Abstract
STUDY QUESTION
Is intake of fatty acids related to semen quality among young men?
SUMMARY ANSWER
The intake of trans fatty acids is inversely related to total sperm count in healthy young men.
WHAT IS KNOWN ALREADY
Spain has seen an increase in the proportion of calories consumed as fat over the same period that a downward trend in semen quality has been observed. In addition, rodent models suggest that trans fat intake may severely affect testicular function.
STUDY DESIGN, SIZE, DURATION
Cross-sectional study of 209 men recruited between October 2010 and November 2011.
PARTICIPANTS/MATERIALS, SETTING, METHODS
A group of 209 healthy young university students 18–23 years of age provided a semen sample and completed a previously validated food frequency questionnaire. The association between intake of fatty acids with semen quality parameters (sperm concentration, motility, morphology and total count) was assessed using multivariate linear regression.
MAIN RESULTS AND THE ROLE OF THE CHANCE
Trans fatty acid intake was inversely related to total sperm count after adjusting for potential confounders (P, trend = 0.03). The multivariate adjusted mean (95% confidence interval) total sperm count in increasing quartiles of trans fat intake was 144 (110–190), 113 (87–148), 100 (18–130) and 89 (69–117). There also was an inverse association between cholesterol intake and ejaculate volume (P, trend = 0.04). No other statistically significant relations were observed.
LIMITATIONS, REASONS FOR CAUTION
The cross-sectional design of the study limits causal inference, we cannot exclude the possibility of unmeasured confounding and there was insufficient statistical power to identify modest associations.
WIDER IMPLICATIONS OF THE FINDINGS
The results of this study, together with previous experimental work in rodents and biomarker studies among infertility patients, suggest that intake of trans fatty acids may be related to lower semen quality. Although the data provide further evidence that diet is a modifiable factor that could impact male fertility, it is not known whether the observed differences in sperm count translate into differences in fertility.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by The Seneca Foundation, Regional Agency of Science and Technology, grant no 00694/PI/04, Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III (FIS), grant no PI10/00985, and grant P30 DK46200 from the National Institutes of Health. The authors have no competing interests to declare.
Keywords: trans fatty acids, fat intake, semen quality, young healthy men
Introduction
Whether sperm counts have been declining in Western countries during recent decades is a matter of ongoing research and debate in reproductive medicine (Carlsen et al., 1992; Fisch et al., 1996; Paulsen et al., 1996; Vierula et al., 1996; Handelsman, 1997; Swan et al., 1997, 2000; Skakkebæk et al., 2006). In southern Spain we have estimated a yearly rate of decline of 1.9% during the last 10 years (Mendiola et al., 2013). However, the potential causes of this downward trend in semen quality remain unclear.
Increasing evidence suggests that diet may have an impact on semen quality (Eskenazi et al., 2005; Mendiola et al., 2009; Gaskins et al., 2012; Mínguez-Alarcón et al., 2012). While this remains an emerging field and data are still scarce, two recent studies have found an inverse association between dietary fats and semen quality (Attaman et al., 2012; Jensen et al., 2013). Specifically, saturated fat intake was inversely related to sperm concentration and total sperm count among US fertility patients (Attaman et al., 2012), and young Danish men (Jensen et al., 2013). In addition, experimental evidence in rodent models suggests that intake of trans fats can cause impaired spermatogenesis and testicular damage (Jensen, 1976; Hanis et al., 1989).
Spain, like the USA (Putnam et al., 2002; Briefel and Johnson, 2004; Nielsen and Popkin, 2004), has seen an increase in the proportion of calories consumed as fat over the same period where a downward trend in semen quality has been observed (Varela-Moreiras et al., 2010). It is unknown, however, whether the findings from the previous studies can be generalized to Spain or other Mediterranean countries. To further examine the potential impact of dietary fat intake on semen quality in young healthy men, we evaluated the relationship between intake of fatty acids and semen quality among young men in southern Spain.
Material and Methods
The Murcia Young Men's Study was a cross-sectional study carried out between October 2010 and November 2011 in the Murcia Region of Spain. It included healthy young university students between 18 and 23 years of age. Written informed consent was obtained from all subjects. The Research Ethics Committee of the University of Murcia approved this study.
Recruitment flyers were posted at university campuses to invite students to participate in this study. To be included in the study men had to be university students, born in Spain after 31 December 1987, and able to contact their mother and ask her to complete a questionnaire regarding her pregnancy with the participant. A total of 240 students contacted the study staff. Of these, 17 subjects were ineligible (5 had not been born in Spain; 9 had not been born after 31 December 1987; and 3 were not able to contact their mother). Of the remaining 223 men (92.9%), 215 completed a study visit and agreed to participate in the study. We further excluded 6 men who reported implausible total caloric intake leaving 209 men (87.1%) for the current analysis.
During the study visit, men completed questionnaires on lifestyle, diet, smoking and quality of life, underwent an andrological examination and provided a semen sample. A €50 gift card was given to men completing the study.
Physical examination
Body weight and height were measured using a digital scale (Tanita SC 330-S, London, UK). BMI was calculated as weight in kilograms divided by squared height in meters. Presence of varicocele or other scrotal abnormalities was also evaluated, and classified as no varicocele, only detected during Valsalva procedure, palpable or visible. Testicular volume was measured using a Prader orchidometer (Andrology Australia, Clayton, Victoria, Australia). All physical examinations were performed by the same investigator (J.M.) to minimize variability in study procedures.
Dietary assessment
We used a validated (Vioque, 1995; Vioque et al., 2007, 2013) 101-food item semi-quantitative food frequency questionnaire (FFQ) to assess the usual intake of foods and nutrients (available at: http://bibliodieta.umh.es/files/2011/07/CFA101.pdf). Men were asked to report how often, on average, they had consumed each food item over the past year. Serving sizes were specified for each food item in the FFQ. The questionnaire offered nine options for frequency of consumption for each food, ranging from never or less than once a month to 6 or more times/day. Nutrient values for each food in the questionnaire were obtained from food composition tables of the US Department of Agriculture and supplemented with Spanish sources (Palma et al., 2008; U.S. Department of Agriculture, 2010). Intake of energy-bearing nutrients was adjusted for total energy intake using the multivariate nutrient density method while non-energy-bearing nutrients were adjusted using the nutrient residual method (Willett, 2013).
The reproducibility and validity of this FFQ is comparable with other widely used FFQs (Willett et al., 1985; Block et al., 1990; Vioque and Gonzalez, 1991; Ocké et al., 1997; Willet, 1998; Subar et al., 2001). The mean correlation coefficients between nutrient intakes estimated using prospectively collected diet records and those estimated with the FFQ were 0.47 for validity and 0.40 for reproducibility (Vioque, 1995). This FFQ also showed satisfactory biochemical validity when compared with plasma levels of carotenoids and vitamin C in an elderly population with a high prevalence of obesity (Vioque et al., 2007).
Semen analysis
Men were asked to abstain from ejaculation for at least 48 h before sample collection, but were not excluded if they failed to follow this instruction (n = 30). Abstinence time was recorded as the time between current and previous ejaculation as reported by the study subject. Men collected semen samples by masturbation at the clinic; no lubricants were used. Ejaculate volume was estimated by specimen weight, assuming a semen density of 1.0 g/ml. Sperm concentration was evaluated by hemocytometer (Improved Neubauer; Hauser Scientific, Inc., Horsham, PA, USA). Spermatozoa were classified as either motile or immotile according to the World Health Organization (WHO) criteria (WHO, 2010) and the percentage of motile sperm (progressive + non-progressive) was calculated. Smears for morphology were prepared, air-dried, fixed, Papanicolaou stained and assessed using strict criteria (Menkveld et al., 1990). Total sperm count (volume × sperm concentration) was also calculated. The same specialist biologist carried out all the semen analyses. An external quality control on semen samples throughout the study period was carried out in collaboration with the University of Copenhagen's Department of Growth and Reproduction. In order to assess inter-laboratory variation in sperm concentration analysis, five sets of duplicate semen samples (600 µl each) were sent by mail during the study period from the University of Copenhagen's Department of Growth and Reproduction to the Murcia Andrology Laboratory. The specimens were blinded, undiluted fresh sperm samples from regular semen donors that were preserved by adding 10 µl of a 3 M sodium azide solution per 1 ml of the ejaculate after liquefaction. No systematic differences in the results were identified. The mean inter-examiner coefficient of variation was 4.0%, ranging between 1.7 and 7.1%.
Statistical analyses
Semen volume, sperm concentration, total sperm count and percentage of morphologically normal sperm showed non-normal distributions and were transformed using the natural log (ln) before analysis. Men were divided into quartiles of dietary fatty acids intake. Men with the lowest intake of each fat category were considered as the reference group. To test for associations across quartiles of intake, Kruskal–Wallis tests were used for continuous variables, and χ2 tests for categorical variables. Linear regression was used to examine the association of each fatty acid category with semen quality parameters. Tests for linear trend were performed using the median values of fat categories in each quartile as a continuous variable and semen parameters as the response variable. Confounding was assessed using a hybrid method that combines previous knowledge using directed acyclic graphs and a statistical method on change in point estimated, in which the potential covariate was not retained in final models, if it resulted in a change in the β-coefficient of <10% (Weng et al., 2009). Using this method, final models included BMI (kg/m2), smoking (current smoker versus not current smoker), ejaculation abstinence time (hours), alcohol intake (g/day) and caffeine intake (mg/day). Total calorie intake, intake of fiber and protein, and the remaining types of fat were included in the model, regardless of statistical significance, to allow the interpretation of the regression parameter of interest as the isocaloric substitution of a specific type of fat for the same amount of energy from carbohydrates (Willett, 2013). Intakes of micronutrients previously related to semen quality in this population (vitamin C, β-cryptoxantine, lycopene and β-carotene) (Mínguez-Alarcón et al., 2012) were also forced into the model. Time to start semen analysis (minutes) was also included in models for sperm motility. We used analysis of covariance (ANCOVA) to calculate adjusted semen parameters for each quartile by relevant covariates. Regression coefficients for outcomes that were log-transformed for analysis (volume, concentration, total count and morphology) were exponentiated (‘back-transformed’) to allow the presentation of adjusted means in the scale variables that were originally measured. Multivariate ANCOVA models were created with continuous semen parameters as dependent variables, and fatty acid categories and covariates as independent variables. We considered that an association was present when we found a statistically significant linear trend across quartiles. All tests were two-tailed and the level of statistical significance was set at 0.05. Statistical analyses were performed with the IBM Statistical Package for the Social Sciences 19.0 (IBM Corporation, Armonk, NY, USA).
Results
Study participants were young [median interquartile range (IQR) = 20.4 (19.6, 21.4) years], predominantly Caucasian (97.6%), and had a median BMI of 23.7 (IQR: 21.8, 25.5) kg/m2 (Table I). Almost one-third were current smokers and all of them considered themselves to be in good or excellent general health. Regular (≥1/week) alcohol consumption has highly prevalent; 55% reported liquor consumption, 26% reported red wine consumption, and 80% reported beer consumption. The median (IQR) values for semen analysis parameters were 42.9 × 106/ml (IQR: 21.9, 72.2 × 106/ml) for sperm concentration; 121.5 (IQR: 65.4, 212.7) × 106 for total sperm count; 8.9% (IQR: 6.0, 13.9%) for morphologically normal sperm; and 57.2% (IQR: 50.7, 63.8%) for sperm motility. Men with higher fat intake were less likely to be smokers, had slightly shorter abstinence time, were slimmer, had lower intakes of vitamin C and β-cryptoxantine and a slightly lower total energy intake.
Table I.
Demographic characteristics of men in the Murcia Young Men's Study among total fat intake quartiles.
| Total cohort (n = 209) | Q1 (n = 54) | Q2 (n = 54) | Q3 (n = 52) | Q4 (n = 49) | P-valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 20.4 | (19.6–21.4) | 20.8 | (19.6–21.4) | 20.6 | (19.3–21.3) | 20.0 | (19.5–21.0) | 20.4 | (19.5–21.9) | 0.35 |
| Caucasian, n (%) | 204 | (97.6) | 53 | (98.1) | 52 | (96.3) | 51 | (98.1) | 48 | (98.0) | 0.82 |
| BMI (kg/m2) | 23.7 | (21.8–25.5) | 24.1 | (22.2–25.8) | 24.1 | (21.9–26.3) | 22.6 | (21.2–25.2) | 23.4 | (21.8–25.5) | 0.25 |
| Current smoker, n (%) | 68 | (32.5) | 24 | (44.4) | 13 | (24.1) | 15 | (28.8) | 14 | (28.6) | 0.13 |
| Abstinence time (h) | 71.0 | (59.5–92.0) | 78.5 | (65.0–92.3) | 66.0 | (53.8–87.0) | 74.0 | (56.8–100.8) | 71.0 | (56.0–93.5) | 0.22 |
| Varicocele, n (%) | 32 | (15.3) | 5 | (9.3) | 8 | (14.8) | 9 | (17.3) | 10 | (20.4) | 0.93 |
| History of cryptorchidism, n (%) | 4 | (1.9) | 0 | (0) | 2 | (3.7) | 1 | (1.9) | 1 | (2.0) | 0.36 |
| Semen volume (ml) | 3.0 | (2.0, 4.0) | 3.2 | (2.5, 4.1) | 2.9 | (1.9, 3.7) | 2.6 | (2.0, 3.4) | 3.2 | (2.3, 4.4) | 0.04 |
| Sperm concentration (millions/ml) | 42.9 | (21.9, 72.2) | 50.4 | (18.9, 71.5) | 43.4 | (27.9, 68.7) | 43.4 | (20.7, 83.1) | 37.0 | (19.3, 73.0) | 0.50 |
| Total sperm count (millions) | 121.5 | (65.4, 212.7) | 134.3 | (73.0, 210.6) | 111.1 | (55.7, 219.2) | 105.6 | (46.5, 190.6) | 130.3 | (75.2, 242.3) | 0.80 |
| Sperm motility (PR + NPR) (%) | 57.2 | (50.7, 63.8) | 54.5 | (48.7, 60.8) | 55.7 | (49.9, 63.0) | 59.2 | (54.0, 64.8) | 58.0 | (50.5, 67.5) | 0.17 |
| Morphologically normal sperm (%) | 8.9 | (6.0, 13.9) | 10.0 | (6–0, 14.0) | 8.9 | (5.5, 14.0) | 8.9 | (6.0, 16.0) | 8.9 | (5.5, 13.9) | 0.81 |
| Testicular volume (ml) | 21.0 | (19.5, 24.0) | 22.0 | (20.0, 24.5) | 22.5 | (20.0, 24.5) | 21.0 | (19.0, 23.38) | 21.0 | (19.0, 23.0) | 0.08 |
| Calories intake (kcal/day) | 2278.0 | (1896.1–2828.6) | 2443.6 | (2107.3–2946.3) | 2132.1 | (1775.1–2958.9) | 2312.8 | (1818.3–2912.0) | 2263.5 | (1899.4–2904.4) | 0.27 |
| Carbohydrates intake (g/day) | 230.2 | (202.0–252.9) | 267.7 | (250.7–297.3) | 242.3 | (224.7–252.0) | 215.5 | (202.6–231.4) | 191.2 | (170.4–203.2) | <0.01 |
| Fiber intake (g/day) | 19.8 | (16.4–23.4) | 22.9 | (17.2–26.7) | 21.5 | (17.9–24.7) | 19.2 | (16.5–22.7) | 17.3 | (14.6–19.8) | <0.01 |
| Protein intake (g/day) | 104.9 | (94.1–115.4) | 99.0 | (87.6–113.6) | 105.1 | (95.6–118.6) | 107.1 | (94.8–119.9) | 108.3 | (98.8–115.1) | 0.17 |
| Saturated fat intake (g/day) | 29.5 | (25.0–33.1) | 23.6 | (20.5–26.4) | 29.1 | (26.8–30.6) | 30.2 | (28.4–33.1) | 37.2 | (31.6–41.6) | <0.01 |
| Monounsaturated fat intake (g/day) | 40.3 | (36.0–45.0) | 33.0 | (30.3–35.8) | 38.8 | (36.8–40.2) | 43.2 | (41.0–45.0) | 47.9 | (46.0–54.4) | <0.01 |
| Polyunsaturated fat intake (g/day) | 14.5 | (12.8–16.4) | 12.6 | (11.3–14.0) | 14.0 | (12.9–15.4) | 15.5 | (14.4–16.9) | 16.7 | (14.1–19.6) | <0.01 |
| Trans fatty acid intake (g/day) | 1.7 | (1.2–2.2) | 1.5 | (1.0–2.0) | 1.7 | (1.3–2.3) | 1.9 | (1.5–2.3) | 1.3 | (0.9–1.8) | <0.01 |
| Vitamin C intake (mg/day) | 113.1 | (77.1–142.3) | 129.1 | (85.2–161.3) | 123.6 | (95.1–154.7) | 105.6 | (77.4–134.1) | 82.2 | (67.1–114.1) | <0.01 |
| Lycopene intake (µg/day) | 3938.7 | (2425.9–5893.4) | 3466.9 | (2359.7–5528.5) | 4623.9 | (2618.8–6105.5) | 4079.5 | (2590.5–5883.5) | 3483.8 | (2176.8–5133.9) | 0.39 |
| Β-carotene intake (µg/day) | 2465.3 | (1488.1–4213.3) | 228.4 | (1357.5–3992.2) | 3112.3 | (1651.6–5036.3) | 2899.4 | (1670.2–4383.6) | 2108.9 | (1520.5–3174.6) | 0.36 |
| Cryptoxantine intake (µg/day) | 276.8 | (160.1–414.9) | 342.8 | (187.0–519.1) | 328.9 | (231.9–429.3) | 278.0 | (164.5–419.2) | 163.9 | (110.9–267.0) | <0.01 |
| Alcohol intake (g/day) | 6.8 | (3.3–13.7) | 9.5 | (3.2–20.4) | 6.1 | (3.4–12.5) | 7.1 | (3.3–12.9) | 4.7 | (1.9–10.6) | 0.09 |
| Caffeine intake (mg/day) | 77.0 | (24.6–159.6) | 83.4 | (37.0–173.1) | 82.2 | (25.7–159.5) | 62.3 | (18.0–106.4) | 44.0 | (24.6–163.7) | 0.17 |
| Physical activity (h/week) | 9.0 | (6.0–13.0) | 9.0 | (6.0–14.0) | 9.0 | (6.0–12.0) | 10.0 | (6.0–14.0) | 7.0 | (4.0–13.5) | 0.31 |
| TV watching (h/week) | 20.0 | (14.0–41.0) | 24.5 | (14.0–36.5) | 25.0 | (14.0–45.5) | 22.5 | (14.0–41.0) | 20.0 | (14.0–35.0) | 0.96 |
Continuous variables are shown as median and interquartile range (IQR) unless otherwise indicated.
PR, progressive; NPR, non-progressive.
aFor continuous variables, Kruskal–Wallis analyses of variance were used to test for associations across quartiles of intake. For categorical variables, χ2 tests were used to test the associations between quartiles of intake.
Total fat intake was positively related to sperm motility in unadjusted analyses (Table II). Compared with men in the lowest quartile (Q1) of total fat, men in Q2, Q3 and Q4 had 2.4 (95% confidence interval (CI) −1.5, 6.3), 4.6 (0.6, 8.6) and 4.0 (0, 8.0) % units higher sperm motility. This association was driven by saturated fat intake (P for trend = 0.02). Adjustment for potential confounders attenuated the associations of total and saturated fat intakes with sperm motility (Table III). Men in the top quartile (Q4) of saturated fat intake had −21% (95% CI −72 to 26%) lower sperm concentration than men in the bottom quartile but this difference was not statistically significant (P = 0.7). When we evaluated the relation of specific fatty acids with semen parameters, intake of stearic acid remained positively related to sperm motility in multivariate models (data not shown). The adjusted percentage of motile sperm (95% CI) for men in increasing quartiles of stearic acid intake was 54.3 (51.0, 57.5), 55.9 (52.9, 58.9), 56.3 (53.4, 59.2) and 61.9 (58.3, 65.6) (P for trend = 0.02).
Table II.
Crude associations of fatty acid and cholesterol intakes with semen parameters (n = 209).
| Median for each quartile | Volume |
Motile sperm |
Morphologically normal sperm |
Sperm concentration |
Total sperm count |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| ml | 95% CI | % | 95% CI | % | 95% CI | 106/ml | 95% CI | 106 | 95% CI | |
| Total fat | ||||||||||
| Q1 (31.44% of calories) | 3.0 | 2.8–3.6 | 54.1 | 51.4–56.9 | 8.8 | 7.4–10.9 | 39.9 | 30.7–51.8 | 120.4 | 94.2–154.2 |
| Q2 (36.33% of calories) | 2.5 | 2.1–3.0 | 56.5 | 53.8–59.3 | 8.5 | 7.1–10.0 | 41.6 | 32.0–54.0 | 104.9 | 82.2–133.2 |
| Q3 (39.97% of calories) | 2.5 | 2.1–2.3 | 58.8 | 55.9–61.6a | 9.2 | 7.7–11.0 | 33.9 | 26.0–44.1 | 96.0 | 74.7–123.3 |
| Q4 (45.45% of calories) | 3.2 | 2.7–3.8 | 58.2 | 55.3–61.1a | 8.2 | 6.9–9.8 | 34.4 | 26.2–45.2 | 122.5 | 94.5–158.7 |
| Ptrend | 0.7 | 0.03 | 0.7 | 0.3 | 0.9 | |||||
| Saturated | ||||||||||
| Q1 (9. 44% of calories) | 3.1 | 2.6–3.6 | 55.3 | 52.5–58.1 | 8.9 | 7.5–10.6 | 41.0 | 31.6–53.3 | 124.2 | 97.0–159.2 |
| Q2 (11.31% of calories) | 2.9 | 2.9–3.4 | 55.6 | 52.8–58.4 | 8.1 | 6.8–9.6 | 29.9 | 23.1–38.8 | 107.7 | 84.0–137.8 |
| Q3 (12.63% of calories) | 2.2 | 1.9–2.6a | 56.8 | 54.0–59.6 | 9.6 | 8.1–11.5 | 41.1 | 31.6–53.4 | 90.5 | 70.8–115.6 |
| Q4 (15.53% of calories) | 3.1 | 2.7–3.7 | 59.8 | 56.9–62.7a | 8.1 | 6.8–9.7 | 39.1 | 29.8–51.4 | 123.2 | 95.5–159.0 |
| Ptrend | 0.9 | 0.02 | 0.6 | 0.8 | 0.9 | |||||
| Monounsaturated | ||||||||||
| Q1 (13.86% of calories) | 2.9 | 2.4–3.4 | 55.5 | 52.7–58.2 | 8.8 | 7.4–10.4 | 39.6 | 30.5–51.5 | 112.8 | 88.2–144.5 |
| Q2 (15.74% of calories) | 2.7 | 2.3–3.2 | 55.9 | 53.1–58.7 | 8.2 | 6.9–9.8 | 39.7 | 30.5–51.7 | 106.5 | 83.2–136.5 |
| Q3 (17.65% of calories) | 2.6 | 2.2–3.1 | 59.5 | 56.7–62.3a | 9.0 | 7.5–10.6 | 38.4 | 29.5–50.0 | 101.6 | 79.4–130.1 |
| Q4 (20.43% of calories) | 3.0 | 2.5–3.6 | 56.5 | 53.6–59.5 | 8.7 | 7.3–10.4 | 32.0 | 24.3–42.0 | 122.4 | 94.1–159.0 |
| Ptrend | 0.7 | 0.3 | 0.9 | 0.3 | 0.8 | |||||
| Polyunsaturated | ||||||||||
| Q1 (4.84% of calories) | 3.2 | 2.7–3.8 | 56.0 | 53.2–58.9 | 8.8 | 7.4–10.4 | 40.9 | 31.4–53.1 | 130.1 | 101.6–166.5 |
| Q2 (5.64% of calories) | 2.6 | 2.2–3.1 | 56.1 | 53.2–58.9 | 9.1 | 7.6–10.8 | 34.4 | 26.5–44.8 | 90.3 | 70.7–115.4a |
| Q3 (6.31% of calories) | 2.7 | 2.6–3.2 | 58.4 | 55.5–61.3 | 9.1 | 7.7–10.8 | 40.2 | 30.9–52.3 | 120.7 | 94.3–154.5 |
| Q4 (7.62% of calories) | 2.7 | 2.3–3.3 | 56.9 | 54.0–59.8 | 7.8 | 6.5–9.3 | 34.4 | 26.2–45.2 | 104.2 | 80.8–134.4 |
| Ptrend | 0.3 | 0.5 | 0.3 | 0.5 | 0.5 | |||||
| Omega-3 | ||||||||||
| Q1 (0.51% of calories) | 2.7 | 2.3–3.2 | 58.5 | 55.8–61.3 | 9.5 | 8.0–11.3 | 43.5 | 33.7–56.2 | 118.2 | 92.9–150.2 |
| Q2 (0.62% of calories) | 2.5 | 2.1–3.0 | 54.7 | 51.8–57.5 | 8.6 | 7.2–10.2 | 32.1 | 24.7–41.6 | 99.99 | 77.9–128.4 |
| Q3 (0.74% of calories) | 3.1 | 2.6–3.6 | 55.3 | 52.5–58.1 | 7.1 | 6.0–8.4a | 29.3 | 22.6–37.9a | 88.06 | 68.9–112.5 |
| Q4 (0.91% of calories) | 3.0 | 2.5–3.5 | 58.9 | 56.0–61.8 | 9.8 | 8.3–11.7 | 48.3 | 37.0–63.1 | 142.45 | 110.9–182.9 |
| Ptrend | 0.3 | 0.7 | 0.9 | 0.6 | 0.3 | |||||
| Omega-6 | ||||||||||
| Q1 (4.08% of calories) | 3.1 | 2.63–3.71 | 54.7 | 51.8–57.5 | 8.3 | 7.0–9.9 | 36.0 | 27.6–47.0 | 111.4 | 86.6–143.3 |
| Q2 (4.87% of calories) | 2.7 | 2.28–3.19 | 57.7 | 54.8–60.5 | 9.6 | 8.1–11.4 | 37.5 | 28.8–78.9 | 101.2 | 79.0–129.0 |
| Q3 (5.42% of calories) | 2.7 | 2.27–3.19 | 58.3 | 55.4–61.2 | 9.4 | 7.9–11.2 | 40.8 | 31.3–53.1 | 124.3 | 96.9–159.5 |
| Q4 (6.73% of calories) | 2.7 | 2.27–3.21 | 56.7 | 53.8–59.6 | 7.5 | 6.3–8.9 | 35.4 | 27.0–46.3 | 105.4 | 81.8–136.1 |
| Ptrend | 0.3 | 0.4 | 0.3 | 0.9 | 0.9 | |||||
| Trans | ||||||||||
| Q1 (0.37% of calories) | 3.0 | 2.5–3.5 | 58.1 | 55.2–60.9 | 7.7 | 6.5–9.1 | 45.4 | 34.9–59.0 | 135.4 | 106.1–173.0 |
| Q2 (0.54% of calories) | 3.0 | 2.6–3.6 | 56.4 | 53.5–59.3 | 8.8 | 7.4–10.4 | 35.2 | 26.9–45.9 | 118.3 | 91.9–152.0 |
| Q3 (0.74% of calories) | 2.6 | 2.2–3.1 | 56.6 | 53.7–59.4 | 9.4 | 7.9–11.2 | 33.8 | 26.0–44.0 | 97.8 | 76.3–125.6 |
| Q4 (1.03% of calories) | 2.6 | 2.2–3.1 | 56.3 | 53.4–59.2 | 8.9 | 7.4–10.6 | 36.1 | 27.7–47.0 | 93.9 | 73.4–120.2a |
| Ptrend | 0.1 | 0.5 | 0.3 | 0.3 | 0.03 | |||||
| Cholesterol | ||||||||||
| Q1 (0.26 g/day) | 3.1 | 2.7–3.7 | 57.6 | 54.8–60.4 | 8.6 | 7.2–10.3 | 32.6 | 25.1–42.3 | 111.3 | 86.7–142.9 |
| Q2 (0.34 g/day) | 2.6 | 2.2–3.1 | 54.3 | 51.4–57.1 | 8.6 | 7.3–10.3 | 39.9 | 30.6–51.9 | 116.3 | 90.6–149.2 |
| Q3 (0.40 g/day) | 2.9 | 2.5–3.5 | 58.2 | 55.3–61.1 | 9.0 | 7.6–10.7 | 37.2 | 28.5–48.7 | 109.2 | 84.9–140.6 |
| Q4 (0.51 g/day) | 2.6 | 2.2–3.1 | 57.2 | 54.4–60.1 | 8.4 | 7.1–10.0 | 40.7 | 31.1–53.1 | 104.4 | 81.1–134.3 |
| Ptrend | 0.2 | 0.8 | 0.9 | 0.3 | 0.7 | |||||
CI, confidence interval.
aSignificantly different to mean in the lowest quartile of intake at P < 0.05.
Table III.
Multivariate adjusteda associations of fatty acid and cholesterol intakes with semen parameters (n = 209).
| Median for each quartile | Volume |
Motile sperm |
Morphologically normal sperm |
Sperm concentration |
Total sperm count |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| ml | 95% CI | % | 95% CI | % | 95% CI | 106/ml | 95% CI | 106 | 95% CI | |
| Total fat | ||||||||||
| Q1 (31.44% of calories) | 3.0 | 2.5–3.6 | 54.2 | 51.1–57.4 | 8.7 | 7.2–10.6 | 44.5 | 33.1–59.9 | 122.9 | 93.4–161.6 |
| Q2 (36.33% of calories) | 2.5 | 2.1–2.9 | 56.7 | 53.8–59.6 | 8.4 | 7.0–10.1 | 40.9 | 31.1–53.8 | 105.0 | 81.8–134.7 |
| Q3 (39.97% of calories) | 2.5 | 2.1–3.0 | 58.6 | 55.6–61.6a | 9.5 | 7.9–11.4 | 33.2 | 25.1–43.9 | 94.1 | 72.7–121.6 |
| Q4 (45.45% of calories) | 3.3 | 2.8–4.0 | 58.8 | 55.6–62.1 | 8.5 | 7.0–10.3 | 31.8 | 23.4–43.1 | 121.9 | 91.9–161.4 |
| Ptrend | 0.7 | 0.06 | 0.6 | 0.6 | 0.9 | |||||
| Saturated | ||||||||||
| Q1 (9. 44% of calories) | 3.1 | 2.6–3.7 | 55.7 | 52.5–58.5 | 8.9 | 7.3–10.7 | 45.0 | 33.4–60.5 | 129.7 | 98.4–170.9 |
| Q2 (11.31% of calories) | 2.9 | 2.4–3.4 | 55.5 | 52.6–58.5 | 7.9 | 6.6–9.4 | 30.9 | 23.5–40.5a | 104.8 | 81.4–135.0 |
| Q3 (12.63% of calories) | 2.2 | 1.9–2.6a | 56.7 | 53.8–59.6 | 9.7 | 8.1–11.6 | 40.5 | 39.9–53.1 | 93.1 | 72.7–119.3 |
| Q4 (15.53% of calories) | 3.2 | 2.6–3.9 | 60.6 | 53.8–63.9 | 8.8 | 7.1–10.8 | 35.6 | 25.9–48.9 | 120.4 | 90.0–161.1 |
| Ptrend | 0.9 | 0.06 | 0.7 | 0.7 | 0.7 | |||||
| Monounsaturated | ||||||||||
| Q1 (13.86% of calories) | 3.0 | 2.4–3.7 | 56.3 | 52.8–59.9 | 8.6 | 6.9–10.8 | 35.8 | 25.6–50.0 | 111.9 | 82.3–152.2 |
| Q2 (15.74% of calories) | 2.8 | 2.3–3.3 | 56.3 | 53.3–59.2 | 8.5 | 7.1–10.2 | 37.8 | 28.6–50.0 | 107.2 | 83.0–138.5 |
| Q3 (17.65% of calories) | 2.5 | 2.1–3.0 | 59.0 | 55.9–62.2 | 9.1 | 7.5–11.0 | 41.8 | 31.2–56.0 | 97.1 | 74.2–127.1 |
| Q4 (20.43% of calories) | 3.0 | 2.5–3.7 | 56.4 | 52.9–59.8 | 8.8 | 7.2–10.9 | 34.6 | 25.1–47.7 | 128.3 | 95.1–173.0 |
| Ptrend | 0.9 | 0.9 | 0.9 | 0.8 | 0.7 | |||||
| Polyunsaturated | ||||||||||
| Q1 (4.84% of calories) | 3.2 | 2.6–3.9 | 56.4 | 53.0–59.9 | 8.8 | 7.2–10.9 | 43.7 | 31.8–60.2 | 148.0 | 110.6–198.0 |
| Q2 (5.64% of calories) | 2.7 | 2.2–3.2 | 56.0 | 53.1–58.9 | 9.3 | 7.8–11.1 | 34.0 | 25.9–44.7 | 93.2 | 73.0–119.2a |
| Q3 (6.31% of calories) | 2.6 | 2.1–3.0 | 58.5 | 55.3–61.6 | 9.1 | 7.5–10.9 | 39.7 | 29.8–52.8 | 114.0 | 87.9–147.8 |
| Q4 (7.62% of calories) | 2.8 | 2.3–3.4 | 57.1 | 53.7–60.6 | 7.8 | 6.4–9.6 | 33.1 | 24.0–45.6 | 93.4 | 69.8–125.1a |
| Ptrend | 0.5 | 0.7 | 0.4 | 0.3 | 0.1 | |||||
| Omega-3 | ||||||||||
| Q1 (0.51% of calories) | 2.7 | 2.2–3.3 | 59.3 | 56.2–62.5 | 9.1 | 7.5–11.0 | 42.1 | 31.4–56.3 | 118.4 | 90.6–154.8 |
| Q2 (0.62% of calories) | 2.6 | 2.1–3.1 | 55.4 | 52.4–58.5a | 8.4 | 7.0–10.1 | 33.6 | 25.5–44.3 | 110.2 | 85.0–142.7 |
| Q3 (0.74% of calories) | 2.9 | 2.4–3.5 | 54.7 | 51.7–57.7 | 7.1 | 5.9–8.5 | 28.0 | 21.1–37.0 | 80.5 | 62.0–104.5 |
| Q4 (0.91% of calories) | 3.0 | 2.5–3.7 | 58.4 | 55.1–61.8 | 10.9 | 8.9–13.2 | 49.6 | 36.6–67.3 | 139.6 | 105.4–184.8 |
| Ptrend | 0.3 | 0.8 | 0.3 | 0.4 | 0.5 | |||||
| Omega-6 | ||||||||||
| Q1 (4.08% of calories) | 3.2 | 2.5–3.9 | 54.8 | 51.2–58.3 | 8.7 | 7.0–10.8 | 37.0 | 26.4–52.0 | 119.2 | 87.0–163.4 |
| Q2 (4.87% of calories) | 2.7 | 2.3–3.2 | 57.4 | 54.5–60.3 | 9.9 | 8.3–11.8 | 36.9 | 28.0–48.7 | 106.1 | 82.5–136.5 |
| Q3 (5.42% of calories) | 2.6 | 2.2–3.2 | 58.5 | 55.4–61.7 | 9.3 | 7.7–11.2 | 40.1 | 81.3–53.7 | 116.8 | 89.2–152.8 |
| Q4 (6.73% of calories) | 2.7 | 2.2–3.3 | 57.3 | 53.9–60.7 | 7.3 | 5.9–8.9 | 35.8 | 29.9–49.4 | 99.7 | 74.1–134.0 |
| Ptrend | 0.5 | 0.5 | 0.1 | 0.8 | 0.4 | |||||
| Trans | ||||||||||
| Q1 (0.37% of calories) | 3.0 | 2.5–3.6 | 58.3 | 55.1–61.6 | 8.2 | 6.7–9.9 | 45.7 | 33.8–61.8 | 144.3 | 109.5–190.0 |
| Q2 (0.54% of calories) | 3.0 | 2.5–2.6 | 56.5 | 53.4–59.6 | 9.2 | 7.6–11.0 | 33.3 | 25.0–44.4 | 113.5 | 87.1–148.1 |
| Q3 (0.74% of calories) | 2.6 | 2.2–3.1 | 56.9 | 53.9–60.0 | 9.5 | 7.9–11.3 | 35.8 | 27.2–47.1 | 100.5 | 77.9–129.8 |
| Q4 (1.03% of calories) | 2.6 | 2.2–3.2 | 56.2 | 53.0–59.4 | 8.3 | 6.9–10.1 | 35.8 | 26.8–47.9 | 89.5 | 68.5–117.0b |
| Ptrend | 0.2 | 0.5 | 0.8 | 0.6 | 0.03 | |||||
| Cholesterol | ||||||||||
| Q1 (0.26 g/day) | 3.6 | 2.9–4.4 | 59.8 | 56.3–63.3 | 7.6 | 6.1–9.5 | 32.7 | 23.4–45.7 | 122.1 | 89.4–166.8 |
| Q2 (0.34 g/day) | 2.6 | 2.2–3.0b | 53.8 | 50.9–56.7b | 8.5 | 7.1–10.2 | 41.3 | 31.3–54.4 | 117.2 | 90.8–151.3 |
| Q3 (0.40 g/day) | 2.8 | 2.3–3.3 | 57.9 | 55.0–60.9 | 9.6 | 8.0–11.5 | 36.0 | 27.1–47.9 | 103.1 | 79.4–133.9 |
| Q4 (0.51 g/day) | 2.4 | 1.9–2.9b | 56.4 | 52.9–59.8 | 9.5 | 7.7–11.7 | 40.4 | 29.2–55.9 | 99.4 | 73.9–133.5 |
| Ptrend | 0.04a | 0.6 | 0.2 | 0.6 | 0.4 | |||||
aAdjusted for BMI (kg/m2), smoking (current smoker versus not current smoker), ejaculation abstinence time (hours), alcohol intake (g/day), caffeine intake (mg/day), total caloric intake (kcal/day), fiber intake (%calories), protein intake (%calories), intakes of the remaining fatty acids (% calories), vitamin C (mg/day), β-cryptoxantine (µg/day), lycopene (µg/day) and β-carotene (µg/day). Models for sperm motility are further adjusted for time to start semen analysis (minutes).
bSignificantly different to mean in the lowest quartile of intake at P < 0.05.
Cholesterol intake was inversely related to ejaculate volume after adjusting for potential confounders (Table III). In the multivariable adjusted model, ejaculate volume was −33% (95% CI −74 to −8%) lower in men in the highest quartile of cholesterol intake compared with men in the lowest quartile, and there was a statistically significant linear trend of lower volume with increasing cholesterol intake (P = 0.04).
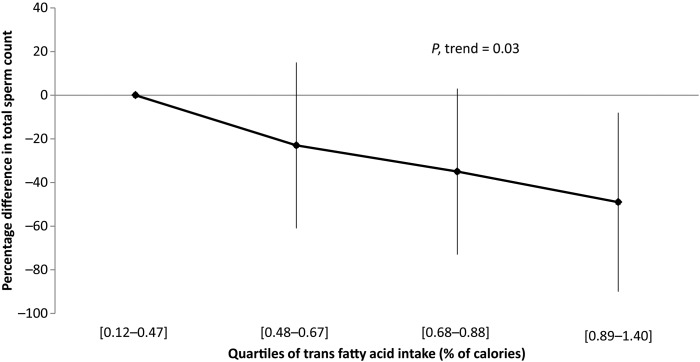
Intake of trans fatty acids, which were primarily derived from French fried potatoes and commercially baked items, was inversely related to total sperm count in unadjusted models (Table II). The total sperm count for men in the second, third and fourth quartiles of intake was 14, 33 and 37% lower than men in the lowest quartile of intake, respectively. The inverse association between trans fatty acids intake and total sperm count remained statistically significant after adjusting for potential confounders (Table III). After adjustments, total sperm count for men in increasing quartiles of trans fat intake was 23, 35 and 49% lower than that of men in the lowest quartile of intake (Fig. 1). There was also a suggestion of an inverse relation between trans fat intake and sperm concentration. Men in the second, third and fourth quartiles of trans fat intake had 28, 22 and 22% lower sperm concentration than men in the bottom quartile but these differences were not statistically significant (P = 0.14, 0.25 and 0.29, respectively). Intakes of mono-unsaturated and poly-unsaturated fatty acids (PUFAs) were unrelated to semen parameters.
Figure 1.
Relative difference in total sperm count in young men by increasing quartiles of trans fatty acid intake. Values represent the percentual (relative) difference and 95% confidence interval in total sperm count for men in the 2nd, 3rd and 4th quartile of trans fat intake when compared with men in the first quartile of intake. Models are adjusted for BMI (kg/m2), smoking (current smoker versus not current smoker), ejaculation abstinence time (hours), alcohol intake (g/day), caffeine intake (mg/day), total caloric intake (kcal/day), fiber intake (%calories), protein intake (%calories), intakes of the remaining fatty acids (% calories), vitamin C (mg/day), β-cryptoxantine (µg/day), lycopene (µg/day) and β-carotene (µg/day).
Discussion
We evaluated the association of fatty acid intake and semen quality among young men from Southern Spain. Intake of trans fatty acids, primarily derived from French fried potatoes and commercially baked items, was inversely related to total sperm count. Intake of trans fats and other major types of fat was comparable with that observed in the general Spanish population (Hulshof et al., 1999; Spanish Food Safety and Nutrition Agency, 2011). We also found a positive association between stearic acid intake and sperm motility and an inverse association between cholesterol intake and ejaculate volume. Intake of other fatty acids was not significantly related to semen parameters.
To our knowledge, this is the first study to report a relation between intake of trans fats and semen quality parameters in humans. These findings are, nevertheless, consistent with previously published experimental and human data. In rodent models, trans fatty acids supplementation resulted in significant reproductive damage. Male rodents fed hydrogenated oils instead of non-hydrogenated vegetable oils accumulate trans fats in the testis (Jensen, 1976; Privett et al., 1977). Furthermore, this type of supplementation with trans fatty acids leads to a number of adverse male reproductive outcomes including decreased fertility, decreased serum testosterone levels, decreased sperm count, motility and normal morphology and, in extreme cases, arrest of spermatogenesis and testicular degeneration (Jensen, 1976; Hanis et al., 1989; Veaute et al., 2007). Our findings are also consistent with a previous report of an inverse association between sperm levels of trans fatty acids and sperm concentration among fertility patients in the USA (Chavarro et al., 2011), in which the amount of trans fatty acids in sperm membranes was inversely related to sperm concentration. Chavarro et al. (2011) did not assess trans fatty acid intake and it was, therefore, not possible to assess the relation between trans fatty acid intake and sperm levels. However, because trans fats cannot be synthesized endogenously, and previous studies have found that trans fatty acid levels in other tissues are good biomarkers of intake (Baylin et al., 2002, 2005; Sun et al., 2007), the authors concluded that their findings suggested a deleterious effect of trans fatty acid intake on spermatogenesis. The results of the current study are in agreement with that interpretation.
In addition to the consistency of our findings with previous work, there are known biologic effects of trans fatty acids that could explain their association with decreased sperm count. In multiple species, including humans, sperm membranes increase the relative amount of PUFAs, particularly of docosahexaenoic acid, during epididymal maturation (Hall et al., 1991; Rana et al., 1991; Haidl and Opper, 1997; Lenzi et al., 2000; Ollero et al., 2000). The increase in the relative amount of PUFAs appears to be mediated to a great extent by local fatty acid metabolism, as evidenced by the high efficiency of Sertoli cells converting 18-carbon PUFAs into 22- and 24-carbon PUFAs (Christophersen et al., 1986; Retterstøl et al., 1998, 2000), as well as the high expression levels of the enzymes necessary for this metabolic process, including Δ5- and Δ6-desaturase (Saether et al., 2003) and fatty acid elongases (Leonard et al., 2000; Tvrdik et al., 2000; Zhang et al., 2001; Mandal et al., 2004) in the testis and epididymis. Trans fatty acids decrease the activity of the Δ5- and Δ6-desaturases (Hill et al., 1982; Larqué et al., 2003) potentially limiting the incorporation of long chain PUFAs into sperm membranes, and, therefore, affecting spermatogenesis. Although it is not known to what extent trans fats could impair these enzymes in the testes, in a mouse model of FADS2 deficiency (the gene encoding Δ6-desaturase, the rate-limiting enzyme in the pathway), FADS2−/− mice had major changes in testicular fatty acid composition and infertility due to a profound impairment of spermatid elongation, evidenced by a complete absence of normal spermatozoa or elongated spermatids in the seminiferous tubules (Stroud et al., 2009). Although this mechanism could explain our observation, it is unclear whether it is at play in human spermatogenesis and to what extent trans fatty acids could impair it.
There was also an inverse relationship between cholesterol intake and ejaculate volume. While we are unaware of previous reports of this association in humans, our findings are in agreement with previous experimental work in rabbits. A cholesterol enriched diet (0.05%) resulted in hypercholesterolemia, increased incorporation of cholesterol into sperm membranes, decreased semen volume, decreased sperm motility, higher frequency of morphological abnormalities, and impaired capacitation and acrosomal reaction (Saez Lancellotti et al., 2010). While our findings for ejaculate volume are in agreement with this study, we did not observe associations with sperm motility or morphology. It is possible that the first effects of a high cholesterol diet might be on ejaculate volume, and longer exposure would be needed to observe effects on motility and morphology. It is also possible that hypercholesterolemia might be necessary for the other reproductive effects observed in rabbits to be evident. While we cannot test directly this hypothesis because we did not measure serum cholesterol levels, study participants were young healthy men and the expected frequency of hypercholesterolemia among these men, given their demographic characteristics, is low. Further research is necessary to evaluate the potentially deleterious effects of dietary cholesterol on spermatogenesis.
We also found a positive association between stearic acid and sperm motility. Attaman and collaborators reported inverse relations of sperm motility with the concentration of stearic acid in spermatozoa and seminal plasma among fertility patients (Attaman et al., 2012). However, stearic acid intake was not related to sperm concentration of this fatty acid in spermatozoa or seminal plasma. Moreover, two previous studies examining the relation between saturated fat intake and semen quality have reported inverse relations with sperm concentration and no relation with sperm motility (Attaman et al., 2012; Jensen et al., 2013). In contrast, men adhering to a traditional Dutch dietary pattern (high in meat products, an important source of saturated fats) had higher sperm concentration (Vujkovic et al., 2009). Our observed relation between stearic acid and sperm motility is unexpected and not consistent with previous work. While it may represent a chance finding, it is important to further evaluate this relation.
The present study has some limitations. First, the cross-sectional design severely limits causal inference. However, by selecting young men with untested fertility as the study population and not communicating the results of the semen analysis to them, participants were blinded to the study outcomes. This minimizes the possibility that men had changed their diet in response to their knowledge of the study outcome leading to reverse causation, a common concern in cross-sectional studies. A related concern is that, as is the case in all observational epidemiologic studies, we cannot exclude the possibility of unmeasured confounding. Nevertheless, we adjusted for a large number of known and suspected confounders. In addition, the associations did not change dramatically between the crude and fully adjusted models suggesting that unmeasured confounding is not likely to explain the entirety of the observed relation. Second, we only obtained one sample of semen for each subject. Nevertheless, others have found that one semen sample may be sufficient for representing a man's semen quality in epidemiological studies (Carlsen et al., 2005; Stokes-Riner et al., 2007). Third, multiple statistical tests could be a potential limitation of the current study. However, all these analyses were based on a priori hypotheses and therefore biologically justifiable. In consequence, and in agreement with the prevailing view and practice in epidemiology, we believe that adjustment for multiple comparisons is not necessary in studies such as this one. Last, although the study is large relative to the usual size of semen quality studies, it did not have sufficient power to identify modest associations such as those observed for saturated and trans fat intake with sperm concentration. In light of these limitations, it is important that the observed relations are further evaluated in other studies. The strengths of our study include the use of a previously validated FFQ (Vioque, 1995; Vioque et al., 2007), the wide range of fat intake observed in the study population and the comprehensive assessment of a wide range of potential confounders. In summary, we found an inverse relation of trans fatty acid intake, derived primarily from hydrogenated oils, with total sperm count among young healthy men in Spain. We also found an inverse relation between cholesterol intake and ejaculate volume. Both findings are in agreement with experimental work in rodents and the relation of trans fats with total sperm count is also in agreement with previous work among fertility patients. Whether the observed relations have any impact on fertility is not known.
Authors' roles
A.M.T.-C. and J.M. were involved in study conception and study design. J.M. was involved in study execution and acquisition of data. J.E.C., L.M.-A. and J.J.L.-E. contributed to data analysis and interpretation. J.E.C., L.M.-A. and A.C.-T. drafted the manuscript. All authors provided substantial intellectual contributions and approved the final version of the manuscript.
Funding
This work was supported by The Seneca Foundation, Regional Agency of Science and Technology, grant no 00694/PI/04, Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III (FIS), grant no PI10/00985, and grant P30 DK46200 from the National Institutes of Health.
Conflict of interest
The authors have no competing interests to declare.
Acknowledgements
The authors gratefully acknowledge Dr M. Roca, C. Ruiz, E. Belmonte, F. Mas and all the Quirón Dexeus Murcia clinic staff for their assistance in data collection; and the young men of the study for their participation. We also thank L. Sarabia and G. Vivero for semen analyses, K. Ruiz and E. Estrella for database management and J. Vioque and E.M. Navarrete-Muñoz for dietary assessment.
References
- Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod. 2012;27:1466–1474. doi: 10.1093/humrep/des065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin A, Kabagambe EK, Siles X, Campos H. Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr. 2002;76:750–757. doi: 10.1093/ajcn/76.4.750. [DOI] [PubMed] [Google Scholar]
- Baylin A, Kim MK, Donovan-Palmer A, Siles X, Dougherty L, Tocco P, Campos H. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol. 2005;162:373–381. doi: 10.1093/aje/kwi213. [DOI] [PubMed] [Google Scholar]
- Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during the past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen E, Swan SH, Petersen JH, Skakkebæk NE. Longitudinal changes in semen parameters in young Danish men from the Copenhagen area. Hum Reprod. 2005;20:942–949. doi: 10.1093/humrep/deh704. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Furtado J, Toth TL, Ford J, Keller M, Campos H, Hauser R. Trans–fatty acid levels in sperm are associated with sperm concentration among men from an infertility clinic. Fertil Steril. 2011;95:1794–1797. doi: 10.1016/j.fertnstert.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophersen BO, Hagve TA, Christensen E, Johansen Y, Tverdal S. Eicosapentaenoic- and arachidonic acid metabolism in isolated liver cells. Scand J Clin Lab Invest Suppl. 1986;184:55–60. [PubMed] [Google Scholar]
- Eskenazi B, Kidd SA, Marks AR, Sloter E, Block G, Wyrobek AJ. Antioxidant intake is associated with semen quality in healthy men. Hum Reprod. 2005;20:1006–1012. doi: 10.1093/humrep/deh725. [DOI] [PubMed] [Google Scholar]
- Fisch H, Goluboff ET, Olson JH, Feldshuh J, Broder SJ, Barad DH. Semen analyses in 1283 men from the United States over a 25-year period: no decline in quality. Fertil Steril. 1996;65:1009–1014. doi: 10.1016/s0015-0282(16)58278-8. [DOI] [PubMed] [Google Scholar]
- Gaskins A, Colaci D, Mendiola J, Swan SH, Chavarro J. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27:2899–2907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidl G, Opper C. Changes in lipids and membrane anisotropy in human spermatozoa during epididymal maturation. Hum Reprod. 1997;12:2720–2723. doi: 10.1093/humrep/12.12.2720. [DOI] [PubMed] [Google Scholar]
- Hall JC, Hadley J, Doman T. Correlation between changes in rat sperm membrane lipids, protein, and the membrane physical state during epididymal maturation. J Androl. 1991;12:76–87. [PubMed] [Google Scholar]
- Handelsman DJ. Sperm output of healthy men in Australia: magnitude of bias due to self-selected volunteers. Hum Reprod. 1997;12:2701–2705. doi: 10.1093/humrep/12.12.2701. [DOI] [PubMed] [Google Scholar]
- Hanis T, Zidek V, Sachova J, Klir P, Deyl Z. Effects of dietary trans-fatty acids on reproductive performance of Wistar rats. Br J Nutr. 1989;61:519–529. doi: 10.1079/bjn19890140. [DOI] [PubMed] [Google Scholar]
- Hill EG, Johnson SB, Lawson LD, Mahfouz MM, Holman RT. Perturbation of the metabolism of essential fatty acids by dietary partially hydrogenated vegetable oil. Proc Natl Acad Sci USA. 1982;79:953–957. doi: 10.1073/pnas.79.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshof KF, van Erp-Baart MA, Anttolainen M, Becker W, Church SM, Couet C, Hermann-Kunz E, Kesteloot H, Leth T, Martins I, et al. Intake of fatty acids in western Europe with emphasis on trans fatty acids: the TRANSFAIR Study. Eur J Clin Nutr. 1999;53:143–157. doi: 10.1038/sj.ejcn.1600692. [DOI] [PubMed] [Google Scholar]
- Jensen B. Rat testicular lipids and dietary isomeric fatty acids in essential fatty acid deficiency. Lipids. 1976;11:179–188. doi: 10.1007/BF02532855. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Heitmann BL, Jensen MB, Halldorsson TI, Andersson AM, Skakkebaek NE, Joensen UN, Lauritsen MP, Christiansen P, Dalgard C, et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am J Clin Nutr. 2013;97:411–418. doi: 10.3945/ajcn.112.042432. [DOI] [PubMed] [Google Scholar]
- Larqué E, García-Ruiz PA, Perez-Llamas F, Zamora S, Gil A. Dietary trans fatty acids alter the compositions of microsomes and mitochondria and the activities of microsome delta6-fatty acid desaturase and glucose-6-phosphatase in livers of pregnant rats. J Nutr. 2003;133:2526–2531. doi: 10.1093/jn/133.8.2526. [DOI] [PubMed] [Google Scholar]
- Lenzi A, Gandini L, Maresca V, Rago R, Sgrò P, Dondero F, Picardo M. Fatty acid composition of spermatozoa and immature germ cells. Mol Hum Reprod. 2000;6:226–231. doi: 10.1093/molehr/6.3.226. [DOI] [PubMed] [Google Scholar]
- Leonard AE, Bobik EG, Dorado J, Kroeger PE, Chuang LT, Thurmond JM, Parker-Barnes JM, Das T, Huang YS, Mukerji P. Cloning of a human cDNA encoding a novel enzyme involved in the elongation of long-chain polyunsaturated fatty acids. Biochem J. 2000;350:765–770. [PMC free article] [PubMed] [Google Scholar]
- Mandal MN, Ambasudhan R, Wong PW, Gage PJ, Sieving PA, Ayyagari R. Characterization of mouse orthologue of ELOVL4: genomic organization and spatial and temporal expression. Genomics. 2004;83:626–635. doi: 10.1016/j.ygeno.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Torres-Cantero AM, Moreno-Grau JM, Ten J, Roca M, Moreno-Grau S, Bernabeu R. Food intake and its relationship with semen quality: a case–control study. Fertil Steril. 2009;91:812–818. doi: 10.1016/j.fertnstert.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Jørgensen N, Mínguez-Alarcón L, Sarabia-Cos L, López-Espín JJ, Vivero-Salmerón G, Ruiz-Ruiz KJ, Fernández MF, Olea N, Swan SH, et al. Sperm counts may have declined in young university students in Southern Spain. Andrology. 2013;1:408–413. doi: 10.1111/j.2047-2927.2012.00058.x. [DOI] [PubMed] [Google Scholar]
- Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5:586–592. doi: 10.1093/oxfordjournals.humrep.a137150. [DOI] [PubMed] [Google Scholar]
- Mínguez-Alarcón L, Mendiola J, López-Espín JJ, Sarabia-Cos L, Vivero-Salmerón G, Vioque J, Navarrete-Muñoz EM, Torres-Cantero AM. Dietary intake of antioxidant nutrients is associated with semen quality in young university students. Hum Reprod. 2012;27:2807–2814. doi: 10.1093/humrep/des247. [DOI] [PubMed] [Google Scholar]
- Nielsen SM, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004;27:205–210. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Ocké MC, Bueno-de-Mesquita HB, Pols MA, Smit HA, van Staveren WA, Kromhout D. The Dutch EPIC food frequency questionnaire. II. Relative validity and reproducibility for nutrients. Int J Epidemiol. 1997;26:S49–S58. doi: 10.1093/ije/26.suppl_1.s49. [DOI] [PubMed] [Google Scholar]
- Ollero M, Powers RD, Alvarez JG. Variation of docosahexaenoic acid content in subsets of human spermatozoa at different stages of maturation: implications for sperm lipoperoxidative damage. Mol Reprod Dev. 2000;55:326–334. doi: 10.1002/(SICI)1098-2795(200003)55:3<326::AID-MRD11>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Palma I, Farran P, Cervera P. Tablas de composición de Alimentos por medidas caseras de consumo habitual en España: CESNID [in Spanish] 4th edn. Spain: McGraw Hill; 2008. [Google Scholar]
- Paulsen CA, Berman NG, Wang C. Data from men in greater Seattle area reveals no downward trend in semen quality: further evidence that deterioration of semen quality is not geographically uniform. Fertil Steril. 1996;65:1015–1020. [PubMed] [Google Scholar]
- Privett OS, Phillips F, Shimasaki H, Nozawa T, Nickell EC. Studies of effects of trans fatty acids in the diet on lipid metabolism in essential fatty acid deficient rats. Am J Clin Nutr. 1977;30:1009–1017. doi: 10.1093/ajcn/30.7.1009. [DOI] [PubMed] [Google Scholar]
- Putnam J, Allshouse J, Scott-Kantor L. US per capita food supply trends: more calories, refine carbohydrates and fats. Food Rev. 2002;25:2–15. [Google Scholar]
- Rana AP, Majumder GC, Misra S, Ghosh A. Lipid changes of goat sperm plasma membrane during epididymal maturation. Biochim Biophys Acta. 1991;1061:185–196. doi: 10.1016/0005-2736(91)90284-f. [DOI] [PubMed] [Google Scholar]
- Retterstøl K, Haugen TB, Woldseth B, Christophersen BO. A comparative study of the metabolism of n-9, n-6 and n-3 fatty acids in testicular cells from immature rat. Biochim Biophys Acta. 1998;1392:59–72. doi: 10.1016/s0005-2760(98)00021-6. [DOI] [PubMed] [Google Scholar]
- Retterstøl K, Haugen TB, Christophersen BO. The pathway from arachidonic to docosapentaenoic acid (20:4n-6 to 22:5n-6) and from eicosapentaenoic to docosahexaenoic acid (20:5n-3 to 22:6n-3) studied in testicular cells from immature rats. Biochim Biophys Acta. 2000;1483:119–131. doi: 10.1016/s1388-1981(99)00166-3. [DOI] [PubMed] [Google Scholar]
- Saether T, Tran TN, Rootwelt H, Christophersen BO, Haugen TB. Expression and regulation of delta5-desaturase, delta6-desaturase, stearoyl-coenzyme A (CoA) desaturase 1, and stearoyl-CoA desaturase 2 in rat testis. Biol Reprod. 2003;69:117–124. doi: 10.1095/biolreprod.102.014035. [DOI] [PubMed] [Google Scholar]
- Saez Lancellotti TE, Boarelli PV, Monclus MA, Cabrillana ME, Clementi MA, Espínola LS, Cid Barría JL, Vincenti AE, Santi AG, Fornés MW. Hypercholesterolemia impaired sperm functionality in rabbits. PLoS One. 2010;5:e13457. doi: 10.1371/journal.pone.0013457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebæk NE, Jørgensen N, Main KM, Rajpert-De Meyts E, Leffers H, Andersson AM, Juul A, Carlsen E, Mortensen GK, Jensen TK, et al. Is human fecundity declining? Int J Androl. 2006;29:2–11. doi: 10.1111/j.1365-2605.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- Spanish Food Safety and Nutrition Agency. 2011. http://www.aesan.msc.es/en/AESAN/web/home.shtml .
- Stokes-Riner A, Thurston SW, Brazil C, Guzick D, Liu F, Overstreet JW, Wang C, Sparks A, Redmon JB, Swan SH. One semen sample or 2? Insights from a study of fertile men. J Androl. 2007;28:638–643. doi: 10.2164/jandrol.107.002741. [DOI] [PubMed] [Google Scholar]
- Stroud CK, Nara TY, Roqueta-Rivera M, Radlowski EC, Lawrence P, Zhang Y, Cho BH, Segre M, Hess RA, Brenna JT, et al. Disruption of FADS2 gene in mice impairs male reproduction and causes dermal and intestinal ulceration. J Lipid Res. 2009;50:1870–1880. doi: 10.1194/jlr.M900039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007;86:74–81. doi: 10.1093/ajcn/86.1.74. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect. 1997;105:1228–1232. doi: 10.1289/ehp.971051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect. 2000;108:961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tvrdik P, Westerberg R, Silve S, Asadi A, Jakobsson A, Cannon B, Loison G, Jacobsson A. Role of a new mammalian gene family in the biosynthesis of very long chain fatty acids and sphingolipids. J Cell Biol. 2000;149:707–718. doi: 10.1083/jcb.149.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. Agricultural Research Service 2010 National Nutrient Database for Standard Reference, Release 23. 2010. Nutrient Data Laboratoryhttp://www.ars.usda.gov/ba/bhnrc/ndl .
- Varela-Moreiras G, Avila JM, Cuadrado C, del Pozo S, Ruiz E, Moreiras O. Evaluation of food consumption and dietary patterns in Spain by the Food Consumption Survey: updated information. Eur J Clin Nutr. 2010;64:37–43. doi: 10.1038/ejcn.2010.208. [DOI] [PubMed] [Google Scholar]
- Veaute C, Andreoli MF, Racca A, Bailat A, Scalerandi MV, Bernal C, Malan Borel I. Effects of isomeric fatty acids on reproductive parameters in mice. Am J Reprod Immunol. 2007;58:487–496. doi: 10.1111/j.1600-0897.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- Vierula M, Niemi M, Keiski A, Saaranen M, Saarikoski S, Suominen J. High and unchanged sperm counts of Finnish men. Int J Androl. 1996;19:11–17. doi: 10.1111/j.1365-2605.1996.tb00427.x. [DOI] [PubMed] [Google Scholar]
- Vioque J. Validez de la evaluación de la ingesta dietética [in Spanish] In: Serra-Majem L, Aranceta J, Mataix J, editors. Nutrición y Salud Pública. Métodos, Bases Científicas y Aplicaciones. Barcelona, Spain: Masson; 1995. [Google Scholar]
- Vioque J, Gonzalez L. Validity of a food frequency questionnaire (preliminary results) Eur J Cancer Prev. 1991;1:19–20. [Google Scholar]
- Vioque J, Weinbrenner T, Asensio L, Castelló A, Young I, Fletcher A. Plasma concentrations of carotenoids and vitamin C are better correlated with dietary intake in normal weight than overweight and obese elderly subjects. Br J Nutr. 2007;97:977–986. doi: 10.1017/S0007114507659017. [DOI] [PubMed] [Google Scholar]
- Vioque J, Navarrete–Muñoz EM, Gimenez–Monzó D, Hera MG, Granado F, Young IS, Ramón R, Ballester F, Murcia M, Rebagliato M, et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr J. 2013;12:26. doi: 10.1186/1475-2891-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujkovic M, de Vries JH, Dohle GR, Bonsel GJ, Lindemans J, Macklon NS, van der Spek PJ, Steegers EA, Steegers-Theunissen RP. Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Hum Reprod. 2009;24:1304–1312. doi: 10.1093/humrep/dep024. [DOI] [PubMed] [Google Scholar]
- Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169:1182–1190. doi: 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- Willett W. Nutritional Epidemiology. 3rd edn. Oxford: Oxford University Press; 2013. [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- Willett W. Nutritional Epidemiology. 2nd edn. Oxford: Oxford University Press; 1998. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th edn. Geneva, Switzerland: WHO Press; 2010. [Google Scholar]
- Zhang K, Kniazeva M, Han M, Li W, Yu Z, Yang Z, Li Y, Metzker ML, Allikmets R, Zack DJ, et al. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat Genet. 2001;27:89–93. doi: 10.1038/83817. [DOI] [PubMed] [Google Scholar]