Cytochromes P450 and Skin Cancer: Role of Local Endocrine Pathways. Anticancer Agents Med Chem. 2013 doi: 10.2174/18715206113139990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slominski A, Zjawiony J, Wortsman J, et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slominski AT, Kim TK, Chen J, et al. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol. 2012;44:2003–2018. doi: 10.1016/j.biocel.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slominski AT, Kim T-K, Shehabi HZ, et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–3915. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slominski AT, Janjetovic Z, Kim TK, et al. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012;32:3733–3742. [PMC free article] [PubMed] [Google Scholar]
- 17.Slominski AT, Zmijewski MA, Semak I, et al. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PloS one. 2009;4:e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key Role of CRF in the Skin Stress Response System. Endocr Rev. 2013;34:827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 20.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301:E484–493. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. The British journal of dermatology. 2013;168:595–601. doi: 10.1111/bjd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skobowiat C, Nejati R, Lu L, Williams RW, Slominski AT. Genetic variation of the cutaneous HPA axis: An analysis of UVB-induced differential responses. Gene. 2013 doi: 10.1016/j.gene.2013.08.035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vukelic S, Stojadinovic O, Pastar I, et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286:10265–10275. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slominski A. On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. The British journal of dermatology. 2009;160:229–232. doi: 10.1111/j.1365-2133.2008.08958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sevilla LM, Latorre V, Sanchis A, Perez P. Epidermal inactivation of the glucocorticoid receptor triggers skin barrier defects and cutaneous inflammation. J Invest Dermatol. 2013;133:361–370. doi: 10.1038/jid.2012.281. [DOI] [PubMed] [Google Scholar]
- 26.Miljkovic Z, Momcilovic M, Miljkovic D, Mostarica-Stojkovic M. Methylprednisolone inhibits IFN-gamma and IL-17 expression and production by cells infiltrating central nervous system in experimental autoimmune encephalomyelitis. J Neuroinflammation. 2009;6:37. doi: 10.1186/1742-2094-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiala I, Suomela S, Huuhtanen J, et al. The CCHCR1 (HCR) gene is relevant for skin steroidogenesis and downregulated in cultured psoriatic keratinocytes. J Mol Med (Berl) 2007;85:589–601. doi: 10.1007/s00109-006-0155-0. [DOI] [PubMed] [Google Scholar]
- 28.Hannen RF, Michael AE, Jaulim A, Bhogal R, Burrin JM, Philpott MP. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem Biophys Res Commun. 2011;404:62–67. doi: 10.1016/j.bbrc.2010.11.059. [DOI] [PubMed] [Google Scholar]


