Abstract
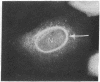
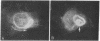
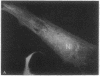
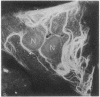
Guinea pig vascular endothelial cells contain naturally occurring rings of intermediate filaments that completely encircle the nucleus. Indirect immunofluorescence staining showed that these perinuclear rings bound antibody prepared against protein from bovine brain 9-nm filaments. In endothelial cells grown in the presence of 1 muM demecolcine (Colcemid) the perinuclear ring "coils" into a juxtanuclear "cap". Throughout this process we could demonstrate staining of the intermediate filaments. Chick cardiac muscle cells in culture stained diffusely with the antibody. After treatment for 24 hr with 1 muM demecolcine the cardiac cells accumulated large bands of intermediate filaments. These bands stained intensely with the antibody. Our findings suggest that intermediate filaments in guinea pig endothelial cells and those induced in chick cardiac muscle cells are antigenically similar to bovine brain filaments. The staining of these filaments is not affected by treatment with demecolcine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN E. R., PEPE F. A. ULTRASTRUCTURE OF DEVELOPING MUSCLE CELLS IN THE CHICK EMBRYO. Am J Anat. 1965 Jan;116:115–147. doi: 10.1002/aja.1001160107. [DOI] [PubMed] [Google Scholar]
- Anderson H. C., Chacko S., Abbott J., Holtzer H. The loss of phenotypic traits by differentiated cells in vitro. VII. Effects of 5-bromodeoxyuridine and prolonged culturing on fine structure of chondrocytes. Am J Pathol. 1970 Aug;60(2):289–312. [PMC free article] [PubMed] [Google Scholar]
- Biberfeld P., Ericsson J. L., Perlmann P., Raftell M. Increased occurrence of cytoplasmic filaments in in vitro propagated rat liver epithelial cells. Exp Cell Res. 1965 Aug;39(1):301–305. doi: 10.1016/0014-4827(65)90034-0. [DOI] [PubMed] [Google Scholar]
- Blose S. H., Chacko S. Rings of intermediate (100 A) filament bundles in the perinuclear region of vascular endothelial cells. Their mobilization by colcemid and mitosis. J Cell Biol. 1976 Aug;70(2 Pt 1):459–466. doi: 10.1083/jcb.70.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko S. DNA synthesis, mitosis, and differentiation in cardiac myogenesis. Dev Biol. 1973 Nov;35(1):1–18. doi: 10.1016/0012-1606(73)90002-x. [DOI] [PubMed] [Google Scholar]
- Cooke P. A filamentous cytoskeleton in vertebrate smooth muscle fibers. J Cell Biol. 1976 Mar;68(3):539–556. doi: 10.1083/jcb.68.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop J., Holtzer H. Response of myogenic and fibrogenic cells to cytochalasin B and to colcemid. I. Light microscope observations. J Cell Biol. 1975 May;65(2):271–285. doi: 10.1083/jcb.65.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE PETRIS S., KARLSBAD G., PERNIS B. Filamentous structures in the cytoplasm of normal mononuclear phagocytes. J Ultrastruct Res. 1962 Aug;7:39–55. doi: 10.1016/s0022-5320(62)80025-2. [DOI] [PubMed] [Google Scholar]
- DeBrabander M., Aerts F., Van de Veire R., Borgers M. Evidence against interconversion of microtubules and filaments. Nature. 1975 Jan 10;253(5487):119–120. doi: 10.1038/253119a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968 Sep;38(3):538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimbow K., Fitzpatrick T. B. Changes in distribution pattern of cytoplasmic filaments in human melanocytes during ultraviolet-mediated melanin pigmentation. The role of the 100-Angstrom filaments in the elongation of melanocytic dendrites and in the movement and transfer of melanosomes. J Cell Biol. 1975 May;65(2):481–488. doi: 10.1083/jcb.65.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkut G. A. Axoplasmic transport. Comp Biochem Physiol A Comp Physiol. 1975 Aug 1;51(4):701–704. doi: 10.1016/0300-9629(75)90041-9. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak L. V., Kato F. Electron microscopic studies of lymphatic capillaries during early inflammation. I. Mild and severe thermal injuries. Lab Invest. 1972 May;26(5):572–588. [PubMed] [Google Scholar]
- Liwnicz B. H., Kristensson K., Wiśniewski H. M., Shelanski M. L., Terry R. D. Observations on axoplasmic transport in rabbits with aluminum-induced neurofibrillary tangles. Brain Res. 1974 Nov 22;80(3):413–420. doi: 10.1016/0006-8993(74)91026-9. [DOI] [PubMed] [Google Scholar]
- Moellmann G., McGuire J. Correlation of cytoplasmic microtubules and 10-nm filaments with the movement of pigment granules in cutaneous melanocytes of Rana pipiens. Ann N Y Acad Sci. 1975 Jun 30;253:711–722. doi: 10.1111/j.1749-6632.1975.tb19240.x. [DOI] [PubMed] [Google Scholar]
- Sajdera S. W., Franklin S., Fortuna R. Matrix vesicles of bovine fetal cartilage: metabolic potential and solubilization with detergents. Fed Proc. 1976 Feb;35(2):154–155. [PubMed] [Google Scholar]
- Schmitt F. O. Fibrous proteins--neuronal organelles. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1092–1101. doi: 10.1073/pnas.60.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Albert S., DeVries G. H., Norton W. T. Isolation of filaments from brain. Science. 1971 Dec 17;174(4015):1242–1245. doi: 10.1126/science.174.4015.1242. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Devine C. E., Somlyo A. V., Rice R. V. Filament organization in vertebrate smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):223–229. doi: 10.1098/rstb.1973.0027. [DOI] [PubMed] [Google Scholar]
- Sutton J. S. Ultrastructural aspects of in vitro development of monocytes into macrophages, epithelioid cells, and multinucleated giant cells. Natl Cancer Inst Monogr. 1967 Sep;26:71–141. [PubMed] [Google Scholar]
- TANAKA Y. FIBRILLAR STRUCTURES IN THE CELLS OF BLOODFORMING ORGANS. J Natl Cancer Inst. 1964 Sep;33:467–485. [PubMed] [Google Scholar]
- Wisniewski H., Shelanski M. L., Terry R. D. Effects of mitotic spindle inhibitors on neurotubules and neurofilaments in anterior horn cells. J Cell Biol. 1968 Jul;38(1):224–229. doi: 10.1083/jcb.38.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewski H., Terry R. D., Hirano A. Neurofibrillary pathology. J Neuropathol Exp Neurol. 1970 Apr;29(2):163–176. [PubMed] [Google Scholar]
- Wuerker R. B., Kirkpatrick J. B. Neuronal microtubules, neurofilaments, and microfilaments. Int Rev Cytol. 1972;33:45–75. doi: 10.1016/s0074-7696(08)61448-5. [DOI] [PubMed] [Google Scholar]
- Yen S. H., Dahl D., Schachner M., Shelanski M. L. Biochemistry of the filaments of brain. Proc Natl Acad Sci U S A. 1976 Feb;73(2):529–533. doi: 10.1073/pnas.73.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]