Abstract
Empirical research on informed consent has shown that study participants often do not fully understand consent information. This study assessed participant understanding of three mock consent approaches describing an HIV-prevention clinical trial in Lilongwe, Malawi prior to trial implementation. Pregnant women (n = 297) were systematically selected from antenatal-care waiting lines and sequentially allocated to receive an enhanced standard consent form (group 1), a context-specific consent form (group 2), or context-specific counseling cards (group 3). Understanding of research concepts and study procedures was assessed immediately postintervention and at 1-week follow-up. At postintervention, participants in groups 2 and 3 understood more about research concepts and study procedures compared with group 1. Group 3 participants also understood more about study procedures compared with group 2. At follow-up, participants in groups 2 and 3 continued to understand more about research concepts and study procedures. Context-specific approaches improved understanding of consent information in this study.
Keywords: Informed consent, Evaluation, Comprehension, Africa
Introduction
Obtaining informed consent from study participants is mandatory in clinical research. Respect for persons, the underlying ethical principle of informed consent, cannot be safeguarded without adequate disclosure of information, sufficient participant understanding of the information, and a voluntary agreement to participate in research [1]. According to U.S. and international ethical guidelines, participant understanding of consent information is a condition that must be sufficiently met before potential study participants can give their informed consent [1–4]. Yet empirical research on informed consent has shown that study participants often do not understand the information provided to them during the consent process, both in resource-rich and resource-limited countries [5–12]. Although barriers to administering informed consent in developing countries have been noted in the literature [13–16], many researchers believe that limited participant understanding of consent information due to differences in cultural backgrounds, health belief systems, and languages as well as barriers due to illiteracy can be overcome [17–19]. Improvements in understanding have been demonstrated in these settings [20–23].
Many studies have evaluated alternative methods of improving participant understanding of consent information or have used alternative methods for informing potential study participants about a research study. Despite these efforts, only some methods have improved participant understanding of consent information [24–26]. With a few exceptions [19, 20], most alternative approaches have focused on simplifying text or using new methods of disclosing information rather than modifying the text to make it meaningful to participants. However, readability formulas are insufficient for ensuring that the information makes sense to potential study participants [27] or is at a low reading level when the information will be translated into another language [28]. In a review of informed consent interventions in the U.S., the authors suggested that it might be more effective to focus on the informational process, such as the words or terms used to explain research concepts, rather than on the method of presenting the information [29, 30]. Similarly, for U.S.-regulated international research conducted in developing countries, the National Bioethics Advisory Commission [17] recommends that researchers focus on disclosing consent information using culturally appropriate and innovative means, which may include both the informational process and method of delivery.
Our decision to conduct research on developing and evaluating a culturally-appropriate informed consent process emerged in response to formative data we collected to inform protocol development for the Breastfeeding, Antiretrovirals, and Nutrition (BAN) Study, a clinical trial in Lilongwe, Malawi, on the safety and efficacy of antiretroviral and nutrition interventions to reduce mother-to-child transmission of HIV during breastfeeding [31]. Objectives of the BAN study included evaluating the benefit of nutritional supplementation given to mothers during breastfeeding, the benefit and safety of antiretroviral medications given either to infants or to their mothers to prevent HIV transmission during breastfeeding, and the feasibility of exclusive breastfeeding, followed by early, rapid breastfeeding cessation. The study design was a 2-by-3 factorial design where mothers received or did not receive a daily nutritional supplement, and mothers, infants, or neither were given antiretrovirals for a 24- to 28-week breastfeeding period. Healthy breastfeeding mothers with a CD4 count >200 cells/μl and hemoglobin ≥7 g/dl and their HIV-negative newborns were eligible to enroll.
The formative research was conducted in June and July 2002 with HIV-positive mothers and other members of the community, and the results suggested that the community had a limited understanding of medical research [32]. Respondents believed that study participants would be assigned to a study arm based on their individual health needs, that they would receive a therapeutic benefit from their participation, and that all medicines provided would already have been tested and known to be safe and efficacious. These data demonstrated that further investigation of participant understanding of research was needed to increase the effectiveness of the informed consent process for the clinical trial. For that reason, in May 2003, we conducted additional formative research with mothers infected with HIV and with other members of the community specifically on how to better explain consent information to potential study participants [33]. Findings from both formative studies were used to modify the informed consent process specifically for the Malawian context.
Here we describe the evaluation of three consent approaches, including two context-specific approaches, to determine which approach obtained the highest level of participant understanding of research concepts and study procedures related to the BAN study. The evaluation was an independent substudy of the BAN study and was conducted several months before recruitment for the clinical trial began.
The research was approved by the National Health Science Research Committee in Malawi and the institutional review boards at the Centers for Disease Control and Prevention and The University of North Carolina at Chapel Hill in the U.S.
Methods
Study Population
Pregnant women with unknown HIV status were recruited from two antenatal clinics that later served as recruitment sites for the BAN study. We excluded women who were known to be infected with HIV because of concern that they might believe they were enrolling in the BAN study rather than participating only in a mock consent evaluation. Another reason for enrolling women of unknown HIV status was that we did not want to inform pregnant women infected with HIV about an upcoming clinical trial for which they would not be eligible because of the timing of their delivery. Because the participants in our study were of unknown HIV status, they would not have considered themselves eligible for the BAN clinical trial. Further, to reduce the possibility that participants would believe they were enrolling in the BAN study, potential participants were informed during screening and twice during the informed consent process that they were not being asked to take part in the BAN study but rather they would hear information and be asked questions about the BAN study in order to find out the best way to explain information about the study to future participants.
Data Collection
Participants were selected from the waiting lines at the antenatal clinics from September to December 2003 using a systematic sampling strategy. First, participants were informed in a group about the study as they waited for the clinic to open. All women typically arrived for care early in the morning; women who were HIV-positive received care separately. Next, potential participants were selected by asking every “nth” woman in line, based on the total number of women in line that day, if she would be interested in participating in the study. If yes, she would meet with study staff to learn more about the study. After giving written informed consent, participants were sequentially allocated to one of three mock consent interventions. With this allocation method, participants were placed into group 1 until the targeted sample size was achieved, and then in groups 2 and 3 following the same approach. This study design was necessary to reduce intervention contamination that might have occurred because the same six nurses delivered the consent information for each group, and groups 2 and 3 included context-specific information. All mock consent interventions were administered individually to the participants on the same day they were recruited from the antenatal care line.
All participants received four bars of household soap and a ½ kg bag of iodized salt for participating in the mock informed consent process and the post-intervention interview; no travel costs were incurred by participants for the first visit as they were already at the clinic for antenatal care. All participants were provided with 200 Malawian Kwacha (approximately $2.00) for transportation costs to the health facility for the follow-up interview.
Participants’ understanding of research concepts and study procedures was assessed immediately after the consent intervention was delivered and again 1 week later. To assess participant understanding of research concepts, we modified an existing validated scale [34] and field-tested it extensively prior to use to ensure that the questions and categories were culturally appropriate. The 20-item scale measured participants’ understanding of the eight basic elements of informed consent as outlined by U.S. regulations [35]. After field-testing, the original response categories of “agree,” “disagree,” and “unsure” were changed to “true,” “not true,” and “unsure”. Following the same scoring procedures as in the original scale, a total of 100 points could be obtained; correct answers were assigned a score of 100, incorrect answers were assigned a score of 0, and the response category “unsure” was assigned a score of 50.
Questions that assessed participants’ understanding of study procedures were informed by the basic procedures described in the protocol and by our formative findings. Twenty-four open- and closed-ended questions were asked, covering participants’ understanding of five domains: eligibility criteria, ARV intervention, nutrition intervention, blood draws, and breastfeeding protocol. Scores indicate the percentage of correct answers. Open-ended questions were coded either correct or incorrect.
For both measures, a higher score indicates better understanding. The questionnaire was field-tested with a total of 24 HIV-positive women and women of unknown HIV status. Appropriate modifications were made to improve clarity and appropriateness.
We also collected data on the time needed to deliver each consent approach; nurses’ perceptions of participants’ attentiveness at the beginning, middle, and end of the mock consent session rated on a 4 point scale (very attentive, moderately attentive, slightly attentive, and not attentive at all); participants’ understanding of consent information 1-week after the intervention; and participants’ and nurses’ perceptions of the different approaches. Nurses and other field staff provided advice on what behaviors would be representative of inattentiveness among Malawian women in a clinic setting. Behaviors included frequent yawning, falling asleep, staring out the window, looking around the room, or nurses having to repeat a question because the participant did not appear to be listening.
Two areas of participants’ perceptions that were of specific interest were their perceptions of the amount of time needed to deliver each approach and the amount of information presented in each approach. To measure participants’ perceptions of time, they were asked whether the time taken to present and discuss the information was “too long,” “just right,” or “too little.” Similarly, for the amount of information presented, participants were asked whether the amount was “too much,” “just right,” or “too little.”
Interventions
The three intervention groups were as follows–
Group 1: For group 1, an enhanced standard informed consent form was used (Table 1). U.S. investigators wrote the form based on a U.S. medical school consent form template that used a question-and-answer format; section headers were based on the eight required elements of informed consent as well as additional elements as appropriate. This form was IRB-approved for the clinical trial. In comparison with the average Flesh-Kincaid Grade Level score of 10.6 reported in a study of 114 medical school consent form templates [36], the enhanced standard form was written with much simpler language (English), rating a score of 7.4. Nurses followed the standard practice for administering the consent process at the study site by summarizing each section after it was read aloud to the participant and asking unstructured questions to gauge participants’ understanding of the section. Nurses did not proceed until they believed the participant understood the information from each section. The Chichewa version was 10 pages in length (9 in English) and contained a total of 3,386 words.
Group 2: For group 2, a context-specific consent form was used that was informed by our formative research findings [32, 33], theory [37, 38], a descriptive framework for developing culturally appropriate interventions [39], and recommendations in the literature [17, 40–42]. “Context-specific” means that consent information was described using local words and sentence structure, meanings, and analogies as discovered through the formative research, that formative data informed areas in which additional explanation was needed because of limited understanding among the study population, that recommendations from the literature for improving and ensuring participant understanding of consent information were followed, and that local health professionals contributed to the design of the consent information. As with group 1, nurses asked participants questions after reading each section of the consent form to gauge the participant’s understanding and did not proceed until they believed the participant understood the section; information was not summarized after each section. Structured questions were used to assess understanding of the previous section as a way to increase the fidelity of the standard method at the site of asking questions throughout the delivery of the consent information and to ensure that information identified as problematic in the formative research was understood. The Chichewa version was 13 pages in length (12 in English) and contained a total of 6,317 words.
Group 3: For group 3, counseling cards were used and they combined the context-specific informational approach of group 2 with culturally appropriate drawings of each stage of the protocol (Fig. 1). Counseling cards are used in Malawi for providing family planning education in the clinics; thus, it is a familiar method for delivering information. The context-specific text was distributed over 46 pages in a flip chart, each with a drawing on the reverse side. The addition of drawings was the only difference between groups 2 and 3.
Table 1.
Excerpt from the enhanced standard consent form that provides an overview of the BAN study
| What is the study about? |
| Mothers who have HIV can pass it to their babies. This can happen during pregnancy, around the time of delivery, and after the baby is born through breastfeeding. This study is looking for ways to prevent HIV transmission during breastfeeding. It is also looking for ways to keep HIV-infected mothers and their babies healthy. |
| There are medicines to help prevent HIV transmission from mothers to babies during pregnancy and around the time of delivery. There are also medicines to treat people infected with HIV when they become very sick. These medicines are called anti-retroviral drugs. |
| In this study we will be comparing different anti-retroviral drugs to prevent HIV transmission from mothers to babies during breastfeeding. HIV-infected mothers who are not very sick will be put in one of three groups. Some mothers will take a combination of drugs while they are breastfeeding. Other mothers will be given a drug for their babies to take every day while they are breastfeeding. The third group of mothers will not be given drugs to take while they are breastfeeding. |
| This study will also give half of the mothers a special food supplement. It will be eaten daily while breastfeeding. The purpose of this part of the study is to see if the special food supplement helps to keep mothers who have HIV healthy while they are breastfeeding. |
| Mothers will be asked to stop breastfeeding when their babies are 6 months old in order to prevent HIV transmission. Once mothers stop breastfeeding, they will be given a special weaning food to feed to their infants. The drugs and mother’s special food supplement will be stopped when the baby is weaned at 6 months. |
The findings from this study will answer three questions that will help the Government of Malawi to provide better health care to HIV-infected mothers and their babies:
|
Fig. 1.
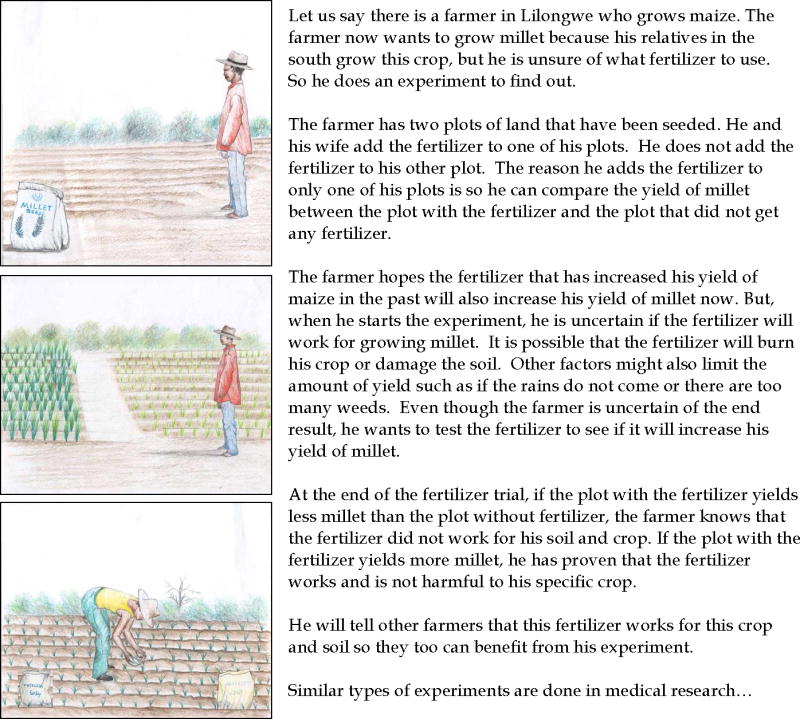
An abbreviated description from the counseling cards regarding an analogy about medical research
Information covering the eight basic elements of informed consent as outlined in U.S. federal regulations [35] was presented to each group. Because of the use of context-specific information, more information was presented to participants in groups 2 and 3 compared to group 1.
Statistical Analyses
We hypothesized that there would be no differences in the level of understanding of research concepts or study procedures by participants in all three groups directly after the consent information was delivered. One-way analysis of variance (ANOVA) was used to compare participant demographics, participant understanding, and time to deliver consent information among the groups. Significant ANOVA tests were followed by a Tukey–Kramer multiple comparisons procedure to determine which groups were different. For the categorical data, multiple pair-wise comparisons of binary data were conducted using the global permutation distribution to produce the Fisher exact test for two-sample comparisons [43]. All P values for group comparisons listed in the tables have been adjusted for multiple comparisons. Student t-tests were used to determine the comparability of postintervention participants with the participants who did not return for follow-up. All statistical tests were conducted with SAS (Version 8.2, SAS Institute Inc., Cary, NC), and P values of 0.05 or less were considered significant.
Results
Participant Demographics
A total of 297 participants were enrolled in the study; 96 participants were assigned to group 1, 101 to group 2, and 100 to group 3. Number of years in school averaged 6.7 for group 1, 6.8 for group 2, and 7.5 for group 3. Participant age averaged 23.2 years for group 1, 23.7 years for group 2, and 23.3 years for group 3. There were no significant differences among participants in the three groups with respect to education (F2,293 = 1.48, P = 0.23) or age (F2,288 = 0.45, P = 0.64). About 10% of women who were invited to join the study declined to participate. The discontinuation rate during the delivery of the intervention and postintervention questionnaire was less than 2%. These rates did not differ by group.
Participant Understanding Post-Intervention
Understanding of research concepts differed significantly among the groups (Table 2). Participants in groups 2 and 3 understood significantly more information about research concepts postintervention compared with participants in group 1. Although the mean score for understanding of research concepts among participants in group 3 was higher than the mean score in group 2, the difference was not significant. Similarly, understanding of study procedures differed significantly among the groups. Participants in groups 2 and 3 understood significantly more information about study procedures postintervention compared with participants in group 1. Additionally, participants in group 3 understood significantly more information compared with participants in group 2.
Table 2.
Mean percentage for correct scores for the postintervention and follow-up assessments
| Item/concept | Mean scores
|
F statistic | P value |
P values between groupsa
|
||||
|---|---|---|---|---|---|---|---|---|
| Group 1: enhanced standard form | Group 2: context-specific form | Group 3: counseling cards | Groups 1 & 2 | Groups 1 & 3 | Groups 2 & 3 | |||
| Postintervention | n = 96 | n = 101 | n = 100 | |||||
| Understanding of research concepts | 57.4 | 66.2 | 69.3 | 26.982,294 | <0.001 | <0.001 | <0.001 | 0.14 |
| Understanding of study procedures | 47.6 | 65.5 | 69.9 | 82.362,294 | <0.001 | <0.001 | <0.001 | 0.039 |
| Follow-up | n = 53 | n = 63 | n = 67 | |||||
| Understanding of research concepts | 58.2 | 68.3 | 69.6 | 16.132,180 | <0.001 | <0.001 | <0.001 | 0.81 |
| Understanding of study procedures | 51.4 | 61.2 | 65.5 | 18.402,180 | <0.001 | 0.002 | <0.001 | 0.14 |
P values are computed for multiple comparisons using the Tukey–Kramer method for multiple comparisons
Supplemental Findings
Time and Amount of Information
Time needed to deliver the consent information averaged 59 min for group 1, 72 min for group 2, and 73 min for group 3. Differences in time among the three groups were significant (F2,294 = 75.41, P <0.001) as were differences between groups 1 and 2 (P <0.001), and groups 1 and 3 (P <0.001). Although more time was needed to deliver the consent information in groups 2 and 3 compared with group 1, participants’ perceptions of whether the amount of time needed to deliver the information was “too long” did not vary significantly among the groups. Moreover, there were no significant differences between groups 1 and 3 in participants’ perceptions that the amount of consent information provided was “too much,” although more information was presented in group 3. Conversely, while the same amount of information was presented in groups 2 and 3, significantly more participants in group 2 perceived the amount of information to be “too much” compared to those in group 1 (Table 3).
Table 3.
Participants’ perceptions of burden and nurses’ perceptions of attentiveness during consent process
| Item/concept | % of Participants
|
P values between groupsa
|
||||
|---|---|---|---|---|---|---|
| Group 1: enhanced standard form (n = 96) | Group 2: context-specific form (n = 101) | Group 3: counseling cards (n = 100) | Groups 1 & 2 | Groups 1 & 3 | Groups 2 & 3 | |
| Participant perceptions | ||||||
| Amount of time taken was “too long” | 40 | 52 | 48b | 0.17 | 0.36 | 0.56 |
| Amount of information was “too much” | 24 | 47 | 32c | 0.002 | 0.23 | 0.066 |
| Nurse perceptions | ||||||
| “Very attentive” at beginning of session | 74 | 73 | 73 | 1.00 | 1.00 | 1.00 |
| “Very attentive” at end of session | 9 | 21 | 27c | 0.042 | 0.003 | 0.28 |
P values are computed for multiple comparisons using the Fisher exact test with stepdown methods
Missing two values
Missing one value
Nurses reported that while a similar percentage of participants in all three groups were perceived to be “very attentive” at the beginning of the consent session, by the end of the session, significantly more participants continued to be “very attentive” in groups 2 and 3 compared to those in group 1. There were no significant differences between groups 2 and 3 in nurses’ perceptions of participants’ attentiveness at the end of the consent session (Table 3).
Participant Understanding at the 1 Week Follow-Up Assessment
Fifty-three participants (55%) from group 1 returned 1 week after the intervention for the follow-up interview, 63 (62%) returned from group 2, and 67 (67%) returned from group 3. There were no significant differences among participants in the three follow-up groups in education (F2,180 = 0.59, P = 0.56) or age (F2,177 = 0.76, P = 0.47). Within each group there were no significant differences in education and age between participants who returned for the follow-up interview and those who did not.
Scores from the follow-up assessment revealed similar patterns to that of the postintervention assessment scores, with understanding of research concepts and study procedures differing significantly among the groups (Table 2). One week after the intervention, participants in groups 2 and 3 understood significantly more information about both research concepts and study procedures compared to participants in group 1. Participants’ understanding of research concepts was not significantly different between groups 2 and 3, and, unlike the postintervention assessment, participants’ understanding of study procedures was not significantly different between groups 2 and 3.
Preferences of Nurses
All six nurses preferred the counseling cards over the context-specific and enhanced standard consent forms. One nurse said, “Because the counseling cards had pictures to illustrate the information, it was easier for the clients to understand and to keep them alert.” Another said, “Counseling cards are suitable for Malawian women. Most Malawian women are illiterate, so the pictures really assisted them.” In fact, nurses said the counseling cards would be best at keeping them motivated to ensure participants’ understanding throughout the clinical trial. As described by one nurse: “Because after asking questions throughout the consent process, clients are responding well, so as a nurse you are motivated.”
Discussion
These data are relevant to the literature on participant understanding of informed consent given that these data are not time-sensitive, and participant understanding of consent information continues to be a concern. The results demonstrated that of the three methods evaluated, the counseling cards were the most effective intervention for improving participant understanding of the consent information. Participants who received the counseling cards intervention understood more information about research concepts and study procedures after the intervention than participants who received the enhanced standard consent form intervention and they understood more information about study procedures than participants who received the context-specific consent form intervention. Moreover, at the follow-up 1 week later, participants who received the counseling cards intervention continued to understand more information about research concepts and study procedures than participants who received the enhanced standard consent form intervention.
Data on participants’ and nurses’ perceptions also support the use of both the counseling cards and the context-specific consent form. Participants’ perceptions of the amount of time needed to present the consent information did not differ among the groups, and therefore the additional time needed to present the context-specific consent form or the counseling cards might not be problematic from the participant’s perspective. In fact, the nurses reported that participants remained significantly more attentive throughout the delivery of the two context-specific interventions compared with that of the enhanced standard consent form intervention. These findings suggest that the use of a context-specific approach may increase participant interest in the consent process.
Further, the addition of drawings may have increased participant engagement with the information given that significant differences were found between groups 1 and 2 in participants’ perception of whether the amount of information presented was “too much” but not between groups 1 and 3, even though the same amount of information was presented in groups 2 and 3. Indeed, two functions of drawings are to increase participant attention to and engagement with information presented [44]. All nurses indicated that they preferred the counseling cards over the other two interventions.
Our findings also support previous research demonstrating that the use of locally-relevant analogies [45] and the inclusion of drawings with text simplification [46] contributed to participants’ continued understanding of concepts over time compared with participants who received information that did not include analogies or drawings with text simplification. In our study, the overall mean follow-up scores for participants who received the context-specific consent form intervention or the counseling cards intervention were at least 10 percentage points higher than the overall mean follow-up score for participants who received the enhanced standard consent form intervention.
The time needed to deliver the context-specific approaches is comparable to phase three clinical trials conducted in developing countries where consent for enrollment may take 1 h or more, depending on the participant’s level of understanding. Yet, a lengthy informed consent process often leads to long clinic visits for participants and may not be needed for comprehension. Research is needed on how to streamline the informed consent process without compromising participant understanding. In a recent study, authors reported that comprehension scores were similar across participants who received either a standard or concise consent form, suggesting that providing less information may not hinder comprehension [47].
Our study enrolled a surrogate population and did not conduct the study with participants in an actual clinical trial. The use of surrogate populations is not uncommon in empirical research on informed consent [29, 48], although some researchers believe that, when appropriate, research on informed consent should be conducted in a realistic context [49]. Because in this study we were testing two experimental informed consent approaches for a complicated 6-treatment-condition clinical trial with three interventions among a vulnerable population, we elected to enroll a surrogate population to evaluate the effectiveness of the consent approaches in improving participant understanding before use in a real setting.
Furthermore, the study population was not infected with HIV, and therefore the consent information may not have been salient to them. Although it is unknown whether HIV status is related to understanding of consent information, participants in our study might not have been as attentive as women infected with HIV because they did not need to consider participation in the BAN study. Conversely, women with HIV might have paid less attention to the information because of anxiety. Because we enrolled a surrogate population of women with unknown HIV status, we chose to measure only the informational aspects of the consent information rather than hypothetical attitudinal factors such as perception of risk or decision making which might differ between women of unknown HIV status and women infected with HIV.
In addition, as is the case with most multicomponent interventions, our design did not permit us to identify the specific contributions of each intervention component to improved participant understanding of consent information. While we know that both context-specific approaches worked better than the enhanced standard form, we cannot attribute specific effects, for example, to the use of drawings versus no drawings or to structured versus unstructured questions.
It is also possible that nurses became more proficient at presenting the consent interventions with each successive intervention because the interventions were assigned sequentially. On the other hand, nurses may have become fatigued over time. However, one indicator of proficiency—the time to deliver the consent information—did not provide any evidence that the nurses’ proficiency changed as the amount of time needed to deliver the consent information did not decrease over time within each group. Lastly, we chose to limit our research on informed consent to the evaluation of comprehension. Other social contextual factors that may influence informed consent—such as limited access to quality health care—were not explored in this study.
Despite these limitations, we believe our study has important implications for informed consent design. Before these research findings can be translated into practice, however, several issues should be considered. First, although the results are not generalizable beyond the BAN study and consent approaches used in this evaluation, the methods we used in conducting the formative research and developing and implementing the context-specific approaches (see [33] and described above) could be replicated not only for other U.S.-regulated international clinical trials but also for clinical trials conducted in the U.S. Previous research has demonstrated the effectiveness of interventions when they were informed of theory and by findings from formative research, were culturally appropriate, or used drawings with text simplifications [46, 50–54]. Given that our context-specific approaches incorporated these factors, we believe these approaches might also be effective in improving participant understanding of consent information in other settings.
Second, our findings suggest that if resources do not permit the development of counseling cards for the informed consent process, the use of a context-specific consent form as described here can improve participant understanding of consent information. At the same time, however, we cannot ignore the fact that approximately half of the participants who received the context-specific consent form intervention perceived the amount of information to be “too much.” Additional research should be conducted to find a balance between providing context-specific information to enhance understanding and maintaining participant satisfaction.
Third, although our results demonstrated that both context-specific approaches improved understanding compared with the enhanced standard form, the mean scores were lower than we would have preferred, and therefore additional improvements should be made to these approaches. Previous research has shown that multiple meetings [23] can improve participant understanding of consent information. Further research should be conducted to determine if combining this method with a context-specific approach would enhance participant understanding of consent information given the amount of additional information that may accompany a context-specific approach.
Fourth, our findings support reports [29, 30] suggesting that approaches for improving participant understanding of consent information may need to focus more on the informational process than on the method of delivery. At the same time, however, our findings also demonstrated that use of the counseling card intervention enhanced participants’ understanding and interest, which suggests the method of presenting the information is also important.
Conclusion
If participants do not understand the information provided during the consent process, their consent is not meaningful. Our results demonstrate that going beyond the use of a generic consent form that is simply stated and structured and using a context-specific approach instead can lead to improvements in participant understanding.
Acknowledgments
The authors would like to thank study staff and participants who contributed their time and effort to this research. This work was funded by the Prevention Research Centers Special Interest Project SIP 13-01 U48-CCU409660-09 and SIP 26-04 U48-DP000059-01, Centers for Disease Control and Prevention; supported by the NIAID P30-AI50410 UNC Center for AIDS Research; DHHS/ NIH/FIC 2-D43 Tw01039-06 AIDS International Training and Research Program; Elizabeth Glaser Pediatric AIDS Foundation Call to Action Award and International Leadership Award, UNICEF, World Food Programme, GlaxoSmithKline, Boehringer-Ingelheim, Roche Pharmaceuticals, BristolMyersSquibb Company, and the Ministry of Health and Population of Malawi. The drawings were created by the Malawian artist Nyangu Andrew Chodola.
Contributor Information
Amy L. Corneli, Email: acorneli@fhi.org, Department of Health Behavior and Health Education, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA. FHI, 2224 E NC Hwy 54, Durham, NC 27713, USA
James R. Sorenson, Department of Health Behavior and Health Education, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Margaret E. Bentley, Department of Nutrition, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Gail E. Henderson, Department of Social Medicine, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
J. Michael Bowling, Department of Health Behavior and Health Education, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Jacqueline Nkhoma, The UNC Project, Lilongwe, Malawi.
Agnes Moses, The UNC Project, Lilongwe, Malawi.
Cynthia Zulu, The UNC Project, Lilongwe, Malawi.
James Chilima, Independent Consultant, Lilongwe, Malawi.
Yusuf Ahmed, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Charles M. Heilig, Centers for Disease Control and Prevention, Atlanta, GA, USA
Denise J. Jamieson, Centers for Disease Control and Prevention, Atlanta, GA, USA
Charles van der Horst, Center for Infectious Diseases, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
References
- 1.National Commission for the Protection of Human Subjects of Biomedical, Behavioral Research. The Belmont report: ethical principles and guidelines for the protection of human subjects of research. Washington, DC: U.S. Government Printing Office; 1979. [Google Scholar]
- 2.Nuremberg Code. Tribunals under control counsel law. 10. Vol. 11. Washington, DC: U.S. Government Printing Office; 1949. Trials of war criminals before the nuremberg military; pp. 181–2. [Google Scholar]
- 3.Council for International Organizations of Medical Sciences (CIOMS) International ethical guidelines for biomedical research involving human subjects. Geneva: CIOMS/WHO; 2002. [PubMed] [Google Scholar]
- 4.World Medical Association (WMA) Declaration of Helsinki: ethical principles for medical research involving human subjects, as amended by the 52nd WMA General Assembly; Edinburgh, Scotland. 2000. [Google Scholar]
- 5.Taiwo O, Kass N. Post-consent assessment of dental subject’s understanding of informed consent in oral health research in Nigeria. BMC Med Ethics. 2009;10:11. doi: 10.1186/1472-6939-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breese P, Burman W, Goldberg S, Weis S. Education level, primary language, and comprehension of the informed consent process. J Empir Res Hum Res Ethics. 2007;2:69–79. doi: 10.1525/jer.2007.2.4.69. [DOI] [PubMed] [Google Scholar]
- 7.Krosin M, Klitzman R, Levin B, Cheng J, Ranney M. Problems in comprehension of informed consent in rural and peri-urban Mali, West Africa. Clin Trials. 2006;3:302–13. doi: 10.1191/1740774506cn150oa. [DOI] [PubMed] [Google Scholar]
- 8.Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent in cancer clinical trials: a cross-sectional survey. Lancet. 2001;358:1772–7. doi: 10.1016/S0140-6736(01)06805-2. [DOI] [PubMed] [Google Scholar]
- 9.Lynöe N, Hyder Z, Chowdhury M, Ekström L. Obtaining informed consent in Bangladesh. N Engl J Med. 2001;344:460–1. doi: 10.1056/NEJM200102083440617. [DOI] [PubMed] [Google Scholar]
- 10.Leach A, Hilton S, Greenwood BM, et al. An evaluation of the informed consent procedure used during a trial of a Haemophilus influenzae type B conjugate vaccine undertaken in The Gambia, West Africa. Soc Sci Med. 1999;48:139–48. doi: 10.1016/s0277-9536(98)00317-7. [DOI] [PubMed] [Google Scholar]
- 11.Daugherty C, Ratain MJ, Grochowsi E, Stocking C, Kodish E, et al. Perceptions of cancer patients and their physicians involved in phase 1 trials. J Clin Oncol. 1995;13:1062–72. doi: 10.1200/JCO.1995.13.5.1062. [DOI] [PubMed] [Google Scholar]
- 12.Riecken HW, Ravich R. Informed consent to biomedical research in Veterans Administration Hospitals. JAMA. 1982;248:344–8. [PubMed] [Google Scholar]
- 13.Mystakidou K, Panagiotou I, Katsaragakis S, Tsilika E, Parpa E. Ethical and practical challenges in implementing informed consent in HIV/AIDS clinical trials in developing or resource-limited countries. SAHARA J. 2009;6:46–57. doi: 10.1080/17290376.2009.9724930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krogstad DJ, Diop S, Diallo A, et al. Informed consent in international research: the rationale for different approaches. Am J Trop Med Hyg. 2010;83:743–7. doi: 10.4269/ajtmh.2010.10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kass P, Hyder A. Ethical and policy issues in international research: clinical trials in developing countries, volume II: commissioned papers and staff analysis. Bethesda, MD: National Bioethics Advisory Commission; 2001. Attitudes and experiences of U.S. and developed country investigators regarding U.S. human subject regulations; pp. B1–B158. [Google Scholar]
- 16.Marshall P. Ethical and policy issues in international research: clinical trials in developing countries, volume II: commissioned papers and staff analysis. Bethesda, MD: National Bioethics Advisory Commission; 2001. The relevance of culture for informed consent in U.S.-funded international health research; pp. C1–C38. [Google Scholar]
- 17.National Bioethics Advisory Commission (NBAC) Report on clinical trials research in developing countries. volume I: report and recommendations of the National Bioethics Advisory Commission. Bethesda, MD: National Bioethics Advisory Commission; 2001. [Google Scholar]
- 18.Ijsselmuiden C, Faden R. Research and informed consent in Africa—another look. In: Mann JM, Gruskin S, Grodin MA, Annas GJ, editors. Health and human rights: a reader. New York: Routledge; 1999. pp. 363–72. [Google Scholar]
- 19.Préziosi M, Yam A, Ndiaye M, Simaga A, Simondon F, Wassilak S. Practical experiences in obtaining informed consent for a vaccine trial in rural Africa. N Engl J Med. 1997;336:370–3. doi: 10.1056/NEJM199701303360511. [DOI] [PubMed] [Google Scholar]
- 20.Vallely A, Lees S, Shagi C, Kasindi S, Soteli S, et al. How informed is consent in vulnerable populations? Experience using a continuous consent process during the MDP301 vaginal microbicide trial in Mwanza, Tanzania. BMC Med Ethics. 2010;11:10. doi: 10.1186/1472-6939-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minnies D, Kawkridge T, Hanekom W, Ehrlich R, London L, Hussey G. Evaluation of the quality of informed consent in a vaccine field trial in a developing country setting. BMC Med Ethics. 2008;9:15. doi: 10.1186/1472-6939-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pace C, Emanuel E, Cheunyam T, et al. The quality of informed consent in a clinical research study in Thailand. IRB. 2005;1:9–17. [PubMed] [Google Scholar]
- 23.Fitzgerald D, Marotte C, Verdier RI, Johnson WD, Pape JW. Comprehension during informed consent in a less-developed country. Lancet. 2002;360:1301–2. doi: 10.1016/S0140-6736(02)11338-9. [DOI] [PubMed] [Google Scholar]
- 24.Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA. 2004;292:593–601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 25.Cohn E, Larson E. Improving participant comprehension in the informed consent process. J Nurs Scholarsh. 2007;39:273–80. doi: 10.1111/j.1547-5069.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- 26.Edwards S, Lilford R, Thornton J, Hewison J. Informed consent for clinical trials: in search of the “best” method. Soc Sci Med. 1998;47:1825–40. doi: 10.1016/s0277-9536(98)00235-4. [DOI] [PubMed] [Google Scholar]
- 27.Ancker J. Assessing patient comprehension of informed consent forms. Control Clin Trials. 2004;25:72–4. doi: 10.1016/j.cct.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Kithinji C, Kass N. Assessing the readability of non-English-language consent forms: the case of Kiswahili for research conducted in Kenya. IRB. 2010;32:1–15. [PMC free article] [PubMed] [Google Scholar]
- 29.Agre P, Rapkin B. Improving informed consent: a comparison of four consent tools. IRB. 2003;25:1–7. [PubMed] [Google Scholar]
- 30.Agre P, Campbell F, Goldman B, et al. Improving informed consent: the medium is not the message. IRB. 2003;25:S11–9. [PubMed] [Google Scholar]
- 31.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corneli A, Piwoz E, Bentley M, et al. Involving communities in the design of clinical trial protocols: The BAN Study in Lilongwe, Malawi. Contemp Clin Trials. 2007;28:59–67. doi: 10.1016/j.cct.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corneli A, Bentley M, Sorenson J, et al. Using formative research to develop a context-specific approach to informed consent for clinical trials. J Empir Res Hum Res Ethics. 2006;1:45–60. doi: 10.1525/jer.2006.1.4.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joffe S, Cook E, Cleary P, Clark J, Weeks J. Quality of informed consent: a new measure of understanding among research subjects. J Natl Cancer Inst. 2001;93:139–47. doi: 10.1093/jnci/93.2.139. [DOI] [PubMed] [Google Scholar]
- 35.Department of Health, Human Services (DHHS) 45 Code of federal regulations 46. Federal Register. 1991;56:28012. [Google Scholar]
- 36.Paasche-Orlow M, Taylor H, Brancati F. Readability standards for informed-consent forms as compared with actual readability. N Engl J Med. 2003;348:721–6. doi: 10.1056/NEJMsa021212. [DOI] [PubMed] [Google Scholar]
- 37.Ausubel D. The psychology of meaningful verbal learning. New York: Grune & Stratton, Inc; 1963. [Google Scholar]
- 38.Ausubel D. The acquisition and retention of knowledge: a cognitive view. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. [Google Scholar]
- 39.Kreuter M, Lukwago S, Bucholtz D, Clark E, Sanders-Thompson V. Achieving cultural appropriateness in health promotion programs: target and tailored approaches. Health Education and Behavior. 2003;30:133–46. doi: 10.1177/1090198102251021. [DOI] [PubMed] [Google Scholar]
- 40.Appelbaum P, Roth L, Lidz C, Benson P, Winslade W. False hopes and best data: consent to research and the therapeutic misconception. Hasting Center Report. 1987;17:20–4. [PubMed] [Google Scholar]
- 41.Lidz C, Appelbaum P. The therapeutic misconception: problems and solutions. Med Care. 2002;40:v55–63. doi: 10.1097/01.MLR.0000023956.25813.18. [DOI] [PubMed] [Google Scholar]
- 42.Moreno J. Abandon all hope? The therapeutic misconception and informed consent. Cancer Invest. 2003;21:481–2. doi: 10.1081/cnv-120018240. [DOI] [PubMed] [Google Scholar]
- 43.Westfall P, Tobias R, Rom D, Wolfinger R, Hochberg Y. Multiple comparisons and multiple tests using SAS. Cary, NC: SAS Institute, Inc; 1999. [Google Scholar]
- 44.Levie W, Lentz R. Effects of text illustrations: a review of research. Educ Commun Technol J. 1982;30:195–232. [Google Scholar]
- 45.Newby T, Ertmer P, Stepich D. Instructional analogies and the learning of concepts. Educ Technol Res Dev. 1995;43:5–18. [Google Scholar]
- 46.Murphy D, O’Keefe H, Kaufman A. Improving comprehension and recall of information for an HIV vaccine trial among women at risk for HIV: reading level simplification and inclusion of pictures to illustrate key concepts. AIDS Educ Prev. 1999;11:389–99. [PubMed] [Google Scholar]
- 47.Stunkel L, Benson M, McLellan L, et al. Comprehension and informed consent: assessing the effectiveness of a short consent form. IRB. 2010;32:1–9. [PMC free article] [PubMed] [Google Scholar]
- 48.Llewellyn-Thomas H, Thiel E, Sem F, Harrison Woermke D. Presenting clinical trial information: a comparison of methods. Patient Educ Couns. 1995;25:97–107. doi: 10.1016/0738-3991(94)00705-q. [DOI] [PubMed] [Google Scholar]
- 49.Lavori P, Sugarman J, Hays M, Feussner J. Improving informed consent in clinical trials: a duty to experiment. Control Clin Trials. 1999;20:187–93. doi: 10.1016/s0197-2456(98)00064-6. [DOI] [PubMed] [Google Scholar]
- 50.Black M, Siegel H, Abel Y, Bentley M. Home and videotape intervention delays early complementary feeding among adolescent mothers. Pediatrics. 2001;107:1–8. doi: 10.1542/peds.107.5.e67. [DOI] [PubMed] [Google Scholar]
- 51.Creed-Kanashiro H, Uribe T, Bartolini R, et al. Improving dietary intake to prevent anemia in adolescent girls through community kitchens in a periurban population of Lima, Peru. J Nutr. 2000;130:459S–461S. doi: 10.1093/jn/130.2.459S. [DOI] [PubMed] [Google Scholar]
- 52.Davis S, Clay T, Smyth M, et al. Pathways curriculum and family interventions to promote healthful eating and physical activity in American Indian schoolchildren. Prev Med. 2003;37:S24–34. doi: 10.1016/j.ypmed.2003.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelley K, Abraham C. RCT of a theory-based intervention promoting healthy eating and physical activity amongst out-patients older than 65 years. Soc Sci Med. 2003;59:787–97. doi: 10.1016/j.socscimed.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 54.Miller C, Edwards L, Kissling G, Sanville L. Evaluation of a theory-based nutrition intervention for older adults with diabetes mellitus. J Am Diet Assoc. 2002;102:1069–81. doi: 10.1016/s0002-8223(02)90242-7. [DOI] [PubMed] [Google Scholar]


