Abstract
Background
Anatomical diversity amongst cerebellar AVMs calls for a classification that is intuitive and surgically informative. Selection tools like the Spetzler-Martin grading system are designed to work best with cerebral AVMs, but have shortcomings with cerebellar AVMs.
Objective
To define subtypes of cerebellar AVMs that clarify anatomy and surgical management, determine results according to subtypes, and compare predictive accuracies of Spetzler-Martin and supplementary systems.
Methods
From a consecutive surgical series of 500 patients, 60 had cerebellar AVMs, 39 had brain stem AVMs and were excluded, and 401 had cerebral AVMs.
Results
Cerebellar AVM subtypes were: 18 vermian, 13 suboccipital, 12 tentorial, 12 petrosal, and 5 tonsillar. Patients with tonsillar and tentorial AVMs fared best. Cerebellar AVMs presented with hemorrhage more than cerebral AVMs (p<0.001). Cerebellar AVMs were more likely to drain deep (p=0.036) and less likely eloquent (p<0.001). The predictive accuracy of supplementary grade was better than that of Spetzler-Martin grade with cerebellar AVMs (areas under the ROC curve 0.74 and 0.59, respectively). The predictive accuracy of the supplementary system was consistent for cerebral and cerebellar AVMs, whereas that of the Spetzler-Martin system was greater with cerebral AVMs.
Conclusion
Patients with cerebellar AVMs present with hemorrhage more than patients with cerebral AVMs, justifying an aggressive treatment posture. The supplementary system is better than the Spetzler-Martin system at predicting outcomes after cerebellar AVM resection. Key components of the Spetzler-Martin system, like venous drainage and eloquence, are distorted by cerebellar anatomy in ways that components of the supplementary system are not.
Keywords: arteriovenous malformation, cerebellum, microsurgical resection, Spetzler Martin grading scale, supplementary grading scale
Introduction
Cerebellar AVMs are a small group of AVMs that differ from cerebral AVMs in their hemorrhagic behavior, clinical presentation, and surgical outcomes. They comprise less than 15% of all brain AVMs1, and this relatively low incidence has led authors to combine them with brain stem AVMs in reports on infratentorial or posterior fossa AVMs2-11. This contamination confuses the clinical description of cerebellar AVMs. In this report, we examined the anatomy, surgical strategies, and operative results with only cerebellar AVMs.
Few authors have tried to categorize cerebellar AVMs into distinct subtypes. Yasargil12 categorized 58 cerebellar AVMs into 7 types: hemispheric (superior and inferior, divided by the horizontal fissure), vermian (superior and inferior, divided by the horizontal fissure), cerebellopontine, giant, and fistula. Rhoton and colleagues described 3 cerebellar surfaces (suboccipital, tentorial, and petrosal) in their anatomical studies, but did not apply this to AVMs specifically13. We adapted these efforts to define 5 distinct subtypes of cerebellar AVMs: suboccipital, vermian, tonsillar, tentorial, and petrosal. We found these subtypes offer an intuitive appreciation of their anatomy and surgical management, and are useful in describing surgical results. A focused analysis of outcomes after cerebellar AVM resection enables assessment of our patient selection. Some neurosurgeons have expressed dissatisfaction with the Spetzler-Martin grading scale as a tool for surgical selection with cerebellar AVMs because deep nuclei are the only eloquent structures in the cerebellum and Galenic venous drainage is not a good indicator of cerebellar AVM depth14. We compared the predictive accuracy of this grading scale with that of the supplementary grading scale, which combines patient age, bleeding, and compactness with the assignment of points in a manner analogous with the Spetzler-Martin grading scale. We hypothesized that key components of the Spetzler-Martin scale, like venous drainage and eloquence, are distorted by cerebellar anatomy in ways that the components of the supplementary scale are not, resulting in greater predictive accuracy with the supplementary scale.
Methods
The study was approved by the University of California, San Francisco Committee on Human Research and conducted in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations.
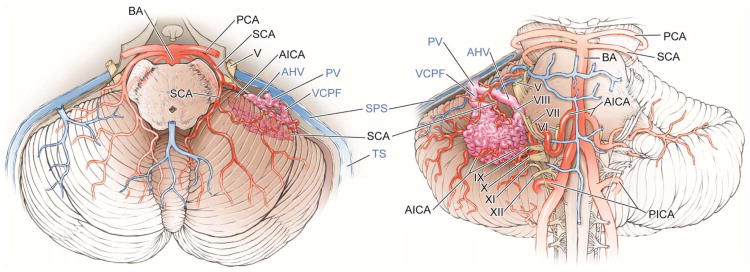
5 Types of Cerebellar AVMs
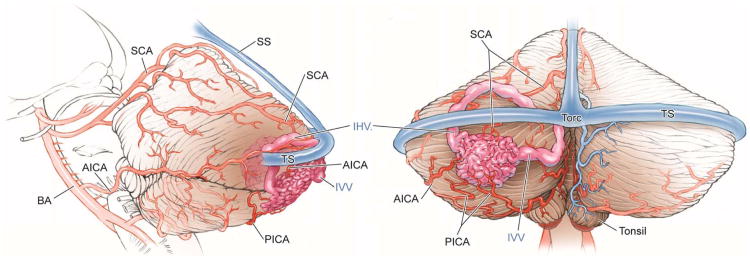
Suboccipital AVMs are based on the posterior cerebellar surface facing the occipital bone, located below and between the transverse and sigmoid sinuses. The hemispheric portion of the suboccipital surface is comprised of the superior semilunar, inferior semilunar, and biventral lobules. The suboccipital surface is divided into superior and inferior parts by its major fissure, the suboccipital fissure. Minor fissures on the suboccipital surface include the petrosal or horizontal fissure (between superior and inferior semilunar lobules), the prebiventral fissure (between inferior semilunar and biventral lobules), and the tonsillobiventral fissure. AVMs based on the vermian portion of the suboccipital surface are categorized as vermian.
Tentorial AVMs are based on the tentorial surface. The hemispheric part of the tentorial surface is comprised of the quadrangular, simple, and superior semilunar lobules. The tentorial surface is divided into anterior and posterior parts by its major fissure, the tentorial or primary fissure. This fissure separates the quadrangular and simple lobules on the hemisphere, and the culmen and declive on the vermis. The postclival fissure separates the simple and superior semilunar lobules. AVMs based on the vermian portion of the tentorial surface are categorized as vermian.
Petrosal AVMs are based on the petrosal surface, the anterior cerebellum that faces the posterior petrous bone. The cerebellopontine angle is the V-shaped cerebellopontine fissure formed where the hemispheric lobules wrap around the pons and middle cerebellar peduncle. The petrosal surface is divided into superior and inferior parts by the petrosal or horizontal fissure, which extends onto the suboccipital surface between the superior and inferior semilunar lobules. The superior and inferior limbs of the cerebellopontine fissure meet laterally at the apex of the CP angle, at the anterior end of the petrosal fissure. The petrosal surface is formed by the anterior surfaces of the quadrangular, simple, semilunar, and biventral lobules, and the flocculi.
Vermian AVMs lie in the midline and may be on the tentorial surface or on the suboccipital surface. The inferior vermis lies in a deep vertical depression in the suboccipital surface called the posterior cerebellar incisura, which also contains the falx cerebelli. The inferior vermis forms the posterior cortical surface within this incisura. In contrast, the superior vermis is the highest point on the cerebellum, occupying the space under the straight sinus where the tentorial leaflets intersect with the falx cerebri. The superior vermis slopes downward from its apex anteriorly to the posterior cerebellar incisura. The tentorial part of the vermian surface includes (from anterior to posterior) the culmen, declive, and folium. The suboccipital part of the vermian surface includes (from superior to inferior) the tuber, pyramid, uvula, and nodule. The nodule is hidden deep to the uvula.
Tonsillar AVMs lie in the tonsils, which are ovoid structures on the inferomedial aspect of the cerebellar hemispheres that attach to cerebellum superolaterally through the tonsillar peduncles. The other tonsillar surfaces are free, with the inferior pole and posterior surfaces in the cisterna magna. The anterior tonsil faces the posterior medulla and is separated by the cerebellomedullary fissure. The medial tonsils face each other and are separated by the vallecula, a cleft that leads into the fourth ventricle. The ventral aspect of the superior tonsil faces the lower half of the roof of the fourth ventricle, which is formed by the tela choroidea, inferior medullary velum, and nodule. The lateral tonsil is separated from the hemisphere by the tonsillobiventral fissure.
Computed tomographic (CT) scans, magnetic resonance imaging (MRI), digital subtraction angiograms (DSA), operative reports, intraoperative photographs, and surgeon notes were systematically reviewed to classify cerebellar AVMs according to the 5 types.
Patients
During a 13-year period, a total of 500 patients with brain AVMs were treated surgically at the UCSF Medical Center by one neurosurgeon (MTL) and recorded in the prospective registry of the UCSF Brain Arteriovenous Malformation Study Project. The registry was searched for patients with cerebellar AVMs and 60 patients were identified for inclusion in this study. Surgical patients with AVMs in the midbrain, pons, and medulla were not included (39 patients). For comparison, the remainder of surgical patients with cerebral AVMs were analyzed (401 patients).
Outcome Evaluation
Neurological outcome was assessed by using the modified Rankin Scale (mRS). Neurological assessments were performed by a nurse clinician, under supervision of a neurologist, preoperatively, postoperatively, and up to 2 years postoperatively. Follow-up information was obtained during routine clinic visits or telephone interviews. Good outcomes were defined as a final mRS score of 0 to 2, and poor outcomes were defined as a final mRS greater than 2. Improvement was defined as a decrease in mRS score (change in the mRS of less than or equal to zero), and deterioration was defined as an increase in mRS score (change in the mRS of greater than zero).
Statistical Analysis
Characteristics of cerebellar versus cerebral AVMs were evaluated using descriptive statistics, including t tests for continuous variables and chi-square tests for categorical variables. Logistic regression analysis was performed using change in mRS score (outcome) versus log-time in combination with either Spetzler-Martin score or the Supplementary score (predictors). Predictive accuracy of the Spetzler-Martin and Supplementary grading scales were quantified by measuring the area under receiver operating characteristic (ROC) curves based on our logistic regression models. An area under the ROC curve of 1.0 indicates perfect discrimination; an area of 0.5 indicates no discrimination; and an area ≥ 0.70 was considered clinically useful. A p-value less than 0.05 was also considered significant for all tests.
Results
Cerebellar AVM Patients
There were 32 women (53%) and 28 men (47%) with a mean age of 41 years (range, 6 to 84). 47 patients (78%) presented with hemorrhage, 7 patients (12%) with headaches or neurological deficits, and 6 patients (10%) had incidental, asymptomatic AVMs. None presented with seizures. Hemorrhagic presentation was more frequent in women (90%) than in men (69%).
The mean cerebellar AVM size was 2.5 cm. The median Spetzler-Martin grade was II, and the median supplementary grade was III (Table 1). Small AVMs (< 3cm) were more likely to present with hemorrhage than larger AVMs (> 3cm) (91% vs. 65%, p < 0.05). In decreasing order of frequency, cerebellar AVM types were as follows: 18 vermian (30%), 13 suboccipital (22%), 12 tentorial (20%), 12 petrosal (20%), and 5 tonsillar (8%) (Table 2). Vermian, tentorial, and tonsillar AVMs had the highest frequency of hemorrhagic presentation (all > 93%).
One patient with hereditary hemorrhagic telangiectasia had a coexisting supratentorial AVM. 12 patients (20%) had intracranial aneurysms, including 10 feeding artery aneurysms and 2 intranidal aneurysms. All 12 aneurysms were diagnosed in patients presenting with hemorrhage. Nine of these patients presented with intracerebellar hemorrhage and the rupture was attributed to the AVM. Three of these patients presented with subarachnoid hemorrhage without intracerebellar hemorrhage, and the rupture was attributed to the aneurysm (unruptured AVM). Therefore, of the 47 patients presenting with posterior fossa bleeding, 44 were from AVM ruptures and 3 were from aneurysm ruptures.
Surgical Management
Patients with suspected cerebellar AVMs were evaluated with preoperative angiography. However, six patients (10%) with large hematomas and brain stem compression were operated on emergently. 4 of these patients underwent hematoma evacuation at outside hospitals before transferring to our institution, deferring AVM resection for later. The other 2 patients presented to our institution and underwent emergency hematoma evacuation and AVM resection without angiography.
Preoperative embolization was performed in 33 patients (55%) (Table 3) using a variety of agents including polyvinyl alcohol (PVA) particles, n-butyl cyanoacrylate (NBCA) glue, and Onyx. Embolic complications occurred in one patient: an AICA perforation resulting in SAH and a pontine infarct with new facial numbness and contralateral hemisensory deficit.
7 different approaches were used for cerebellar AVMs, consisting of mainly suboccipital craniotomy, far lateral craniotomy, retrosigomoid craniotomy, or combination craniotomies (Table 2). Vermian AVMs were typically resected through torcular craniotomies (89%), improving access to the superior vermian surface, relative to the standard subocciptial craniotomy, by removing the ledge of overhanging bone and lift the transverse sinuses with tacking sutures on the dural flap. Tentorial AVMs were also resected through torcular craniotomies (58%) because of this improved access to the tentorial cerebellar surface. Petrosal AVMs were resected through extended retrosigmoid craniotomies (67%), with skeletonization of the sigmoid sinus from transverse-sigmoid junction to jugular bulb for wider access to the petrosal surface and CP angle. Tonsillar AVMs were resected through standard midline suboccipital craniotomies (80%). Suboccipital AVMs had the widest variety of surgical approach, the most common being a lateral suboccipital craniotomy (31%). Combination approaches were used for larger AVMs at the margins of the suboccipital surface, with the far lateral approach adding to the inferolateral exposure, the extended retrosigmoid approach adding to the superolateral exposure, and the torcular approach adding to the superior exposure.
Complete AVM resection was achieved in all 60 patients and confirmed angiographically (surgical obliteration rate, 100%). All AVMs were resected in a single stage. Complications included a postoperative intracerebellar hematoma that required evacuation in one patient, and a superficial wound infection that required debridement in another patient. All three ruptured aneurysms were treated: 2 SCA aneurysms with preoperative endovascular coiling and 1 AICA aneurysm with simultaneous clipping.
Patient Outcomes
3 patients died in the perioperative period (surgical mortality, 5%). Two of these patients had ruptured AVMs, presented in coma, and failed to improve after aggressive management. One of these patients had a Spetzler-Martin grade IV AVM, difficult intraoperative bleeding, and significant postoperative deficits, and support was withdrawn. 12 patients had neurological deficits perioperatively that resolved completely at late follow-up (transient neurological morbidity, 20%).
Follow-up evaluations (mean duration, 1.1 years) were performed in all but 3 patients. Of the remaining patients, 3 additional patients died. One death was caused by a delayed cerebellar abscess in a patient that had done well with surgery (unchanged at early follow-up). Two deaths occurred 1 and 5 years after surgery in patients who were worse postoperatively, but living dependently. Overall, 42 patients (74%) had good outcomes (mRS 0 – 2), 9 patients (16%) had poor outcomes (mRS 3 – 4), and 6 patients died (11%) (Table 3). Relative to preoperative neurological condition, 44 patients were improved or unchanged after surgery (77%) and 13 patients (23%) were worsened or dead. Of the 7 patients who were worsened, 6 patients had good outcomes (2 patients, mRS 1 and 4 patients, mRS 2).
Patients with tonsillar and tentorial AVMs fared best (improved/unchanged in 100% and 92%, respectively). These patients also had the lowest Spetzler-Martin and supplementary grades (Table 2). Patients with petrosal and vermian AVMs fared worst (improved/unchanged in 60% and 72%, respectively). Patients with petrosal AVMs had the highest Spetzler-Martin and supplementary grades (Table 2).
Comparison of Cerebellar and Cerebral AVMs
Differences in patient age and sex were not significant with cerebellar and cerebral AVMs (p=0.543). However, cerebellar AVMs are more likely than cerebral AVMs to present with hemorrhage (78% vs. 53%, p<0.001). Increased hemorrhagic presentation accounted for worse pre-surgical mRS scores in patients with cerebellar AVMs (Table 3, p<0.001). Hemorrhagic presentation with cerebellar AVMs is not attributable to differences in AVM size, as measured by mean diameter or by size scores on the Spetzler-Martin scale (Table 1). However, cerebellar AVMs are more likely than cerebral AVMs to have deep venous drainage (57% vs. 41%, p=0.036) and less likely to have eloquence (39% vs. 70%, p<0.001). Overall differences in Spetzler-Martin grades were not significant (p=0.096).
Cerebellar AVMs were no more compact or diffuse than cerebral AVMs (p=0.322). Overall differences in supplementary AVM grades were not significant (p=0.298). Deep perforator supply was twice as frequent with cerebral AVMs than with cerebellar AVMs (21% vs. 10%, p=0.050). Cerebral AVMs were more likely to require multiple stages than cerebellar AVMs (9% vs. 0%, p=0.033). Patients with cerebral AVMs tended to have more favorable outcomes than those with cerebellar AVMs (Table 3, p=0.014).
Predictive Accuracy of Grading Scales with Cerebellar AVMs
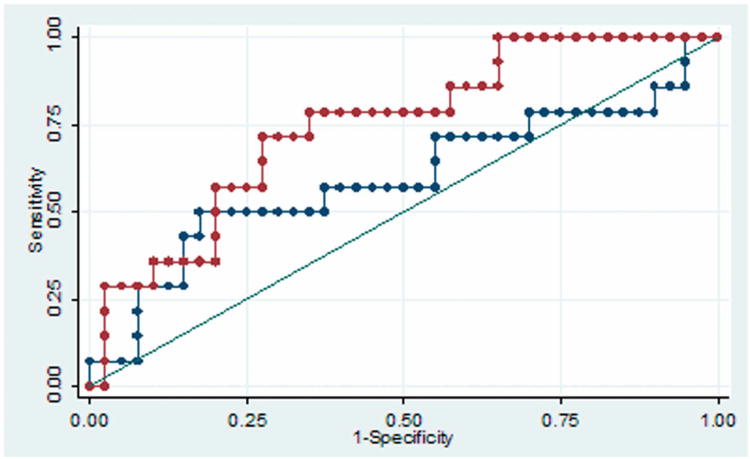
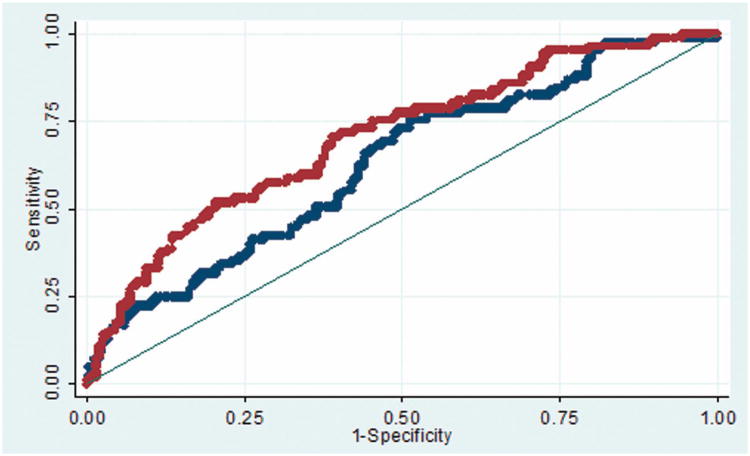
The predictive accuracy of the supplementary AVM grade was better than that of the Spetzler-Martin grade with both cerebellar (Figure 1A) and cerebral AVMs (p = 0.05, Figure 1B). For cerebellar AVMs, the areas under the ROC curve were 0.74 and 0.59 for supplementary and Spetzler-Martin scores, respectively. For cerebral AVMs, the areas under the ROC curve were 0.70 and 0.63 for supplementary and Spetzler-Martin scores, respectively.
Figure 1.
(A) The predictive accuracy of the supplementary AVM grade (red curve) was better than that of the Spetzler-Martin grade (blue curve) with cerebellar AVMs. The areas under the ROC curve were 0.74 and 0.59 for supplementary and Spetzler-Martin scores, respectively. (B) The predictive accuracy of the supplementary AVM grade (red curve) was better than that of the Spetzler-Martin grade (blue curve) with cerebral AVMs. The areas under the ROC curve were 0.70 and 0.63 for supplementary and Spetzler-Martin scores, respectively.
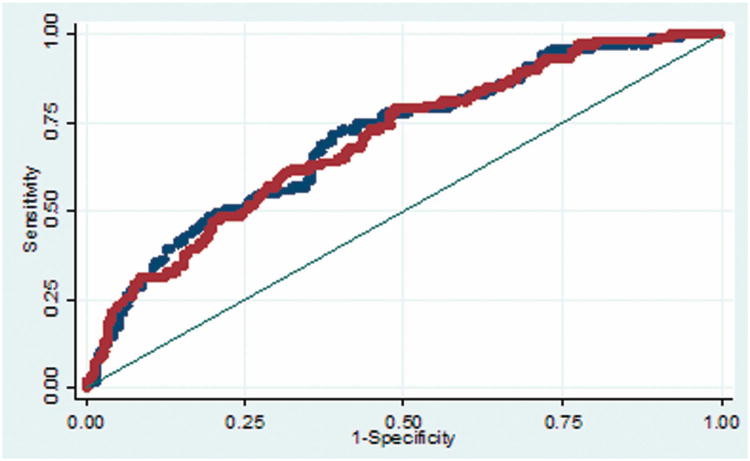
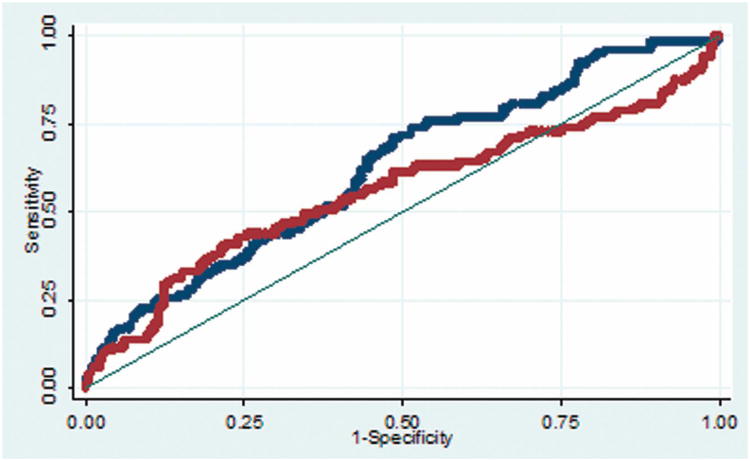
The predictive accuracy of the supplementary AVM grading scale is consistent for cerebral and cerebellar AVMs (Figure 2A), with areas under the ROC curve of 0.70 and 0.70, respectively. In contrast, the predictive accuracy of the Spetzler-Martin grade is greater with cerebral AVMs than with cerebellar AVMs (Figure 2B), with areas under the ROC curve of 0.62 and 0.56, respectively. These analyses demonstrate that the supplementary AVM grading scale has greater predictive accuracy than the Spetzler-Martin scale, and that its accuracy is consistent with all AVMs, including cerebellar AVMs. The greatest predictive accuracy from the grading systems comes from combining the two scores in a supplemented Spetzler-Martin score (Table 4).
Figure 2.
(A) The predictive accuracy of the supplementary AVM grading scale is consistent for cerebellar AVMs (red curve) and cerebral AVMs (blue curve), with areas under the ROC curve of 0.70 and 0.70, respectively. (B) The predictive accuracy of the Spetzler-Martin grade is greater with cerebral AVMs (blue curve) than with cerebellar AVMs (red curve), with areas under the ROC curve of 0.62 and 0.56, respectively.
Discussion
Hemorrhage Risk with Cerebellar AVMs
This report describes a large surgical experience with 60 cerebellar AVMs, accounting for 12% of all AVMs. Patients with cerebellar AVMs are significantly more likely to present with hemorrhage than patients with cerebral AVMs (three quarters vs. one half). Hemorrhagic presentation results in more neurological deficits both preoperatively and at late follow-up3,15-17. The explanation for increased bleeding from cerebellar AVMs is unclear18,19. Deep venous drainage is associated with increased rupture risk and was significantly increased in our cerebellar AVMs. Small nidus size is also associated with increased rupture risk, but there were no differences in size between cerebellar and cerebral AVMs. Significant differences were observed with eloquence and deep perforator supply, but these factors are not associated with AVM rupture. Patients with cerebellar AVMs and associated aneurysms did not have a higher incidence of hemorrhage.
One important difference between cerebellar and cerebral AVMs is that cerebellar AVMs do not present with seizures. Overall, 23% of patients with cerebral AVMs presented with seizures, which approximated the difference in hemorrhagic presentation between patients with cerebellar and cerebral AVMs. Therefore, the increased rupture risk with cerebellar AVMs may be unrelated to their anatomy, and instead may be explained by their lack of seizures. Hemodynamic differences between cerebellar and cerebral AVMs may be relevant, but are difficult to measure and are beyond the scope of this report. Nonetheless, the increased hemorrhagic behavior of cerebellar AVMs supports a more aggressive management posture1-3,15,20. We recommend resection in most patients presenting with ruptured AVMs, including 13% with high Spetzler-Martin grades. We recommend treatment in most patients with unruptured cerebellar AVMs as well, selecting patients with low and intermediate grade AVMs for surgery and high grade AVMs for radiosurgery21. We are less inclined to recommend treatment in patients with unruptured cerebral AVMs, particularly the high-grade AVMs presenting incidentally or with seizures21-23.
AVM Grading
This experience with cerebellar AVMs offers some insights into AVM grading. Our analysis of receiver operating characteristic curves revealed that the Spetzler-Martin grading system tended not to be as good as the supplementary grading system at predicting patient outcomes after resection of cerebellar AVMs (Figure 1). Areas under the ROC curve exceeded thresholds for clinical utility with the supplementary scale, but not with the Spetzler-Martin scale. This was also true with cerebral AVMs. The predictive accuracy of the supplementary scale was the same for cerebellar and cerebral AVMs, demonstrating consistency with all AVMs. In contrast, the predictive accuracy of the Spetzler-Martin scale decreased with cerebellar AVMs (Figure 2).
The Spetzler-Martin grading system is well designed for cerebral AVMs where venous drainage to the Galenic system is an excellent indicator of AVM depth24. Similarly, eloquence is an important determinant of surgical risk in the cerebral hemispheres where there are numerous eloquent areas in the motor cortex, sensory cortex, visual cortex, basal ganglia, thalamus, and hypothalamus25. These two components of the Spetzler-Martin grading scale are distorted by cerebellar anatomy. First, venous drainage to the Galenic system is not a reliable indicator of cerebellar AVM depth. Superior vermian and precentral cerebellar veins are the only cerebellar veins draining to the Galenic complex and these veins are superficial, relative to the cerebellum as a whole. Cerebellar AVMs drained frequently through the Galenic complex (57%), which was significantly increased compared to cerebral AVMs (41%). Assessment of deep venous drainage specifically and cerebellar AVM depth generally are artificially elevated by the Spetzler-Martin system, which defines deep venous drainage as everything except “cerebellar hemispheric veins that drain directly into the straight sinus or transverse sinus” 24. Conversely, draining veins at the depth of the cerebellar nuclei often connected to non-Galenic veins that course to the straight sinus, torcula, and transverse sinuses, which are considered superficial and would not be assigned a point on the scale. Therefore, the conventional assignments of venous drainage scores can misjudge cerebellar AVM depth. Second, the deep nuclei are the only eloquent structures in the cerebellum. As a result, eloquence with cerebellar AVMs was half that of cerebral AVMs (30% and 61%, respectively). Therefore, the importance of eloquence as a risk predictor with cerebellar AVMs is reduced in the Spetzler-Martin scale. It is interesting to note that there were no Spetzler-Martin grade V AVMs in the_cerebellum. Only one AVM was greater than 6 cm in diameter, and this low likelihood of large AVM size limits Spetzler-Martin grades in the posterior fossa to grades I – IV.
In contrast, the supplementary grading system embodies three factors (patient age, bleeding, and compactness) that are not susceptible to anatomical differences between the cerebellum and cerebrum. Compactness (or diffuseness) relates the surgical plane of dissection around the AVM to patient outcome and does not depend on AVM location22,26. Age is a clinical factor that is also independent of AVM location22,27. The supplementary scale incorporates the increased incidence of hemorrhagic presentation already discussed. Therefore, it is not surprising that the supplementary scale outperformed the Spetzler-Martin scale in the cerebellum, and was consistently accurate for all AVMs and all locations.
Cerebellar AVM Types
The subocciptal surface is the largest and most accessible of the cerebellar surfaces. AVM exposure depends on its location on the surface: medial or lateral, superior or inferior. Suboccipital AVMs (Figure 3) had the widest variety of surgical approach, the most common being a lateral suboccipital craniotomy (31%). Combination approaches were used for larger AVMs at the margins of the suboccipital surface, with the far lateral approach adding to the inferolateral exposure, the extended retrosigmoid approach adding to the superolateral exposure, and the torcular approach adding to the superior exposure. The skin incision was typically a linear midline incision for more medial craniotomies and a “hockey stick” or lateral horseshoe incision for more lateral or combination craniotomies. The approach is perpendicular, with good access to all sides of the nidus. Subarachnoid dissection is minimal because suboccipital AVMs are supplied by distal cortical branches from SCA superiorly, PICA inferiorly, and AICA laterally, with relative contributions of each depending on the nidus size and location in the hemisphere. SCA feeders are identified as they course inferiorly over the superior semilunar lobule. AICA feeders are identified as they course medially over the superior and inferior semilunar lobules. PICA feeders are identified as they emerge from the tonsillobiventral fissure and course superiorly over the biventral lobule. Cortical feeders are occluded with the circumscribing incision around the AVM, and are typically unilateral. Deep perforating arteries are encountered along the deep plane of the nidus. The draining veins are typically superficial, including the inferior vermian veins and inferior hemispheric veins that drain to the torcula or transverse sinuses. Larger AVMs can have draining veins that cross to the contralateral hemisphere or travel anteriorly to the vein of Galen. Suboccipital AVMs have no associated cranial nerves and are noneloquent, unless they are large and extend down to the deep cerebellar nuclei.
Figure 3.
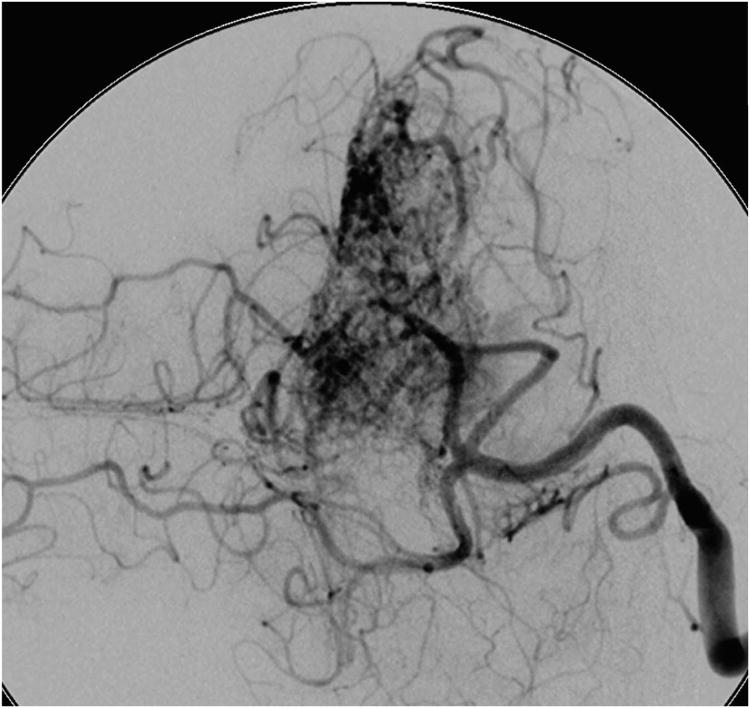
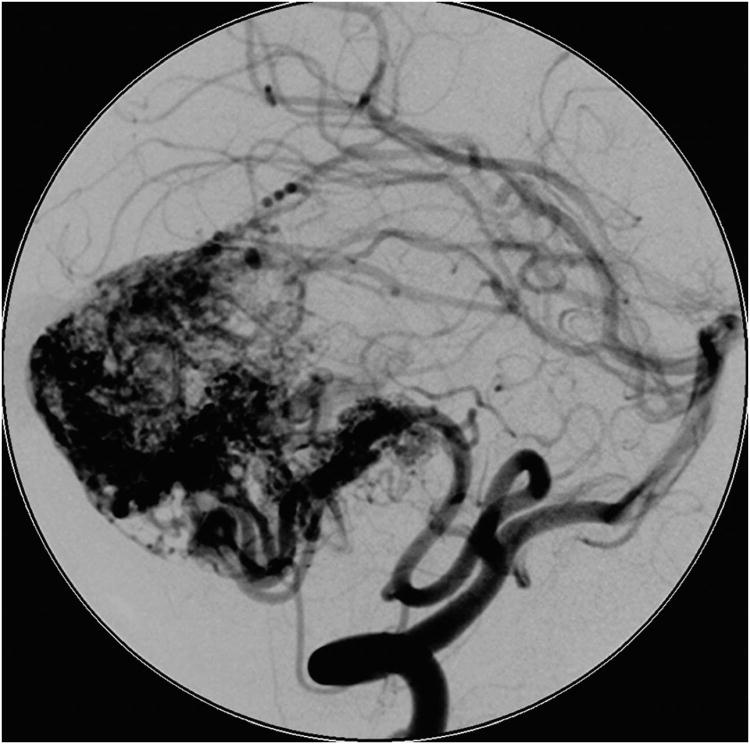
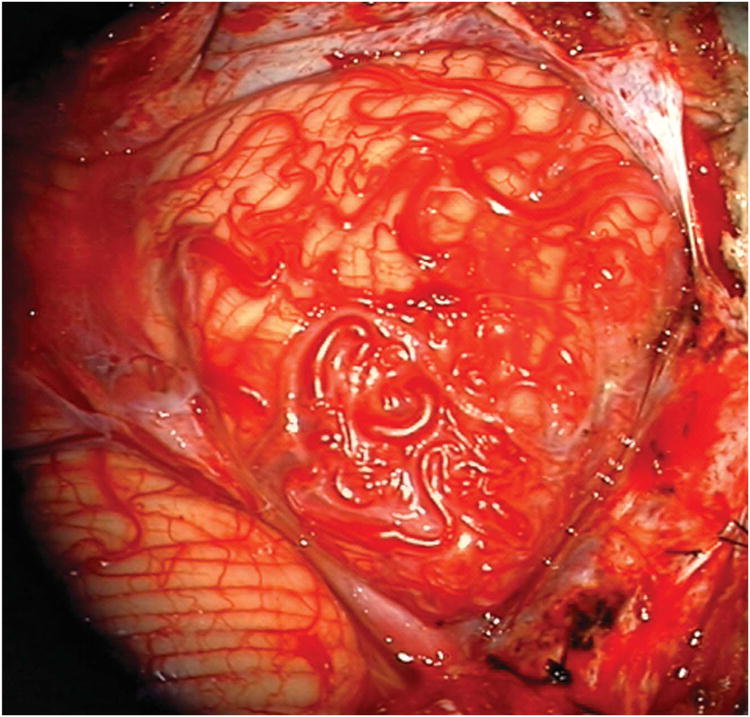
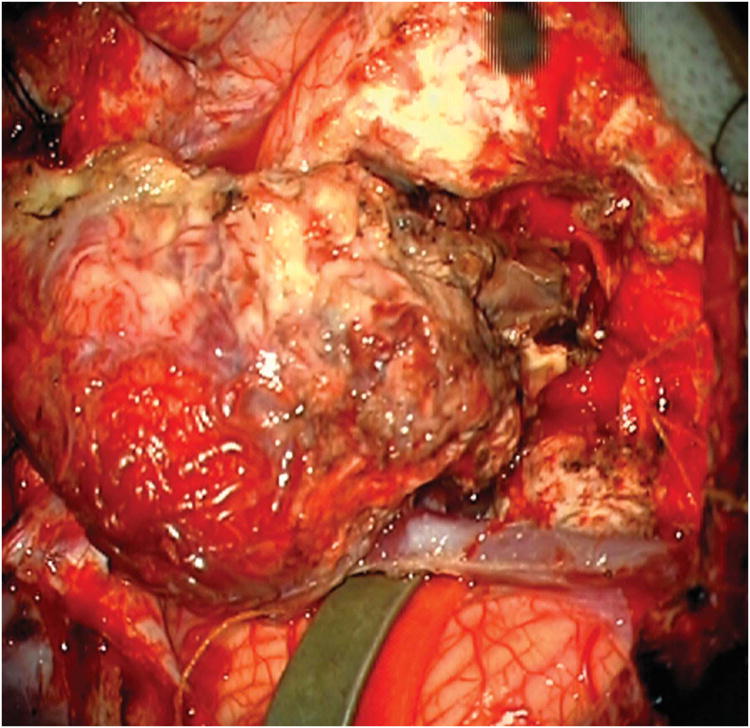
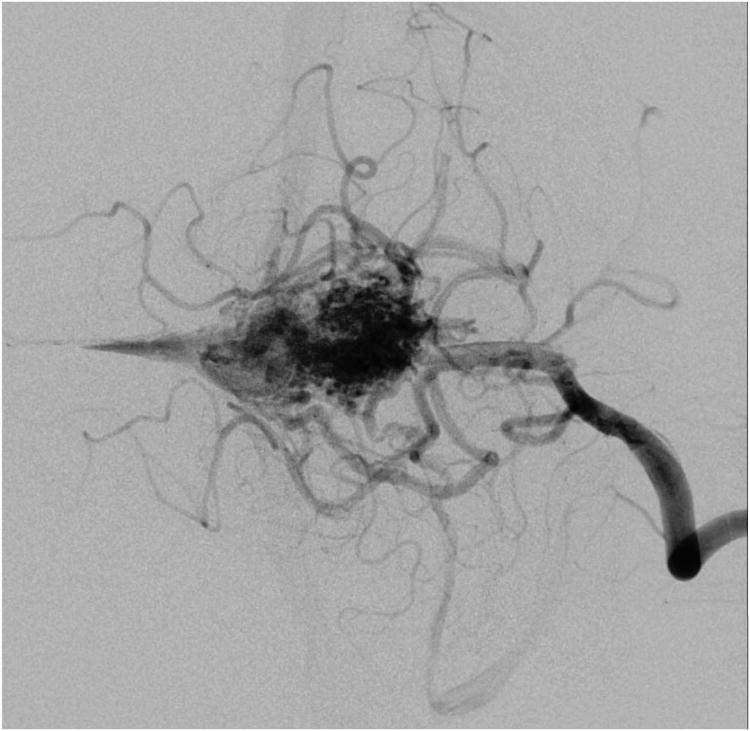
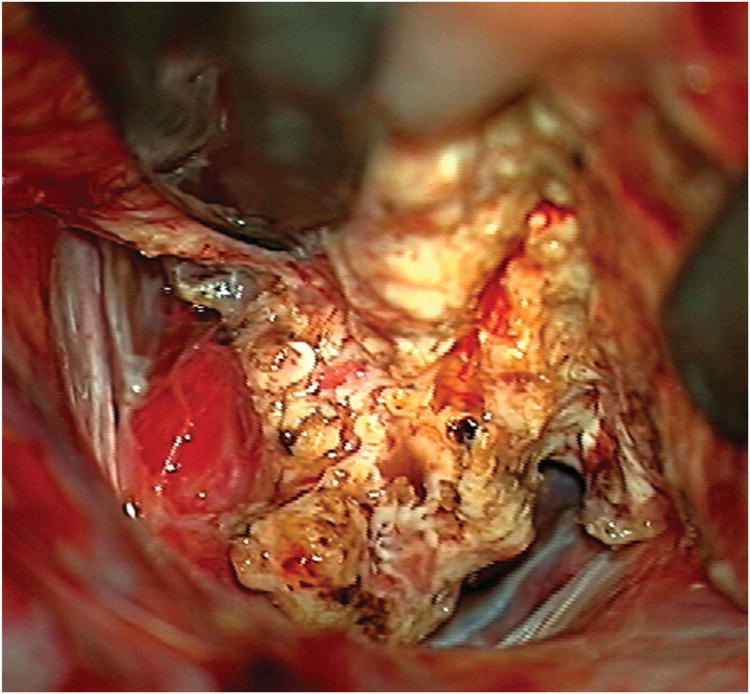
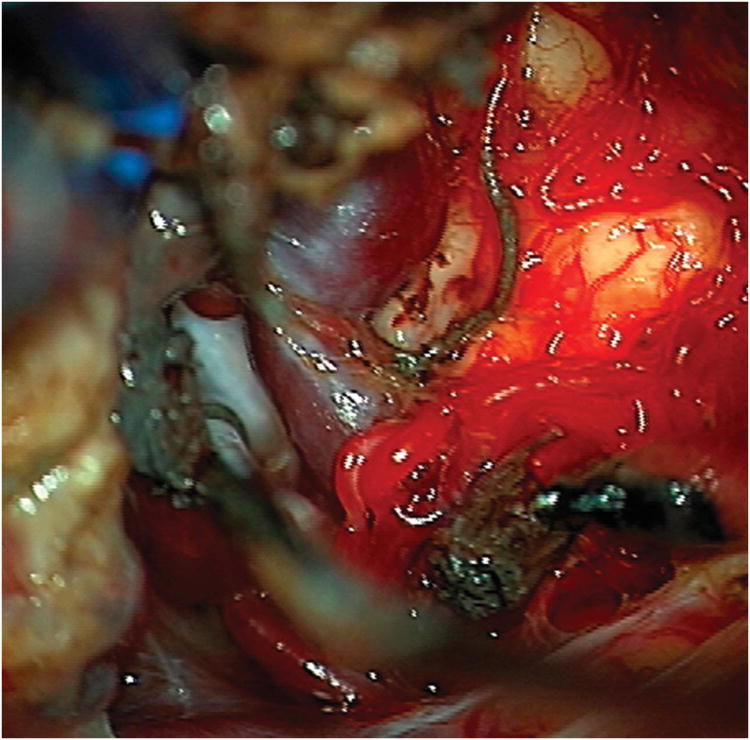
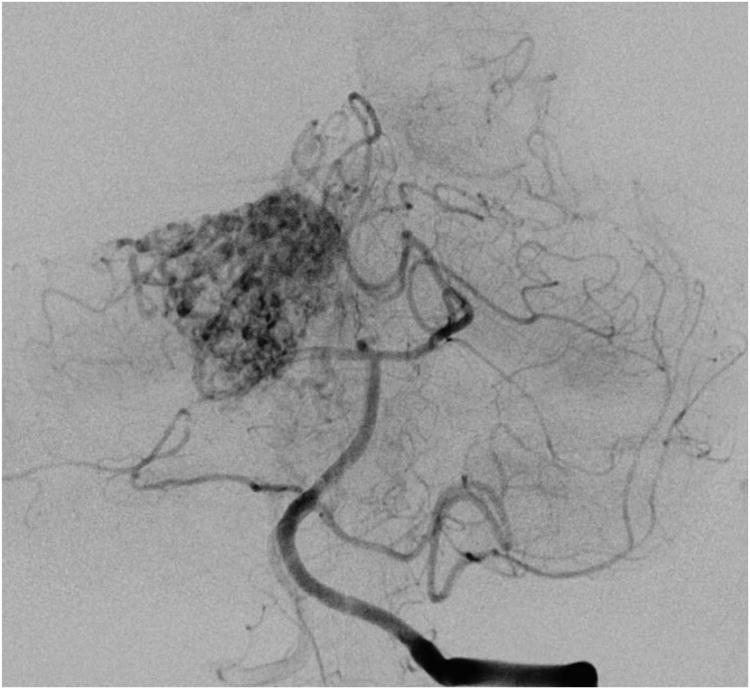
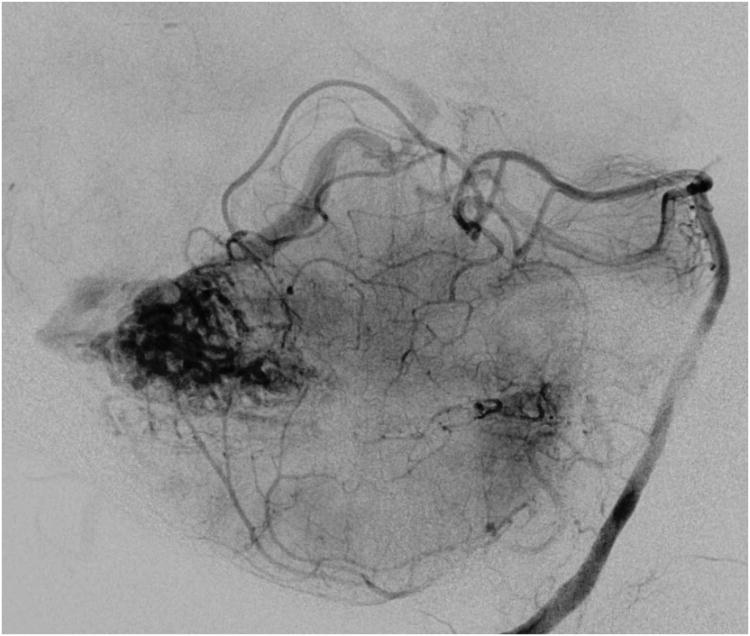
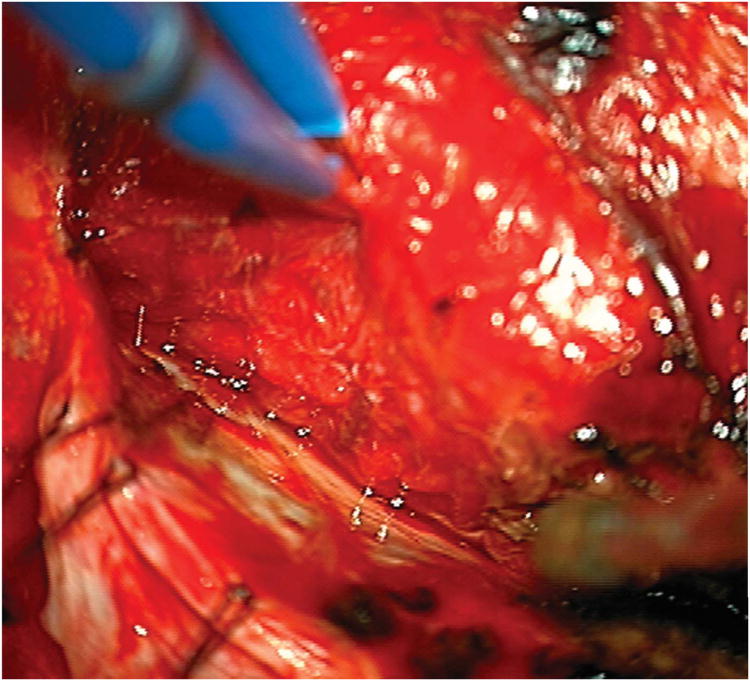
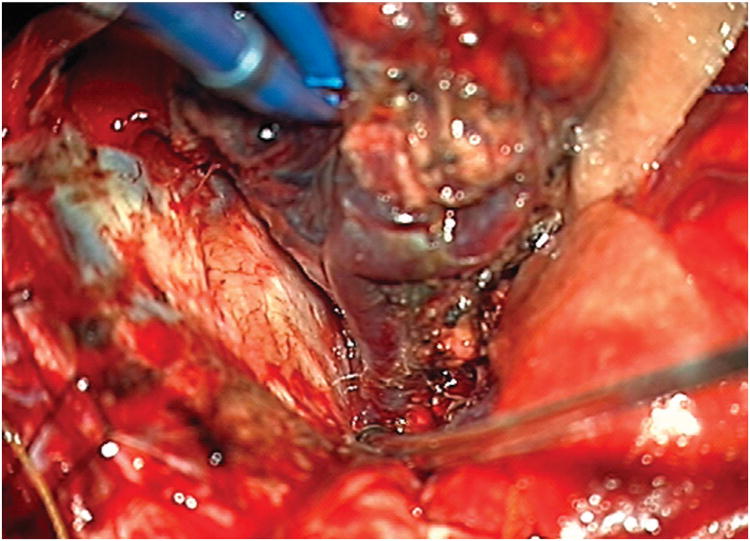
Cerebellar AVM, suboccipital subtype. The suboccipital AVM is based on the posterior cerebellar surface facing the occipital bone, below the transverse sinus, as seen in (A) left lateral and posterior views. The AVM is supplied by distal cortical branches from SCA superiorly, PICA inferiorly, and AICA laterally. This suboccipital AVM in a 6 year-old boy (Spetzler-Martin grade IV (S2V1E1), supplementary grade II (A1U0D1)) demonstrates unilateral supply from the left posterior inferior cerebellar artery (PICA) and superior cerebellar artery (SCA), as seen on left vertebral artery digital subtraction angiography ((B) anteroposterior and (C) lateral views). (D) The entire suboccipital surface and all margins of the AVM were exposed through a suboccipital/torcular craniotomy that crossed the transverse sinuses and torcula. This AVM was diffuse, requiring a wide pia incision and dissection through some normal appearing parenchyma. The hematoma was encountered at the depth of the dissection. (E) The venous pedicle drained superficially to the torcula. Abbreviations: BA = basilar artery; AICA = anterior inferior cerebellar artery; PICA = posterior inferior cerebellar artery; SCA = superior cerebellar artery; SS = straight sinus; TS = transverse sinus; IVV = inferior vermian vein; IHV = inferior hemispheric vein; Torc = torcula.
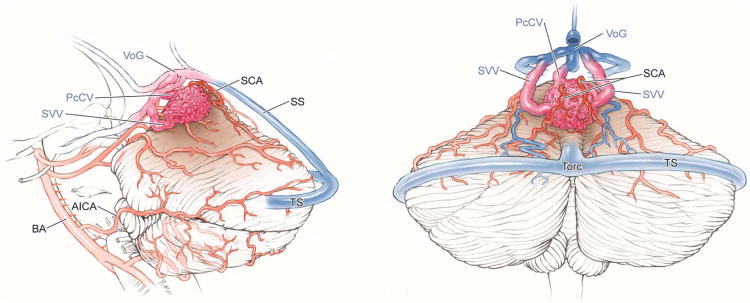
Vermian AVMs (Figure 4) are located in the midline and exposed with a torcular craniotomy to gain access to both the suboccipital and tentorial surfaces. The suboccipital part of the vermis (tuber, pyramid, uvula, and nodule) is superficial and easily accessed, but the tentorial part (culmen, declive, and folium) is deep and requires subarachnoid dissection to open the supracerebellar-infratentorial plane. The ascending slope of the tentorial part of the vermis requires significant neck flexion when positioning the head, tucking the chin two finger breadths from the manubrium in the prone position to align the tentorium vertically. Alternatively, small AVMs at the apex of the vermis or anteriorly in the quadrigeminal cistern can be approached with the patient in the sitting position, which allows gravity to retract the cerebellum and open the supracerebellar-infratentorial plane. Vermian AVMs attract bilateral feeding arteries, with superior vermian AVMs supplied by SCAs and inferior vermian AVMs supplied by PICAs. Superior vermian AVMs are much more common than inferior vermian AVMs (90% and 10%, respectively). Surgical exposure is perpendicular with inferior vermian AVMs but tangential with superior vermian AVMs, requiring some transgression of the posterior vermis to access the inferior margins. The SCA feeders are identified by incising the posterior arachnoid of the quadrigeminal cistern on both sides of the vermian apex and opening the cerebellomesencephalic fissure where the cortical branches (s4 segments) emerge28. Feeders are traced to the AVM margin and coagulated, carefully preserving arteries to the tectum and posterior midbrain. PICA feeders originate beyond its cranial loop along the distal telovelotonsillar (p4) and cortical (p5) segments28. Venous drainage is through superior vermian veins, which drain to the Galenic complex (unlike inferior vermian veins). Vermian AVMs are not considered eloquent unless they extend to the cerebellar nuclei, and can be near but not associated with the trochlear nerve.
Figure 4.
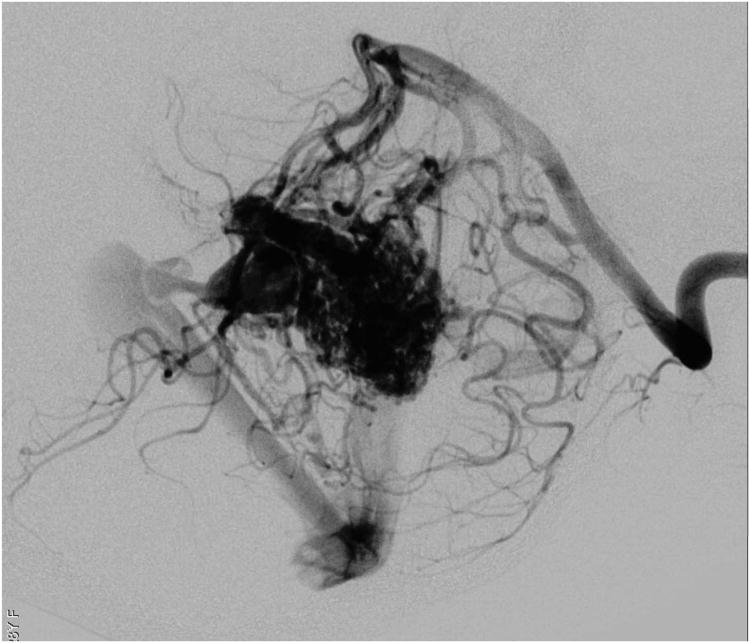
Cerebellar AVM, vermian subtype. The vermian AVM is midline and may be located on the suboccipital vermis or the tentorial vermis, as shown here in (A) left lateral and posterior views. The AVM is supplied by distal SCA branches bilaterally as they emerge from the cerebellomesencephalic fissure and course over the tentorial surface of the cerebellum. This vermian AVM in a 28 year-old woman (Spetzler-Martin grade III (S2V1E0), supplementary grade II (A2U0D0)) demonstrates bilateral supply from both SCAs, as seen on right vertebral artery digital subtraction angiography ((B) anteroposterior and (C) lateral views). Venous drainage into the precentral cerebellar vein/vein of Galen and the petrosal vein/superior petrosal sinus. (D) Torcular craniotomy in the prone position exposed the superior vermis, through which AVM was accessed. SCA feeding arteries were exposed under the tentorium, anterior to the nidus, and interrupted along the superior and lateral margins of the AVM. (E) The AVM remained attached to its ascending venous pedicle, which was traced to the vein of Galen. Abbreviations: BA = basilar artery; AICA = anterior inferior cerebellar artery; PICA = posterior inferior cerebellar artery; SS = straight sinus; TS = transverse sinus; SVV = superior vermian vein; PcCV = precentral cerebellar vein; VoG = vein of Galen; Torc = torcula.
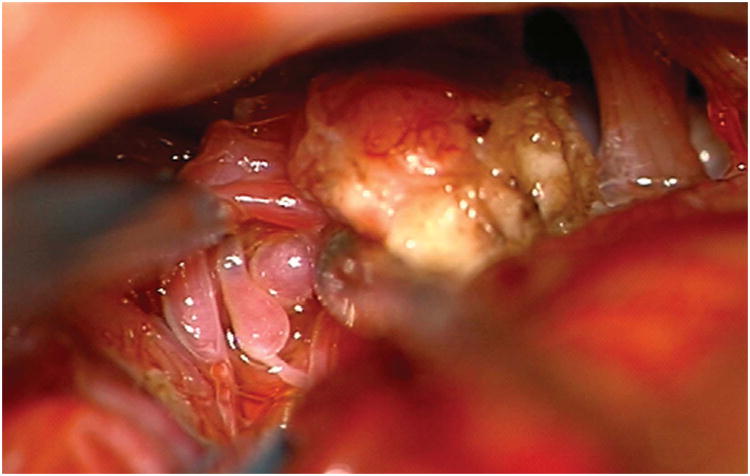
Tonsillar AVMs (Figure 5) are paramedian and small, and a standard suboccipital craniotomy provides good perpendicular exposure. Subarachnoid dissection includes opening cisterna magna, widening the vallecula to separate the tonsils, opening the cerebellomedullary fissure, and splitting the tonsillobiventral fissure. These maneuvers circumferentially mobilize the tonsil and gain control of PICA as it courses around the medulla. Upward retraction on the inferior pole of the tonsillar AVM exposes PICA proximally for early interruption of feeding arteries. Distal en passage arteries are preserved, but PICA frequently terminates in the AVM. Drainage courses through the tonsillar veins: medial and lateral tonsillar veins drain to superior and inferior retrotonsillar veins, which collect in inferior vermian veins that drain to the torcula or transverse sinuses. Tonsillar AVMs are noneloquent, but adjacent to the medulla and glossopharyngeal, vagus, and spinoaccessory nerves.
Figure 5.
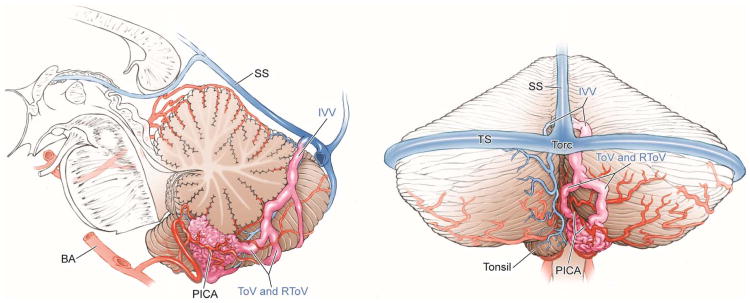
Cerebellar AVM, tonsillar subtype. The tonsillar AVM is a small, paramedian AVM in the tonsil, easily circumscribed in the cisterna magna by separating the tonsils, opening the cerebellomedullary fissure, and splitting the tonsillobiventral fissure. The AVM is fed unilaterally by PICA (left lateral view), which can be interrupted early in the dissection. It is drained by tonsillar and inferior vermian veins (posterior view). Abbreviations: BA = basilar artery; PICA = posterior inferior cerebellar artery; SS = straight sinus; TS = transverse sinus; Torc = torcula; IVV = inferior vermian vein; ToV = tonsillar vein; RToV = retrotonsillar vein.
Tentorial AVMs (Figure 6) on the tentorial surface of the cerebellum are accessed tangentially through supracerebellar-infratentorial dissection. When the craniotomy exposes the torcula and transverse sinus, the dural flap elevates the sinuses and widens this plane. The breadth of the craniotomy (lateral suboccipital, torcular, or extended retrosigmoid) depends on AVM location and size. Patient position is prone with the chin tucked as described for vermian AVMs. Release of dense arachnoid adhesions between the posterior border of the tentorial surface and the tentorium begins the dissection over the cerebellum. Bridging veins to the tentorium can be sacrificed if they do not drain the AVM. Dissection continues to the AVM or its draining vein, which can be visualized on the tentorial surface. Tentorial AVMs are fed by distal cortical SCA branches (s4 segment), usually unilaterally, which can be interrupted early in the dissection28. The draining vein is often prominent in the surgical field and dissection proceeds around it to deeper borders. This being a tangential approach, it requires a cortical incision into cerebellum that helps better visualize the posterior and inferior margins. Evacuation of associated hemispheric hematomas facilitates this tangential dissection. The AVM is lifted up out of the hemisphere, towards the tentorium. Venous drainage is typically through superior hemispheric veins that course superficially to the tentorium/tentorial sinus, transverse sinus or torcula, or course deep to the vein of Galen. The vein is an obstacle during the resection, but must be carefully preserved. Tentorial AVMs are noneloquent, but anteriorly located AVMs are near midbrain and trochlear nerve.
Figure 6.
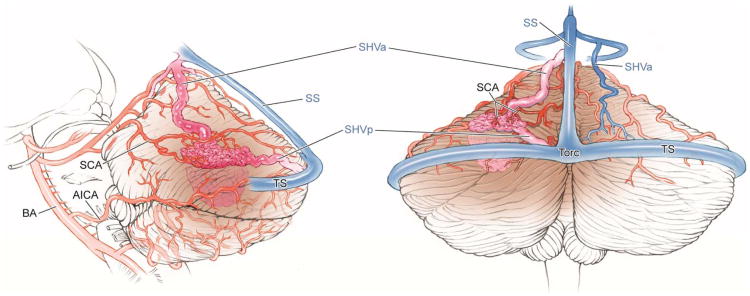
Cerebellar AVM, tentorial subtype. The tentorial AVM is based on the tentorial surface, as seen in (A) left lateral and posterior views. The AVM is supplied by distal branches of the SCA unilaterally, and can drain superficially to transverse or straight sinuses, deep to vein of Galen, or both. This tentorial cerebellar AVM in a 62 year-old man presented with a subarachnoid hemorrhage from a feeding artery aneurysm (Spetzler-Martin grade III (S2V1E0), supplementary grade IV (A3U0D1)). Left vertebral artery digital subtraction angiography demonstrated SCA supply ((B) anteroposterior and (C) lateral views). Venous drainage was deep via a superior hemispheric vein. (D) A torcular craniotomy provided access to the tentorial surface, but the AVM was not visible on the suboccipital surface. The AVM was only visible after dissecting the supracerebellar-infratentorial plane and exposing the tentorial surface. (E) As the nidus was dissected circumferentially, the draining superior vermian vein darkened. Abbreviations: BA = basilar artery; AICA = anterior inferior cerebellar artery; SCA = superior cerebellar artery; SS = straight sinus; TS = transverse sinus; SHVa = supferior hemispheric vein, anterior; SHVp = superior hemispheric vein, posterior; Torc = torcula.
Petrosal AVMs (Figure 7) on the petrosal surface of the cerebellum are accessed through an extended retrosigmoid approach that skeletonizes the sigmoid sinus from the transverse-sigmoid junction to the jugular bulb, allowing the dural flap to pull the sinus anteriorly and maximize the opening into the CP angle. This craniotomy minimizes the need for fixed retraction29. The approach trajectory is tangential rather than perpendicular, which means that the lateral and posterior AVM margins are accessed at the expense of some overlying cerebellum. Subarachnoid dissection opens the lateral arachnoid of the prepontine cistern to identify the vestibulocochlear and facial nerves (CN VII/VIII) and AICA, the main feeder to these petrosal AVMs. Subarachnoid dissection extends superiorly to the trigeminal nerve (CN V) when SCA contributes to the AVM, and inferiorly to the lower cranial nerves (CN IX/X/XI) when PICA contributes. Petrosal AVMs are cerebellar rather than pontine, and therefore reside lateral to CN VII/VIII. AICA feeders arising from the flocculopeduncular (a3) and cortical (a4) segments are interrupted early by dissecting medial to the nidus, but many of these medial feeders cannot be visualized early in the resection28. The tangential nature of this exposure requires an incision in the cerebellar cortex lateral to the AVM and resection of some intervening lobule to reach the lateral AVM margin. In hemorrhagic cases, this route can access hemispheric hematomas for early evacuation and relaxation of swollen cerebellum. The AVM is then circumscribed, dissecting around the back side and rolling it anteriorly away from the middle cerebellar peduncle and cranial nerves deep to the nidus. Scooping the AVM from behind leaves deep feeders until the end of the resection, and draining veins must be meticulously preserved. AVM drainage is via anterior hemispheric veins and the vein of the cerebellopontine fissure, which course to the petrosal vein (Dandy's vein) and superior petrosal sinus. Petrosal AVMs are noneloquent but adjacent to pons, middle cerebellar peduncle, and CN VII/VIII. The dissection must remain lateral to the cranial nerves to avoid entry into the brain stem.
Figure 7.
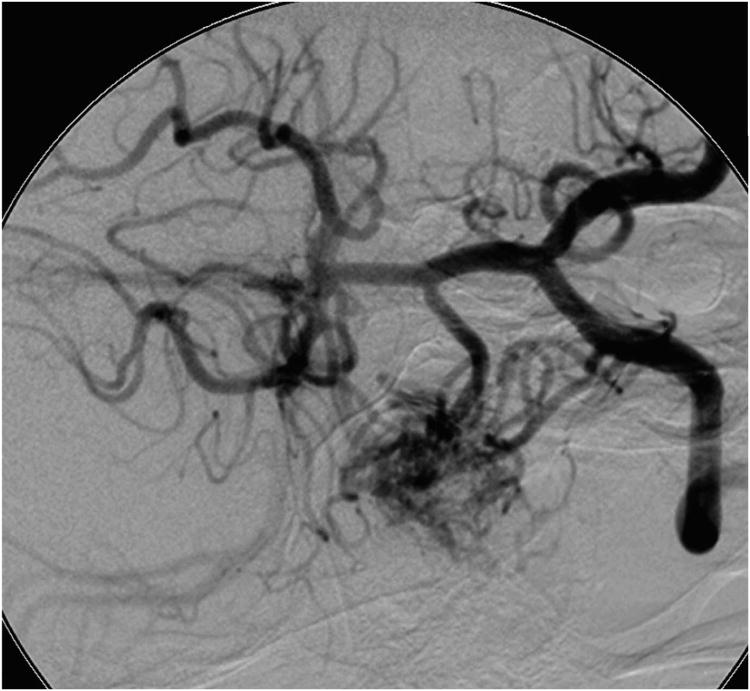
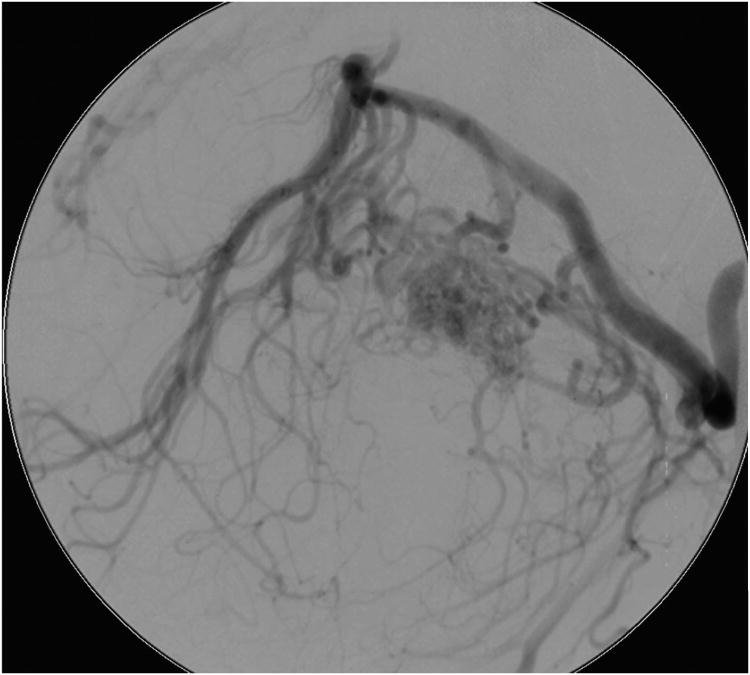
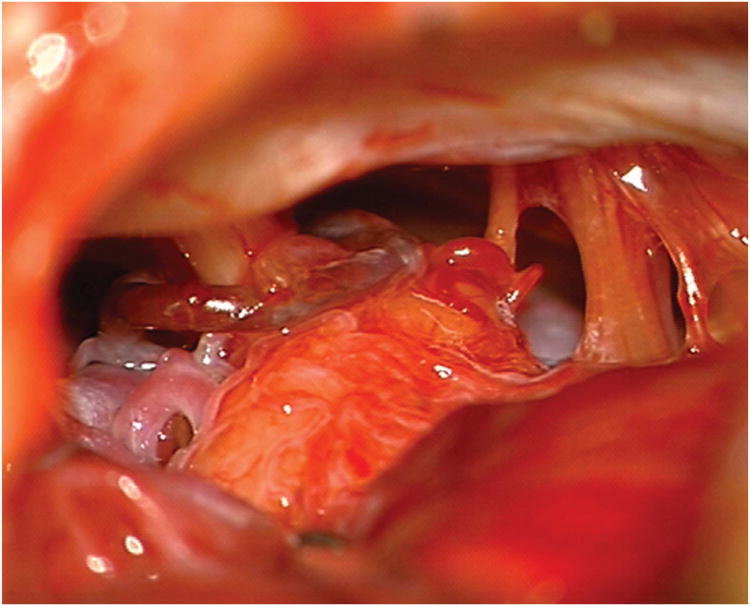
Cerebellar AVM, petrosal subtype. The petrosal AVM lies in the anterior cerebellum facing the petrous bone, lateral to cranial nerves VII and VIII, and extending into the cerebellopontine angle, as seen on these (A) superior and anterior views. AICA is the main arterial feeder to these AVMs. This petrosal cerebellar AVM in a 57 year-old man (Spetzler-Martin grade III- (S1V1E1), supplementary grade III (A3U0D0)) was fed by a large right anterior inferior cerebellar artery, as seen on left vertebral artery digital subtraction angiography ((B) anteroposterior and (C) lateral views). (D) An extended retrosigmoid craniotomy exposed the AVM in the cerebellopontine angle around cranial nerves VII and VIII. Note CN IX, X, and XI below the nidus. (E) Dissection through the flocculus exposed the lateral margin and feeding arteries along the superior and inferior margins. The medial margin was accessed by deepening the dissection to and along the pontine surface.
Abbreviations: BA = basilar artery; AICA = anterior inferior cerebellar artery; PICA = posterior inferior cerebellar artery; SCA = superior cerebellar artery; PCA = posterior cerebral artery; SS = straight sinus; SPS = superior petrosal sinus;PV = petrosal vein; AHV = anterior hemispheric vein; VCPF = vein of the cerebellopontine fissure; V = trigeminal nerve; VI = abducens nerve; VII = facial nerve; VIII = vestibulocochlear nerve; IX = glossopharyngeal nerve; X = vagus nerve; XI = accessory nerve; XII = hypoglossal nerve.
Conclusion
Patients with cerebellar AVMs are significantly more likely to present with hemorrhage than patients with cerebral AVMs, resulting in more neurological deficits preoperatively and at late follow-up, and justifying a more aggressive treatment posture. The supplementary AVM grading system is better than the Spetzler-Martin system at predicting outcomes after cerebellar AVM resection, and its accuracy is consistent with all AVMs, cerebellar and cerebral. Key components of the Spetzler-Martin system, like venous drainage and eloquence, are distorted by cerebellar anatomy in ways that the components of the supplementary system are not, resulting in greater predictive accuracy with supplementary grades.
Acknowledgments
This research is funded in part by the National Institutes of Health, R01 NS034949 (WLY) and P01 NS044155 (Center for Cerebrovascular Research). Dr. Ana Rodríguez-Hernández is supported by a grant from “Obra Social La Caixa.”
Footnotes
Disclosures: Dr. Michael T. Lawton receives a royalty for microsurgical instruments from Mizuho America, Inc. The other authors have no personal or financial interests in the drugs, materials, or devices described in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arnaout OM, Gross BA, Eddleman CS, Bendok BR, Getch CC, Batjer HH. Posterior fossa arteriovenous malformations. Neurosurg Focus. 2009;26(5):E12. doi: 10.3171/2009.2.FOCUS0914. [DOI] [PubMed] [Google Scholar]
- 2.Batjer H, Samson D. Arteriovenous malformations of the posterior fossa: clinical presentation, diagnostic evaluation and surgical treatment. Neurosurg Rev. 1986;9:287–296. doi: 10.1007/BF01743635. [DOI] [PubMed] [Google Scholar]
- 3.Da Costa L, Thines L, Dehdashti AR, Wallace MC, Willinsky RA, Tymianski M, Schwartz ML, ter Brugge KG. Management and clinical outcome of posterior fossa arteriovenous malformations: report on a single-centre 15-year experience. J Neurol Neurosurg Psychiatry. 2009;80(4):376–379. doi: 10.1136/jnnp.2008.152710. [DOI] [PubMed] [Google Scholar]
- 4.Drake CG, Friedman AH, Peerless SJ. Posterior fossa arteriovenous malformations. J Neurosurg. 1986;64:1–10. doi: 10.3171/jns.1986.64.1.0001. [DOI] [PubMed] [Google Scholar]
- 5.Kelly ME, Guzman R, Sinclair J, Bell-Stephens TE, Bower R, Hamilton S, Marks MP, Do HM, Chang SD, Adler JR, Levy RP, Steinberg GK. Multimodality treatment of posterior fossa arteriovenous malformations. J Neurosurg. 2008;108(6):1152–1161. doi: 10.3171/JNS/2008/108/6/1152. [DOI] [PubMed] [Google Scholar]
- 6.Khaw AV, Mohr JP, Sciacca RR, Schumacher HC, Hartmann A, Pile-Spellman J, et al. Association of infratentorial brain arteriovenous malformations with hemorrhage at initial presentation. Stroke. 2004;35:660–663. doi: 10.1161/01.STR.0000117093.59726.F9. [DOI] [PubMed] [Google Scholar]
- 7.Neacsu A, Ciurea AV. General considerations on posterior fossa arteriovenous malformations (clinics, imaging and therapy). Actual concepts and literature review. J Med Life. 2010;3(1):26–35. [PMC free article] [PubMed] [Google Scholar]
- 8.O'Shaughnessy BA, Getch CC, Bendok BR, Batjer HH. Microsurgical resection of infratentorial arteriovenous malformations. Neurosurg Focus. 2005;19(2):E5. doi: 10.3171/foc.2005.19.2.6. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair J, Kelly ME, Steinberg GK. Surgical management of posterior fossa arteriovenous malformations. Neurosurgery. 2006;58(4 Suppl 2):189–201. doi: 10.1227/01.NEU.0000204641.38419.2D. [DOI] [PubMed] [Google Scholar]
- 10.Symon L, Tacconi L, Mendoza N, Nakaji P. Arteriovenous malformations of the posterior fossa: a report on 28 cases and review of the literature. Br J Neurosurg. 1995;9:721–732. doi: 10.1080/02688699550040675. [DOI] [PubMed] [Google Scholar]
- 11.Vilalta J, Topezewski T, Añez JD, Arikan F, Guitart JM, Rubio E. Arteriovenous malformations of the posterior fossa. Clinical features, treatment and results. Rev Neurol. 2001;32(12):1124–1128. Spanish. [PubMed] [Google Scholar]
- 12.Yasargil MG. Microneurosurgery. IIIB. New York: Thieme Medical Publishers, Inc.; 1988. [Google Scholar]
- 13.Rhoton AL., Jr Cerebellum and fourth ventricle. Neurosurgery. 2000;47(3 Suppl):S7–27. doi: 10.1097/00006123-200009001-00007. [DOI] [PubMed] [Google Scholar]
- 14.Fine AD, Beauregard CL, Day AL. Arteriovenous Malformations of the Cerebellar Vermis and Hemispheres. In: Stieg PE, Batjer HH, Samsom D, editors. Intracranial Arteriovenous Malformations. New York: Informa Health Care; 2007. pp. 285–297. [Google Scholar]
- 15.De Oliveira E, Tedeschi H, Raso J. Comprehensive management of arteriovenous malformations. Neurol Res. 1998;20(8):673–683. doi: 10.1080/01616412.1998.11740583. [DOI] [PubMed] [Google Scholar]
- 16.Lawton MT, Du R, Tran M, et al. Effect of presenting hemorrhage on outcome after microsurgical resection of brain arteriovenous malformations. Neurosurgery. 2005;56(3):485–493. doi: 10.1227/01.neu.0000153924.67360.ea. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz F, Clavel P, Molet J, Castaño C, de Teresa S, Solivera J, de Quintana C, Tresserras P, Rodríguez R, Bartumeus F. Current management of arteriovenous malformations. Retrospective study of 31 cases and literature review. Neurocirugia (Astur) 2007;18(5):394–404. Spanish. [PubMed] [Google Scholar]
- 18.Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359:863–873. doi: 10.1016/S0140-6736(02)07946-1. [DOI] [PubMed] [Google Scholar]
- 19.Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66(9):1350–1355. doi: 10.1212/01.wnl.0000210524.68507.87. [DOI] [PubMed] [Google Scholar]
- 20.Halim AX, Johnston SC, Singh V, et al. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke. 2004;35(7):1697–1702. doi: 10.1161/01.STR.0000130988.44824.29. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Mejia RO, McDermott MW, Tan J, Kim H, Young WL, Lawton MT. Radiosurgery facilitates resection of brain arteriovenous malformations and reduces surgical morbidity. Neurosurgery. 2009;64(2):231–238. doi: 10.1227/01.NEU.0000338068.44060.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawton MT, Kim H, McCulloch CE, Mikhak B, Young WL. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery. 2010;66(4):702–713. doi: 10.1227/01.NEU.0000367555.16733.E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawton MT. UCSF AVM Study Project: Spetzler-Martin Grade III arteriovenous malformations: surgical results and a modification of the grading scale. Neurosurgery. 2003;52(4):740–748. doi: 10.1227/01.neu.0000053220.02268.9c. [DOI] [PubMed] [Google Scholar]
- 24.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. Journal of Neurosurgery. 1986;65:476–483. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 25.Vates GE, Lawton MT, Wilson CB, et al. Magnetic source imaging demonstrates altered cortical distribution of function in patients with arteriovenous malformations. Neurosurgery. 2002;51(3):614–623. [PubMed] [Google Scholar]
- 26.Du R, Keyoung HM, Dowd CF, Young WL, Lawton MT. The effects of diffuseness and deep perforating artery supply on outcomes after microsurgical resection of brain arteriovenous malformations. Neurosurgery. 2007;60(4):638–646. doi: 10.1227/01.NEU.0000255401.46151.8A. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Mejia RO, Chenaputi S, Gupta N, Fullerton HJ, Young WL, Lawton MT. Superior outcomes in children versus adults after microsurgical resection of brain arteriovenous malformations. Journal of neurosurgery. 2006;105(2 Suppl):82–87. doi: 10.3171/ped.2006.105.2.82. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-Hernández A, Rhoton AL, Jr, Lawton MT. Segmental anatomy of cerebellar arteries: a proposed nomenclature. Laboratory investigation. J Neurosurg. 2011;115(2):387–397. doi: 10.3171/2011.3.JNS101413. [DOI] [PubMed] [Google Scholar]
- 29.Quiñones-Hinojosa A, Chang EF, Lawton MT. The extended retrosigmoid approach: an alternative to radical cranial base approaches for posterior fossa lesions. Neurosurgery. 2006;58(4 Suppl 2):208–214. doi: 10.1227/01.NEU.0000192714.15356.08. [DOI] [PubMed] [Google Scholar]