Hypoxia can induce adenosine-independent T-cell suppression in vivo and in vitro
Keywords: IFN-γ, immunosuppression, inflammation, oxygen, tumor microenvironment
Abstract
Tissue hypoxia plays a key role in establishing an immunosuppressive environment in vivo by, among other effects, increasing the level of extracellular adenosine, which then signals through A2A adenosine receptor (A2AR) to elicit its immunosuppressive effect. Although the important role of the adenosine–A2AR interaction in limiting inflammation has been established, the current study revisited this issue by asking whether hypoxia can also exert its T-cell inhibitory effects even without A2AR. A similar degree of hypoxia-triggered inhibition was observed in wild-type and A2AR-deficient T cells both in vitro and, after exposure of mice to a hypoxic atmosphere, in vivo. This A2AR-independent hypoxic T-cell suppression was qualitatively and mechanistically different from immunosuppression by A2AR stimulation. The A2AR-independent hypoxic immunosuppression strongly reduced T-cell proliferation, while IFN-γ-producing activity was more susceptible to the A2AR-dependent inhibition. In contrast to the sustained functional impairment after A2AR-mediated T-cell inhibition, the A2AR-independent inhibition under hypoxia was short lived, as evidenced by the quick recovery of IFN-γ-producing activity upon re-stimulation. These data support the view that T-cell inhibition by hypoxia can be mediated by multiple mechanisms and that both A2AR and key molecules in the A2AR-independent T-cell inhibition should be targeted to overcome the hypoxia-related immunosuppression in infected tissues and tumors.
Introduction
The immune system has various mechanisms to discourage potentially tissue-damaging immune activation, e.g. anti-inflammatory cells (regulatory T cells, myeloid-derived suppressor cells), anti-inflammatory cytokines (TGF-β, IL-10) and anti-inflammatory small molecules (adenosine, lipoxins, vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide) (1–3). The counterbalance of these mechanisms can prevent overactivation of immune responses and consequent excessive tissue injury, but it can also diminish immune responses to fight infectious pathogens and cancer cells. These immunoregulatory mechanisms are crucial in controlling the pro- and anti-inflammatory balance.
Immune cells express different types of receptors for adenosine. T cells predominantly express the A2A subtype of adenosine receptor (A2AR), which strongly represses T-cell activation (3–5). Studies using A2AR−/− mice demonstrated the pathophysiological importance of immunoregulation through adenosine–A2AR interaction. Induction of inflammation in A2AR−/− mice resulted in highly exaggerated inflammatory tissue damage (6, 7). Therapeutically promising, blockade of the adenosine–A2AR interaction resulted in a significant improvement in tumor regression as observed in A2AR−/− mice and in wild-type (WT) mice treated with A2AR antagonist (8). These studies established adenosine as an indispensable regulator of the immune system.
Generation of the immunoregulatory concentration of adenosine in the extracellular space is accomplished by the cell surface enzymes, CD39 and CD73, which catalyze degradation of ATP to AMP (CD39) and to adenosine (CD73). The expression of CD39 and CD73 is inducible by lowering oxygen tension (9, 10). Hypoxia not only promotes degradation of ATP to adenosine but also inhibits conversion of adenosine to AMP by adenosine kinase (11, 12). Therefore, tissue hypoxia is believed to be important in the local accumulation of extracellular adenosine. Indeed, whole body exposure to a hypoxic atmosphere increased adenosine concentration in vivo (7, 13). Local hypoxia can result from inflammation due to the massive accumulation of inflammatory effector cells and inflammatory damage to blood vessels. Taken together, these findings imply the significance of the hypoxia-triggered negative feedback mechanism to prevent excessive inflammatory tissue damage.
Hypoxia induces cellular stress responses in order to adapt to energy deprivation and to diverse cell type-specific changes in cellular functions. Studies have shown that A2AR antagonists were capable of blocking many of these hypoxia-induced changes, suggesting the involvement of adenosine-A2AR signaling downstream from hypoxia. These instances include in vitro membrane depolarization, neurite outgrowth, up-regulation of glucose transporter-1, vascular endothelial growth factor (VEGF) and erythropoietin (14–18). There are also in vivo studies suggesting A2AR dependence of hypoxia-induced cerebral vasodilation, bradycardia and hypertension (19, 20). In immune cells, more severe inflammation in A2AR−/− mice breathing a hypoxic atmosphere suggested an involvement of adenosine-A2AR signaling in the anti-inflammatory effect of hypoxia (7, 13). Although the adenosine-A2AR pathway plays a role downstream from hypoxia, cellular stress produced by hypoxia may also be able to suppress certain functions in an A2AR-independent mechanism. Indeed, hypoxia-induced erythropoietin in mice did not change in the absence of A2AR or by adenosine deprivation by the inhibition of CD73 (21).
In this study, we focused on the immunosuppressive action of hypoxia in T cells and examined the A2AR dependence of the mechanism. As a result, we found that hypoxia can suppress T-cell activation independent of A2AR. Comparison of the A2AR-dependent and -independent pathways revealed important immunological differences between these two hypoxia-driven immunosuppressive mechanisms.
Methods
Mice
Female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA, USA). RAG1−/− mice and B6.PL-Thy1a/CyJ mice (Thy1.1+ C57BL/6 mice) were from Jackson Laboratory (Bar Harbor, ME, USA). A2AR−/− mice were backcrossed 12 times to C57BL/6 mice (22). Mice were used at 8–12 weeks of age. Experiments were approved by the Northeastern University Institutional Animal Care and Use Committee and were carried out in accordance with the institutional animal care guidelines.
Stimulation of T cells
Spleen cells (5×105 cells) were stimulated by adding anti-CD3 mAb (145-2C11; BD Biosciences, San Diego, CA, USA) at 0.1 μg ml−1 in RPMI1640 media supplemented with 10% FCS. The cells were cultured in a normoxic (21% oxygen) or hypoxic (1% oxygen) atmosphere supplemented with 5% carbon dioxide. A2AR agonist CGS21680 (CGS) was added at concentrations between 10−9 and 10−5 M. For adenosine receptor antagonists, DPCPX (A1 adenosine receptor antagonist) and KW6002 (A2AR-specific antagonist) were used at 0.1 μM; ZM241385 (A2A/A2B adenosine receptor antagonist), MRS1754 (A2B adenosine receptor antagonist) and MRS1191 (A3 adenosine receptor antagonist) were used at 1 μM. CD73 inhibitor α,β-methylene adenosine-5′-diphosphate (APCP) was added up to 100 μM. CGS, DPCPX and ZM241385 were from Tocris (Ellisville, MO, USA). MRS1754, MRS1191 and APCP were from Sigma (St Louis, MO, USA). KW6002 was synthesized by Dr G.J. (Department of Chemistry, Northeastern University). Culture supernatant was collected after 24h for determination of IFN-γ levels by ELISA (R&D Systems, Minneapolis, MN, USA).
Cell proliferation assays
Proliferative activity of the cells at 24h was examined by thymidine incorporation assay. The cells were incubated for 4h with 1-μCi [3H] thymidine (American Radiolabeled Chemicals, St Louis, MO, USA), and incorporated radioactivity was counted. To monitor total cell proliferation, spleen cells were prelabeled with CFSE (Molecular Probes, Eugene, OR, USA) as described previously (5). Forty hours after the stimulation of the CFSE-labeled cells with anti-CD3 mAb, cell division was analyzed for CD4+ and CD8+ cells using a FACSCalibur flow cytometer (BD Biosciences).
Based on CFSE analysis, the proliferation index was calculated as follows:
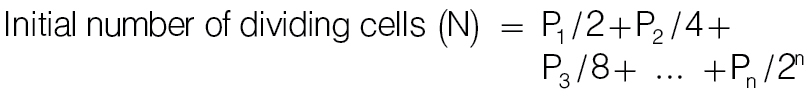 |
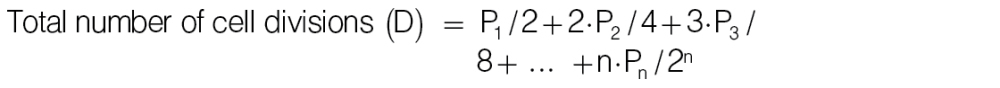 |
where, n, times of cell division; Pn, cell number in peak n, e.g. P1 and P2 are numbers of cells divided once and twice, respectively.
Proliferation index represents times of cell division per dividing cells (= D/N).
Quantification of adenosine
Culture supernatants were collected 48h after culturing spleen cells with anti-CD3 mAb under a 21 or 1% oxygen atmosphere. To prevent degradation of adenosine, erythro-9-(2-hydroxy-3-nonyl)adenine (final concentration 10 μM) was immediately added to the collected samples. Then, perchloric acid was added at 5% (v/v), and precipitated materials were removed by centrifugation. Concentrations of adenosine were determined by high-performance liquid chromatography as described previously (13).
T-cell stimulation in vivo
T cells were stimulated in vivo by i.v. injection of anti-CD3 mAb. WT and A2AR−/− mice were exposed to a hypoxic atmosphere containing 8% oxygen for 1h, injected with anti-CD3 mAb (50 μg kg−1) and further exposed to 8% oxygen. Whole body exposure of mice to hypoxia was carried out using airtight modular incubation chambers (Billups-Rothenberg, San Diego, CA, USA). Expression of activation markers on T cells was analyzed by flow cytometry 2h after injection of anti-CD3 mAb.
For T-cell proliferation in vivo, a mixture of spleen cells from A2AR−/− (Thy1.2+) and Thy1.1+ WT C57BL/6 mice were labeled with CFSE and injected i.v. to RAG1−/− mice (2×107 cells). Two hours after the transfer of CFSE-labeled spleen cells, the recipient mice received an i.v. injection of anti-CD3 mAb (50 μg kg−1), and spleen cells were analyzed by flow cytometry for the extent of proliferation after 40h.
Mixed lymphocyte culture
To induce antigen-specific cytotoxic T cells, spleen cells from C57BL/6 mice (responder; H-2b) were co-cultured with BALB/c spleen cells (stimulator; H-2d) as described previously (23). Spleen cells from BALB/c mice were pre-treated with mitomycin C (Sigma). Cells were incubated for 5 days under either 21 or 1% oxygen.
Cytotoxicity
After developing effector T cells by mixed lymphocyte culture, cytotoxicity of T cells was determined by a 51Cr release assay. Effector cells were prepared 5 days after the initiation of mixed lymphocyte culture and incubated with the P815 mastocytoma cells (H-2d) for 4h. The extent of target cell lysis was determined by the radioactivity of 51Cr in the supernatant.
Re-stimulation of activated T cells
Two days after initial stimulation, the activated T cells were washed twice. To evaluate cytokine-producing activity, activated T cells (2×105 cells) were re-stimulated with immobilized anti-CD3 mAb in the absence of A2AR agonist. IFN-γ levels in the culture supernatant were analyzed after 24h by ELISA.
Statistics
Data represent mean ± SD. Statistical calculations were performed using Student’s t-test. Statistical significance was accepted for P values <0.05.
Results
Hypoxia can inhibit T-cell activation independent of adenosine-A2AR signaling
Compared with cells cultured in a normoxic atmosphere, T cells stimulated at 1% oxygen significantly reduced their proliferative activity (Supplementary Figure 1A, available at International Immunology Online). In parallel assays, exposure of T cells to hypoxia also diminished IFN-γ production to approximately half the level in normoxic cells (Supplementary Figure 1B, available at International Immunology Online). Data obtained from the current experimental system confirm and extend previous studies showing hypoxia-induced impairment of T-cell activation (24–28).
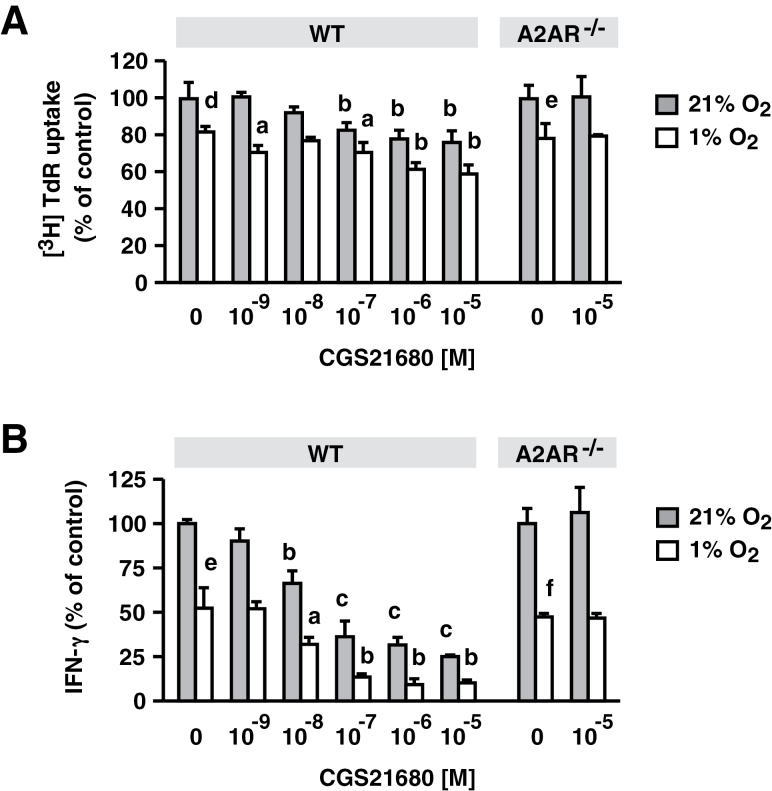
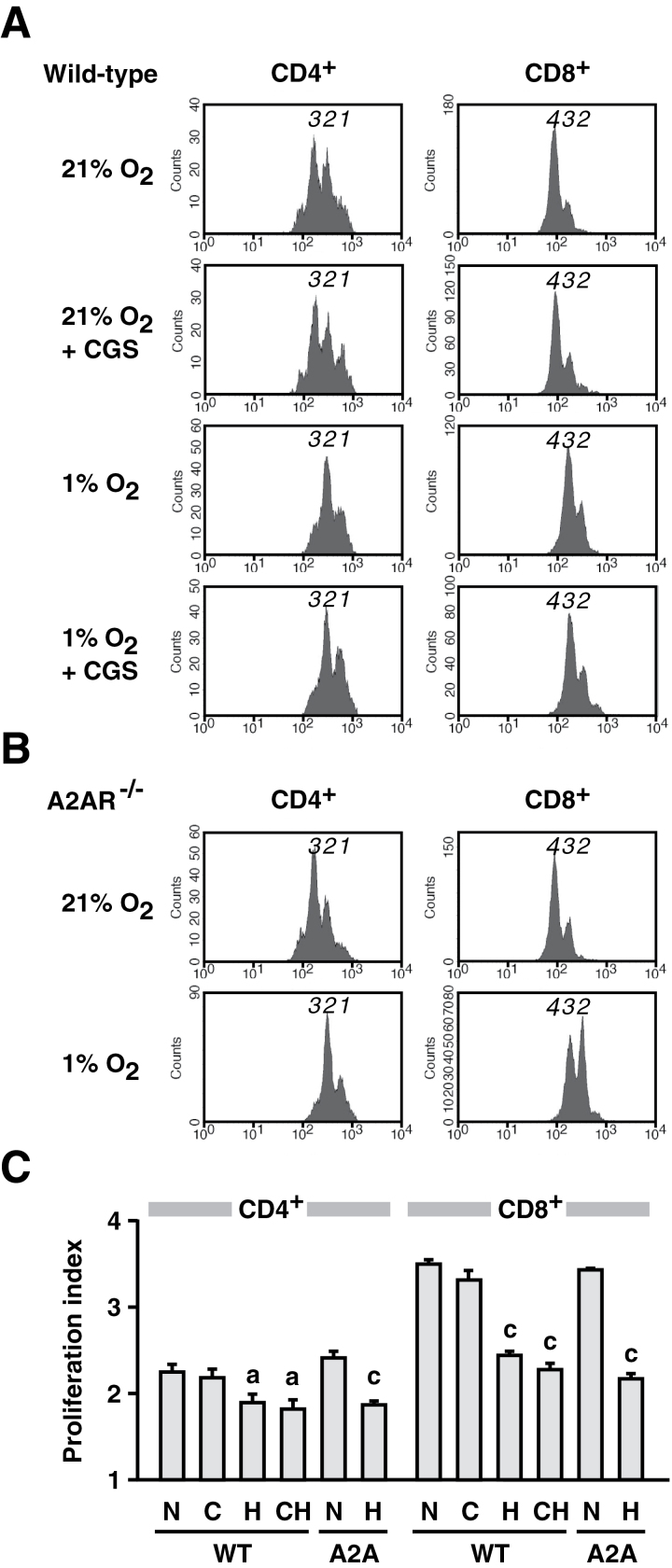
Previous studies have shown that the adenosine-A2AR pathway mediates many of the hypoxia-induced changes in cellular functions in vivo and in vitro (7, 13–20). We evaluated whether the adenosine-A2AR pathway is the only mechanism involved in the suppression of T-cell activation under hypoxia. To this end, T cells from A2AR−/− mice were stimulated under hypoxia, and their responses were compared with WT T cells. Interestingly, cell proliferation and IFN-γ-producing activity in A2AR−/− T cells were also sensitive to hypoxia, and the extent of inhibition in A2AR−/− T cells was quite similar to that in WT T cells (Fig. 1). The CFSE assay showed major peak shifts under hypoxia, confirming strong inhibition of both WT and A2AR−/− T-cell proliferation (Fig. 2). Since there was no difference in the inhibitory effects on WT and A2AR−/− T cells, hypoxia in this in vitro culture system seemed to regulate T-cell activation by a mechanism independent of the adenosine-A2AR pathway.
Fig. 1.
Suppression of T-cell functions by hypoxia did not require involvement of A2AR. Spleen cells from WT and A2AR−/− mice were stimulated with anti-CD3 mAb for 24h in the presence of various concentrations of CGS. Cell proliferative activity was monitored by [3H] thymidine uptake after 24h (A). IFN-γ levels in the supernatant were also determined after 24h (B). Hypoxia significantly inhibited cell proliferation and IFN-γ production in both WT and A2AR−/− T cells. Inhibition by CGS was observed only in WT cells. Data represent average ± SD of triplicate samples. a, P < 0.05; b, P < 0.01; c, P < 0.001 versus no CGS. d, P < 0.05; e, P < 0.01; f, P < 0.001; 21 versus 1% oxygen (no CGS).
Fig. 2.
Hypoxia strongly suppressed T-cell proliferation as compared with the effect of A2AR stimulation by CGS. Proliferation of CFSE-labeled CD4+ and CD8+ cells after 36h was monitored separately for WT (A) and A2AR−/− (B) T cells. Peaks with diminished fluorescence intensity (shown by numbers) indicate dilution of CFSE in proliferated cells. Results for normoxic culture, hypoxic culture and CGS (10−5 M) treatment shown here are representative of four separate experiments with similar results. (C) Proliferation index calculated as in Methods. The index represents average times of cell division in cells that divided at least once. Therefore, the minimum number of proliferation index is one. WT, WT mice; A2A, A2AR−/− mice; N, normoxic atmosphere (21% oxygen); H, hypoxic atmosphere (1% oxygen); C, CGS (10−5 M); CH, CGS + 1% oxygen. Data represent average ± SD of triplicate samples. a, P < 0.05; c, P < 0.001 versus normoxia.
Next, we considered a possibility that, although A2AR was not essential to T-cell suppression under hypoxia, other types of adenosine receptors, i.e. A1, A2B and A3, could be involved in immunosuppression by extracellular adenosine that might be produced in the hypoxic cell culture. To examine the possible contribution of other adenosine receptors, we tested the effects of various adenosine receptor antagonists: DPCPX (A1 receptor), ZM241385 (A2A/A2B receptor), KW6002 (A2AR), MRS1754 (A2B receptor) and MRS1191 (A3 receptor) (29). A2AR antagonists, ZM241385 and KW6002, did not reverse hypoxic suppression of IFN-γ production and T-cell proliferation (Supplementary Figure 2, available at International Immunology Online), and that finding is consistent with the observation in A2AR−/− mice. Moreover, none of these adenosine receptor antagonists could block the inhibitory effect of hypoxia. The inhibition of extracellular adenosine production by a CD73 inhibitor, APCP, also had no effect (Supplementary Figure 2, available at International Immunology Online). These results suggest hypoxic T-cell inhibition in this culture system does not require extracellular adenosine in the mechanism. Indeed, extracellular adenosine levels in T-cell culture supernatant showed no significant increase under hypoxia (Supplementary Figure 2, available at International Immunology Online).
A2AR-independent T-cell suppression in vivo when exposed to hypoxia
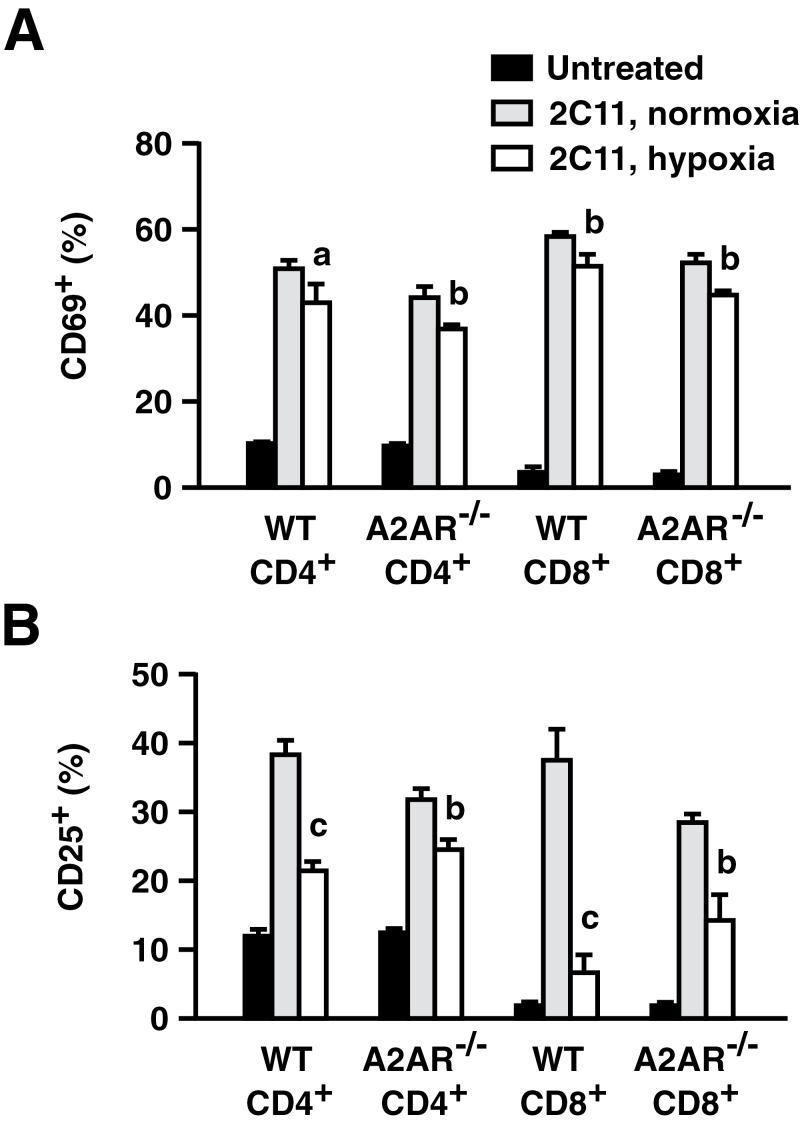
To study hypoxic T-cell suppression in vivo, the extent of T-cell activation was compared in mice exposed to normoxic versus hypoxic atmosphere. After injection of anti-CD3 mAb, T cells rapidly up-regulated surface expression of activation markers, CD69 and CD25 (Fig. 3). Hypoxia significantly reduced the proportion of activated T cells in both WT and A2AR−/− mice. The effect of hypoxia in A2AR−/− mice indicated that hypoxia could inhibit T-cell activation in vivo independent of A2AR. Although hypoxic T-cell inhibition was significant in both WT and A2AR−/− mice, the inhibitory effect of hypoxia was relatively weaker in A2AR−/− mice, especially for CD25 expression. Indeed, hypoxia treatment reduced CD25+ cells by 19% (CD4) and 31% (CD8) in WT mice, but it was 7% (CD4) and 14% (CD8) in A2AR−/− mice (Fig. 3B). This difference may imply that both A2AR-dependent and -independent mechanisms contributed to T-cell inhibition under hypoxia in vivo.
Fig. 3.
Hypoxia inhibited early activation of T cells in vivo even in the absence of A2AR. WT and A2AR−/− mice received injection of anti-CD3 mAb while breathing a normoxic or hypoxic (8% oxygen) atmosphere. Changes in CD69 (A) and CD25 (B) expression on T cells in the spleen were analyzed 2h after the injection of anti-CD3 mAb. Percentages of CD69-positive or CD25-positive cells in the CD4+ or CD8+ population are shown. Data represent average ± SD of 3–5 mice. a, P < 0.05; b, P < 0.01; c, P < 0.001 versus anti-CD3 mAb + normoxia.
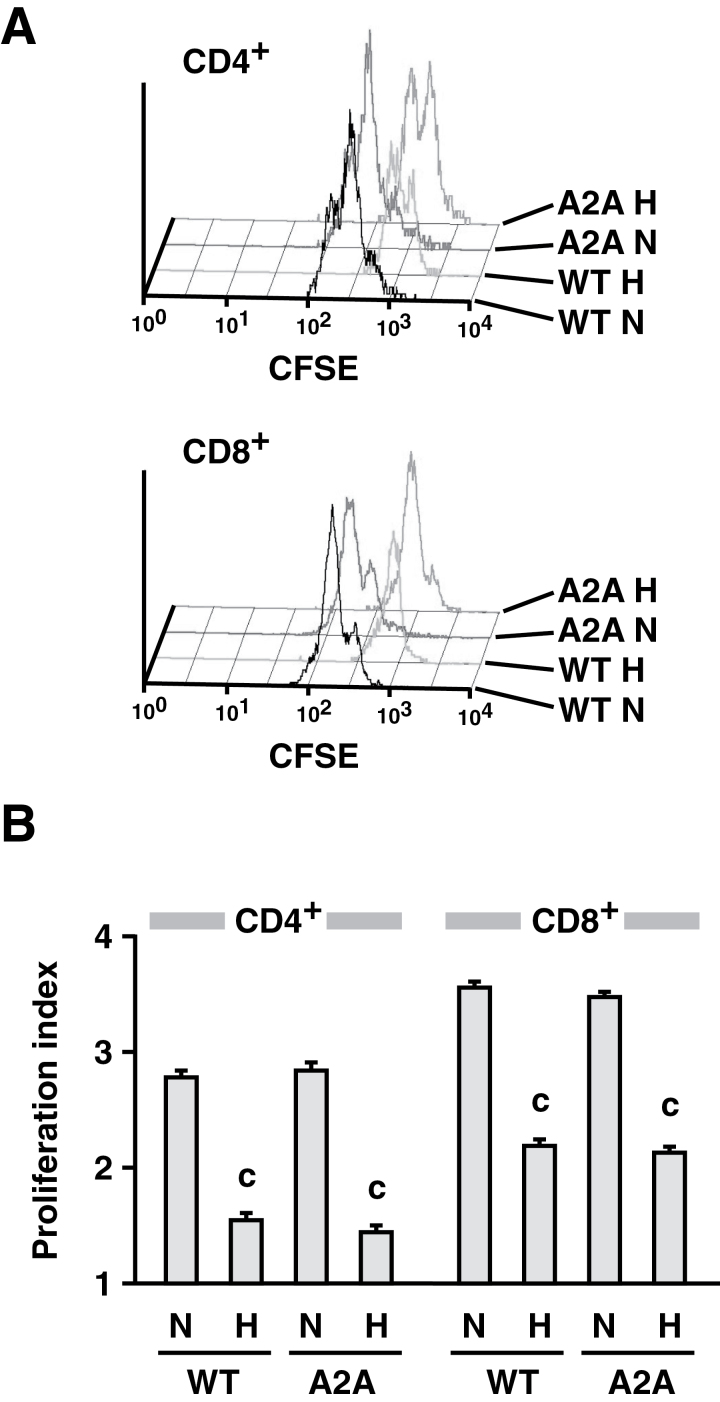
A2AR-independent repression of T-cell function in mice under hypoxic conditions was examined by monitoring cell proliferation in vivo. A mixture of T cells from WT and A2AR−/− mice was labeled with CFSE, injected into RAG1−/− mice and stimulated by i.v. injection of anti-CD3 mAb. Aggressive T-cell proliferation, which was observed in normoxic mice, was strongly diminished when mice were exposed to a hypoxic atmosphere (Fig. 4). Hypoxia inhibited proliferation of WT and A2AR−/− T cells to the same extent, demonstrating that the A2AR-independent mechanism of hypoxic T-cell inhibition is biologically relevant in vivo.
Fig. 4.
A2AR-independent inhibition of T-cell proliferation in mice exposed to a hypoxic atmosphere. After the transfer of CFSE-labeled spleen cells from WT and A2AR−/− (A2A) mice, the recipient RAG1−/− mice received an injection of anti-CD3 mAb and were maintained under an either normoxic (N) or hypoxic (H; 8% oxygen) atmosphere. After 40h, the CFSE pattern in the spleen cells was analyzed by flow cytometry. T cells derived from WT and A2AR−/− mice were discriminated by the expression of Thy1.1 and Thy1.2 markers. (A) Representative pattern of cell proliferation. (B) Proliferation index calculated as in Methods. Data represent average ± SD of three mice. c, P < 0.001 versus normoxia.
Differential effects of the A2AR-dependent and -independent mechanisms on T-cell functions
This A2AR-independent T-cell suppression by hypoxia was compared with A2AR-mediated immunosuppression. The addition of CGS, an A2AR agonist, suppressed cell proliferation and IFN-γ production in a concentration-dependent manner (Fig. 1). CGS did not affect the function of A2AR−/− T cells, thereby confirming the A2AR dependence of the inhibition. Although both CGS and hypoxia inhibited thymidine uptake to a similar extent, analysis of total cell proliferation by the CFSE assay showed a clear difference in the inhibitory effects of these two mechanisms. CGS caused only minor changes in peak heights in the CFSE assay, e.g. augmentation of peak 1 of CD4+ and peak 3 of CD8+ cells (Fig. 2A). In contrast to the marginal decrease in cell proliferation by A2AR stimulation, hypoxia caused a remarkable reduction in cell proliferation. Under normoxic conditions, the majority of T cells showed extensive proliferation as represented by peak 3 of CD4+ and peak 4 of CD8+ cells (Fig. 2A). However, hypoxia strongly diminished these major peaks. The majority belonged to peak 2 of CD4+ and peak 3 of CD8+ cells (Fig. 2A), indicating much stronger inhibition of T-cell proliferation than A2AR-dependent immunosuppression (Fig. 2C). This decrease in cell proliferation was reflected in a 45% reduction in cell number in the hypoxic culture (data not shown).
In contrast to the stronger antiproliferative effect of hypoxia as compared with CGS, IFN-γ production was rather more sensitive to CGS than to hypoxia. CGS decreased IFN-γ levels to 25% of control, while 55% of IFN-γ remained after hypoxia in the same experiment (Fig. 1B). Different effects on each T-cell function show that the profile of T-cell inhibition by the A2AR-independent pathway is distinct from the A2AR-dependent immunosuppression. Importantly, adding CGS to the hypoxic culture further enhanced T-cell inhibition by hypoxia. This indicates that A2AR-mediated T-cell inhibition is additive to the A2AR-independent mechanism triggered by hypoxia (Figs 1 and 2).
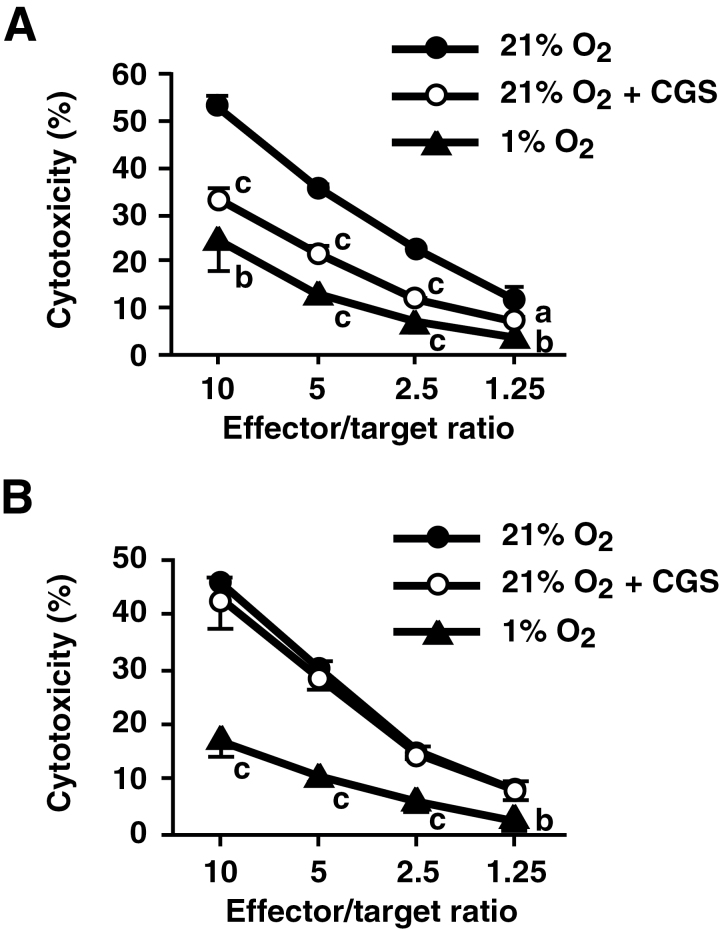
Experiments of CTL development under hypoxic conditions also supported involvement of the A2AR-independent mechanism of immunosuppression. CTL were induced by mixed lymphocyte culture at a normoxic or hypoxic atmosphere. CTL development in the presence of either CGS or hypoxia significantly reduced cytotoxicity against allogenic target cells (Fig. 5A). While CGS treatment reduced cytotoxic activity by approximately half, T cells cultured under hypoxia were four times less effective than control. Hypoxia impaired A2AR−/− CTL development to the same extent as WT CTL and again produced four times less cytotoxic effectors (Fig. 5B). This result suggests that hypoxia also inhibited CTL development by the A2AR-independent mechanism.
Fig. 5.
Decrease in cytotoxicity of effector T cells induced by mixed lymphocyte culture under hypoxic atmosphere. C57BL/6 WT (A) and A2AR−/− (B) spleen cells (H-2b) were cultured with BALB/c spleen cells (H-2d) for 5 days under 21 or 1% oxygen. Cytotoxicity against allogenic target cells was determined by a 51Cr release assay using P815 cells (H-2d). Data represent average ± SD of triplicate samples. a, P < 0.05; b, P < 0.01; c, P < 0.001 versus 21% oxygen.
Hypoxic T-cell inhibition by the A2AR-independent mechanism is a transient change
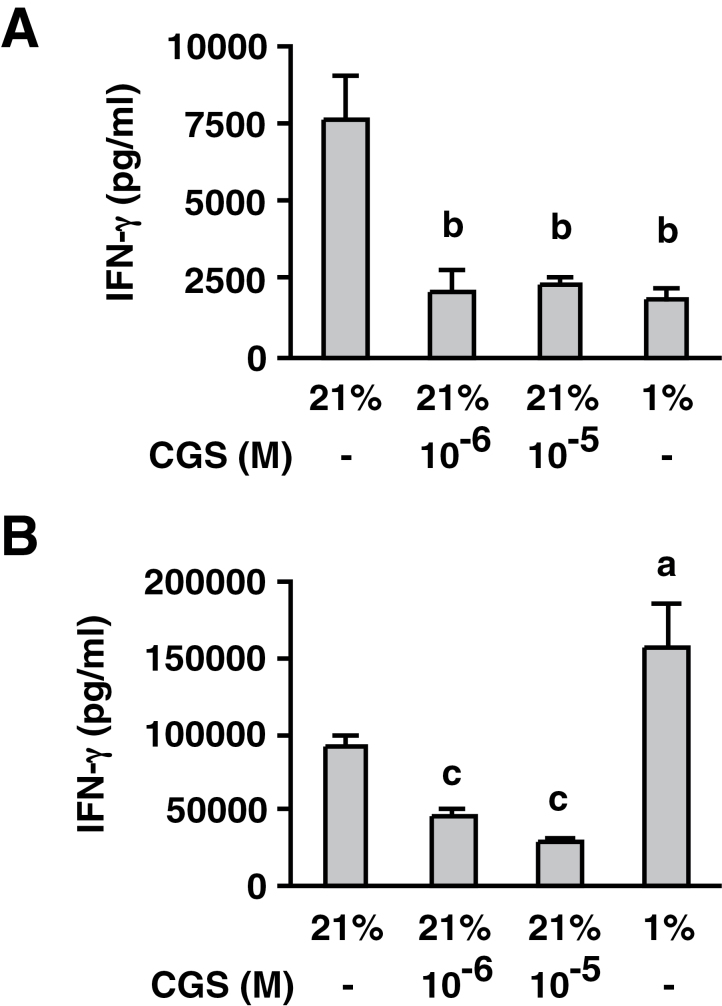
A2AR stimulation at the time of T-cell activation is known to induce prolonged impairment of effector functions in these T cells (4, 5). Our previous study showed that T cells activated in the presence of CGS could produce only a limited amount of IFN-γ even after the removal of CGS (5). In the current study, T-cell activation was induced in either a normoxic or hypoxic environment. The activated T cells were washed and re-stimulated for the evaluation of IFN-γ-producing activity. When the same number of cells was re-stimulated at the same normoxic condition, T cells pre-activated under hypoxia were able to produce as large an amount of IFN-γ as normoxic T cells (Fig. 6). In sharp contrast, T-cell activation in the presence of CGS impaired the IFN-γ-producing activity of the resultant T cells (Fig. 6). This result suggests that the A2AR-dependent mechanism of immunosuppression persistently inhibits T-cell activity; however, the A2AR-independent T-cell inhibition by hypoxia may be transient, and T cells can regain their normal function after the resolution of hypoxia.
Fig. 6.
Activated T cells developed in the presence of CGS retain an immunosuppressed phenotype, but the inhibitory effect of hypoxia was temporary and T cells recovered effector function. T-cell activation was induced by anti-CD3 mAb with CGS (10−6, 10−5 M) or hypoxia (1% oxygen) for 40h. After washing, the cells were re-stimulated for 24h with immobilized anti-CD3 mAb for the measurement of IFN-γ levels. (A) IFN-γ levels after 40-h primary stimulation. (B) IFN-γ levels after re-stimulation. Data represent average ± SD of triplicate samples. a, P < 0.05; b, P < 0.01; c, P < 0.001 versus control.
Discussion
Studies of tissue oxygen tension in vivo revealed low oxygen levels in lymphoid organs (26, 28, 30). While most in vitro cell culture studies have been conducted in well-oxygenated conditions, physiological T-cell activation takes place in a less oxygenated environment (<4.5% oxygen). Pathological conditions such as tissue inflammation and tumors induce an even more hypoxic environment within the tissue (31, 32). T-cell culture in a hypoxic atmosphere is expected to provide an important insight for T-cell biology in vivo especially in pathophysiological conditions.
To mimic more physiological conditions, some studies introduced lower oxygen tension in the T-cell culture. The majority of these studies has demonstrated hypoxia-related down-regulation of T-cell activities including proliferation, cytokine production and cytotoxicity (24–28), although a smaller number of papers sporadically provided opposite results (33–35). In this study, we decided to reconcile these outcomes and we standardized the assays by stimulating resting T cells with anti-CD3 mAb. The experiments confirmed the suppressive effects of hypoxia on T-cell proliferation and effector functions when cultured at 1% oxygen (Figs 1, 2 and 5). Inhibition of T-cell activation in mice exposed to a hypoxic atmosphere further supported the T-cell inhibitory effect of hypoxia (Figs 3 and 4).
Hypoxia provokes accumulation of extracellular adenosine, and its binding to A2AR has been shown to mediate various effects of hypoxia both in vivo and in vitro (7, 13–20). Since T-cell activation is quite sensitive to A2AR stimulation (4, 5), this scenario was likely a mechanism of T-cell inhibition by hypoxia. However, our experiments showed that hypoxia inhibited T-cell activation regardless of A2AR expression. The presence of the A2AR-independent pathway is evident from not only in vitro experiments (Figs 1, 2 and 6) but also T-cell stimulation in vivo (Figs 3 and 4). These results strongly suggest the significance of the A2AR-independent mechanism in the inhibition of T-cell activation by hypoxia.
Although whole body exposure of mice to hypoxia increases blood adenosine levels (7, 13), ex vivo hypoxic cell culture in this study did not significantly increase adenosine levels in the culture supernatant. Taking account of the increased rate of cellular adenosine release under hypoxia (11, 12), this result may implicate immediate degradation of adenosine by adenosine deaminase activity in FCS (36). Even though adenosine levels did not increase in the hypoxic cell culture, effects of local and transient increase of adenosine might not be excluded. To test this possibility, we used various antagonists of adenosine receptors and an inhibitor of adenosine formation, but none of them could reverse T-cell inhibition under hypoxia (Supplementary Figure 2, available at International Immunology Online). Nonetheless, equal susceptibility of WT and A2AR−/− T cells to hypoxia strongly suggested the presence of an adenosine-independent mechanism of T-cell suppression. Corresponding to the increase of extracellular adenosine levels in mice exposed to hypoxia (7, 13), the inhibition of CD25 induction under hypoxia was relatively weaker in A2AR−/− mice (Fig. 3), suggesting partial contribution of A2AR-dependent T-cell inhibition in vivo.
Both the A2AR-dependent inhibition mechanism and the A2AR-independent mechanism under hypoxia involve distinct profiles of T-cell inhibition. Consistent with our previous paper (5), A2AR stimulation caused only a minor decrease in T-cell proliferation. This A2AR-dependent change is not enough to explain the much stronger antiproliferative effect of hypoxia (Fig. 2). In contrast, the inhibitory effect of A2AR stimulation on IFN-γ production was very strong and it usually exceeded that of hypoxia (Fig. 1). Furthermore, there was also a difference in the persistence of the immunosuppressive effect. When T-cell activation was induced in the presence of an A2AR agonist, the resultant T cells ‘memorized’ the immunosuppressive effect of A2AR stimulation and were functionally impaired even after the removal of the A2AR agonist (Fig. 6) (4, 5). The persistence of A2AR-dependent T-cell inhibition is consistent with the previously published concept of A2AR-mediated memory of cells’ exposure to extracellular adenosine (37). However, T cells did not remember the immunosuppression by hypoxia and recovered normal IFN-γ-producing activity as they returned to a normoxic environment. Thus, T-cell activation under hypoxia did not impair the IFN-γ-producing activity of activated cells but significantly delayed their proliferation. The temporary inhibition only during hypoxic exposure may represent a control of energy-consuming cellular events, e.g. cell proliferation. In contrast, a high concentration of extracellular adenosine may provoke active immunosuppression targeting T-cell effector functions and producing activated T cells with functional impairment.
Tumors establish immunosuppressive environments and evade anti-tumor immune responses (2, 38, 39). A countermeasure to intratumoral immunosuppression is prerequisite for successful tumor immunotherapy. A hypoxic environment, which is frequently observed in tumors, may be fundamental to a tumor’s self-protection in vivo (40, 41). Consistent with severe hypoxia within the tumor, tumors also contain high levels of extracellular adenosine (8, 42). Previously, we found that inactivation of A2AR improved T-cell-mediated tumor eradication, suggesting the significance of the adenosine–A2AR pathway in the immunosuppressive tumor microenvironment (8). However, the current study suggests the existence of the alternative immunosuppressive mechanism downstream from hypoxia. Thus, hypoxia is likely to evoke immunosuppression by multiple mechanisms, A2AR-dependent and -independent pathways. Interruption of intratumoral adenosine–A2AR interaction by A2AR antagonists could significantly, but partially, relieve T cells from hostility in tumors (8); however, the second mechanism of hypoxic immunosuppression may still negatively affect anti-tumor responses. Identification of key molecule(s) in the A2AR-independent pathway may further improve the efficacy of immunotherapy.
Consideration of the mechanism of A2AR-independent hypoxic T-cell suppression should include the possible effects of oxygen deprivation that severely limit cellular oxidative energy production. To focus on survival of this critically stressful condition, cells may shut down various functions that are less important for survival and facilitate adaptation to the new environment until sufficient oxygenation is reinstated (43, 44). Naive T cells are known to contain a large number of mitochondria and rely on oxidative metabolism for energy production (45). Therefore, when exposed to hypoxia, T cells may suspend activation and retard cell proliferation in order to save ATP. Indeed, hypoxia induces a switch of the main ATP production pathway from oxidative phosphorylation to glycolysis. In parallel, cells produce factors inducing angiogenesis and erythropoiesis to facilitate reoxygenation. Molecules such as glycolytic enzymes, VEGF and erythropoietin are regulated by hypoxia-inducible factor-1α (HIF-1α) (43, 44). Immediately after sensing hypoxia, stabilized HIF-1α activates transcription of various genes to promote adaptation to hypoxia. This increase of HIF-1α, however, may be inhibitory to T-cell activation as suggested by previous reports showing higher IFN-γ production and stronger cytotoxicity in T cells lacking HIF-1α (46, 47). Stabilization of HIF-1α was also reported to diminish signals from T-cell receptors (48). Both ATP shortage and the metabolic switch under hypoxia could render T-cell inhibition without an adenosine–A2AR interaction. Changes in ATP supply and cellular metabolism would be a temporary reaction to the stress and become normal upon resolution of hypoxia. This is also consistent with the reversible T-cell inhibition observed in the A2AR-independent mechanism (Fig. 6).
When cells are damaged under stressful conditions, they may release their contents to the extracellular space. These components include adenine nucleotides and NAD+ (45, 49, 50). Not only do these molecules serve as a source of extracellular adenosine, NAD+ is also known to suppress T-cell proliferation, cytotoxicity and cytokine production (51, 52). NAD+ can inhibit T-cell activities through ADP ribosylation of P2X7 receptor (53, 54), in an A2AR-independent manner. This pathway may require attention as a possible scenario of T-cell inhibition under hypoxia.
Another possible cause of T-cell inhibition is oxidative stress. Hypoxia was shown to promote reactive oxygen species formation from mitochondria (55, 56) and to decrease levels of reduced glutathione (57, 58). Induction of oxidative stress leads to impairment of T-cell activation (59, 60). A recent paper showed augmentation of oxidative stress in T cells under hypoxia concomitant with a decrease in T-cell proliferation and an increase in T-cell apoptosis (61).
In conclusion, while tissue hypoxia-driven accumulation of extracellular adenosine and signaling through A2AR are known to be strongly immunosuppressive, hypoxia could suppress T-cell function even in the absence of A2AR. T-cell inhibition provided by the A2AR-independent mechanism is different from A2AR stimulation, most notably in its reversible and transient nature as compared with long-lasting T-cell impairment after A2AR stimulation. The current study implicates different immunoregulatory roles of hypoxia depending on whether hypoxia accompanies an extensive increase of extracellular adenosine or not. Analysis of the molecular mechanism of the A2AR-independent pathway may enable intervention to hypoxic immunosuppression. When T cells are liberated from both A2AR-dependent and -independent mechanisms, there will be further enhancement of T-cell activities, which is desirable for improving the immunotherapy of cancer and infectious diseases.
Supplementary data
Supplementary data are available at International Immunology Online.
Funding
National Institutes of Health (R01 CA112561, R01 CA111985 to M.S.).
Supplementary Material
Acknowledgement
The authors thank Ms Susan Ohman for her careful reading of this manuscript.
References
- 1. Lawrence T., Willoughby D. A., Gilroy D. W. 2002. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2:787. [DOI] [PubMed] [Google Scholar]
- 2. Mellor A. L., Munn D. H. 2008. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat. Rev. Immunol. 8:74. [DOI] [PubMed] [Google Scholar]
- 3. Sitkovsky M. V., Lukashev D., Apasov S., et al. 2004. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 22:657. [DOI] [PubMed] [Google Scholar]
- 4. Zarek P. E., Huang C. T., Lutz E. R., et al. 2008. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 111:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohta A., Ohta A., Madasu M., et al. 2009. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. J. Immunol. 183:5487. [DOI] [PubMed] [Google Scholar]
- 6. Ohta A., Sitkovsky M. 2001. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414:916. [DOI] [PubMed] [Google Scholar]
- 7. Thiel M., Chouker A., Ohta A., et al. 2005. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 3:e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohta A., Gorelik E., Prasad S. J., et al. 2006. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl Acad. Sci. USA 103:13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Synnestvedt K., Furuta G. T., Comerford K. M., et al. 2002. Ecto-5’-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 110:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deaglio S., Robson S. C. 2011. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv. Pharmacol. 61:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Decking U. K., Schlieper G., Kroll K., Schrader J. 1997. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ. Res. 81:154. [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi S., Zimmermann H., Millhorn D. E. 2000. Chronic hypoxia enhances adenosine release in rat PC12 cells by altering adenosine metabolism and membrane transport. J. Neurochem. 74:621. [DOI] [PubMed] [Google Scholar]
- 13. Choukèr A., Thiel M., Lukashev D., et al. 2008. Critical role of hypoxia and A2A adenosine receptors in liver tissue-protecting physiological anti-inflammatory pathway. Mol. Med. 14:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takagi H., King G. L., Robinson G. S., Ferrara N., Aiello L. P. 1996. Adenosine mediates hypoxic induction of vascular endothelial growth factor in retinal pericytes and endothelial cells. Invest. Ophthalmol. Vis. Sci. 37:2165. [PubMed] [Google Scholar]
- 15. Kobayashi S., Conforti L., Pun R. Y., Millhorn D. E. 1998. Adenosine modulates hypoxia-induced responses in rat PC12 cells via the A2A receptor. J. Physiol. 508(Pt 1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takagi H., King G. L., Aiello L. P. 1998. Hypoxia upregulates glucose transport activity through an adenosine-mediated increase of GLUT1 expression in retinal capillary endothelial cells. Diabetes 47:1480. [DOI] [PubMed] [Google Scholar]
- 17. Fisher J. W., Brookins J. 2001. Adenosine A(2A) and A(2B) receptor activation of erythropoietin production. Am. J. Physiol. Renal Physiol. 281:F826. [DOI] [PubMed] [Google Scholar]
- 18. O’Driscoll C. M., Gorman A. M. 2005. Hypoxia induces neurite outgrowth in PC12 cells that is mediated through adenosine A2A receptors. Neuroscience 131:321. [DOI] [PubMed] [Google Scholar]
- 19. Coney A. M., Marshall J. M. 1998. Role of adenosine and its receptors in the vasodilatation induced in the cerebral cortex of the rat by systemic hypoxia. J. Physiol. 509(Pt 2):507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koos B. J., Maeda T. 2001. Adenosine A(2A) receptors mediate cardiovascular responses to hypoxia in fetal sheep. Am. J. Physiol. Heart Circ. Physiol. 280:H83. [DOI] [PubMed] [Google Scholar]
- 21. Grenz A., Zhang H., Weingart J., et al. 2007. Lack of effect of extracellular adenosine generation and signaling on renal erythropoietin secretion during hypoxia. Am. J. Physiol. Renal Physiol. 293:F1501. [DOI] [PubMed] [Google Scholar]
- 22. Chen J. F., Huang Z., Ma J., et al. 1999. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 19:9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ohta A., Kjaergaard J., Sharma S., et al. 2009. In vitro induction of T cells that are resistant to A2 adenosine receptor-mediated immunosuppression. Br. J. Pharmacol. 156:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loeffler D. A., Juneau P. L., Masserant S. 1992. Influence of tumour physico-chemical conditions on interleukin-2-stimulated lymphocyte proliferation. Br. J. Cancer 66:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naldini A., Carraro F., Silvestri S., Bocci V. 1997. Hypoxia affects cytokine production and proliferative responses by human peripheral mononuclear cells. J. Cell. Physiol. 173:335. [DOI] [PubMed] [Google Scholar]
- 26. Caldwell C. C., Kojima H., Lukashev D., et al. 2001. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J. Immunol. 167:6140. [DOI] [PubMed] [Google Scholar]
- 27. Atkuri K. R., Herzenberg L. A., Herzenberg L. A. 2005. Culturing at atmospheric oxygen levels impacts lymphocyte function. Proc. Natl Acad. Sci. USA 102:3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohta A., Diwanji R., Kini R., Subramanian M., Ohta A., Sitkovsky M. 2011. In vivo T cell activation in lymphoid tissues is inhibited in the oxygen-poor microenvironment. Front. Immunol. 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fredholm B. B., IJzerman A. P., Jacobson K. A., Linden J., Müller C. E. 2011. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors–an update. Pharmacol. Rev. 63:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hale L. P., Braun R. D., Gwinn W. M., Greer P. K., Dewhirst M. W. 2002. Hypoxia in the thymus: role of oxygen tension in thymocyte survival. Am. J. Physiol. Heart Circ. Physiol. 282:H1467. [DOI] [PubMed] [Google Scholar]
- 31. Braun R. D., Lanzen J. L., Snyder S. A., Dewhirst M. W. 2001. Comparison of tumor and normal tissue oxygen tension measurements using OxyLite or microelectrodes in rodents. Am. J. Physiol. Heart Circ. Physiol. 280:H2533. [DOI] [PubMed] [Google Scholar]
- 32. Karhausen J., Haase V. H., Colgan S. P. 2005. Inflammatory hypoxia: role of hypoxia-inducible factor. Cell Cycle 4:256. [PubMed] [Google Scholar]
- 33. Krieger J. A., Landsiedel J. C., Lawrence D. A. 1996. Differential in vitro effects of physiological and atmospheric oxygen tension on normal human peripheral blood mononuclear cell proliferation, cytokine and immunoglobulin production. Int. J. Immunopharmacol. 18:545. [DOI] [PubMed] [Google Scholar]
- 34. Carswell K. S., Weiss J. W., Papoutsakis E. T. 2000. Low oxygen tension enhances the stimulation and proliferation of human T lymphocytes in the presence of IL-2. Cytotherapy 2:25. [DOI] [PubMed] [Google Scholar]
- 35. Roman J., Rangasamy T., Guo J., et al. 2010. T-cell activation under hypoxic conditions enhances IFN-gamma secretion. Am. J. Respir. Cell Mol. Biol. 42:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bynum J. W. 1980. Characterization of adenosine-induced cytostasis in melanoma cells. Cancer Res. 40:2147. [PubMed] [Google Scholar]
- 37. Koshiba M., Kojima H., Huang S., Apasov S., Sitkovsky M. V. 1997. Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J. Biol. Chem. 272:25881. [DOI] [PubMed] [Google Scholar]
- 38. Gajewski T. F., Meng Y., Blank C., et al. 2006. Immune resistance orchestrated by the tumor microenvironment. Immunol. Rev. 213:131. [DOI] [PubMed] [Google Scholar]
- 39. Rabinovich G. A., Gabrilovich D., Sotomayor E. M. 2007. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 25:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harris A. L. 2002. Hypoxia–a key regulatory factor in tumour growth. Nat. Rev. Cancer 2:38. [DOI] [PubMed] [Google Scholar]
- 41. Brown J. M., Wilson W. R. 2004. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 4:437. [DOI] [PubMed] [Google Scholar]
- 42. Blay J., White T. D., Hoskin D. W. 1997. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 57:2602. [PubMed] [Google Scholar]
- 43. Semenza G. L. 2007. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem. J. 405:1. [DOI] [PubMed] [Google Scholar]
- 44. Majmundar A. J., Wong W. J., Simon M. C. 2010. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kominsky D. J., Campbell E. L., Colgan S. P. 2010. Metabolic shifts in immunity and inflammation. J. Immunol. 184:4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lukashev D., Klebanov B., Kojima H., et al. 2006. Cutting edge: hypoxia-inducible factor 1alpha and its activation-inducible short isoform I.1 negatively regulate functions of CD4+ and CD8+ T lymphocytes. J. Immunol. 177:4962. [DOI] [PubMed] [Google Scholar]
- 47. Guo J., Lu W., Shimoda L. A., Semenza G. L., Georas S. N. 2009. Enhanced interferon-gamma gene expression in T cells and reduced ovalbumin-dependent lung eosinophilia in hypoxia-inducible factor-1-alpha-deficient mice. Int. Arch. Allergy Immunol. 149:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Neumann A. K., Yang J., Biju M. P., et al. 2005. Hypoxia inducible factor 1 alpha regulates T cell receptor signal transduction. Proc. Natl Acad. Sci. USA 102:17071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Belenky P., Bogan K. L., Brenner C. 2007. NAD+ metabolism in health and disease. Trends Biochem. Sci. 32:12. [DOI] [PubMed] [Google Scholar]
- 50. Adriouch S., Hubert S., Pechberty S., Koch-Nolte F., Haag F., Seman M. 2007. NAD+ released during inflammation participates in T cell homeostasis by inducing ART2-mediated death of naive T cells in vivo. J. Immunol. 179:186. [DOI] [PubMed] [Google Scholar]
- 51. Wang J., Nemoto E., Kots A. Y., Kaslow H. R., Dennert G. 1994. Regulation of cytotoxic T cells by ecto-nicotinamide adenine dinucleotide (NAD) correlates with cell surface GPI-anchored/arginine ADP-ribosyltransferase. J. Immunol. 153:4048. [PubMed] [Google Scholar]
- 52. Okamoto S., Azhipa O., Yu Y., Russo E., Dennert G. 1998. Expression of ADP-ribosyltransferase on normal T lymphocytes and effects of nicotinamide adenine dinucleotide on their function. J. Immunol. 160:4190. [PubMed] [Google Scholar]
- 53. Ohlrogge W., Haag F., Löhler J., et al. 2002. Generation and characterization of ecto-ADP-ribosyltransferase ART2.1/ART2.2-deficient mice. Mol. Cell. Biol. 22:7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Seman M., Adriouch S., Scheuplein F., et al. 2003. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity 19:571. [DOI] [PubMed] [Google Scholar]
- 55. Chandel N. S., Maltepe E., Goldwasser E., Mathieu C. E., Simon M. C., Schumacker P. T. 1998. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl Acad. Sci. USA 95:11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chandel N. S., McClintock D. S., Feliciano C. E., et al. 2000. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275:25130. [DOI] [PubMed] [Google Scholar]
- 57. Chang S. W., Stelzner T. J., Weil J. V., Voelkel N. F. 1989. Hypoxia increases plasma glutathione disulfide in rats. Lung 167:269–276 [DOI] [PubMed] [Google Scholar]
- 58. Mansfield K. D., Simon M. C., Keith B. 2004. Hypoxic reduction in cellular glutathione levels requires mitochondrial reactive oxygen species. J. Appl. Physiol. 97:1358. [DOI] [PubMed] [Google Scholar]
- 59. Cemerski S., Cantagrel A., Van Meerwijk J. P., Romagnoli P. 2002. Reactive oxygen species differentially affect T cell receptor-signaling pathways. J. Biol. Chem. 277:19585. [DOI] [PubMed] [Google Scholar]
- 60. Klemke M., Wabnitz G. H., Funke F., Funk B., Kirchgessner H., Samstag Y. 2008. Oxidation of cofilin mediates T cell hyporesponsiveness under oxidative stress conditions. Immunity 29:404. [DOI] [PubMed] [Google Scholar]
- 61. Larbi A., Cabreiro F., Zelba H., et al. 2010. Reduced oxygen tension results in reduced human T cell proliferation and increased intracellular oxidative damage and susceptibility to apoptosis upon activation. Free Radic. Biol. Med. 48:26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.