Summary
Hsp90 is a homo-dimeric ATPase that is essential in eukaryotes for the maturation of client proteins frequently involved in signal transduction including many kinases and nuclear steroid hormone receptors. Competitive inhibitors of ATP binding to Hsp90 prevent client maturation and show promise as anti-cancer agents in clinical trials. However, the role of ATP binding and hydrolysis in each subunit of the Hsp90 dimer has been difficult to investigate because of an inability to assemble and study dimers of defined composition. We used protein engineering to generate functional Hsp90 subunits that preferentially assemble as heterodimers. We analyzed dimers where one subunit harbors a disruptive mutation and observed that ATP binding by both subunits is essential for function in yeast, while ATP hydrolysis is only required in one subunit. These findings demonstrate important functional contributions from both symmetric and asymmetric Hsp90 dimers, and provide valuable reagents for future investigations of Hsp90 mechanism.
Introduction
Interest in the chaperone mechanism of Hsp90 blossomed with the discovery that inhibitors targeting Hsp90 could revert the transforming potential of the v-src oncogene in cell culture (Whitesell et al., 1994). Subsequent studies demonstrated that many kinases require active Hsp90 along with the kinase-specific Cdc37 co-chaperone for efficient maturation (Hartson et al., 1996; Mimnaugh et al., 1996; Schneider et al., 1996; Schulte et al., 1996; Stepanova et al., 1996), and that roughly 60% of human kinases bind to Hsp90 in cells (Taipale et al., 2012). In addition to kinases, Hsp90 is also required for efficient activation of many nuclear steroid hormone receptors (Pratt and Toft, 1997). The maturation of both kinases and steroid receptors requires ATPase activity by Hsp90 (Grenert et al., 1999; Wayne et al., 2010). Competitive inhibitors of ATP binding to Hsp90 have shown promise as anti-cancer agents in clinical trials (Jhaveri et al., 2012), suggesting that cancer cells have a stronger dependence on Hsp90 than normal cells. Despite the central role of Hsp90 in signal transduction and cancer and the therapeutic promise of competitive ATP inhibitors, the mechanism by which ATP hydrolysis is linked to client maturation remains poorly understood.
Our current understanding of the ATPase mechanism of Hsp90 is based largely on in vitro studies performed in the absence of clients. Hsp90 is a member of the GHKL family of proteins that couple ATP hydrolysis to conformational changes that drive diverse biochemical functions (Dutta and Inouye, 2000). Structurally, Hsp90 is a homodimer with each monomer containing three domains (Ali et al., 2006). The N-terminal (N) domain contains an ATP binding site (Prodromou et al., 1997), the Middle (M) domain contains client binding sites (Meyer et al., 2003), and the C-terminal (C) domain contains a stable dimerization domain (Harris et al., 2004; Richter et al., 2001). Dimerization of Hsp90 is critical for in vivo function (Wayne and Bolon, 2007). In purified form, conformational shifts in dimeric Hsp90 are mediated by ATP binding and hydrolysis, with an open state observed in the absence of nucleotide and a closed state when both subunits are bound to ATP (Ali et al., 2006; Shiau et al., 2006; Southworth and Agard, 2008). The nucleotide free state of Hsp90 is dimerized only at the C-domain and forms a V-shaped conformation (Shiau et al., 2006; Southworth and Agard, 2008). In contrast, the ATP-bound conformation is dimerized at both the N and C domains, and the N and M domains reposition to orient catalytic side chains for efficient ATP hydrolysis (Ali et al., 2006; Hessling et al., 2009). Studies of Hsp90 in the absence of clients have provided valuable insights into the Hsp90 ATPase cycle. However, many critical questions remain regarding how Hsp90 functions in the presence of clients.
In vitro studies of client maturation are challenging both because client proteins tend to aggregate in purified form and because efficient Hsp90 function in cells involves many different co-chaperones that can be difficult to recapitulate in vitro. Biochemical and genetic approaches have identified Hsp90 interactions with over 20 different co-chaperone proteins that assist and/or modulate Hsp90 function (Rohl et al., 2013; Zhao et al., 2005). In vitro studies with combinations of purified chaperones have been utilized to mature progesterone receptor to a ligand competent form and shown that in addition to Hsp90, five other proteins are required: Hsp40, Hsp70, Hop, p23, and FKBP52 (Pratt and Toft, 1997). In vitro studies with chaperone combinations have also been used to mature immobilized Chk2 kinase and shown that the co-chaperone Cdc37 is critical for this reaction (Arlander et al., 2006). Motivated by the challenges of native clients, model client systems have been explored and among other findings, implicated the middle domain in client binding (Hagn et al., 2011; Rudiger et al., 2002; Street et al., 2011; Street et al., 2012).
A number of studies have investigated communication between Hsp90 subunits in vitro with variable conclusions. In purified form, the ATPase activity of each Hsp90 subunit appears biochemically independent (McLaughlin et al., 2004). In addition, single molecule studies indicate that ATP binding in one subunit does not promote nucleotide binding in the trans subunit (Ratzke et al., 2012), and that ATP binding only modestly affects conformational preferences (Mickler et al., 2009). However, crystallographic observations (Ali et al., 2006) indicate that ATP-binding induces N-terminal dimerization, that could positively couple ATP binding at each subunit in the Hsp90 dimer. Furthermore, dimers of Hsp90 where one subunit lacks an N-domain are deficient for ATP hydrolysis (Richter et al., 2001), and mutations in Hsp90 have been identified that perturb ATPase activity in the trans subunit in vitro (Cunningham et al., 2008). Thus, conclusions regarding the mechanism of ATP hydrolysis in each Hsp90 subunit depend to some extent on experimental variables.
In vivo, it was unclear if ATP must bind or hydrolyze in both Hsp90 subunits simultaneously, or if the function of Hsp90 can be supported by binding and/or hydrolysis of ATP by a single subunit of the Hsp90 dimer (Figure 1). To address this question, we designed Hsp90 subunits that preferentially assemble as heterodimers in cells. Based on the in vivo function of Hsp90 heterodimers harboring ATP binding or hydrolysis defective mutations in a single subunit, we propose a model of the essential Hsp90 ATPase cycle where binding of ATP to both Hsp90 subunits is required, but where ATP hydrolysis by one subunit of Hsp90 is sufficient for maturation of essential clients. These results provide a unifying mechanistic framework for understanding Hsp90 function.
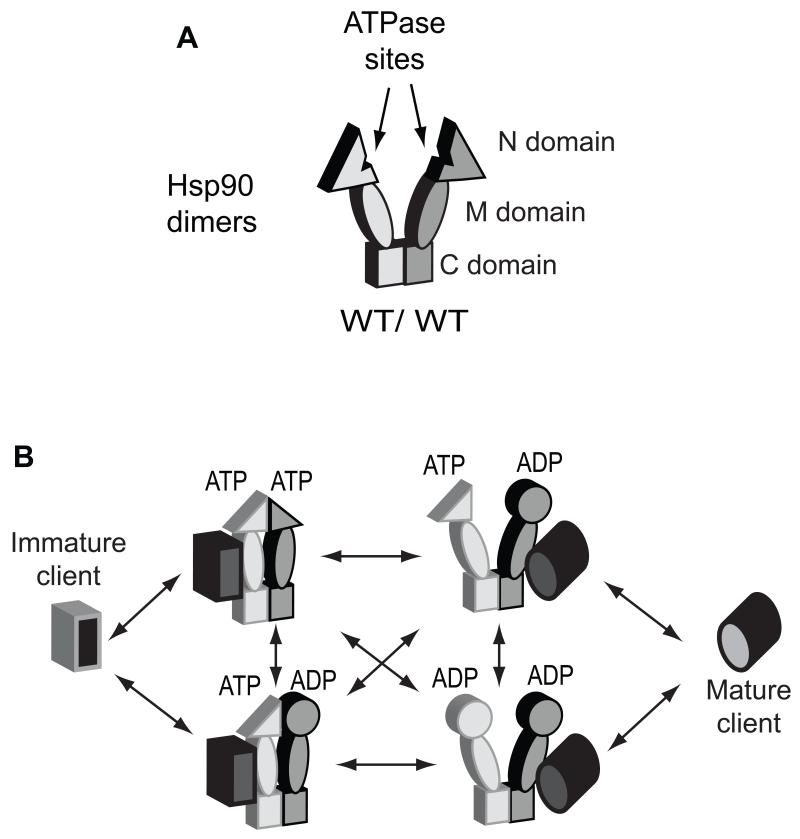
Figure 1.
Models of potential ATP binding and hydrolysis pathways in Hsp90 dimers. (A) Cartoon representation of an Hsp90 homodimer illustrating the three domains within each subunit and the sites of ATP binding. (B) Potential pathways by which Hsp90 might bind to and hydrolyze ATP in the process of client maturation.
Results and Discussion
Designed heterodimeric Hsp90 subunits assemble into asymmetric dimers in vitro
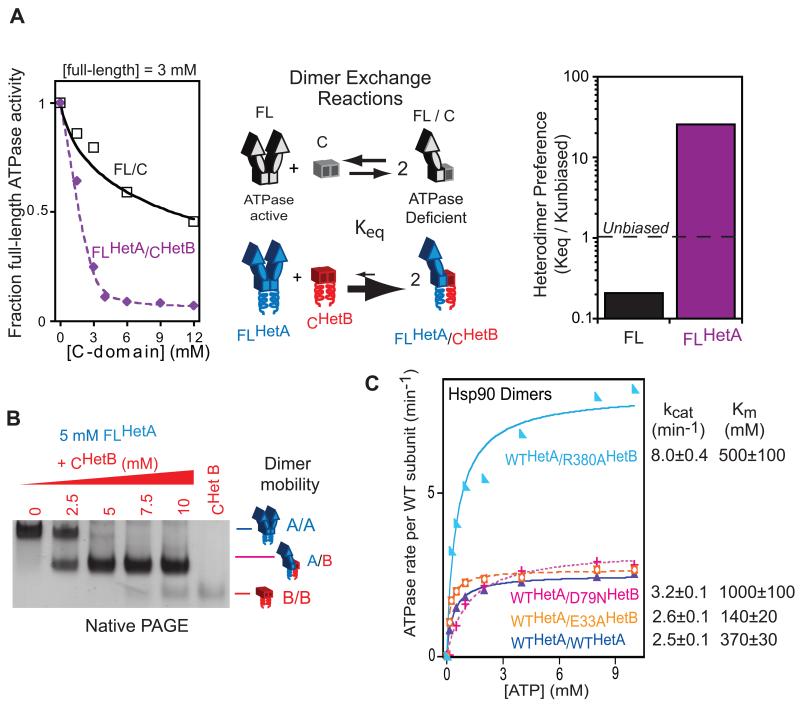
We designed heterodimeric Hsp90 subunits through fusion to coiled-coil sequences previously optimized to form thermodynamically stabilized heterodimers (Havranek and Harbury, 2003). Fusion of these coiled-coils to Hsp90 is fully compatible with ATPase activity (Figure S1A, B), indicating that chaperone function is not compromised. We examined the propensity of wild type (WT) and designed heterodimer Hsp90 subunits to cross dimerize under equilibrium conditions (Figure 2A). We utilized a dimer exchange reaction between ATPase active, full-length (FL) Hsp90 and ATPase inactive C-domain. Previous studies have demonstrated that heterodimers of full-length and C-domain (FL/C) are deficient in ATPase activity (Richter et al., 2001; Wayne and Bolon, 2007). Thus, the extent of FL/C heterodimer formation can be followed by monitoring ATPase activity. If all three possible dimer species (C/C, FL/C, and FL/FL) have equivalent thermodynamic stability, they should form at a 1:2:1 ratio when both subunits are present at the same concentration causing a 50% decrease in observed ATPase activity. As previously observed (Wayne and Bolon, 2007), at equimolar concentration of the wild type FL and C-domain subunits of Hsp90, ATPase activity was decreased about 20% (Figure 2A). This result indicates that the heterodimer of wild type FL and C-domain subunits (FL/C) is thermodynamically disfavored relative to the FL/FL dimers, consistent with dimer stabilizing contacts between the N-domains in ATP-bound FL/FL homodimers (Ali et al., 2006). We generated heterodimeric FL (FLHetA) and C-domain (CHetB) constructs and examined their propensity to form heterodimers (Figure 2A). The ATPase activity of the FLHetA decreased to near-baseline levels in the presence of an equimolar concentration of CHetB (Figure 2A), indicating that the FLHetA/CHetB was thermodynamically preferred relative to homodimers. Fitting the data to an equilibrium dimer exchange model indicates that the designed Hsp90-coil fusions provide a 100 fold heterodimer preference relative to constructs without the coils. This equates to a free energy preference of 2.5 kcal/mol that is identical to that measured for the coils in isolation (Havranek and Harbury, 2003).
Figure 2.
Designed Hsp90 subunits preferentially assemble as heterodimers in vitro. (A) Dimer exchange reactions monitored based on the ATPase activity from full-length homodimers. Fitting the titrations to an equilibrium model indicates that designed Hsp90 subunits assemble with a roughly 100-fold heterodimer preference relative to wild type controls. (B) Native PAGE confirms the preferential assembly of designed Hsp90 subunits as heterodimers. (C) ATPase activity of Hsp90 heterodimers with ATP binding (D79N) or hydrolysis (E33A, R380A) defective mutations in one subunit. The Michaelis-Menten model was fit to the data to estimate kcat and Km along with errors from the fitting procedure. See also Figure S1.
As heterodimer formation is central to our investigations, we used two independent experiments to augment our ATPase based investigations of dimer species. First, we analyzed the designed constructs by Native PAGE, taking advantage of the distinct mobility of FL/FL, FL/C and C/C dimers (Figure 2B). Consistent with our ATPase studies, we observed the preferential accumulation of FLHetA/CHetB heterodimers by Native PAGE. Second, we used size exclusion chromatography (SEC) to investigate dimerization preference. SEC also indicated that the designed Hsp90 constructs strongly favor heterodimer formation (Figure S1C). From these three independent biochemical analyses, we concluded that the designed Hsp90 constructs preferentially assemble as heterodimers in vitro.
We analyzed the in vitro ATPase activity of full length Hsp90 heterodimers with one WT subunit and one subunit containing a mutation that prevents either ATP binding (D79N), or hydrolysis (E33A and R380A). Consistent with their distant location from the stable dimerization regions in the C-domain, each of the ATPase mutants (E33A, D79N, and R380A) readily cross dimerize in our system (Figure S1D, E). Having confirmed that the ATPase mutants form preferential heterodimers, we investigated the ATPase activity in vitro of WTHetA/MutantHetB Hsp90 dimers (Figure 2C). The ATPase rate (kcat) per WT subunit was similar for WT/WT homodimers (2.5 min−1), as well as WT/E33A (2.6 min−1), and WT/D79N (3.2 min−1) heterodimers, while it was elevated about three fold for WT/R380A (8.0 min−1). The R380 mutation impacts Hsp90 conformation in addition to ATPase efficiency (Cunningham et al., 2012), suggesting that conformational properties of the ‘trans’ subunit can impact ATP hydrolysis in the ‘cis’ subunit. Therefore, our results indicate that ATP hydrolysis in one Hsp90 subunit was sensitive to the biochemical properties of the partner subunit, but not completely dependent on the nucleotide bound state of the partner subunit. We observed differences in Km for Hsp90 heterodimers (ranging from 140 μM to 1000 μM) that we cannot precisely interpret due to the multiple steps involved in ATP hydrolysis including conformational rearrangements (Hessling et al., 2009). However, the Km differences we observed indicate that the ATP-bound state of the partner subunit influences either ATP binding and/or conformational rearrangement kinetics involved in ATPase activity.
Designed Hsp90 subunits preferentially assemble as heterodimers in vivo
Investigating the essential function of Hsp90 in yeast can be challenging because the endogenous expression level of Hsp90 is far above that required for robust growth (Borkovich et al., 1989; Jiang et al., 2013). In order to clearly interpret functional studies of Hsp90 heterodimers in yeast, it is critical to reduce background functional homodimer concentrations to trace levels. To accomplish this, we generated yeast strain DBY519 that expresses chromosomally integrated FLHetA Hsp90 to levels just above critical levels for robust yeast growth (Figure 3 and Figure S2A-E). The level of Hsp90 in these cells is reduced to the point where it causes a minor decrease in growth (Figure S2A). While this level of Hsp90 is ideal for analyzing effects on yeast growth, it is poorly suited to investigating traditionally studied substrates including v-src and glucocorticoid receptor that require high levels of Hsp90. The DBY519 strain also harbors a URA3-marked plasmid expressing wild type Hsp90 to near endogenous levels that can be negatively selected using fluoroorotic acid (FOA). To examine heterodimers in DBY519 cells, we introduced plasmid encoded Hsp90HetB constructs and treated with FOA to swap out the wild type Hsp90 plasmid. To reduce functional FLHetA/FLHetA levels below that required for growth, we expressed Hsp90HetB subunits in excess to Hsp90HetA subunits (Figure S2C). In this setup, excess Hsp90HetB homodimers should accumulate in cells. For Hsp90HetB constructs where HetB homodimers are non-toxic and non-functional (both properties of ATPase mutants), strain growth under FOA selection depends on the function of Hsp90HetA/HetB heterodimers.
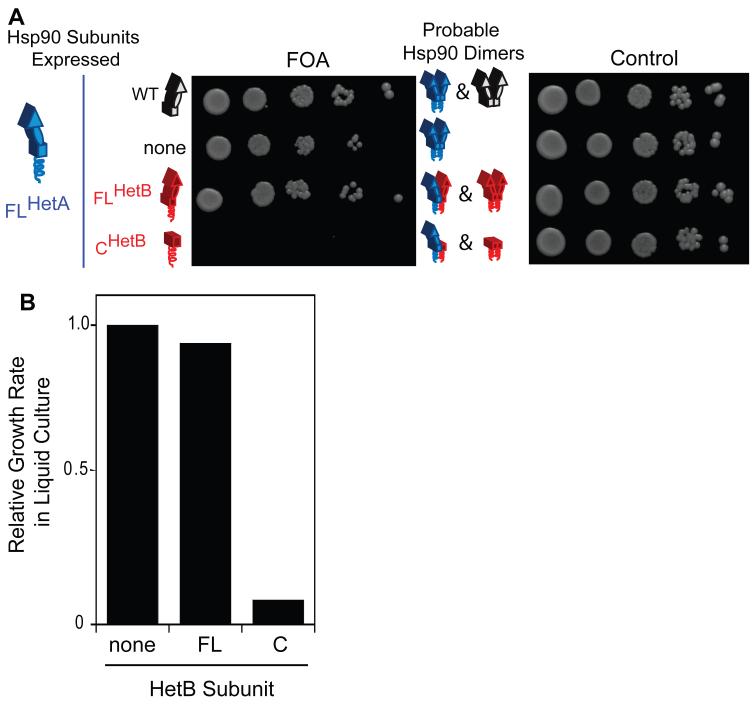
Figure 3.
Yeast system to study the function of Hsp90 dimers of defined composition. (A) Under selective conditions, the growth of the engineered strain is strictly dependent on the function of A/B Hsp90 heterodimers: full-length heterodimers support robust growth but known null heterodimers lacking the N and M domains in one subunit do not support growth. Control cells grown without FOA all support growth indicating that none of the constructs are toxic. (B) Growth rates in liquid culture parallel growth observed on plates. See also Figure S2.
To determine the robustness of the designed Hsp90 heterodimer system in yeast, we investigated the function of FLHetA/CHetB Hsp90 (Figure 3). Our previous work (Wayne and Bolon, 2007), demonstrates that analogous single chain FL-C Hsp90 constructs fail to support yeast viability. Thus, if FLHetA assembled predominantly into FLHetA/CHetB Hsp90 heterodimers in our yeast system it should lead to a strong growth defect under FOA selection. The FLHetA/CHetB dimer serves as a stringent test of heterodimer preference as the absence of potential inter-subunit N-domain contacts provides a homodimer bias (Figure 2A). In control DBY519 cells that only express FLHetA, growth in the presence of FOA is close to that of control cells expressing endogenous levels of WT Hsp90 (Figure S2A), indicating that the level of FLHetA homodimers in these cells is sufficient to support robust growth. By contrast, the co-expression of FLHetA and CHetB subunits dramatically reduces growth in independent experiments on plates (Figure 3A), and in liquid culture (Figure 3B and Figure S2B). These findings demonstrate that the designed system provides a biological readout (yeast growth) that is directly dependent on the chaperone function of Hsp90 heterodimers and provides a valuable approach to further investigate essential elements of Hsp90 mechanism in vivo.
Asymmetric Hsp90 dimers with ATP deficient mutations in one subunit reveal essential elements of the ATPase cycle
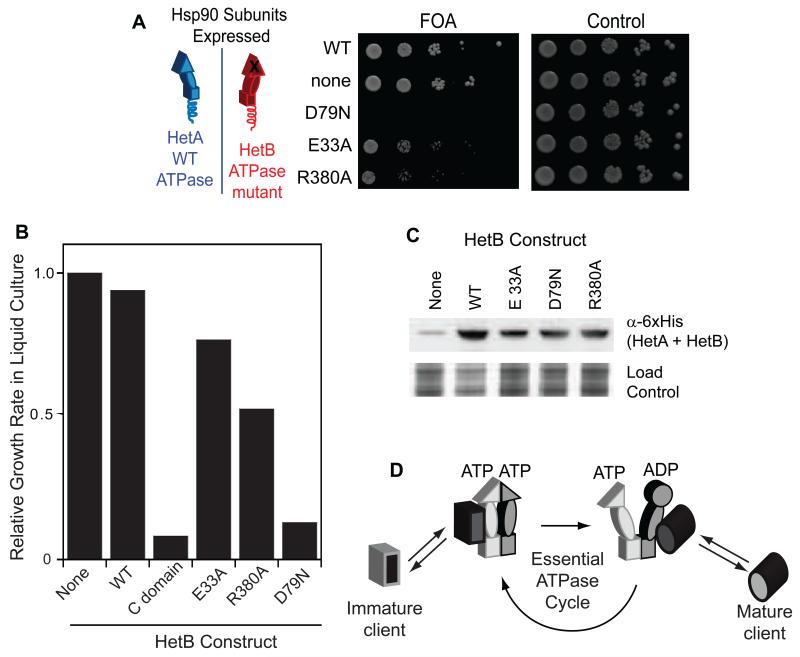
To investigate how ATP binding and hydrolysis in each subunit of Hsp90 contributes to function, we generated FLHetB constructs containing either the D79N mutation that prevents ATP binding, or the E33A or R380A mutations that prevent ATP hydrolysis (Meyer et al., 2003; Panaretou et al., 1998). Both biochemical and structural studies indicate that the side chain of E33 in Hsp90 poises a water molecule for attack on the scissile bond of ATP (Ali et al., 2006; Obermann et al., 1998). The side chain of R380, which comes from the M-domain, contacts the γ-phosphate and is implicated in both stabilizing the leaving phosphate and promoting nucleotide-dependent conformational changes in the Hsp90 dimer (Cunningham et al., 2012; Meyer et al., 2003). In structures of Hsp90, the side chain of D79 forms a hydrogen bond to the N6 atom of adenosine that is critical for ATP binding (Ali et al., 2006; Prodromou et al., 1997). All three of these ATPase deficient mutants (E33A, D79N, and R380A) fail to support yeast viability as the sole Hsp90 (Figure S3A,B) and thus are compatible with our approach. We observed null-growth for WTHetA/D79NHetB Hsp90 heterodimers, indicating that ATP binding in each Hsp90 subunit is critical for function in vivo (Figure 4 and Figure S3C). This observation also demonstrates that WTHetA homodimers are depleted to trace levels consistent with the design strategy. Both WTHetA/E33AHetB and WTHetA/R380AHetB Hsp90 dimers support yeast viability, albeit with growth defects of about 20% and 50% respectively relative to controls (Figure 4B and Figure S3C).
Figure 4.
Contributions of ATP binding and hydrolysis in each subunit of the Hsp90 dimer to function in vivo. (A) Growth of yeast supported by Hsp90 heterodimers harboring one subunit with a mutation that prevents ATP binding (D79N), or hydrolysis (E33A or R380A). Growth on control plates lacking FOA demonstrates that none of the constructs are toxic. (B) Quantification of growth rates observed in liquid culture. (C) Western blot demonstrating that Hsp90 mutants deficient for ATP binding or hydrolysis express to similar levels as wild type. (D) Model of the essential Hsp90 ATPase cycle required for client maturation in yeast. See also Figure S3.
Because of the important potential mechanistic implications of our observations, we explored a number of features to ascertain the robustness of our findings. First, we examined the expression level of each ATPase HetB variant in our heterodimer experiments (Figure 4C) as well as the same HetB constructs in cells with WT Hsp90 (Figure S3B). In both of these experiments, all ATPase mutants expressed to similar levels. The observation that D79NHetB abolished growth in DBY519 cells indicates that the expression level of HetB variants was sufficient to deplete HetA homodimers to trace levels. As reduced Hsp90 levels can activate a stress response including induction of the Hsp90 activator Aha1, we examined Aha1 level in our strains (Figure S3D). We find that all ATPase heterodimer strains had levels of Aha1 similar to unstressed cells, suggesting that the heat shock response was not strongly induced. To investigate potential sensitivity to the expression level of the HetB subunits in our system, we examined the growth properties of DBY519 yeast harboring HetB variants expressed from a roughly three-fold weaker (Jiang et al., 2013) promoter (Figure S3E,F). Increases in HetB expression were not pursued because these levels of expression caused a growth defect for WT Hsp90. The growth properties of Hsp90 heterodimers with one ATPase deficient subunit were similar when the expression of the HetB subunit was decreased, indicating that the biochemical properties of Hsp90 heterodimers were the primary factor mediating growth rates.
Our observations indicate that ATP hydrolysis in a single Hsp90 subunit is sufficient for its essential function in yeast (Figure 4D). The strict in vivo requirement for ATP binding in both Hsp90 subunits, but for hydrolysis in only one subunit provides a mechanistic framework for understanding the ATPase driven Hsp90 conformation cycle during client maturation.
Mechanistic implications
The in vivo requirement for ATP binding in both subunits is consistent with structural observations that nucleotide binding causes large conformational rearrangements in purified Hsp90 that are conserved across eukaryotic lineages (Ali et al., 2006; Southworth and Agard, 2008). The evolutionary conservation of this conformational response to ATP binding indicates that it is under strong selection in natural populations and suggests that it is critical to function. These inferences from structure and evolution are supported by our observations that preventing ATP binding in one subunit causes a severe functional defect in yeast. While on the surface this finding contradicts some results with purified Hsp90 that indicate independent ATPase or negatively cooperative ATP binding in each subunit (McLaughlin et al., 2004; Ratzke et al., 2012; Richter et al., 2001), there is growing evidence that binding to co-chaperones and clients dramatically impact the conformation and biochemical properties of Hsp90 (Southworth and Agard, 2011; Street et al., 2011; Vaughan et al., 2006). In this light, structural investigations of purified Hsp90 reveal important insights into some of the conformational ensembles available to bind to co-chaperones and clients, but suggest that further investigations in the presence of co-chaperones, clients, and post-translational modifications may be required to delineate the Hsp90 conformational cycle during client maturation.
The in vivo functionality of Hsp90 dimers impaired for ATP hydrolysis in one subunit demonstrates the mechanistic importance of asymmetric chaperone conformations. In particular, the energy from ATP hydrolysis in one subunit appears sufficient to drive the critical Hsp90 conformational changes required for client maturation. This mechanistic observation is consistent with in vitro studies where the ATPase activity in each subunit is independent (McLaughlin et al., 2004; Richter et al., 2001), and the co-chaperone Aha1 stimulates ATPase preferentially in one Hsp90 subunit (Retzlaff et al., 2010). The modest in vivo defects that we observed for heterodimers with ATP hydrolysis mutants (Figure 4C) suggest that chaperone efficiency is somewhat compromised relative to Hsp90 dimers that can hydrolyze ATP in both subunits. While not conclusive, this observation suggests that the efficiency of client maturation may depend on which subunit in the Hsp90 dimer hydrolyzes ATP, perhaps through conformational asymmetry introduced through client and/or co-chaperone binding. In addition, the stronger functional defect observed for R380A compared to E33A (Figure 4C) is consistent with the dual role of R380A in both stimulating hydrolysis and N-M conformational rearrangements (Cunningham et al., 2012).
Conclusions and Future Directions
This study provides a valuable mechanistic framework for both unifying previous findings and guiding future studies. While this work indicates critical steps involving ATP binding and hydrolysis in the Hsp90 dimer, it also provides valuable reagents to further investigate Hsp90 mechanism. In yeast, our system can be used to investigate mutants that are null as homodimers. This type of mutant has been frequently observed in mutational scans of regions of Hsp90 (Hietpas, 2013; Hietpas et al., 2011; Jiang et al., 2013). In addition our system can be utilized to assemble heterodimers for in vitro analyses. The ability to assemble Hsp90 dimers of defined composition will likely be valuable in determining the structural and biochemical basis for client maturation. For example, defined heterodimers could be used to directly test our current speculation that client binding may require ATP binding in both Hsp90 subunits (Figure 4D), which is based on observations that disrupting nucleotide binding in both subunits impairs client binding in vitro (Grenert et al., 1999). Hsp90 dimers of defined subunit composition will likely also be valuable in structurally and biochemically delineating chaperone mechanism through investigations of the functional impacts of client binding, co-chaperone binding, and post-translational modifications to each Hsp90 subunit.
EXPERIMENTAL PROCEDURES
Further details are described in Supplemental Experimental Procedures.
Yeast studies
The DBY519 strain (trp1 ura3 hsp82::Leu2 hsc82::Leu2 ho::pTef1-Hsp90HetA-His3 pKAT6) used in this study is a W303 derivative that was generated from Ecu cells (Nathan et al., 1997) by integration of the Hsp90HetA construct at the HO locus. The designed Hsp90 heterodimers were generated by inserting coiled coil sequences (Havranek and Harbury, 2003) and a His6 tag (GGHHHHHHGGH) after amino acid 678 in Hsp82 (yeast Hsp90). These constructs retain the native Hsp90 C terminus including the MEEVD motif. Yeast manipulations used standard approaches and media (Gietz et al., 1995).
Studies with purified proteins
Hsp90 constructs were expressed and purified individually by immobilized metal affinity, as well as hydrophobic and ion exchange chromatography. Samples were analyzed by analytical SEC on a Superdex200 column (GE). Hydrolysis of ATP was enzymatically linked to NADH oxidation and monitored spectroscopically as previously described (Norby, 1988). Samples were analyzed on native gels (30 mM Hepes, 30 mM imidazole, pH 7.0, 6% polyacrylamide, 1 mM EDTA) that were run at 30 mAmp for one hour using Coomassie Brilliant Blue staining to visualize bands.
Supplementary Material
Highlights.
Protein engineering transforms Hsp90 from a homodimer to a functional heterodimer.
ATP binding is essential in both Hsp90 subunits.
ATP hydrolysis is required in only one Hsp90 subunit.
Symmetric and asymmetric Hsp90 subunit conformations are important for function.
Acknowledgments
We thank B. Roscoe, R. Hietpas, and L. Jiang for helpful discussions. This work was supported in part by National Institutes of Health Grant R01-GM083038 to D.N.A.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, Prodromou C, Pearl LH. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440:1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlander SJ, Felts SJ, Wagner JM, Stensgard B, Toft DO, Karnitz LM. Chaperoning checkpoint kinase 1 (Chk1), an Hsp90 client, with purified chaperones. J Biol Chem. 2006;281:2989–2998. doi: 10.1074/jbc.M508687200. [DOI] [PubMed] [Google Scholar]
- Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989;9:3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CN, Krukenberg KA, Agard DA. Intra- and intermonomer interactions are required to synergistically facilitate ATP hydrolysis in Hsp90. J Biol Chem. 2008;283:21170–21178. doi: 10.1074/jbc.M800046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CN, Southworth DR, Krukenberg KA, Agard DA. The conserved arginine 380 of Hsp90 is not a catalytic residue, but stabilizes the closed conformation required for ATP hydrolysis. Protein Sci. 2012;21:1162–1171. doi: 10.1002/pro.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, Inouye M. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem Sci. 2000;25:24–28. doi: 10.1016/s0968-0004(99)01503-0. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH, Willems AR, Woods RA. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- Grenert JP, Johnson BD, Toft DO. The importance of ATP binding and hydrolysis by hsp90 in formation and function of protein heterocomplexes. J Biol Chem. 1999;274:17525–17533. doi: 10.1074/jbc.274.25.17525. [DOI] [PubMed] [Google Scholar]
- Hagn F, Lagleder S, Retzlaff M, Rohrberg J, Demmer O, Richter K, Buchner J, Kessler H. Structural analysis of the interaction between Hsp90 and the tumor suppressor protein p53. Nat Struct Mol Biol. 2011;18:1086–1093. doi: 10.1038/nsmb.2114. [DOI] [PubMed] [Google Scholar]
- Harris SF, Shiau AK, Agard DA. The crystal structure of the carboxy-terminal dimerization domain of htpG, the Escherichia coli Hsp90, reveals a potential substrate binding site. Structure (Camb) 2004;12:1087–1097. doi: 10.1016/j.str.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Hartson SD, Barrett DJ, Burn P, Matts RL. Hsp90-mediated folding of the lymphoid cell kinase p56lck. Biochemistry. 1996;35:13451–13459. doi: 10.1021/bi961332c. [DOI] [PubMed] [Google Scholar]
- Havranek JJ, Harbury PB. Automated design of specificity in molecular recognition. Nat Struct Biol. 2003;10:45–52. doi: 10.1038/nsb877. [DOI] [PubMed] [Google Scholar]
- Hessling M, Richter K, Buchner J. Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat Struct Mol Biol. 2009;16:287–293. doi: 10.1038/nsmb.1565. [DOI] [PubMed] [Google Scholar]
- Hietpas RT, Bank C, Jensen JD, Bolon DN. Shifting Fitness Landscapes In Response To Altered Enviornments. In Evolution. 2013 doi: 10.1111/evo.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietpas RT, Jensen JD, Bolon DN. Experimental illumination of a fitness landscape. Proc Natl Acad Sci U S A. 2011;108:7896–7901. doi: 10.1073/pnas.1016024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 2012;1823:742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Mishra P, Hietpas RT, Zeldovich KB, Bolon DN. Latent effects of hsp90 mutants revealed at reduced expression levels. PLoS Genet. 2013;9:e1003600. doi: 10.1371/journal.pgen.1003600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SH, Ventouras LA, Lobbezoo B, Jackson SE. Independent ATPase activity of Hsp90 subunits creates a flexible assembly platform. J Mol Biol. 2004;344:813–826. doi: 10.1016/j.jmb.2004.09.055. [DOI] [PubMed] [Google Scholar]
- Meyer P, Prodromou C, Hu B, Vaughan C, Roe SM, Panaretou B, Piper PW, Pearl LH. Structural and functional analysis of the middle segment of hsp90: implications for ATP hydrolysis and client protein and cochaperone interactions. Mol Cell. 2003;11:647–658. doi: 10.1016/s1097-2765(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Mickler M, Hessling M, Ratzke C, Buchner J, Hugel T. The large conformational changes of Hsp90 are only weakly coupled to ATP hydrolysis. Nat Struct Mol Biol. 2009;16:281–286. doi: 10.1038/nsmb.1557. [DOI] [PubMed] [Google Scholar]
- Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem. 1996;271:22796–22801. doi: 10.1074/jbc.271.37.22796. [DOI] [PubMed] [Google Scholar]
- Nathan DF, Vos MH, Lindquist S. In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc Natl Acad Sci U S A. 1997;94:12949–12956. doi: 10.1073/pnas.94.24.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norby JG. Coupled assay of Na+,K+-ATPase activity. Methods Enzymol. 1988;156:116–119. doi: 10.1016/0076-6879(88)56014-7. [DOI] [PubMed] [Google Scholar]
- Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU. In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol. 1998;143:901–910. doi: 10.1083/jcb.143.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaretou B, Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. Embo J. 1998;17:4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Ratzke C, Berkemeier F, Hugel T. Heat shock protein 90’s mechanochemical cycle is dominated by thermal fluctuations. Proc Natl Acad Sci U S A. 2012;109:161–166. doi: 10.1073/pnas.1107930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzlaff M, Hagn F, Mitschke L, Hessling M, Gugel F, Kessler H, Richter K, Buchner J. Asymmetric activation of the hsp90 dimer by its cochaperone aha1. Mol Cell. 2010;37:344–354. doi: 10.1016/j.molcel.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Richter K, Muschler P, Hainzl O, Buchner J. Coordinated ATP hydrolysis by the Hsp90 dimer. J Biol Chem. 2001;276:33689–33696. doi: 10.1074/jbc.M103832200. [DOI] [PubMed] [Google Scholar]
- Rohl A, Rohrberg J, Buchner J. The chaperone Hsp90: changing partners for demanding clients. Trends Biochem Sci. 2013;38:253–262. doi: 10.1016/j.tibs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Rudiger S, Freund SM, Veprintsev DB, Fersht AR. CRINEPT-TROSY NMR reveals p53 core domain bound in an unfolded form to the chaperone Hsp90. Proc Natl Acad Sci U S A. 2002;99:11085–11090. doi: 10.1073/pnas.132393699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Sepp-Lorenzino L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen N, Hartl FU. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci U S A. 1996;93:14536–14541. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte TW, Blagosklonny MV, Romanova L, Mushinski JF, Monia BP, Johnston JF, Nguyen P, Trepel J, Neckers LM. Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1-MEK-mitogen-activated protein kinase signalling pathway. Mol Cell Biol. 1996;16:5839–5845. doi: 10.1128/mcb.16.10.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau AK, Harris SF, Southworth DR, Agard DA. Structural Analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell. 2006;127:329–340. doi: 10.1016/j.cell.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Southworth DR, Agard DA. Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol Cell. 2008;32:631–640. doi: 10.1016/j.molcel.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southworth DR, Agard DA. Client-loading conformation of the Hsp90 molecular chaperone revealed in the cryo-EM structure of the human Hsp90:Hop complex. Mol Cell. 2011;42:771–781. doi: 10.1016/j.molcel.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova L, Leng X, Parker SB, Harper JW. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996;10:1491–1502. doi: 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- Street TO, Lavery LA, Agard DA. Substrate binding drives large-scale conformational changes in the Hsp90 molecular chaperone. Mol Cell. 2011;42:96–105. doi: 10.1016/j.molcel.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street TO, Lavery LA, Verba KA, Lee CT, Mayer MP, Agard DA. Cross-monomer substrate contacts reposition the Hsp90 N-terminal domain and prime the chaperone activity. J Mol Biol. 2012;415:3–15. doi: 10.1016/j.jmb.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, Lindquist S. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CK, Gohlke U, Sobott F, Good VM, Ali MM, Prodromou C, Robinson CV, Saibil HR, Pearl LH. Structure of an Hsp90-Cdc37-Cdk4 complex. Mol Cell. 2006;23:697–707. doi: 10.1016/j.molcel.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne N, Bolon DN. Dimerization of Hsp90 is required for in vivo function. Design and analysis of monomers and dimers. J Biol Chem. 2007;282:35386–35395. doi: 10.1074/jbc.M703844200. [DOI] [PubMed] [Google Scholar]
- Wayne N, Lai Y, Pullen L, Bolon DN. Modular control of cross-oligomerization: analysis of superstabilized Hsp90 homodimers in vivo. J Biol Chem. 2010;285:234–241. doi: 10.1074/jbc.M109.060129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, Krogan N, Cagney G, Mai D, Greenblatt J, et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120:715–727. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.