Abstract
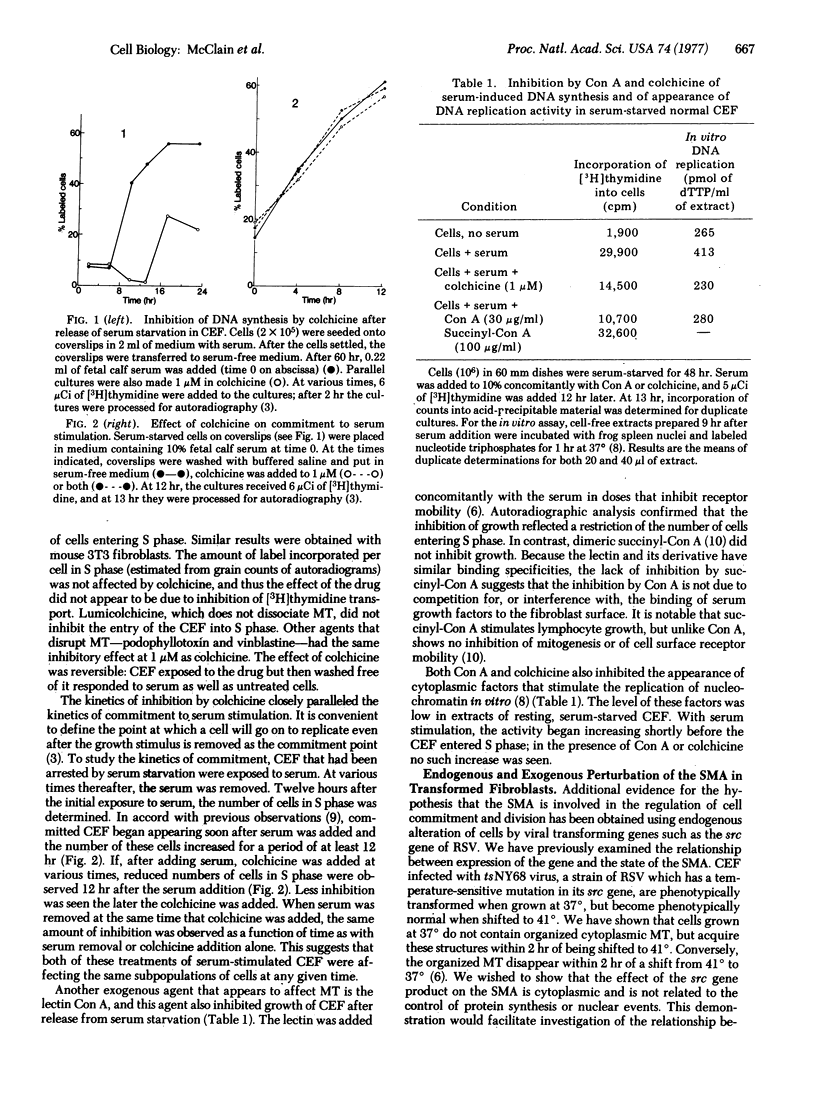
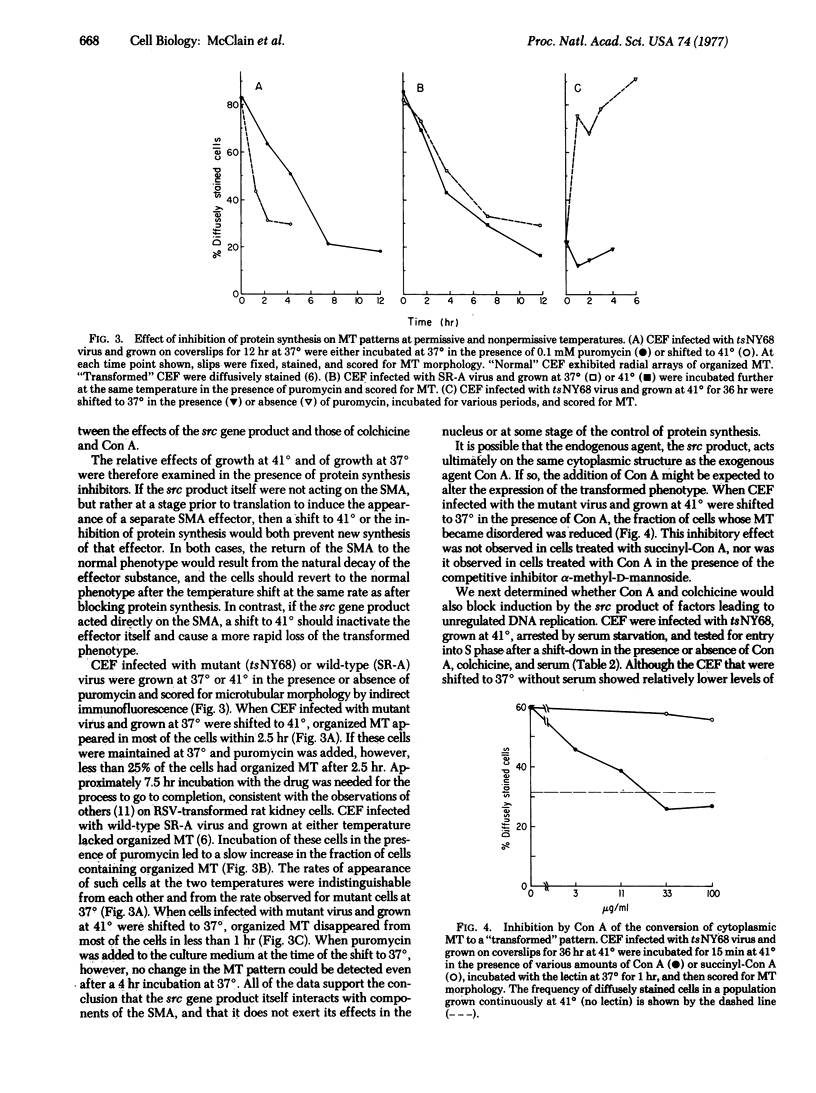
Cellular microtubules, microfilaments, and surface receptors have been postulated to form a surface modulating assembly that regulates surface receptor mobility and cell growth. To test this hypothesis, we examined three agents known to affect cell growth [colchicine, concanavalin A (Con A), and the src gene product of Rous sarcoma virus] for their effects on chick embryo fibroblasts. Individual cells from serum-starved normal fibroblast populations became committed to enter S phase at various times over a 12 hr period after exposure to serum. Colchicine and other microtubule-disrupting agents blocked entry into S phase at a point close to the commitment point for each cell. The lectin Con A also blocked entry into the S phase when present in doses sufficient to modulate surface receptor mobility. In contrast, succinyl-Con A, which does not induce surface modulation, had no effect. Both Con A and colchicine blocked the appearance of cytoplasmic factors capable of stimulating DNA replication in a cell-free system. To study endogenous effects on the surface modulating assembly, we infected fibroblasts with a Rous sarcoma virus (tsNY68) having a temperature-sensitive mutation in the transforming (src) gene. We have previously shown that microtubular and microfilamentous structures of the surface modulating assembly are direct or indirect targets of the src gene product with consequent reduction in the capacity of Con A to induce surface modulation. TsNY68-infected fibroblasts shifted to the non-permissive temperature acquired normal microtubular morphology more rapidly (2 hr) than cells grown at the permissive temperature in the presence of protein synthesis inhibitors (7.5 hr). This suggests that the src gene product acts directly on the surface modulating assembly rather than via the nucleus or at the level of protein synthesis. Furthermore, "transformation" of the surface modulating assembly was partly blocked by treatment of the infected cells with Con A but not succinyl-Con A. Both Con A and colchicine inhibited entry into the S phase following a shift from nonpermissive to permissive growth conditions. All of these observations are in accord with the hypothesis that the surface modulating assembly acts as a signal regulator in growth control.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash J. F., Vogt P. K., Singer S. J. Reversion from transformed to normal phenotype by inhibition of protein synthesis in rat kidney cells infected with a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3603–3607. doi: 10.1073/pnas.73.10.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. E. Colchicine inhibits mitogenesis in C1300 neuroblastoma cells that have been arrested in G0. Nature. 1976 Aug 26;262(5571):785–786. doi: 10.1038/262785a0. [DOI] [PubMed] [Google Scholar]
- Bell J. G., Wyke J. A., Macpherson I. A. Transformation by a temperature sensitive mutant of Rous sarcoma virus in the absence of serum. J Gen Virol. 1975 May;27(2):127–134. doi: 10.1099/0022-1317-27-2-127. [DOI] [PubMed] [Google Scholar]
- Chen K., Heller J., Canellakis E. S. Studies on the regulation of ornithine decarboxylase activity by the microtubules: the effect of colchicine and vinblastine. Biochem Biophys Res Commun. 1976 Jan 26;68(2):401–408. doi: 10.1016/0006-291x(76)91159-1. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Edelman G. M. Kinetics of colchicine inhibition of mitogenesis in individual lymphocytes. Exp Cell Res. 1976 Mar 1;98(1):15–22. doi: 10.1016/0014-4827(76)90457-2. [DOI] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M., Wang J. L., Edelman G. M. Initiation of replication in chromosomal DNA induced by extracts from proliferating cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2231–2235. doi: 10.1073/pnas.73.7.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Edelman G. M. Analysis of the stimulation-inhibition paradox exhibited by lymphocytes exposed to concanavalin A. J Exp Med. 1976 Dec 1;144(6):1494–1508. doi: 10.1084/jem.144.6.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Martin L. Do cells cycle? Proc Natl Acad Sci U S A. 1973 Apr;70(4):1263–1267. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Stimulation by serum of multiplication of stationary chicken cells. J Cell Physiol. 1971 Oct;78(2):161–170. doi: 10.1002/jcp.1040780202. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Electron microscopic analysis of the modulation of lymphocyte receptor mobility. Exp Cell Res. 1975 Mar 1;91(1):125–142. doi: 10.1016/0014-4827(75)90150-0. [DOI] [PubMed] [Google Scholar]


