Abstract
Proteasomes are the major ATP-dependent proteolytic machines in the eukaryotic and archaeal domains of life. To execute protein degradation, the 20S core peptidase combines with the AAA+ ring of the 19S regulatory particle in eukarya or with the AAA+ PAN ring in some archaea. Here, we find that Cdc48 and 20S from the archaeon Thermoplasma acidophilum interact to form a functional proteasome. Cdc48 is an abundant and essential double-ring AAA+ molecular machine ubiquitously present in archaea, where its function has been uncertain, and in eukarya where Cdc48 participates by largely unknown mechanisms in diverse cellular processes including multiple proteolytic pathways. Thus, proteolysis in collaboration with the 20S peptidase may represent an ancestral function of the Cdc48 family.
In all domains of life, ATP-dependent protein-unfolding machines and self-compartmentalized peptidases carry out intracellular protein degradation (1). The unfoldases belong to the AAA+ superfamily (ATPases Associated with a variety of cellular Activities) and function as hexameric rings. The barrel-shaped peptidases have an internal chamber containing the proteolytic active sites. All AAA+ proteases share common operating principles, including coaxial unfoldase-peptidase docking and translocation of unfolded substrates through the axial pores into the degradation chamber. The major degradation machine in eukaryotes is the 26S proteasome, consisting of the 20S core peptidase and 19S regulatory particles, which contain AAA+ Rpt1–6 unfolding rings (2,3). The 20S peptidase, which has an α7β7β7α7 architecture, is also ubiquitous in archaea, where it functions with the hexameric AAA+ PAN unfoldase (4). Paradoxically, PAN is absent from some archaea (Table S1) and is not required for viability of other archaea in which 20S is essential (5). Thus, we reasoned that an unidentified pathway for proteasomal protein degradation must exist.
Cdc48/p97/VCP is double-ring AAA+ machine (6), which is highly conserved but has no clear function in archaea. By contrast, eukaryotic Cdc48 and specific adaptor proteins participate in diverse cellular processes, including proteolytic pathways, but by largely unknown molecular mechanisms (6). Intriguingly, many archaeal and eukaryotic Cdc48 enzymes have C-terminal HbYX (hydrophobic, tyrosine, any residue) motifs, which are also present in PAN and Rpt1–6 subunits and dock into binding pockets on the 20Sα ring (7).
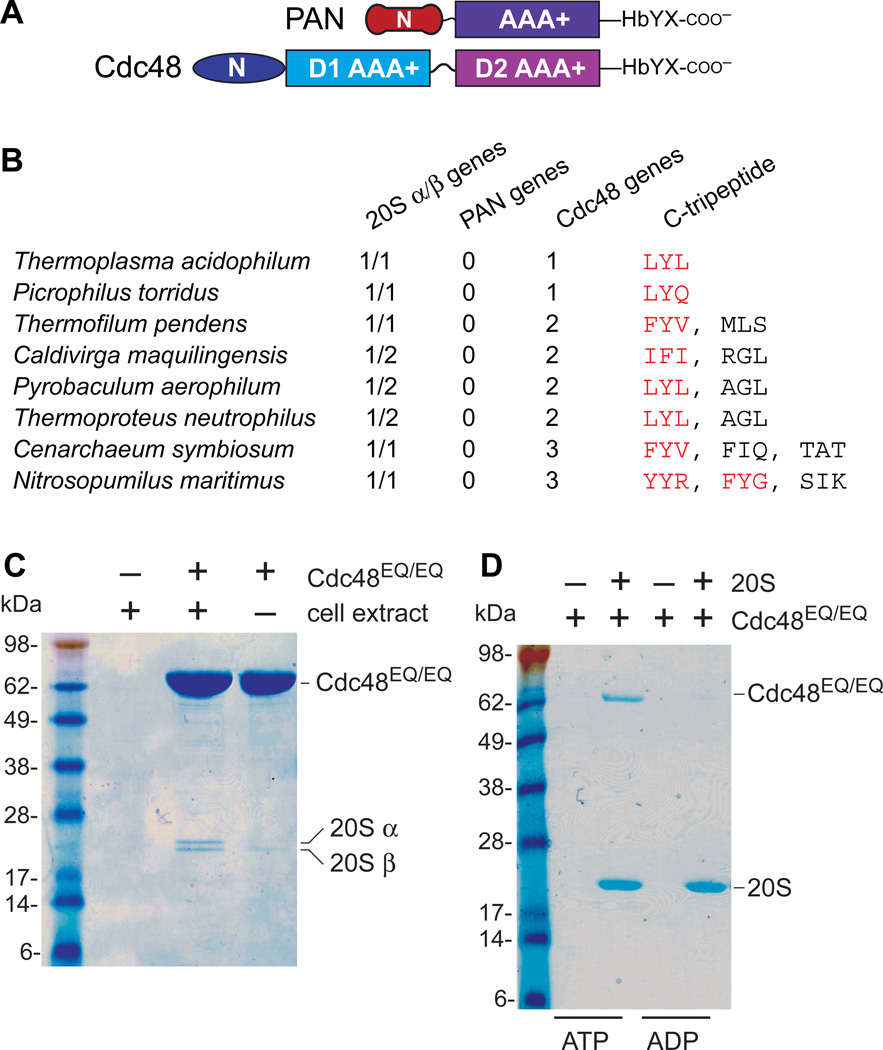
The single AAA+ module of PAN is highly homologous to both AAA+ modules of Cdc48, but these proteins have distinct N domains (Fig. 1A) (8,9). Searches of complete archaeal genomes revealed that ~15% contained no PAN genes, but did contain 20S genes and at least one Cdc48 gene encoding an HbYX or related C-terminal sequence (Fig. 1B; Table S1). Docking the crystal structures of T. acidophilum 20S and mouse Cdc48/p97 showed that the C-terminal tails could be positioned to interact with the HbYX-binding clefts in the 20Sα ring (Fig. S1).
Fig. 1. Cdc48 binds the 20S peptidase.
(A) PAN and Cdc48 have distinct N domains but homologous AAA+ modules and HbYX motifs. (B) Examples of archaea in which PAN was absent but 20S and Ccd48 were present. Red Cdc48 tripeptides match HbYX or related sequences. (C) Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) showed that purified T. acidophilum His6-Cdc48EQ/EQ pulled down two proteins from a T. acidophilum cell extract, which were identified as the 20S α and β subunits (Fig. S2). (D) Using purified T. acidophilum proteins, 20S with a His6-tagged β subunit pulled down roughly stoichiometric quantities of untagged Cdc48EQ/EQ in the presence of 2 mM ATP but not ADP.
For interaction studies, we used T. acidophilum His6-Cdc48EQ/EQ, a variant with E291Q/E568Q substitutions in the Walker-B motifs of the D1 and D2 rings to prevent ATP hydrolysis. When His6-Cdc48EQ/EQ was added to a T. acidophilum cell extract, it pulled down two proteins (Fig. 1C), identified as the 20S α and β subunits (Fig. S2). Using purified His6-tagged 20S and purified Cdc48EQ/EQ, we also observed a direct and approximately stoichiometric interaction in the presence of ATP but not ADP (Fig. 1E).
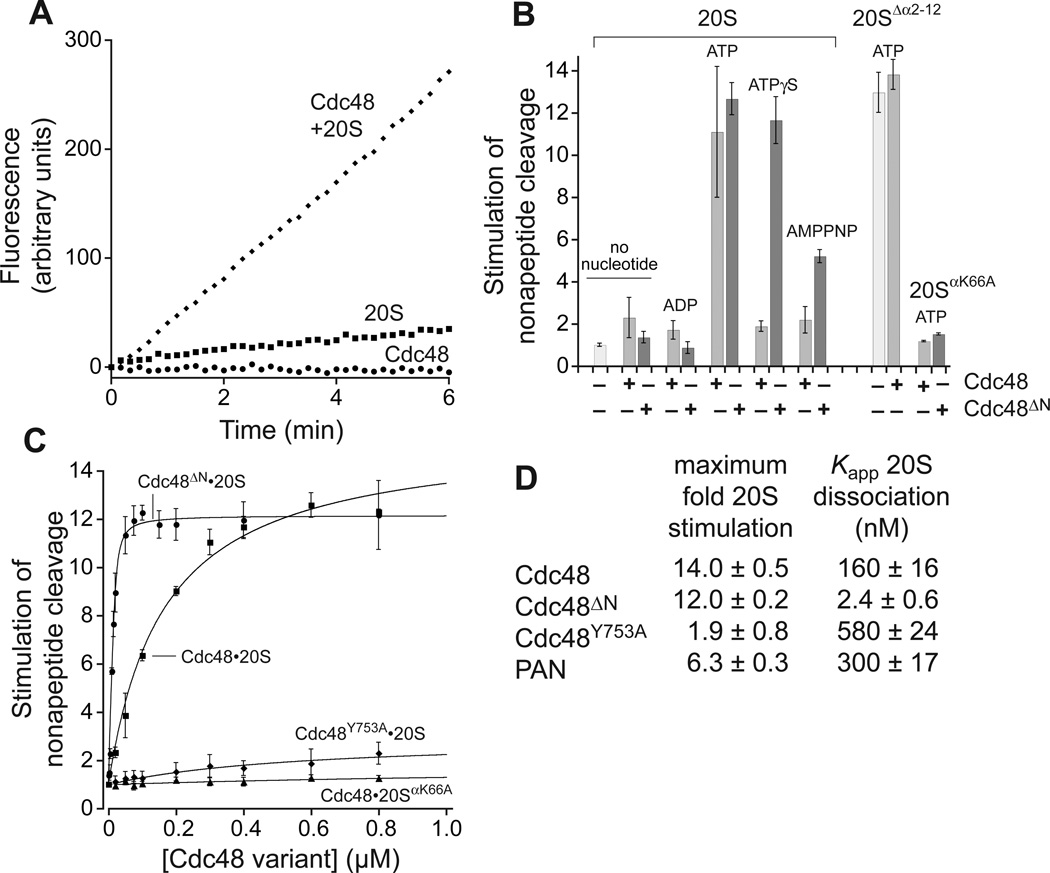
The N termini of the α subunits gate or restrict entry of peptides longer than 5–7 residues into free 20S, and such peptides are cleaved at enhanced rates by PAN•20S or the 26S proteasome (10). With ATP present, Cdc48 also had gate-opening activity, stimulating 20S cleavage of a nonapeptide but not a tetrapeptide (Figs. 2A, S3). When the gate residues were deleted (10), 20SΔα2–12 alone cleaved the nonapeptide at a rate similar to Ccd48•20S, and added Cdc48 did not increase 20SΔα2–12 activity (Fig. 2B). Cdc48ΔN, which lacks the N domain, stimulated nonapeptide cleavage in the presence of ATP and two poorly hydrolyzable analogs, ATPγS (adenosine 5′-[γ-thio]triphosphate) and AMPPNP (adenosine 5′-[β,γ-imido]triphosphate), whereas these analogs supported very weak gate opening by Cdc48 (Fig. 2B).
Fig. 2. Cdc48 activates 20S gate opening.
(A) T. acidophilum Cdc48 (0.4 µM) and 20S (10 nM) cleaved a nonapeptide (10 µM) rapidly as assayed by increased fluorescence with 2 mM ATP. Cleavage by Cdc48 alone was undetected and by 20S alone was slow. (B) Cdc48 or Cdc48ΔN (0.4 µM) stimulation of nonapeptide cleavage by 20S, 20SΔα2–10, or 20SαK66A (10 nM) in the presence of different nucleotides (2 mM). Values are averages ± SD (N ≥ 3) divided by the 20S cleavage rate. (C) Nonapeptide cleavage by 20S or 20SαK66A (10 nM) as a function of increasing concentrations of Cdc48 or variants. Solid lines (R2 ≥ 0.98) are fits to a hyperbolic equation for all curves except Cdc48ΔN•20S, which was fit to a quadratic equation for near-stoichiometric binding. (D) Values for maximum stimulation of cleavage and Kapp were calculated from the fits in panel C or Fig. S4.
Apparent equilibrium dissociation constants of ~160 nM for Cdc48•20S and ~300 nM for PAN•20S were determined by measuring the Cdc48/PAN dependence of 20S nonapeptide cleavage (Figs. 2C, S4). Cdc48ΔN bound 20S substantially more tightly, with an affinity of ~2 nM (Fig. 2C).
A Cdc48 variant with the Y753A mutation in the HbYX motif bound 20S and stimulated peptide cleavage (Fig. 2C), but with a ~3.5-fold decrease in affinity and ~7-fold reduction in maximum activity compared to Cdc48 (Fig. 2D). Thus, binding and gate opening are not fully coupled in this mutant. In the binding pocket on 20Sα, Lys66 forms a salt bridge with the α-carboxylate of PAN (11). Cdc48 or Cdc48ΔN stimulated nonapeptide cleavage by 20SαK66A poorly (Fig. 2B, 2C), suggesting that interactions involving the Cdc48 tail and Lys66 facilitate gate opening.
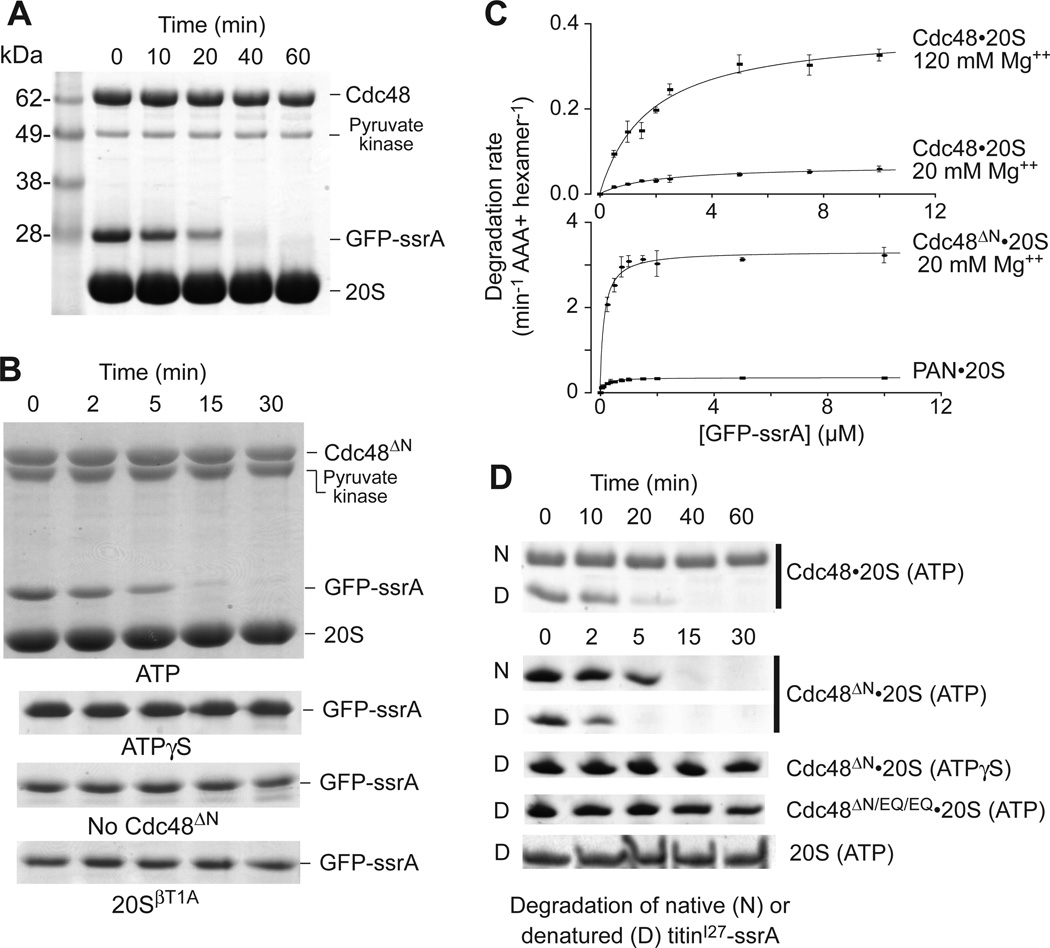
T. acidophilum Cdc48 unfolds GFP bearing the ssrA degron, with unfolding and ATP hydrolysis being stimulated by 120 mM Mg++ or N-domain deletion (12). We found that Cdc48•20S completely degraded native GFP-ssrA in the presence of ATP and 120 mM Mg++ (Fig. 3A). Cdc48ΔN•20S also efficiently degraded GFP-ssrA with ATP and 20 mM Mg++, but no degradation was observed with ATPγS, without Cdc48ΔN, or with proteolytically inactive 20SβT1A (Fig. 3B). Michaelis-Menten analysis of GFP-ssrA degradation by Cdc48•20S showed that KM was ~2 µM in high and low Mg++, whereas Vmax increased from 0.07 to 0.39 min−1 enz−1 as Mg++ was raised (Fig. 3C; Table S2). For Cdc48ΔN•20S in low Mg++, KM was 0.15 µM and Vmax was 3.2 min−1 enz−1. Thus, the N domain of Cdc48 represses proteolytic activity both by increasing KM and decreasing Vmax. For comparison, PAN•20S degraded GFP-ssrA with a KM of 0.15 µM and Vmax of 0.35 min−1 enz−1 (Fig. 3C, Table S2).
Fig. 3. Protein degradation by the Cdc48•20S proteasome.
(A) SDS-PAGE assay of GFP-ssrA (10 µM) degradation by Cdc48 (1 µM) and 20S (2 µM) with 2 mM ATP, 120 mM MgCl2, and an ATP-regeneration system. (B) Upper panel is SDS-PAGE assay of GFP-ssrA (5 µM) degradation by 20S (0.9 µM) and Cdc48ΔN (0.3 µM), 2 mM ATP, 20 mM MgCl2, and a regeneration system. Lower strips show GFP-ssrA in otherwise comparable assays using 2 mM ATPγS (no regeneration), without Cdc48ΔN, or with catalytically inactive 20SβT1A. (C) Michaelis-Menten plots of GFP-ssrA degradation by 20S (0.9 µM) and Cdc48 (0.3 µM; left panel) or 20S (0.9 µM) with Cdc48ΔN or PAN (0.3 µM; right panel). Values are averages ± SD (N ≥ 3). Fitted parameters are listed in Table S2. (D) Degradation of 10 µM native titinI27-ssrA or denatured CM-titinI27-ssrA. The top two gel strips show degradation by Cdc48 (1.2 µM) and 20S (0.4 µM) in 120 mM Mg++ buffer. The next four strips show degradation by Cdc48ΔN or Cdc48ΔN/EQ/EQ (1.2 µM) and 20S (0.4 µM) in 20 mM Mg++ buffer. The bottom strip shows degradation by 20S (0.4 µM) in 20 mM Mg++ buffer. Reactions contained 5 mM ATP and a regeneration system or 5 mM ATPγS without regeneration.
Cdc48•20S degraded CM-titinI27-ssrA, a variant denatured by carboxymethylation of normally buried cysteines (13), faster than native titinI27-ssrA (Fig. 3D). Cdc48ΔN•20S also degraded the denatured substrate more rapidly, in a reaction that required ATP hydrolysis (Fig. 3D). In principle, Cdc48 might unfold and release substrates into solution for subsequent capture and degradation by free 20S. However, degradation of unfolded CM-titinI27-ssrA by 20S alone was much slower than degradation with Cdc48 or Cdc48ΔN (Fig. 3D), suggesting that ATP-fueled translocation from Cdc48 into the associated 20S peptidase is responsible for most degradation. Moreover, a PAN variant that unfolded GFP-ssrA but did not bind 20S failed to support degradation (Figs. S4, S5). Thus, folded and unfolded substrates are only degraded efficiently by complexes of 20S with Cdc48 or PAN.
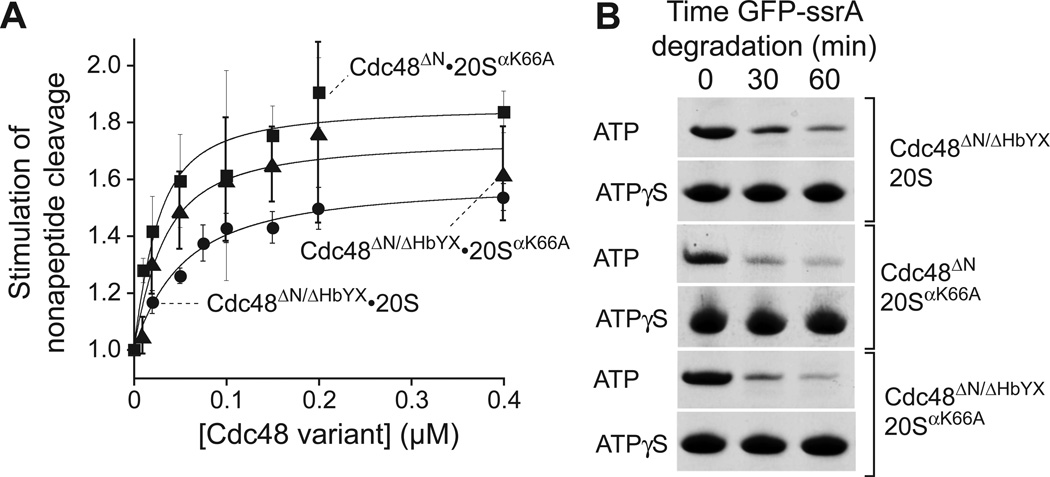
A ΔHbYX Cdc48ΔN variant that terminates with Asp-Gln-Gly supported a low level of gateopening (Fig. 4A) and 20S degradation of GFP-ssrA (Fig. 4B). The affinity of Cdc48ΔN/ΔHbYX for 20S was surprisingly strong (40 nM), albeit ~20-fold weaker than Cdc48ΔN. Because the HbYX motif stabilizes but is not required for 20S binding, other Cdc48 regions must make major contributions to binding. We also observed weak gate opening (Fig. 4A) and GFP-ssrA degradation (Fig. 4B) using 20SαK66A in combination with either Cdc48ΔN or Cdc48ΔN/ΔHbYX, showing that K66 recognition of an α-carboxylate is not essential for binding and degradation. Moreover, the affinity effects of the Cdc48 ΔHbYX and 20S αK66A mutations were nonadditive, as expected for interacting groups. How can the protein degradation activities of Cdc48ΔN/ΔHbYX•20S, Cdc48ΔN•20SαK66A, and Cdc48ΔN/ΔHbYX•20SαK66A, which require an open 20S gate, be reconciled with the poor stimulation of peptide cleavage by these enzymes? The 20S gate dynamically adopts a variety of conformations (14), and an actively translocating polypeptide might trap a transiently open gate with the spooling chain preventing closure.
Fig. 4. The HbYX motif is not required for 20S binding and degradation.
(A) Stimulation of peptide cleavage by 20S or 20SαK66A (10 nM) as a function of Cdc48ΔN/HbYX or Cdc48ΔN concentration. Values are averages (N=3) ± SD. Solid lines (R2 ≥ 0.948) are fits to a quadratic equation for near-stoichiometric binding, yielding Kapp values of 41 ± 4 nM for Cdc48ΔN/HbYX•20S, 21 ± 9 nM for Cdc48ΔN•20SαK66A, and 15 ± 6 nM for Cdc48ΔN/HbYX•20SαK66A. (B) Gel strips showing ATP-dependent degradation of GFP-ssrA (5 µM) by Cdc48ΔN/ΔHbYX•20S, Cdc48ΔN•20SαK66A, or Cdc48ΔN/HbYX•20SαK66A. Assays were performed in 20 mM Mg++ using 5 mM ATP and regeneration or 5 mM ATPγS; 20S/20SαK66A (0.4 µM); Cdc48ΔN/Cdc48ΔN/HbYX (1.2 µM).
We find that archaeal Cdc48 binds to the proteasomal 20S peptidase, induces gate opening, and unfolds and actively translocates protein substrates into the 20S chamber for proteolysis. Cdc48 is the only 20S partner in ~15% of archaea, and Cdc48 and PAN are both present in the remaining ~85%. Based on their C-terminal sequences (Table S1), the fact that Cdc48 binding is not strictly HbYX dependent, and our finding that Cdc48 and PAN bind 20S with similar affinities (Fig. 2D), it appears that either enzyme could combine with 20S to form alternative proteasomes in most archaea. The N domain of Cdc48 is a tethering site for adaptors and possibly substrates and there is evidence that it controls hexamer conformation and ATPhydrolysis rates (12,15–17). In our experiments, deleting the N domain of T. acidophilum Cdc48 strengthened 20S binding and increased proteolytic activity. Thus, N-domain modification or Ndomain binding of substrates and adaptors may regulate intracellular degradation by the Cdc48•20S proteasome.
Supplementary Material
Acknowledgments
We thank C. Hill, A. Horwich, H. Huber, S. Kim, K. Schmitz, B. Stinson, and M. Wohlever for materials and discussions. Supported by NIH grant AI-16892. The data reported in this manuscript are presented in the main paper and supplementary materials.
Footnotes
Supplementary Materials
Materials and Methods
Figs. S1 to S5
Tables S1 and S2
References (18–23)
References and Notes
- 1.Sauer RT, Baker TA. AAA+ proteases: ATP-fueled machines of protein destruction. Annu. Rev. Biochem. 2011;80:587–612. doi: 10.1146/annurev-biochem-060408-172623. [DOI] [PubMed] [Google Scholar]
- 2.Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- 3.Lander GC, et al. Complete subunit architecture of the proteasome regulatory particle. Nature. 2012;482:186–191. doi: 10.1038/nature10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zwickl P, Ng D, Woo KM, Klenk HP, Goldberg AL. An archaebacterial ATPase, homologous to ATPases in the eukaryotic 26 S proteasome, activates protein breakdown by 20 S proteasomes. J. Biol. Chem. 1999;274:26008–26014. doi: 10.1074/jbc.274.37.26008. [DOI] [PubMed] [Google Scholar]
- 5.Zhou G, Kowalczyk D, Humbard MA, Rohatgi S, Maupin-Furlow JA. Proteasomal components required for cell growth and stress responses in the haloarchaeon Haloferax volcanii. J. Bacteriol. 2008;190:8096–8105. doi: 10.1128/JB.01180-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat. Cell Biol. 2012;14:117–123. doi: 10.1038/ncb2407. [DOI] [PubMed] [Google Scholar]
- 7.Stadtmueller BM, Hill CP. Proteasome activators. Mol. Cell. 2011;41:8–19. doi: 10.1016/j.molcel.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies JM, Brunger AT, Weis WI. Improved structures of full-length p97, an AAA ATPase: implications for mechanisms of nucleotide-dependent conformational change. Structure. 2008;16:715–726. doi: 10.1016/j.str.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F, et al. Structural insights into the regulatory particle of the proteasome from Methanocaldococcus jannaschii. Mol. Cell. 2009;34:473–484. doi: 10.1016/j.molcel.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith DM, et al. ATP binding to PAN or the 26S ATPases causes association with the 20S proteasome, gate opening, and translocation of unfolded proteins. Mol. Cell. 2005;20:687–698. doi: 10.1016/j.molcel.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Stadtmueller BM, et al. Structural models for interactions between the 20S proteasome and its PAN/19S activators. J. Biol. Chem. 2010;285:13–17. doi: 10.1074/jbc.C109.070425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerega A, et al. VAT, the thermoplasma homolog of mammalian p97/VCP, is an N domainregulated protein unfoldase. J. Biol. Chem. 2005;280:42856–42862. doi: 10.1074/jbc.M510592200. [DOI] [PubMed] [Google Scholar]
- 13.Kenniston JA, Baker TA, Fernandez JM, Sauer RT. Linkage between ATP consumption and mechanical unfolding during the protein processing reactions of an AAA+ degradation machine. Cell. 2003;114:511–520. doi: 10.1016/s0092-8674(03)00612-3. [DOI] [PubMed] [Google Scholar]
- 14.Religa TL, Sprangers R, Kay LE. Dynamic regulation of archaeal proteasome gate opening as studied by TROSY NMR. Science. 2010;328:98–102. doi: 10.1126/science.1184991. [DOI] [PubMed] [Google Scholar]
- 15.Stolz A, Hilt W, Buchberger A, Wolf DH. Cdc48: a power machine in protein degradation. Trends Biochem. Sci. 2011;36:515–523. doi: 10.1016/j.tibs.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Tang WK, et al. A novel ATP-dependent conformation in p97 N-D1 fragment revealed by crystal structures of disease-related mutants. EMBO J. 2010;29:2217–2229. doi: 10.1038/emboj.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niwa H, et al. The Role of the N-Domain in the ATPase Activity of the Mammalian AAA ATPase p97/VCP. J. Biol. Chem. 2012;287:8561–8570. doi: 10.1074/jbc.M111.302778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benaroudj N, Goldberg AL. PAN, the proteasome-activating nucleotidase from archaebacteria, is a protein-unfolding molecular chaperone. Nat. Cell Biol. 2000;2:833–839. doi: 10.1038/35041081. [DOI] [PubMed] [Google Scholar]
- 19.Martin A, Baker TA, Sauer RT. Protein unfolding by a AAA+ protease is dependent on ATP-hydrolysis rates and substrate energy landscapes. Nat. Struct. Mol. Biol. 2008;15:139–145. doi: 10.1038/nsmb.1380. [DOI] [PubMed] [Google Scholar]
- 20.Norby JG. Coupled assay of Na+,K+-ATPase activity. Methods Enzymol. 1988;156:116–119. doi: 10.1016/0076-6879(88)56014-7. [DOI] [PubMed] [Google Scholar]
- 21.Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal. Biochem. 1988;171:266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- 22.Zwickl P, Kleinz J, Baumeister W. Critical elements in proteasome assembly. Nat. Struct. Biol. 1994;1:765–770. doi: 10.1038/nsb1194-765. [DOI] [PubMed] [Google Scholar]
- 23.Smith DM, et al. Docking of the proteasomal ATPases' carboxyl termini in the 20S proteasome's alpha ring opens the gate for substrate entry. Mol. Cell. 2007;27:731–744. doi: 10.1016/j.molcel.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.