Abstract
O-Polysaccharides (OPS) were isolated from purified Burkholderia pseudomallei and Burkholderia mallei lipopolysaccharides by mild-acid hydrolysis and gel-permeation chromatography. 1-D and 2-D 1H and 13C NMR spectroscopy experiments revealed that the OPS antigens were unbranched heteropolymers with the following structures:
Collectively, our results demonstrate that the predominant OPS antigens expressed by B. pseudomallei and B. mallei isolates are structurally more complex than previously described and provide evidence that different capping residues are used by these closely related pathogens to terminate chain elongation. Additionally, they confirm that Burkholderia thailandensis and B. pseudomallei express OPS antigens that are essentially identical to one another.
Keywords: Burkholderia pseudomallei, Burkholderia mallei, Burkholderia thailandensis, Lipopolysaccharide, O-polysaccharide, Structure
Burkholderia pseudomallei and Burkholderia mallei, the etiologic agents of melioidosis and glanders, respectively, are both CDC Tier 1 select agents.1–3 These facultative intracellular, Gram-negative pathogens are highly infectious via the respiratory route, and can cause severe diseases in humans and animals.4–7 Diagnosis and treatment of these diseases can be challenging, and in the absence of optimal chemotherapeutic intervention, acute human disease is frequently fatal.8–10 Melioidosis and glanders are emerging/re-emerging infectious diseases for which no licensed vaccines currently exist.11–13 Due to the potential misuse of B. pseudomallei and B. mallei as agents of biological warfare and terrorism, as well as their impact on public health in endemic regions, there is significant interest in developing vaccines for immunization against melioidosis and glanders.12,14,15 Because of this, one of the long term objectives of our research is to identify and characterize protective antigens expressed by these pathogens and use them to develop efficacious vaccine candidates.
Several studies have demonstrated that B. pseudomallei and B. mallei express a number of important virulence determinants that are required for survival in animal models of infection. Included among these are the Bsa type III secretion system, the VirAG two-component regulatory system, the cluster 1 type VI secretion system, and a capsular polysaccharide.16–21 Importantly, studies in our lab and others have also shown that the O-polysaccharide (OPS) components of B. pseudomallei and B. mallei lipopolysaccha-rides (LPS) are both virulence determinants and protective antigens.22–26 Consequently, these carbohydrate moieties have become important components of the various glycoconjugate vaccines that we are currently developing for immunization against melioidosis and glanders.
Unlike other Gram-negative pathogens, B. pseudomallei and B. mallei isolates appear to express only a limited repertoire of structurally diverse OPS antigens.27–29 At present, the significance of these observations with regard to virulence and evasion of host immune responses remains to be defined. Nevertheless, this phenomenon certainly bodes well from a vaccine development standpoint. Previous studies have shown that the predominant OPS serotype expressed by B. pseudomallei is an unbranched polymer consisting of disaccharide repeats having the structure →3)-β-d-glucopyranose-(1→3)-6-deoxy-α-l-talopyranose-(1→ in which the 6-deoxy-α-l-talopyranose (6dTal) residues possess 2-O-acetyl (2-O-Ac) or 2-O-methyl (2-O-Me) and 4-O-acetyl (4-O-Ac) modifications.30 Interestingly, studies have also suggested that B. mallei expresses an OPS antigen that is structurally similar to that expressed by B. pseudomallei except that the 6dTal residues lack acetyl modifications at the O-4 position.31
Recently, we demonstrated that the predominant OPS serotype expressed by the closely related, but non-pathogenic species, Burkholderia thailandensis, was structurally more complex than initially reported.32,33 Based upon these findings, we initiated the present study to reinvestigate the structural characteristics of the predominant OPS species expressed by B. pseudomallei and B. mallei.
Results
The B. pseudomallei RR2808 OPS sample examined in this study is essentially identical to the B. thailandensis E264 OPS antigen recently characterized by our laboratories.33 Similar to previous reports, the RR2808 OPS consists of a →3)-β-d-glucopyranose-(1→3)-6-deoxy-α-l-talopyranose-(1→ disaccharide repeat. Earlier investigations by Perry et al. indicated that the 6dTal residue can be 2-O-acetylated (~67%) or 2-O-methylated and 4-O-acetylated (~33%).30 The 1-D and 2-D spectra obtained in the present study confirmed these two residues, but indicated the presence of additional substitution patterns that have not been previously detected in B. pseudomallei OPS (Figs. 1–3 and Table 1). Thus, O-2 can be unsubstituted or substituted with acetyl or methyl, O-3 can be glycosylated with β-glucose (in all the internal residues) or methylated (in the non-reducing end residue), and O-4 can be unsubstituted or acetylated. We found all possible combinations of these substitutions, except residues with two methyl groups and residues that were unsubstituted on O-2 and acetylated on O-4 (Table 1). Table 2 details the exact percentages of the differently substituted 6dTal residues.
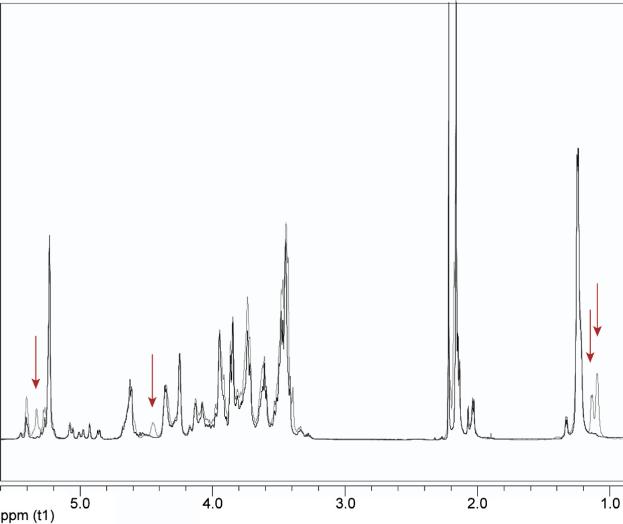
Figure 1.
Overlaid 1-D proton spectra of B. pseudomallei RR2808 (gray) and B. mallei BM2308 (black) OPS. The red arrows emphasize peaks present only in B. pseudomallei OPS (all of which belong to the 4-O-acetylated 6dTal residues).
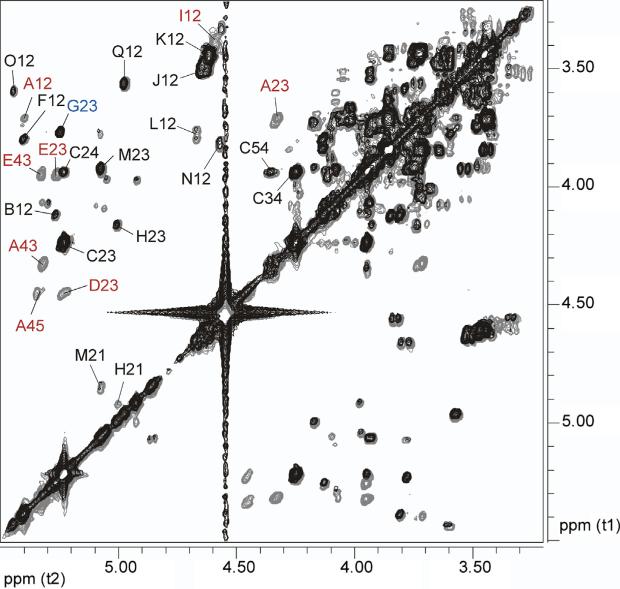
Figure 3.
Overlaid partial gHSQC spectra of B. pseudomallei RR2808 (gray) and B. mallei BM2308 (black) OPS. Red labels indicate peaks present only in B. pseudomallei OPS; blue labels indicate peaks present only in B. mallei OPS (refer to Table 1 for the labeling).
Table 1.
Chemical shift assignments of B. pseudomallei and B. mallei OPS
| Residue | Chemical shift (ppm) |
NOE HMBC | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| A | →3)-2-O-Me-4-O-Ac- α-L-6dTalp-(1→ | 5.40 | 3.71 | 4.33 | 5.32 | 4.44 | 1.09 | Glc-3a |
| 100.5 | 80.4 | 74.3 | 72.4 | 68.2 | 17.8 | Glc-3a | ||
| B | →3)-α-L-6dTalp-(1→ | 5.27 | 4.12 | 4.08 | 3.93 | 4.31 | 1.24 | Glc-3a |
| 103.8 | 72.2 | 76.4 | 72.6 | 70.2 | 18.0 | Glc-3a | ||
| C | →3)-2-O-Ac-a-L-6dTalp-(1 → | 5.23 | 5.23 | 4.25 | 3.95 | 4.36 | 1.24 | Glc-3a |
| 101.2 | 72.9 | 75.7 | 71.2 | 69.8 | 18.0 | Glc-3a | ||
| D | →3)-2,4-di-O-Ac-α-L-6dTalp-(1 → | 5.23 | 5.23 | 4.46 | 5.35 | 4.52 | 1.13 | Glc-3a |
| 101.2 | 71.9 | 73.8 | 72.4 | 68.4 | 17.6 | Glc-3a | ||
| E | 3-O-Me-2,4-di-O-Ac-α-L-6dTalp-(1→ | 5.27 | 5.27 | 3.96 | 5.32 | 4.52 | 1.13 | Glc-3a |
| 101.2 | 70.2 | 75.8 | 72.2 | 68.4 | 17.6 | Glc-3a | ||
| F | →3)-2-O-Me-α-L-6dTalp-(1 → | 5.40 | 3.80 | 4.13 | 3.85 | 4.29 | 1.22 | Glc-3a |
| 100.5 | 81.3 | 76.6 | 72.2 | 70.4 | 18.1 | Glc-3a | ||
| G | 3-O-Me-2-O-Ac-α-L-6dTalp-(1 → | 5.26 | 5.25 | 3.78 | 3.94 | ND | ND | Glc-3a |
| 101.1 | 70.8 | 76.4 | 70.3 | ND | ND | Glc-3a | ||
| H | →3)-2-O-Ac-α-L-6dTalp-(1 → | 4.93 | 5.01 | 4.17 | 3.93 | 4.36 | 1.24 | L-3 |
| 101.8 | 73.1 | 75.9 | 71.2 | 69.8 | 18.0 | L-3 | ||
| I | →3)-β-D-Glcp-(1→ A | 4.59 | 3.40 | 3.62 | 3.46 | 3.43 | 3.85/3.73 | A-3 |
| 103.3 | 76.2 | 84.8 | 70.5 | 78.5 | 63.3 | A-3 | ||
| J | →3)-β-D-Glcp-(1→ B | 4.65 | 3.50 | 3.62 | 3.46 | 3.43 | 3.85/3.73 | B-3 |
| 103.8 | 75.9 | 84.8 | 70.7 | 78.5 | 63.3 | B-3 | ||
| K | →3)-β-D-Glcp-(1→ C | 4.61 | 3.46 | 3.61 | 3.48 | 3.45 | 3.85/3.73 | C-3 |
| 104.3 | 76.2 | 84.6 | 70.5 | 78.5 | 63.3 | C-3 | ||
| L | →3)-β-D-GlcpNAc | 4.67 | 3.79 | 3.61 | 3.45 | 3.42 | 3.85/3.79 | M-3 |
| 104.3 | 58.1 | 84.8 | 70.5 | 78.5 | 63.3 | M-3 | ||
| M | →3)-2-O-Ac-α-L-Rhap | 4.86 | 5.07 | 3.93 | 3.53 | 4.06 | 1.24 | |
| 101.0 | 74.5 | 81.1 | 73.6 | 71.5 | 19.0 | |||
| N | →3)-β-D-QuipNAc | 4.59 | 3.84 | 3.54 | 3.27 | 3.49 | 1.32 | |
| 103.8 | 58.2 | 84.6 | 76.4 | 74.7 | 19.4 | |||
| O | α-D-Galp | 5.44 | 3.59 | 3.85 | 4.08 | ND | ND | S-3 |
| 100.1 | 70.7 | 71.2 | 71.6 | ND | ND | |||
| P | Hepp | 5.05 | 3.97 | 3.86 | 3.86 | 3.75 | ND | |
| 100.4 | 72.8 | 74.4 | 68.6 | 74.9 | ND | |||
| Q | α-D-Glcp | 4.98 | 3.57 | 3.73 | 3.43 | 3.86 | ND | |
| 100.8 | 74.5 | 75.9 | 72.4 | ND | ND | |||
| R | Hepp | 4.92 | 3.98 | 3.64 | 3.86 | ND | ND | |
| 103.5 | 72.6 | 74.3 | 68.6 | ND | ND | |||
| S | →3)-α-Rhap | 5.21 | 4.25 | 4.00 | 3.73 | 3.98 | 1.23 | |
| 101.2 | 72.3 | 82.4 | 75.0 | 68.7 | 18.5 | |||
The glucose signal comprises several spin systems, each slightly different according to the neighboring residues. The H-1 set of signals has NOE and HMB correlations to all of the H-3/C-3 signals of the various 6dTal residues; ND-not determined.
Table 2.
Intensity comparisons of the 6dTal anomeric signals in the HSQC spectra of B. pseudomallei, B. mallei, and B. thailandensis OPS as a percent of the sum of all 6dTal anomerics
| Residue | B. pseudomallei OPS | B. mallei OPS | B. thailandensis OPS | |
|---|---|---|---|---|
| A | →3)-2-O-Me-4-O-Ac-α-L-6dTalp-(1→ | 14.7 | ND | 23.0 |
| B | →3)-α-L-6dTalp-(1→ | 8.8 | 11.6 | 7.7 |
| C | →3)-2-O-Ac-α-L-6dTalp-(1 → | 43.0 | 59.1 | 33.3 |
| D | →3)-2,4-di-O-Ac-α-L-6dTalp-(1 → | 5.4 | ND | 10.1 |
| E | 3-O-Me-2,4-di-O-Ac-α-L-6dTalp-(1→ | 11.3 | ND | 18.7 |
| F | →3)-2-O-Me-α-L-6dTalp-(1 → | 14.6 | 16.4 | 3.5 |
| G | 3-O-Me-2-O-Ac-α-L-6dTalp-(1 → | ND | 10.2 | ND |
| H | →3)-2-O-Ac-α-L-6dTalp-(1 → | 2.2 | 2.7 | 3.7 |
| Total | 100 | 100 | 100 |
ND-not detected.
The B. mallei BM2308 OPS sample examined in this study gave simpler spectra compared to the RR2808 OPS. This was due to lack of any 4-O-acetylated 6dTal residues as intimated in the 1-D proton spectrum by the absence of signals at 5.33, 4.45, and 1.09 ppm (H-4, H-5, and H-6 of 2-O-Me-4-O-Ac-6dTal), and 1.13 ppm (H-6 of 2,4-di-O-Ac-6dTal and 3-O-Me-2,4-di-O-Ac-6dTal).34 Analysis of the 2-D COSY, TOCSY, and HSQC spectra confirmed the absence of these residues and revealed the 3-O-methylated non-reducing end residue without a 4-O-acetyl group (Figs. 1–3 and Table 1).
The 6dTal residues F, G, and H have not previously been described in the Burkholderia literature (Tables 1 and 2). F is 2-O-methylated and like A is characterized by an upfield H-2 and a downfield C-2, but in contrast to A its H-4 is not shifted down-field, indicating the lack of a 4-O-acetyl substituent. G is a non-reducing end terminal residue with an O-methyl group in its 3-position, analogous to E, but lacking 4-O-acetylation, as evidenced by its upfield H-4 signal. G was detected in B. mallei OPS, but not in B. pseudomallei OPS. Residue H has the same constitution and nearly identical carbon chemical shifts as Residue C, but showed upfield displacement of its H-1 and H-2 relative to C. The 1-bond C-H coupling constant of its anomeric position is 173 Hz, clearly identifying it as having α-configuration. Methylation in the anomeric position could account for an upfield shift of H-1 and H-2, but there is no indication from HMBC or NOESY that this residue is a methyl glycoside. Conversely, NOESY and HMBC show correlations of H-H-1 to a signal at 3.61/84.8 ppm, which corresponds to H/C-3 of β-Glc (I, J, and K). However, this signal resonates at the same position as H/C-3 of L, a 3-β-GlcNAc residue.
We used the CASPER website (URL: http://www.casper.organ.su.se/casper/) to investigate if it is plausible that the upfield shifts of H-1 and H-2 of H are due to its being linked to β-GlcNAc instead of β-Glc.35,36 Since 6dTal is not included in the CASPER database, we used α-l-Rhap-(1→3)-β-d-Glcp-(1→3)-β-l-Rhap-(1→3)-β-d-Glcp (Oligosaccharide 1) and α-l-Rhap-(1→3)-β-d-Glcp-(1→3)-α-l-Rhap-(1→3)-β-d-GlcpNAc (Oligosaccharide 2) as model structures to determine if substitution of GlcNAc for Glc would cause upfield displacement of H-1 and H-2 of the penultimate residue (bolded). The calculated chemical shifts of these protons in Oligosaccharide 1 were 5.11 and 4.30 ppm, respectively, compared to 4.90 (chemical shift difference ΔδH-1 = 0.21 ppm) and 4.06 ppm (ΔδH-2 = 0.24 ppm) in Oligosaccharide 2. These chemical shift differences are in close agreement to those between Residues C and H (ΔδH-1 = 0.30 and ΔδH-2 = 0.22 ppm). We therefore propose that Residue H is 1-3-linked to β-GlcNAc (Residue L). This could mean that H represents the reducing end residue of the OPS, which is linked to the core oligosaccharide through a β-GlcNAc residue or that some of the O-chain Glc residues are replaced by GlcNAc. The intensity of H is only about 20% of that of the 3-O-methylated residues from the non-reducing end (E or G; Table 2). The low abundance, however, seems to dispute the option of H as the reducing end residue, which would be expected to have the same intensity as the non-reducing end residue.
In this study we identified several core residues associated with the RR2808 and BM2308 samples (Table 1). However, the presence of the O-chain residues prevented us from performing a detailed characterization of the core structures. To do so will require the isolation and NMR analysis of rough mutants, separation of low-molecular weight OPS from the strains used here, or possibly enzymatic degradation of the OPS. What we were able to determine though was that both the B. pseudomallei and B. mallei samples featured a similar or equal core oligosaccharide, which appears to be different from that expressed by B. thailandensis.33 Studies are ongoing to further investigate the importance of Residue H as well as elucidate the structures of the core moieties expressed by B. pseudomallei, B. mallei, and B. thailandensis.
In the present study, we have shown that B. pseudomallei RR2808 (1026b derivative) and B. mallei BM2308 (ATCC 23344 derivative) express unbranched OPS antigens with the following structures:
Our results demonstrate that the predominant OPS antigens expressed by B. pseudomallei and B. mallei isolates are structurally more complex than previously described as well as confirm that B. thailandensis and B. pseudomallei express essentially identical OPS antigens.30,31,33 Interestingly, they also indicate that these three bacterial species utilize 3-O-methylated 6dTal residues to terminate OPS chain elongation. Unlike B. pseudomallei and B. thailandensis, however, it appears that the capping residue used by B. mallei lacks acetyl modifications at the O-4 position.33 Collectively, these findings help to explain the ability of researchers to generate B. pseudomallei and B. thailandensis or B. mallei OPS-specific monoclonal antibodies as well as the presence of B. mallei-specific bacteriophages recognizing smooth but not rough LPS strains.27,28,37 Based upon the results of this study, it will be interesting to determine if subunit vaccines consisting of one of these OPS species can provide cross-protection against both melioidosis and glanders or if a combination of these antigens will be required given their significant structural differences.
Experimental
Bacterial strains, growth conditions and reagents
B. pseudomallei strains were grown at 37 °C on LB agar or in LB broth supplemented with thiamine (5 μg/mL) and adenine (100 μg/mL). B. mallei strains were grown at 37 °C on LB4G agar or in LB4G broth. B. pseudomallei RR2808 (CPS deficient derivative of the select agent excluded strain Bp82, which is ΔpurM derivative of 1026b) and B. mallei BM2308 (CPS deficient derivative of ATCC 23344) were constructed via sacB-based allelic exchange mutagenesis essentially as previously described.3,16,38,39 Both of the mutant strains harbor markerless, in-frame deletions in their wcbB genes.17,18 Bacterial stocks were maintained at –80 °C as 20% glycerol suspensions. All studies utilizing viable B. mallei were conducted in a CDC select agent certified biosafety level three containment facility.
LPS and OPS purification
Broth in 2 L baffled Erlenmeyer flasks was inoculated with B. pseudomallei RR2808 or B. mallei BM2308 and incubated overnight at 37 °C with vigorous shaking. Cell pellets were obtained by centrifugation and extracted using a modified hot aqueous-phenol procedure. Purified LPS and OPS antigens were then obtained essentially as previously described.30,34
NMR spectroscopy
The samples were deuterium exchanged by dissolving in D2O and lyophilizing and then were dissolved in 0.27 mL D2O containing 1 μL acetone. 1-D Proton and 2-D gradient-enhanced COSY (gCOSY), TOCSY, NOESY, gHSQC, and gHMBC spectra were obtained on a Varian Inova-600 MHz spectrometer at 50 °C using standard Varian pulse sequences. The spectral width was 3.17 kHz in the 1H dimension and 18.1 kHz in the 13C dimension. The number of scans and increments was 4 and 400 for gCOSY, 8 and 200 for TOCSY and NOESY, 64 and 128 for gHSQC, and 128 and 200 for gHMBC. Acquisition times were 2 s for 1-D 1H, 137 ms for gCOSY, TOCSY, and NOESY, 150 ms for gHSQC, and 128 ms for gHMBC. Mixing times for TOCSY and NOESY experiments were 120 and 300 ms, respectively. Proton chemical shifts were measured relative to internal acetone (δH = 2.218 ppm, δC = 33.0 ppm).40
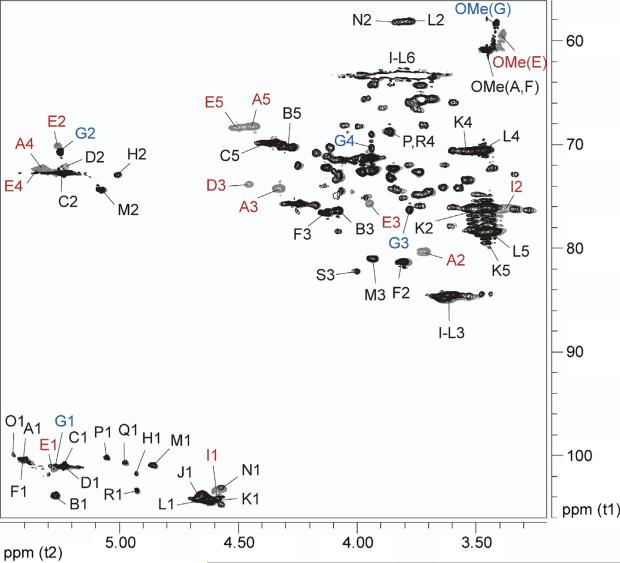
Figure 2.
Overlaid partial gCOSY spectra of B. pseudomallei RR2808 (gray) and B. mallei BM2308 (black) OPS. Red labels indicate peaks present only in B. pseudomallei OPS while blue labels indicate peaks present only in B. mallei OPS (refer to Table 1 for the labeling).
Acknowledgements
This research was supported in part by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant R21AI088418) and the Department of Energy-funded Center for Plant and Microbial Complex Carbohydrates (grant DE-FG09-93ER-20097).
References
- 1.Howe C, Miller WR. Ann. Intern. Med. 1947;26:93–115. doi: 10.7326/0003-4819-26-1-93. [DOI] [PubMed] [Google Scholar]
- 2.Redfearn MS, Palleroni NJ, Stanier RY. J. Gen. Microbiol. 1966;43:293–313. doi: 10.1099/00221287-43-2-293. [DOI] [PubMed] [Google Scholar]
- 3.Yabuuchi E, Kosako Y, Oyaizu H, Yano I, Hotta H, Hashimoto Y, Ezaki T, Arakawa M. Microbiol. Immunol. 1992;36:1251–1275. doi: 10.1111/j.1348-0421.1992.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 4.Galyov EE, Brett PJ, DeShazer D. Annu. Rev. Microbiol. 2010;64:495–517. doi: 10.1146/annurev.micro.112408.134030. [DOI] [PubMed] [Google Scholar]
- 5.Limmathurotsakul D, Thammasart S, Warrasuth N, Thapanagulsak P, Jatapai A, Pengreungrojanachai V, Anun S, Joraka W, Thongkamkoon P, Saiyen P, Wongratanacheewin S, Day NP, Peacock SJ. Emerg. Infect. Dis. 2012;18:325–327. doi: 10.3201/eid1802.111347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitlock GC, Estes DM, Torres AG. FEMS Microbiol. Lett. 2007;277:115–122. doi: 10.1111/j.1574-6968.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- 7.Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. Nat. Rev. Microbiol. 2006;4:272–282. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- 8.Khan I, Wieler LH, Melzer F, Elschner MC, Muhammad G, Ali S, Sprague LD, Neubauer H, Saqib M. Transbound. Emerg. Dis. 2013;60:204–221. doi: 10.1111/j.1865-1682.2012.01342.x. [DOI] [PubMed] [Google Scholar]
- 9.Meumann EM, Cheng AC, Ward L, Currie BJ. Clin. Infect. Dis. 2012;54:362–369. doi: 10.1093/cid/cir808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White NJ. Lancet. 2003;361:1715–1722. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- 11.Bondi SK, Goldberg JB. Expert Rev. Vaccines. 2008;7:1357–1365. doi: 10.1586/14760584.7.9.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peacock SJ, Limmathurotsakul D, Lubell Y, Koh GC, White LJ, Day NP, Titball RW. PLoS Negl. Trop. Dis. 2012;6:e1488. doi: 10.1371/journal.pntd.0001488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkar-Tyson M, Titball RW. Clin. Ther. 2010;32:1437–1445. doi: 10.1016/j.clinthera.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. Emerg. Infect. Dis. 2002;8:225–230. doi: 10.3201/eid0802.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voskuhl GW, Cornea P, Bronze MS, Greenfield RA. J. Okla. State Med. Assoc. 2003;96:214–217. [PubMed] [Google Scholar]
- 16.Burtnick MN, Brett PJ, Harding SV, Ngugi SA, Ribot WJ, Chantratita N, Scorpio A, Milne TS, Dean RE, Fritz DL, Peacock SJ, Prior JL, Atkins TP, Deshazer D. Infect. Immun. 2011;79:1512–1525. doi: 10.1128/IAI.01218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeShazer D, Waag DM, Fritz DL, Woods DE. Microb. Pathog. 2001;30:253–269. doi: 10.1006/mpat.2000.0430. [DOI] [PubMed] [Google Scholar]
- 18.Reckseidler SL, DeShazer D, Sokol PA, Woods DE. Infect. Immun. 2001;69:34–44. doi: 10.1128/IAI.69.1.34-44.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schell MA, Ulrich RL, Ribot WJ, Brueggemann EE, Hines HB, Chen D, Lipscomb L, Kim HS, Mrazek J, Nierman WC, Deshazer D. Mol. Microbiol. 2007;64:1466–1485. doi: 10.1111/j.1365-2958.2007.05734.x. [DOI] [PubMed] [Google Scholar]
- 20.Stevens MP, Wood MW, Taylor LA, Monaghan P, Hawes P, Jones PW, Wallis TS, Galyov EE. Mol. Microbiol. 2002;46:649–659. doi: 10.1046/j.1365-2958.2002.03190.x. [DOI] [PubMed] [Google Scholar]
- 21.Ulrich RL, DeShazer D. Infect. Immun. 2004;72:1150–1154. doi: 10.1128/IAI.72.2.1150-1154.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryan LE, Wong D, Woods DE, Dance DA, Chaowagul W. Can. J. Infect. Dis. 1994;5:170–178. doi: 10.1155/1994/856850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeShazer D, Brett PJ, Woods DE. Mol. Microbiol. 1998;30:1081–1100. doi: 10.1046/j.1365-2958.1998.01139.x. [DOI] [PubMed] [Google Scholar]
- 24.Ho M, Schollaardt T, Smith MD, Perry MB, Brett PJ, Chaowagul W, Bryan LE. Infect. Immun. 1997;65:3648–3653. doi: 10.1128/iai.65.9.3648-3653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson M, Prior JL, Lever MS, Jones HE, Atkins TP, Titball RW. J. Med. Microbiol. 2004;53:1177–1182. doi: 10.1099/jmm.0.45766-0. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Feng SH, Li B, Kim HY, Rodriguez J, Tsai S, Lo SC. Clin. Vaccine Immunol. 2011;18:825–834. doi: 10.1128/CVI.00533-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anuntagool N, Sirisinha S. Microbiol. Immunol. 2002;46:143–150. doi: 10.1111/j.1348-0421.2002.tb02679.x. [DOI] [PubMed] [Google Scholar]
- 28.Neubauer H, Sprague LD, Zacharia R, Tomaso H, Al Dahouk S, Wernery R, Wernery U, Scholz HC. J. Vet. Med. B. Infect. Dis. Vet Public Health. 2005;52:201–205. doi: 10.1111/j.1439-0450.2005.00855.x. [DOI] [PubMed] [Google Scholar]
- 29.Pitt TL, Aucken H, Dance DA. J. Infect. 1992;25:139–146. doi: 10.1016/0163-4453(92)93920-l. [DOI] [PubMed] [Google Scholar]
- 30.Perry MB, MacLean LL, Schollaardt T, Bryan LE, Ho M. Infect. Immun. 1995;63:3348–3352. doi: 10.1128/iai.63.9.3348-3352.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burtnick MN, Brett PJ, Woods DE. J. Bacteriol. 2002;184:849–852. doi: 10.1128/JB.184.3.849-852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brett PJ, DeShazer D, Woods DE. Int. J. Syst. Bacteriol. 1998;48:317–320. doi: 10.1099/00207713-48-1-317. [DOI] [PubMed] [Google Scholar]
- 33.Heiss C, Burtnick MN, Black I, Azadi P, Brett PJ. Carbohydr. Res. 2012;363:23–28. doi: 10.1016/j.carres.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brett PJ, Burtnick MN, Heiss C, Azadi P, DeShazer D, Woods DE, Gherardini FC. Infect. Immun. 2011;79:961–969. doi: 10.1128/IAI.01023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jansson PE, Stenutz R, Widmalm G. Carbohydr. Res. 2006;341:1003–1010. doi: 10.1016/j.carres.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 36.Lundborg M, Widmalm G. Anal. Chem. 2011;83:1514–1517. doi: 10.1021/ac1032534. [DOI] [PubMed] [Google Scholar]
- 37.Woods DE, Jeddeloh JA, Fritz DL, DeShazer D. J. Bacteriol. 2002;184:4003–4017. doi: 10.1128/JB.184.14.4003-4017.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burtnick MN, DeShazer D, Nair V, Gherardini FC, Brett PJ. Infect. Immun. 2010;78:88–99. doi: 10.1128/IAI.00985-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Propst KL, Mima T, Choi KH, Dow SW, Schweizer HP. Infect. Immun. 2010;78:3136–3143. doi: 10.1128/IAI.01313-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, Oldfield E, Markley JL, Sykes BD. J. Biomol. NMR. 1995;6:135–140. doi: 10.1007/BF00211777. [DOI] [PubMed] [Google Scholar]