Abstract
Background
There are three isocitrate dehydrogenases (IDHs) in the pancreatic insulin cell; IDH1 (cytosolic) and IDH2 (mitochondrial) use NADP(H). IDH3 is mitochondrial, uses NAD(H) and was believed to be the IDH that supports the citric acid cycle.
Methods
With shRNAs targeting mRNAs for these enzymes we generated cell lines from INS-1 832/13 cells with severe (80%–90%) knockdown of the mitochondrial IDHs separately and together in the same cell line.
Results
With knockdown of both mitochondrial IDH’s mRNA, enzyme activity and protein level, but not with knockdown of one mitochondrial IDH, glucose- and BCH (an allosteric activator of glutamate dehydrogenase)-plus-glutamine-stimulated insulin release were inhibited. Cellular levels of citrate, α-ketoglutarate, malate and ATP were altered in patterns consistent with blockage at the mitochondrial IDH reactions. We were able to generate only 50% knockdown of Idh1 mRNA in multiple cell lines (without inhibition of insulin release) possibly because greater knockdown of IDH1 was not compatible with cell line survival.
Conclusions
The mitochondrial IDHs are redundant for insulin secretion. When both enzymes are severely knocked down, their low activities (possibly assisted by transport of IDH products and other metabolic intermediates from the cytosol into mitochondria) are sufficient for cell growth, but inadequate for insulin secretion when the requirement for intermediates is certainly more rapid. The results also indicate that IDH2 can support the citric acid cycle.
General Significance
As almost all mammalian cells possess substantial amounts of all three IDH enzymes, the biological principles suggested by these results are probably extrapolatable to many tissues.
Keywords: shRNA, Mitochondrial isocitrate dehydrogenase, cytosolic isocitrate dehydrogenase, Stable knockdown of isocitrate dehydrogenase, Insulin secretion, INS-1 832/13 cell line
1.0. Introduction
The original purpose of the work reported here was to explore the possible redundancy of function among the three isocitrate dehydrogenase (IDH) enzymes in insulin secretion by using shRNA to generate beta cell lines with stable knockdown of each of the three IDH isoforms. There are three mammalian IDHs; two mitochondrial enzymes and one cytosolic enzyme in most mammalian cells. These enzymes catalyze the reversible reaction: NAD(P) + isocitrate ↔ NAD(P)H + α-ketoglutarate. The cytosolic enzyme (IDH1)1 is an NADP-dependent enzyme that is highly homologous (70%) to the mitochondrial NADP-IDH (IDH2). These two enzymes are homo-dimers. The other mitochondrial IDH, IDH3, is a multimeric NAD-dependent enzyme encoded by three separate genes: IDH3a, IDH3b, and IDH3c. This enzyme is not homologous with either of the NADP IDHs in any of its subunits. The three subunits appear to share substrate binding and enzyme activity, but cannot substitute for each other.
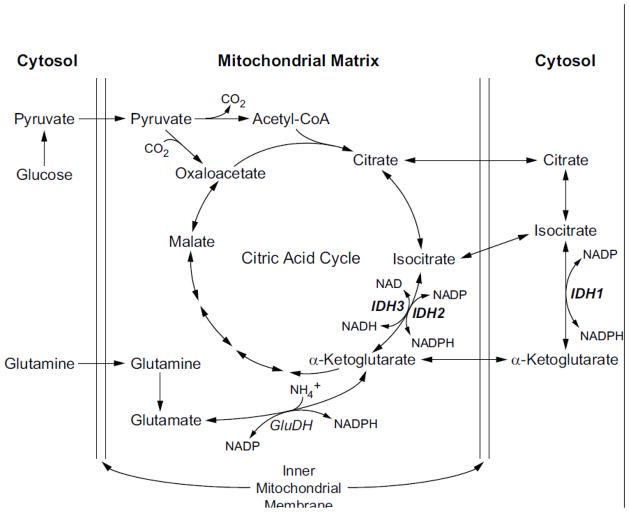
Recent evidence from non-beta cells indicates there could be redundancy in function among the IDH enzymes. The IDH3 enzyme was thought to be an essential element of the citric acid cycle, catalyzing the oxidation of isocitrate to α-ketoglutarate with the reduction of NAD to NADH. However, individuals have been reported who have homozygous mutations of the IDH3c subunit, resulting in essentially complete loss of IDH3 enzyme activity. Surprisingly, loss of this enzyme is apparently detrimental only in the eye, as the only reported finding is retinitis pigmentosa [1]. This suggests that the two mitochondrial enzymes could be substantially redundant in function. It is also possible that the cytosolic IDH enzyme (IDH1) reaction might compensate for loss of the mitochondrial IDH2 or IDH3 by producing metabolites in the cytosol that can be transported into mitochondria, as there is rapid transport of citrate, isocitrate, α-ketoglutarate and other metabolites between the cytosol and mitochondria. Any precursor, substrate or product of the IDH reaction, can be (re)imported into the mitochondrion and used in the citric acid cycle and for other mitochondrial pathways (Figure 1).
Figure 1. Pathways of precursors for substrates and products of the three isocitrate dehydrogenase reactions in the pancreatic beta cell.
Abbreviations: GluDH, glutamate dehydrogenase; IDH, isocitrate dehydrogenase.
In addition to a possible redundancy among the three IDH reactions, there also exists the possibility of redundancy of function between the IDHs and non-IDH enzymes. For example, the movement of citric acid cycle intermediates between the mitochondria and the cytosol has been proposed to participate in reactions that produce NADPH in the cytosol and play a role in insulin secretion by the pancreatic beta cell [2–6]. These reactions occur in cycles or shuttles in which the exported metabolite is oxidized by NADP to produce NADPH in the cytosol and the oxidized metabolite is reimported into the mitochondria for reduction and re-exportation to the cytosol. These shuttles of oxidized and reduced metabolites carry pyridine nucleotide NAD(H) and NADP(H) equivalents across the inner mitochondrial membrane and have evolved because pyridine nucleotides cannot penetrate the inner mitochondrial membrane. An isocitrate shuttle involving either or both of the mitochondrial IDHs and the cytosolic IDH has been proposed as one NADPH shuttle [3, 6]. Previously NADPH shuttles involving mitochondrially exported malate and the cytosolic malic enzyme (the pyruvate malate shuttle) [2, 3] and citrate exported to the cytosol involving the cytosolic enzymes ATP citrate lyase, cytosolic malate dehydrogenase and the cytosolic malic enzyme (the classic citrate pyruvate shuttle) were proposed to exist in the beta cell and there is fairly good evidence for each of these [2–5].
With one exception there is no report of knockdown of any of the IDH isoforms in the beta cell. One group reported that the knockdown of the cytosolic IDH (IDH1) mRNA 78% with siRNA, but with only 39% knockdown of IDH1 enzyme activity, in the INS-1 832/13 cell line, inhibited glucose-stimulated insulin secretion 59% [6]. In view of the redundancy of the IDH1 reaction in the cytosol with the IDH2 and IDH3 reactions in the mitochondria that each can catalyze the interconversion of α-ketoglutarate and isocitrate (Figure 1), as well as other cytosolic enzymes that can participate in a NADP(H) shuttle (the cytosolic malic enzyme) [2–5, 7], it is puzzling why knockdown of a single IDH enzyme should impair insulin secretion unless it plays a unique and nonredundant role in insulin secretion.
To further explore the possible redundancy of IDH enzymes in insulin secretion we attempted to generate INS-1 832/13-derived cell lines with knockdown of all three IDH isoforms and with knockdown of more than one of the IDHs in a single cell line. We were only partially successful even though we used a very efficient method of gene targeting (Tol2). We generated separate cell lines with strong stable knockdown of the individual mRNAs, proteins and enzyme activities of each of the mitochondrial IDHs, but glucose-stimulated insulin release was not inhibited in these cell lines. However, when we generated a cell line with severe knockdown of both mitochondrial isocitrate dehydrogenases together, we did observe inhibition of glucose- and BCH-plus glutamine-stimulated insulin release. We were unable to generate viable beta cell lines with more than 50% knockdown of the cytosolic NADP IDH (Idh1) mRNA. IDH1 enzyme activity was normal and insulin release was not inhibited in these cell lines. In addition, in an attempt to knockdown both NADP IDHs in a single cell line, the mitochondrial NADP-IDH enzyme (IDH2) was severely knocked down, but the cytosolic NADP-IDH (Idh1) mRNA was not knocked down. We also attempted to knock down the cytosolic malic enzyme and the cytosolic IDH in the same cell line. Malic enzyme activity was severely knocked down, but the cytosolic IDH enzyme activity was normal. We concluded that the severe knockdown of the cytosolic NADP IDH might not have permitted the development of viable cell lines. Herein we report a study of these cell lines.
2.0. Material and Methods
2.1. Construction of targeted clones
shRNA vectors were constructed in the vector pSilencer 2.1U6 Hygro + Tol2 [7, 8]. All insert sequences were confirmed by sequencing, and the resulting plasmids were transfected into the cell line INS-1 832/13 using Fugene HD (Roche) according to product directions. Cell populations were selected at least 3–4 weeks with Hygromycin (150 μg/ml, Invivogen) prior to testing, as described previously [7]. For double knockouts, pSilencer 2.1-U6 Puro (Applied Biosystems) was similarly modified to contain the Tol2 transposition site, and was used to create stable transferent populations with reduced Idh2 levels. Cells were first selected for at least 3–4 weeks with puromycin (200 ng/ml, Invivogen), and were subsequently transfected with the pSilencer 2.1-U6 Hygro + Tol2 plasmids targeting other genes and selected for at least 3–4 weeks. Selection was maintained until testing. All transfections included an equimolar amount of the pCMV-Tol2 vector, which encodes the Tol2 transposase, to increase efficiency of transfection [7, 8]. Target sequences are shown in Supplemental Table 1 online.
2.2. Preparation of mitochondria
Cells were harvested by trypsinization from three or four 75 cm2 flasks, resuspended in culture medium and washed twice in phosphate buffered saline. The cell pellet was resuspended in 1 ml of solution containing 220 mM mannitol, 70 mM sucrose, 5 mM potassium HEPES, pH 7.5 (KMSH) and 1 mM dithiothreitol and homogenized with 40 strokes of a Potter-Elvehjem homogenizer. The homogenate was centrifuged at 600 × g for 10 minutes at 4°C, and the resulting supernatant fraction was carefully removed to a new test tube and the centrifugation at 600 × g was repeated. The resulting supernatant fraction was put into another test tube and centrifuged at 10,000 × g for 10 minutes. The 10,000 × g supernatant fraction (“cytosol”) was removed and saved and the pellet was washed with 0.5 ml of the homogenization buffer and the 10,000 × g × 10 min centrifugation was repeated. After removing the supernatant, the pellet (“mitochondria”) was resuspended in 50–200 μl of KMSH solution containing 1 mM dithiothreitol and vortexed vigorously. The mitochondrial fraction was frozen at −20 C and thawed once prior to assay in order to disrupt the mitochondrial membranes.
2.3. Enzyme assays
IDH enzymes were assayed according to our modifications [7, 9–11] of standard assays [12]. The cytosolic NADP IDH (IDH1) and the mitochondrial NADP IDH (IDH2) were assayed in a reaction mixture of 2 mM MgCl2, 0.1 mM dithiothreitol, 0.3 mM NADP+, with or without 0.5 mM DL-isocitrate in 50 mM Tris HCl buffer, pH 7.4. Eight microliters of mitochondrial or cytosol preparation was added to 200 μl of the enzyme reaction mixture and the slopes read for up to 10 minutes at 340 nM and 37° C on the plate reader of the SpectraMax M2 spectrophotometer (Molecular Devices). The mitochondrial NAD IDH (IDH3) was assayed in the presence of 2 mM MgCl2, 1 mM ADP, and 0.3 mM NAD+, with or without 5 mM DL-isocitrate in 50 mM Tris-HCl buffer, pH 7.4. Eight microliters of the mitochondrial preparation was added to 200 μl of the enzyme reaction mixture and read as above. IDH3 has been reported to be unstable in buffers at cold temperatures, so it was assayed immediately after the freeze-thaw of the mitochondria. Any background rate seen in the absence of isocitrate was subtracted from the rate in the presence of isocitrate to give the rate attributable to the enzyme.
2.4. Immunoblots
All immunoblots were of mitochondrial preparations with 15 μg of mitochondrial protein loaded per lane. Blots were blocked with 5% powdered milk. IDH2 was detected with anti-IDH2 (Catalog no. SC-55668), used at a dilution of 1:200 and peroxidase-conjugated donkey anti-goat antibody at a dilution of 1:3500 (Catalog no. SC-2033) (both from Santa Cruz Biotechnologies, Inc.). IDH3a was detected with anti-IDH3A (Catalog no. GTX114486S, GeneTex, Irvine, CA) and peroxidase-conjugated goat anti-rabbit IgG antibody at a dilution of 1:20,000 (Catalog no. 31460, Thermo Scientific). The mitochondrial glycerol phosphate dehydrogenase (GPD2) was used as a loading control for mitochondrial protein and was detected using our rabbit anti-rat GPD2 antibody at a dilution of 1:10,000 [13]. Chemiluminescent HRP substrate (Catalog no. WBKLS0100, Millipore) was used for visualizing all blots.
2.5. RNA quantitation
RNA was prepared with the RNeasy Mini kit (Qiagen) with on-column DNAse digestion (RNase-Free DNAse Set, Qiagen). cDNA was prepared with the ImPromII Reverse Transcription System (Promega) using oligo(dT) primers. Real time quantitative PCR was performed using the BioRad MyIQ instrument, and ABsolute Blue SYBR Green Fluorescein reagent (Catalog no. AB-4219/B, Thermo Scientific). A dilution curve was prepared with cDNA from the control line, and values were normalized using Glud1 as an internal control, as previously reported [7].
2.6. Metabolite assays
Citrate, malate and α-ketoglutarate levels were measured by alkali-enhanced fluorescence as previously described [14, 15]. Fluorescence values in each assay were compared to a standard curve of 0.01 to 0.15 nmol of the respective metabolite and also NAD(H). ATP was measured in an absorbance assay at 340 nm in a reaction mixture of 0.5 mM NADP, 1 mM glucose, 5 mM MgCl2, 0.5 U of glucose-6-phosphate dehydrogenase (from yeast), 0.75 U of hexokinase (from yeast) in 40 mM Tris-chloride buffer, pH 8 [15]. Values in blanks containing all assay ingredients except the enzyme(s) catalyzing the final step of the analytic reaction, were subtracted from values obtained from the complete assay mixture to give values attributable to the metabolite concentration.
2.7. Insulin release studies
Insulin release studies were performed as previously described [7]. Cells were maintained in RPMI 1640 cell culture medium (contains 11.1 mM glucose), 1 mM pyruvate and 50 μM beta-mercaptoethanol and 10% fetal calf serum. Twenty hours before an experiment the glucose concentration in the medium was adjusted to 5 mM. Insulin release was measured in 24 well plates in 1 ml Krebs Ringer bicarbonate Hepes buffer, pH 7.3, after a 1 h incubation period at 37° as previously described [7].
2.8. Statistics
Statistical significance was calculated with student’s t test. The modified z-score test, the Grubbs test and the Dixon test were used to identify outliers in the citrate data and ATP data [16, 17]. It is noteworthy that omission of the outliers did not influence the major conclusion of the manuscript because they were not present in the glucose-stimulated HygC control data set or the glucose-stimulated Idh2-598/Idh3a-251 cell line data set (See Supplementary Material online) which were data sets central to the interpretation of the inhibited insulin release in the double mitochondrial IDH knockdown cell line.
3.0. Results
3.1. Knockdown of IDHs, RNA measurements
Stable knockdown of the individual Idh2 (Idh2 cell lines 598, 423 and 845) and Idh3a (Idh3a cell lines 251 and 587) genes that encode the mitochondrial NADP IDH and the 3a subunit of the mitochondrial NAD-IDH, respectively, were achieved with 85–95% lowering of mRNAs without knockdown of the untargeted Idh mRNAs (Figure 2). Knockdown of Idh1, which encodes the cytosolic NADP-IDH, was only partially successful with only 40–60% lowering of Idh1 mRNA with five different shRNA constructs (Idh1-1191, 57% (Figure 3A); Idh1-75, 59%; Idh1-552, 43%; Idh1-569, 61%; and Idh1-820, 65% (data not shown) vs the CHS non-targeting control cell line. Three additional Idh1 shRNA constructs did not yield any clones. IDH1 enzyme activity was not lowered in any of these cell lines and insulin release stimulated by glucose or BCH-plus-glutamine was not inhibited in these cell lines (Figure 3C and data not shown).
Figure 2. Knockdown of Idh2 and Idh3a mRNA levels individually or both mRNAs in the same cell line with stable transfection of shRNA in INS-1 832/13-derived cell lines without knockdown of untargeted Idh mRNAs.
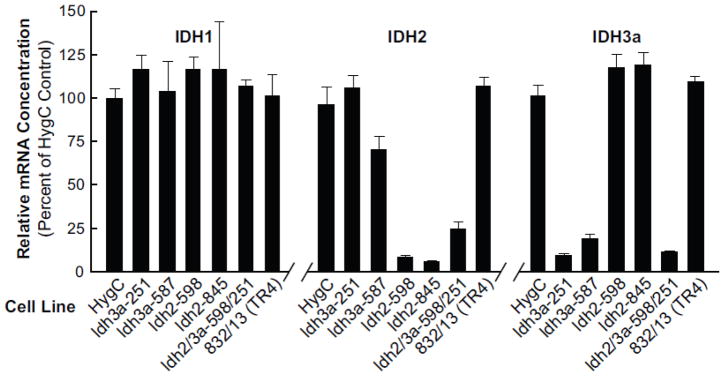
mRNA levels are expressed relative to the HygC, a nontargeting vector control. Results are the mean ± SE of four or more cell preparations. All values < 25% of the HygC control were significant to the p < 0.001 level.
Figure 3. Moderate knockdown of cytosolic NADP isocitrate dehydrogenase (Idh1) mRNA with stable transfection of shRNA in INS-1 832/13 cells does not lower its enzyme activity or glucose- or BCH-plus-glutamine-stimulated insulin release.
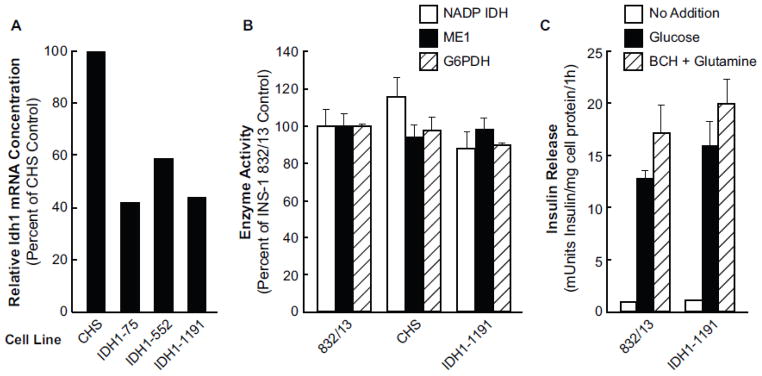
A. mRNA measurements are the average of two measurements. mRNA levels were measured by qRT-PCR and were normalized to glutamate dehydrogenase mRNA. B. Enzyme activity of IDH1 and, as controls, two other NADP requiring enzymes, malic enzyme and glucose-6-phosphate dehydrogenase. Activities are expressed as a percent of the control INS-1 832/13 equal to 100% and are the mean ± SE of measurements on 3 or 4 cell preparations. C. Insulin release measurements were the averages of average values from eight separate experiments with cells incubated with no insulin secretagogue, 11.1 mM glucose or BCH (10 mM)-plus-glutamine (10 mM). There were four replicate incubations per condition per each experiment. Results are expressed as the mean ± SE.
We were able to severely knock down Idh2 mRNA in combination with Idh3a mRNA in the same cell line. Idh2 mRNA was reduced to 5–10% in the single knock-down cell lines and the Idh2 mRNA in the Idh2/Idh3 double knockdown cell line Idh2-598/Idh3a-251was about 25% that of the scrambled vector control HygC (Figure 2). The Idh3a mRNA level remained very low when knocked down in combination with knockdown of Idh2 (Figure 2). In an attempt at knockdown of both the Idh1 and Idh2 genes, the Idh2 mRNA level remained at 11% of the control, but the Idh1 mRNA was not knocked down (data not shown). In an attempt to knock down IDH1 and the cytosolic malic enzyme (ME1) in the same cell line, the Me1 mRNA was knocked down about 90%, but Idh1 mRNA was not decreased.
3.2. Knockdown of IDH proteins
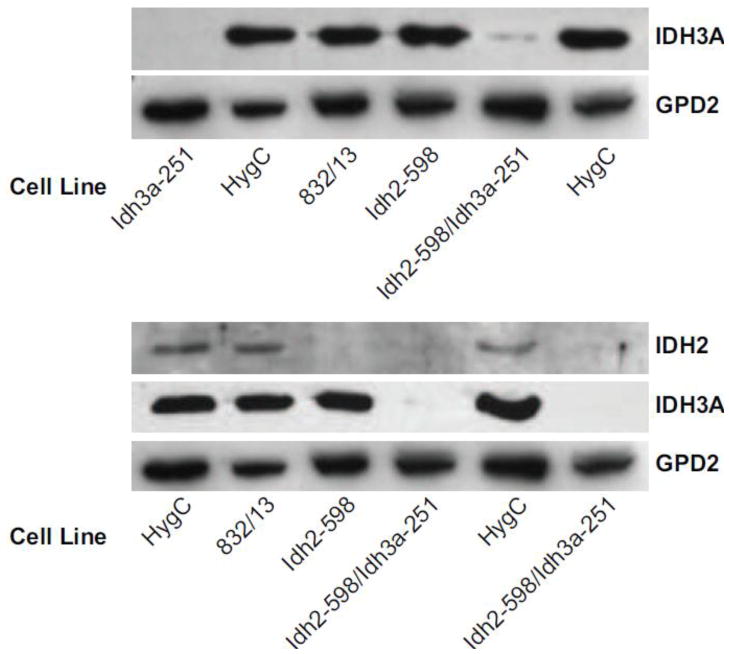
Immunoblot analysis confirmed the strong knockdown of IDH2 and IDH3A proteins in the single target cell lines and with knockdown of only the targeted enzyme, as well as strong knockdown of both enzymes in the double target knockdown cell line (Figure 4).
Figure 4. Severe single and double lowering of IDH2 and IDH3A proteins using stable knockdown with shRNA in INS-1 832/13-derived cell lines.
Immunoblots of IDH2 and IDH3A protein and the mitochondrial glycerol phosphate dehydrogenase (GPD2) as a protein loading control in cell lines. INS-1 832/13 is the parent cell line. HygC is the nontargeting vector control cell line. Blots are representative of 4 repetitions with IDH3A and 2 repetitions with IDH2.
3.3. Knockdown of IDH enzyme activities
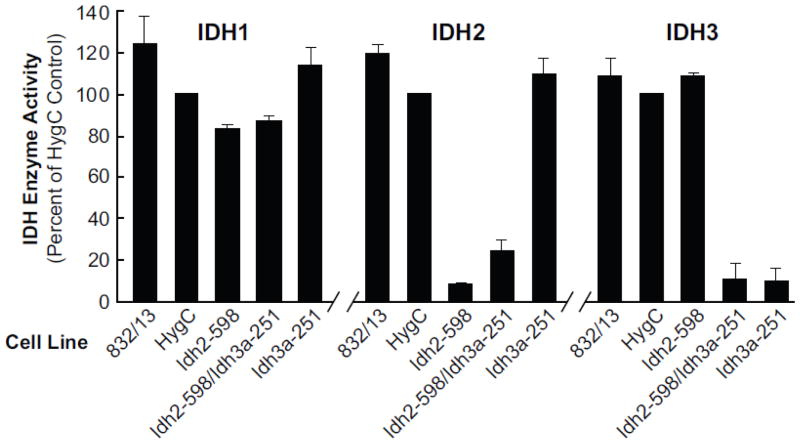
Measurements of enzyme activities of the three IDH enzymes showed knockdown of the targeted mitochondrial IDH enzymes in single target knockdown cell lines and knockdown of both IDH2 and IDH3A activities together in the double target cell line without significant knockdown of the activities of the nontargeted IDH enzyme(s). The decreases in enzyme activities (Figure 5) were severe and comparable to the decreases in Idh mRNA and IDH protein (Figures 2 and 4). IDH3 enzyme activity was decreased about 90% in the single (Idh3a-251) and double (Idh2-598/Idh3a-251) target cell lines and IDH2 activity was decreased 90% in the single target cell line (Idh2-598) and about 80% in the double target (Idh2-598/Idh3a-251) cell line. Fifty percent knockdown of Idh1 mRNA did not result in decreased IDH1 enzyme activity (Figure 3B).
Figure 5. Severe lowering of IDH2 and/or IDH3 enzyme activity with shRNA stable knockdown of Idh2 and/or Idh3a mRNAs in INS-1 832/13 derived cell lines.
Activity is presented relative to that of the cell line containing a nontargeting vector HygC as a control. The average enzyme activities of IDH1, IDH2 and IDH3 in the HygC control cell line were, respectively, 32, 125 and 24 nmol NAD(P)H/min/mg cytosol protein (IDH1) or mitochondrial protein (IDH2 and IDH3). On a total cell basis, calculated from the total activity in each compartment, IDH1 activity accounts for 69% of the activity in these control cells and IDH2 and IDH3 account for 26% and 5%, respectively. Results are the mean ± SE of three or four separate cell preparations. Values < 20% were significant to the p < 0.001 level vs the HygC control.
3.4. Changes in metabolite levels in the presence of IDH knockdown
3.4.1. α-Ketoglutarate
The glucose-stimulated α-ketoglutarate level was lowered about 70% with knockdown of Idh3a alone, not lowered with knockdown of Idh2 alone and lowered about 90% with knockdown of both Idh3a and Idh2 (Figure 6). As both IDH2 and IDH3 are mitochondrial enzymes, this is consistent with decreased formation of α-ketoglutarate, the product of the IDH reaction in the direction of the citric acid cycle, in mitochondria. It appears that there was partial compensation by IDH2 activity when only IDH3 was knocked down. The low level of α-ketoglutarate in the single target IDH3 knockdown cell line and the even lower level in the double target IDH2 and IDH3 cell line, but without lower α-ketoglutarate with knockdown of IDH2 alone, is consistent with what is believed to be known about IDH3; that is, it is the primary IDH that is used in the citric acid cycle. BCH is an allosteric activator of glutamate dehydrogenase that enhances the formation of α-ketoglutarate from glutamine-derived glutamate in the glutamate dehydrogenase reaction in mitochondria [18–21]. Also consistent with the idea that α-ketoglutarate is derived from the IDH reaction in glucose-stimulated cells (i.e. from the direction of the citric acid cycle), α-ketoglutarate was not decreased in any of the cell lines when the cell lines were stimulated with glutamine in the presence of BCH (Figure 6).
Figure 6. Low level of glucose-stimulated α-ketoglutarate and/or malate and increased citrate in INS-1 832/13-derived cell lines with knocked down mitochondrial NAD-IDH (IDH3) and/or mitochondrial NADP-IDH (IDH2).
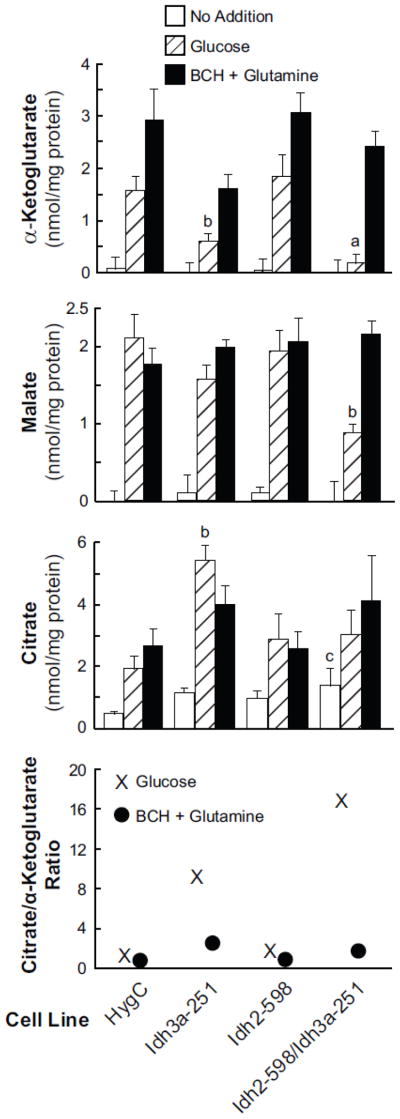
Cells were incubated with 11.1 mM glucose or glutamine (10 mM)-plus-BCH (10 mM) for 30 min. Results are the mean ± SE from five separate experiments. ap < 0.001, bp < 0.01 and cp < 0.05 vs HygC control same condition.
3.4.2. Malate
The level of malate was lowered about 60% in the glucose-stimulated cell line with IDH2 and IDH3 knocked down together compared to the similarly stimulated HygC control cell line. Malate was not lower with glucose stimulation in either of the cell lines with knockdown of one mitochondrial IDH alone or in the presence of glutamine-plus-BCH stimulation in the cell line with both of the mitochondrial IDHs knocked down (Figure 6). The lower level of malate only in the presence of knockdown of both mitochondrial IDH enzyme activities and only in the presence of glucose stimulation suggests malate was lowered from decreased flux through the span of the citric acid cycle prior to malate formation. Thus these malate data support the α-ketoglutarate data which suggest that, although IDH3 is the primary catalyzer of the conversion of isocitrate to α-ketoglutarate in the citric acid cycle under normal conditions, IDH2 can compensate for decreased IDH3 activity. This suggests that the two mitochondrial IDH enzymes can perform redundant functions in respect to α-ketoglutarate formation even though IDH3 uses NAD and IDH2 uses NADP as a cofactor. The lack of decreased malate in the presence of glutamine-plus-BCH can be explained by the fact that α-ketoglutarate formed from glutamine-derived glutamate enters the citric acid cycle beyond the block at the IDH step.
3.4.3. Citrate
Citrate levels, which are in rapid equilibrium with isocitrate levels, were measured instead of isocitrate because isocitrate levels are quite low in the beta cell as in most tissues and thus difficult to measure accurately. Citrate levels were increased in glucose-stimulated cells in the presence of single knockdown of IDH3 activity (Figure 6). Although the average citrate level was 50–60% higher than the control with glucose or BCH-plus-glutamine stimulation in the cell line Idh2-598/Idh3a-251 in which both mitochondrial IDH enzymes were knocked down, this increase was not statistically significant. It is likely that the lower availability of malate in this double mitochondrial IDH knockdown cell line limited citrate production decreasing the amount of oxaloacetate for condensation with acetyl-CoA to form citrate.
The ratio of citrate to α-ketoglutarate was calculated to generate a “crossover” plot for each of the cell lines in the presence of glucose- or BCH-plus-glutamine-stimulation. This showed a very high ratio for the cell line Idh3a-251 and an even higher ratio for the cell line Idh2-598/Idh3a-251 in the presence of glucose stimulation (Figure 7, bottom panel). This is consistent with inhibition of the IDH reaction in the direction of the citric acid cycle in these cell lines when they are stimulated with glucose. As expected, since BCH-plus-glutamine generates α-ketoglutarate, the citrate/α-ketoglutarate ratio was not increased in any of the cell lines stimulated with BCH-plus-glutamine.
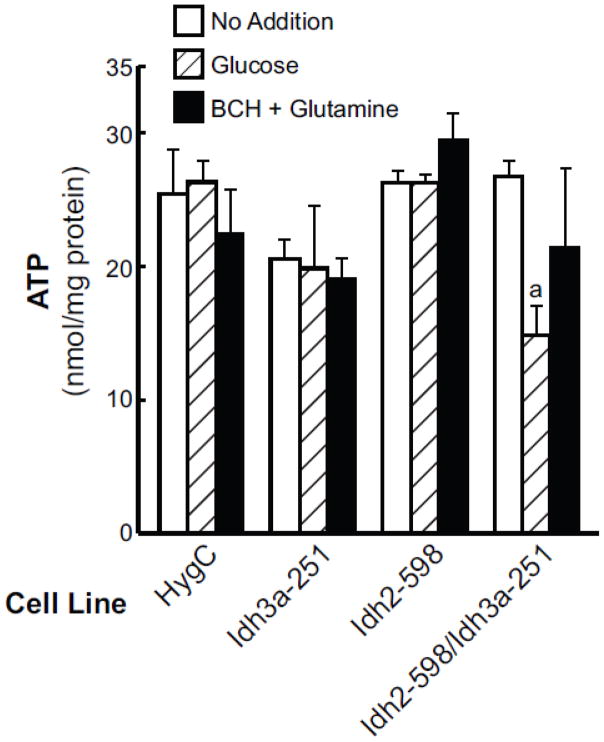
Figure 7. Lower ATP levels in the glucose-stimulated cell line Idh2-5981/Idh3a-251 with IDH2 and IDH3 enzyme activities knocked down.
Cells were incubated 30 min with or without the presence of an insulin secretagogue as in Figure 6. Results are expressed as the mean ± SE of five experiments. ap < 0.006 vs HygC, glucose.
3.5. Lower ATP levels in the double mitochondrial IDH knockdown cell line
Consistent with the lower α-ketoglutarate levels and a crossover point at the IDH reaction that suggested a compromised citric acid cycle in the cell line with knockdown of both IDH2 and IDH3 enzyme activities together, the ATP levels were 40% lower with glucose stimulation in this cell line than the ATP levels in the glucose-stimulated control cell line HygC (Figures 8). The average ATP levels were not lower in the BCH-plus-glutamine-stimulated double mitochondrial IDH knockdown cell line compared to the control cell line (Figure 8).
Figure 8. Inhibition of glucose- or glutamine-plus-BCH-stimulated insulin release in the cell line Idh2-598/Idh3a-251 with both mitochondrial IDH enzymes knocked down.
Cells were incubated with no insulin secretagogue, 11.1 mM glucose or BCH (10 mM)-plus-glutamine (10 mM) for 1 h. Results are the mean ± SE of 3–9 experiments calculated from average values of quadruplicate incubations for each condition per experiment. ap < 0.05 and bp < 0.01 vs HygC control same condition.
3.6. Lowering of insulin release with knockdown of both mitochondrial IDHs
Glucose-stimulated and glutamine-plus-BCH-stimulated insulin release were each decreased about 50% in the cell line with knockdown of both IDH2 and IDH3 enzyme activities compared to the HygC cell line that contains a scrambled shRNA. Knockdown of either enzyme alone did not inhibit insulin release and secretagogue-stimulated insulin release in the Idh2 knockdown cell line was slightly increased compared to the control values (Figure 9).
4.0. Discussion
4.1. Severe knockdown of both mitochondrial IDHs inhibits insulin secretion
The loss of almost all of the activities (80–95%) of the two mitochondrial IDH enzymes together in the same cell line Idh2-598/Idh3a-251 allowed normal growth of the cells but inhibited glucose- and BCH-plus-glutamine-stimulated insulin release (Figure 9). The survival and normal rate of growth of the cell line with knockdown of both mitochondrial IDH enzymes might indicate that the very low mitochondrial IDH activities were sufficient for growth of the cells. Or it might indicate that in addition the cytosolic IDH (IDH1) catalyzed the formation of α-ketoglutarate and isocitrate in the cytosol and that the transport of citrate, isocitrate and α-ketoglutarate from the cytosol across the inner mitochondrial membrane was adequate to supply the citric acid cycle in the mitochondria with substrate for growth, but not for the stimulation of insulin release when more rapid flux of substrates through the citric acid cycle and biosynthesis pathways would be required. In addition, the medium in which cells were maintained for cell culture would enable the cells to grow because it contains a high concentration of glutamine that supplies glutamate-derived α-ketoglutarate to produce energy by metabolism through the second span of the citric acid cycle and for biosynthesis pathways (Figure 1). In line with the idea that the total mitochondrial IDH activity was inadequate to support the citric acid cycle for insulin release in the glucose-stimulated cells with knockdown of both mitochondrial IDHs, ATP levels were decreased compared to the similarly stimulated HygC control (Figure 8). With glucose stimulation the level of α-ketoglutarate, the product of the IDH reaction, was lower (Figure 6) and the citrate level was higher to produce a “crossover point” consistent with the blockage at the IDH reactions in this cell line (Figure 7). The decreased level of malate in this cell line (Figure 6) is also consistent with inhibited flux through the citric acid cycle downstream of α-ketoglutarate formation. The lack of inhibited insulin release with knockdown of either the IDH2 enzyme or the IDH3 enzyme alone can be explained by the fact that both enzymes are mitochondrial enzymes and that either enzyme can catalyze α-ketoglutarate formation in mitochondria when the activity of the other one is low.
Insulin release stimulated by BCH-plus-glutamine was inhibited to the same relative extent as the glucose-stimulated insulin release in the double mitochondrial IDH knockdown cell line. When this cell line was stimulated with glutamine-plus-BCH, the α-ketoglutarate level was not decreased, yet the flux through the first span of the citric acid cycle would still be expected to be impaired because of the low total mitochondrial IDH activity. Because glutamine substantially increases the cellular glutamate level [19] in beta cells and BCH allosterically activates glutamate dehydrogenase to form α-ketoglutarate from glutamine-derived glutamate [18–21], the level of α-ketoglutarate would not be expected to be low and Figure 6 shows this is the case. In addition, because α-ketoglutarate would supply substrate to the second span of the citric acid cycle distal to the IDH reaction, this might enable the cell to function with a less compromised citric acid cycle in the presence of BCH-plus-glutamine- than in the presence of glucose-stimulation. Although the average level of ATP was not decreased significantly in this cell line in the presence of BCH-plus-glutamine, ATP was moderately decreased in this cell line in three of five experiments (data not shown).) Mitochondrial metabolism supports insulin secretion in ways besides the generation of ATP, such as anaplerosis and cataplerosis [2–5, 14]. In addition, as in many cells [22, 23], the IDH reaction in the beta cell mitochondria is reversible (M.J. MacDonald, unpublished data) enabling α-ketoglutarate derived from glutamate via the glutamate dehydrogenase reaction in BCH-plus-glutamine-stimulated cells to be converted to isocitrate and citrate in normal cells (Figure 1). It is likely that anaplerosis and cataplerosis were also impaired in the double mitochondrial IDH knockdown cell line and might have contributed to the impairment of insulin secretion in the presence of BCH-plus-glutamine, as well as in the presence of glucose stimulation.
4.2. Failure to obtain a viable IDH1 knockdown cell line
We originally intended to knock down expression of all three of the isocitrate dehydrogenase genes and two of them in various combinations in a single cell line and measure the effects on insulin release and metabolite profiles. We were unable to derive cell lines with Idh1 mRNA levels of lower than 50% of the control level. This may indicate greater knockdown of Idh1 mRNA is detrimental to survival and growth of the INS-1 832/13 cell line. Furthermore, 50% knockdown of Idh1 mRNA did not result in decreased IDH1 enzyme activity or inhibition of glucose- or BCH-plus-glutamine-stimulated insulin release (Figure 3).
IDH1 may perform an essential role in cellular function such that cells with severely depressed IDH1 may not grow well or even survive. We obtained fewer viable clones than expected with Idh1-targeted shRNAs delivered with Tol2 vectors that provide very efficient integration of shRNA into cellular DNA. When the Newgard laboratory used virally delivered siRNA that targeted Idh1 in this same cell line, more severe (78%) knockdown of Idh1 mRNA was achieved with slight (39%) knockdown of IDH1 enzyme activity and 59% inhibition of glucose-stimulated insulin release [6]. The inhibition of insulin release in their experiment and no inhibition of insulin release in our experiment might be explained by the difference in the time from treatment to testing of shRNA-treated stable cell lines compared to siRNA-treated cells. The production of stable cell lines containing integrated shRNA requires cell growth and replication for at least several weeks prior to testing. Cells that survive the selection and grow and divide will be lost from the culture during this period. Cells treated with siRNA are usually tested within two or three days, so cells that are unable to grow and divide may still be present at high concentrations in the culture.
Since isocitrate and α-ketoglutarate, the substrate and the product of IDH enzymes, can be formed in mitochondria and can be rapidly transported out of mitochondria to the cytosol and back into the mitochondria, knockdown of IDH1 in the cytosol alone would not be expected to inhibit cellular function enough to inhibit survival and growth of a cell line. In addition, if NADPH is important for insulin secretion, it can be formed by malic enzyme or other enzymes in the cytosol [2–7]. That the Newgard laboratory saw inhibition of insulin release with slight knockdown of IDH1 activity and we were unable to obtain a viable cell line with severely knocked down Idh1 mRNA might suggest that IDH1 performs an important function in addition to catalyzing the IDH reaction in the cytosol of the beta cell.
Interestingly, the Idh1 gene encodes for a single protein that possesses a tripeptide C-terminus peroxisomal target sequence (PTS-1). The IDH1 protein co-localizes to both the peroxisome and the cytosol of mammalian [24] and yeast [25] cells. De Duve [26], one of the first to design a method for the isolation of pure peroxisomes, found NADP IDH activity in the peroxisomal fraction of rat liver and this has been confirmed many times. Because NADPH formed in the cytosol cannot penetrate the peroxisomal membrane, IDH1 or another enzyme that can form NADPH needs to be present inside the peroxisome. IDH1 can supply NADPH from isocitrate oxidation of NADP for reductases that catalyze metabolism of fatty acids with double bonds at even numbered positions inside the peroxisomes [27, 28]. This essential function may be related to our inability to obtain viable cell lines with a high degree of Idh1 knockdown using stably integrated shRNA targeted against the Idh1 gene. There are other enzymes besides IDH1 present in the cytosol of the cell that can form NADPH, such as malic enzyme 1 and glucose-6-phosphate dehydrogenase. Although glucose-6-phosphate dehydrogenase enzyme activity has been detected in peroxisomes [29, 30], its concentration in peroxisomes might be lower than that of IDH1. Its C-terminus sequence tripeptide (histidine-lysine-leucine) suggests that, compared to the C-terminus tripeptide sequence of the IDH1 protein (alanine-lysine-leucine), it would be a poor ligand for PEX5, a receptor that functions to import proteins into peroxisomes [31, 32]. In line with this idea, we have found that the ratio of peroxisomal to cytosolic IDH1 enzyme activity is 2-10 times higher than the same ratio of glucose-6-phosphate dehydrogenase in INS-1 832/13 cells (MJM unpublished data).
4.3. Conclusions
Several biological principles that are probably extrapolatable to non-beta cells can be learned from the current study as almost all mammalian cells possess substantial amounts of the three IDH enzymes. The first is that the mitochondrial NADP IDH (IDH2) can compensate for the mitochondrial NAD IDH (IDH3) and participate in the citric acid cycle when IDH3 activity is low. IDH3 was until recently thought to be the only IDH that participates in the citric acid cycle. The current work supports the observation that individuals with genetic mutations that cause almost complete loss of IDH3 enzyme activity are normal except for problems with the retina of the eye [1]. Second, when the activities of both mitochondrial IDH enzymes have been severely lowered, the low activities and/or transport of substrate and product of the IDH reactions or their precursors between the mitochondria and cytosol is apparently rapid enough to support normal cell growth and survival but insufficient to support processes that require rapid fuel consumption, such as insulin secretion. Finally, our inability to obtain viable cell lines with severely knocked down cytosolic IDH, combined with the report from another group of inhibited insulin release associated with only mild knockdown of this enzyme [6], might suggest that this enzyme performs a key function in the extramitochondrial space of the cell that cannot be assumed by other enzymes.
Supplementary Material
Highlights.
Mitochondrial isocitrate dehydrogenases were knocked down with shRNA in beta cells
Lowering one mitochondrial isocitrate dehydrogenase did not inhibit insulin release
Lowering both mitochondrial IDHs in a single cell line inhibited insulin release
Mitochondrial NADP-isocitrate dehydrogenase can support the citric acid cycle
Mitochondrial isocitrate dehydrogenases play redundant roles in insulin secretion
Acknowledgments
This work was supported by NIH grant DK28348 and the Nowlin Family Trust of the Lutheran Community Foundation. The authors thank Jens Eickhoff for statistical advice and analyses of outliers.
Footnotes
Abbreviations: BCH, 2-aminobicyclo [2,2,1]heptane-2-carboxylic acid; IDH1, cytosolic NADP isocitrate dehydrogenase; IDH2, mitochondrial NADP isocitrate dehydrogenase; IDH3, mitochondrial NAD isocitrate dehydrogenase; shRNA, short hairpin RNA; siRNA, small interfering RNA;
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hartong DT, Dange M, McGee TL, Berson EL, Dryja TP, Colman RF. Insights from retinitis pigmentosa into the roles of isocitrate dehydrogenases in the Krebs cycle. Nat Genet. 2008;40:1230–1234. doi: 10.1038/ng.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacDonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J Biol Chem. 1995;270:20051–20058. [PubMed] [Google Scholar]
- 3.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab. 2005;288:E1–15. doi: 10.1152/ajpendo.00218.2004. [DOI] [PubMed] [Google Scholar]
- 4.Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49:718–726. doi: 10.2337/diabetes.49.5.718. [DOI] [PubMed] [Google Scholar]
- 5.Flamez D, Berger V, Kruhøffer M, Orntoft T, Pipeleers D, Schuit FC. Critical role for cataplerosis via citrate in glucose-regulated insulin release. Diabetes. 2002;51:2018–2024. doi: 10.2337/diabetes.51.7.2018. [DOI] [PubMed] [Google Scholar]
- 6.Ronnebaum SM, Ilkayeva O, Burgess SC, Joseph JW, Lu D, Stevens RD, Becker TC, Sherry AD, Newgard CB, Jensen MV. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem. 2006;281:30593–30602. doi: 10.1074/jbc.M511908200. [DOI] [PubMed] [Google Scholar]
- 7.Brown LJ, Longacre MJ, Hasan NM, Kendrick MA, Stoker SW, MacDonald MJ. Chronic reduction of the cytosolic or mitochondrial NAD(P)P-malic enzyme does not affect insulin secretion in a rat insulinoma cell line. J Biol Chem. 2009;284:35359–35367. doi: 10.1074/jbc.M109.040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balciunas D, Wangensteen KJ, Wilber A, Bell J, Geurts A, Sivasubbu S, Wang X, Hackett PB, Largaespada DA, McIvor RS, Ekker SC. Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genet. 2006;2:e169. doi: 10.1371/journal.pgen.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald MJ. Flavin content of intracellular compartments of pancreatic islets compared with acinar tissue and liver. Endocrinology. 1981;108:1899–1902. doi: 10.1210/endo-108-5-1899. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald MJ, Marshall LK. Survey of normal appearing mouse strain which lacks malic enzyme and NAD+-linked glycerol phosphate dehydrogenase: normal pancreatic beta cell function, but abnormal metabolite pattern in skeletal muscle. Mol Cell Biochem. 2001;220:117–125. doi: 10.1023/a:1010821821921. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald MJ. Differences between mouse and rat pancreatic islets: succinate responsiveness, malic enzyme, and anaplerosis. Am J Physiol Endo Metab. 2002;283:E302–E310. doi: 10.1152/ajpendo.00041.2002. [DOI] [PubMed] [Google Scholar]
- 12.Plaut GW, Aogaichi T. Purification and properties of diphosphopyridine nucleotide-linked isocitrate dehydrogenase of mammalian liver. J Biol Chem. 1968;243:5572–5583. [PubMed] [Google Scholar]
- 13.Brown LJ, MacDonald MJ, Lehn DA, Moran SM. Sequence of rat mitochondrial glycerol-3-phosphate dehydrogenase cDNA. Evidence for EF-hand calcium-binding domains. J Biol Chem. 1994;269:14363–14366. [PubMed] [Google Scholar]
- 14.MacDonald MJ. The export of metabolites from mitochondria and anaplerosis in insulin secretion. Biochim Biophys Acta. 2003;1619:77–88. doi: 10.1016/s0304-4165(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 15.Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. Academic Press; New York: 1972. [Google Scholar]
- 16.Hawkins DM. Identification of Outliers. Chapman and Hall; London: 1980. [Google Scholar]
- 17.Barnett V, Lewis T. Outliers in Statistical Data. 3. John Wiley and Sons; Chichester: 1994. [Google Scholar]
- 18.Gylfe E. Comparison of the effects of leucine, non-metabolizable leucine analogues and other insulin secretagogues on the activity of glutamate dehydrogenase. Acta Diabetol. 1976;13:20–24. doi: 10.1007/BF02591577. [DOI] [PubMed] [Google Scholar]
- 19.Sener A, Malaisse WJ. L-leucine and a nonmetabolized analogue activate pancreatic islets glutamate dehydrogenase. Nature. 1980;288:187–189. doi: 10.1038/288187a0. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald MJ, Hasan NM, Longacre MJ. Studies with leucine, beta-hydroxybutyrate and ATP citrate lyase-deficient beta cells support the acetoacetate pathway of insulin secretion. Biochim Biophys Acta. 2008;1780:966–972. doi: 10.1016/j.bbagen.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacDonald MJ, Fahien LA. Glutamate is not a messenger in insulin secretion. J Biol Chem. 2000;275:34025–34027. doi: 10.1074/jbc.C000411200. [DOI] [PubMed] [Google Scholar]
- 22.Des Rosiers C, Di Donato L, Comte B, Laplante A, Marcoux C, David F, Fernandez CA, Brunengraber H. Isopotomer analysis of citric acid cycle and gluconeogenesis in rat liver. Reversibility of isocitrate dehydrogenase and involvement of ATP-citrate lyase in gluconeogenesis. J Biol Chem. 1995;270:10027–10036. doi: 10.1074/jbc.270.17.10027. [DOI] [PubMed] [Google Scholar]
- 23.Des Rosiers C, Fernandez CA, David F, Brunengraber H. Reversibility of the mitochondrial isocitrate dehydrogenase reaction in the perfused rat liver. Evidence form isopotomer analysis of citric acid cycle intermediates. J Biol Chem. 1994;269:27179–27182. [PubMed] [Google Scholar]
- 24.Geisbrecht BV, Gould SJ. The human PICD gene encodes a cytoplasmic and peroxisomal NADP(+)-dependent isocitrate dehydrogenase. J Biol Chem. 1997;274:30527–30533. doi: 10.1074/jbc.274.43.30527. [DOI] [PubMed] [Google Scholar]
- 25.Lu Q, McAlister-Henn L. Peroxisomal localization and function of NADP+-specific isocitrate dehydrogenases in yeast. Arch Biochem Biophys. 2010;493:125–134. doi: 10.1016/j.abb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leighton F, Poole B, Beaufay H, Baudhuin P, Coffey JW, Fowler S, De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968;37:482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henke B, Girzalsky W, Berteaux-Lecellier V, Erdmann R. IDP3 encodes a peroxisomal NADP-dependent isocitrate dehydrogenase required for the beta-oxidation of unsaturated fatty acids. J Biol Chem. 1998;273:3702–3711. doi: 10.1074/jbc.273.6.3702. [DOI] [PubMed] [Google Scholar]
- 28.van Roermund CW, Hettema EH, Kal AJ, van den Berg M, Tabak HF, Wanders RJ. Peroxisomal beta-oxidation of polyunsaturated fatty acids in Saccharomyces cerevisiae: isocitrate dehydrogenase provides NADPH for reduction of double bonds at even positions. EMBO J. 1998;17:677–687. doi: 10.1093/emboj/17.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panchenko LF, Antonenkov VD. Glucose-6-phosphate dehydrogenase of rat liver peroxisomes. Experientia. 1984;40:467–468. doi: 10.1007/BF01952389. [DOI] [PubMed] [Google Scholar]
- 30.Patel BN, Mackness MI, Connock MJ. Peroxisomal localization of glucose-6-phosphate dehydrogenase and pyrophosphate-stimulated dihydroxyacetone-phosphate acyltransferase in mouse kidney. Biochem J. 1987;244:443–448. doi: 10.1042/bj2440443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gatto GJ, Jr, Maynard EL, Guerrerio AL, Geisbrecht BV, Gould SJ, Berg JM. Correlating structure and affinity for PEXS:PTS1 complexes. Biochemistry. 2003;42:1660–1666. doi: 10.1021/bi027034z. [DOI] [PubMed] [Google Scholar]
- 32.Neuberger G, Maurer-Stroh S, Eisenhaber B, Hartig A, Eisenhaber F. Motif refinement of the peroxisomal targeting signal 1 and evaluation of taxon-specific differences. J Mol Biol. 2003;328:567–579. doi: 10.1016/s0022-2836(03)00318-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.