Abstract
Background. Hepatitis C virus (HCV) entry involves scavenger receptor B1 (SRB1). In vitro, SRB1 inhibition by ITX5061 impedes HCV replication.
Methods. Multicenter study to assess safety/activity of ITX5061 in previously untreated, noncirrhotic, HCV genotype 1 infected adults. Design included sequential cohorts of 10 subjects with ITX5061 (n = 8) or placebo (n = 2) to escalate duration (3 to 14 to 28 days) or deescalate dose (150 to 75 to 25 mg) based on predefined criteria for safety and activity (≥4 of 8 subjects with HCV RNA decline ≥1 log10 IU/mL).
Results. Thirty subjects enrolled in 3 cohorts: ITX5061 150 mg/day by mouth for 3 (A150), 14 (B150), and 28 (C150) days. Six subjects had grade ≥3 adverse events (one in placebo); none were treatment related. One of the 7 C150 subjects (14.3%, 95% confidence interval [CI], .7%–55.4%) had ≥1 log10 IU/mL decline in HCV RNA (1.49 log10 IU/mL), whereas none of the 6 placebo, 8 A150 or 8 B150 subjects showed such decline.
Conclusions. Oral ITX5061 150 mg/day for up to 28 days was safe and well tolerated. In the 28-day cohort, 1 of 7 subjects showed antiviral activity; however, predefined criteria for antiviral activity were not met at the doses and durations studied.
Keywords: HCV, SRB1, entry inhibitors
Chronic hepatitis C virus (HCV) infection is a major cause of liver-related morbidity and mortality due to cirrhosis and hepatocellular carcinoma (HCC) [1]. Current HCV therapy is suboptimal due to toxicity, lack of broad efficacy, and poor compliance [2–4]. Although over 2 dozen drugs are being developed against the replicative enzymes of HCV (eg, NS3/4A protease and NS5B polymerase), the area of entry inhibitors is largely unexplored [5]. The use of the in vitro full length JFH-1 (HCV genotype 2a) virus replication system as well as the pseudotyped viruses has allowed characterization of HCV entry [6]. Although many details remain unclear, cellular receptors necessary for HCV entry include scavenger receptor class B, type1 (SR-B1), cluster of differentiation 81 (CD81), claudin-1, and occluding [7–10]. ITX5061 is a high-potency small molecule inhibitor of SR-B1, which is an integral transmembrane protein found in numerous cell types, including the liver. It is a functionally active cell surface high-density lipoprotein (HDL) receptor and is an important component of the reverse cholesterol transport pathway, mediating selective HDL delivery from plasma HDL to the liver [11–13]. Because inhibition of SR-B1 is associated with increases in plasma HDL, ITX5061 was evaluated for treatment of hypoalphalipoproteinemia [14, 15]. Although subsequent trials did not support this, ITX5061 was administered to approximately 280 humans with good safety and reproducible pharmacokinetics (unpublished data).
In vitro, ITX5061 differentially binds to SR-B1 expressing cells and specifically inhibits the binding of soluble HCV E2. Further, ITX5061 inhibits HCV cell culture derived (HCVcc) infection at subnanomolar potency, with a therapeutic window >10 000-fold [14]. In vitro, it acts additively with interferon, ribavirin and the protease inhibitor, telaprevir and, based on its unique mechanism of action, no cross-resistance is expected between ITX5061 and HCV polymerase or protease inhibitors [16].
The objective of A5277 was to assess the safety and antiviral activity of ITX5061 in patients infected with HCV genotype 1 who had not previously received HCV treatment.
METHODS
Study Design
A5277 was a randomized, double-blind, placebo-controlled study to find a safe dose of ITX5061 with antiviral activity. The trial consisted of 3 parts designed to evaluate anti-HCV activity of ITX5061 over 3 drug exposure durations: 3 days (part A), 14 days (part B), and 28 days (part C). The planned treatment durations were based on mathematical modeling of the effect of blocking HCV entry on viral decay in the blood through the loss of infected hepatocytes, which suggested that 4 weeks might be required to observe a significant decline in viremia (Dr Alan Perelson, personal communication). Based on this modeling, tempered with the concern for selecting resistant variants during monotherapy, a range of durations were explored from 3 days to 4 weeks in a sequential fashion. Within each part (A, B, and C), up to 3 doses of ITX5061 given by mouth once daily could be tested (150, 75, and 25 mg). Based on the extensive, human safety data, dosing in each part began with the highest ITX5061 dose (150 mg).The rationale for this approach was that if antiviral activity was demonstrated for the high dose at a given duration, then dose deescalation within the same duration cohort could be performed to establish a minimum effective dose.
The initial cohort, A150, assessed ITX5061 150 mg/day for 3 days in 10 subjects (8 active and 2 placebo). The study design planned for subsequent cohorts to be enrolled based on the safety and antiviral activity observed in previous cohorts. An acceptable safety profile in 8 subjects treated with active drug in each cohort was defined as ≤2 subjects with grade 3 events and none with grade 4 or grade 5 (death) events attributed to the study drug; attribution took into account site opinion, other potential causes and timing of the event. Antiviral activity in 8 subjects treated with active drug in each cohort was defined as ≥4 subjects with serum HCV RNA ≥1 log10 IU/mL decrease from baseline (day 0) at the end of treatment.
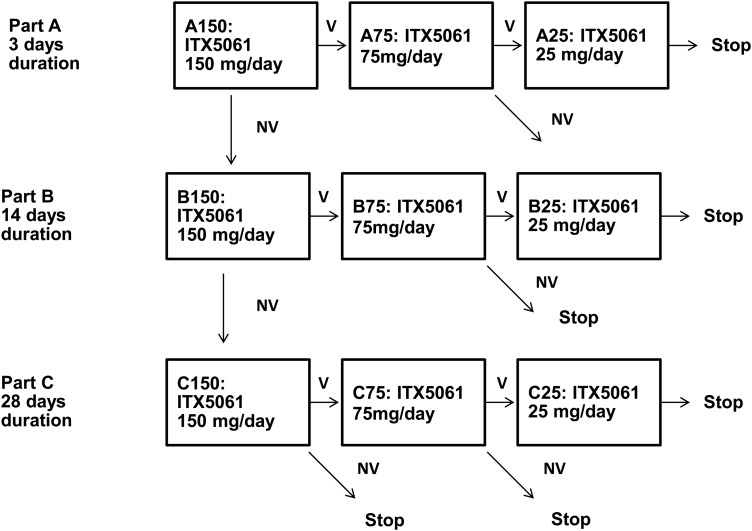
After the completion of each dose/duration cohort, the safety and antiviral activity data were reviewed by the Cohort Review Group (protocol leadership team, an independent clinician and the study statisticians) to determine the next steps. With the exception of the statisticians, CRG members were blinded to the treatment assignment. If the safety criteria were not met, additional cohorts would not be enrolled. If the safety criteria were met, the next cohort to be enrolled would depend on the observed antiviral activity. For the first cohort (150 mg for 3 days, A150), if both safety and antiviral activity criteria were met, the next cohort to enroll would be the next lower dose (75 mg) of ITX5061 administered for the same duration; however, if safety was observed but antiviral activity was not demonstrated, the next cohort to enroll would be the same dose of ITX5061 for longer treatment duration (14 days). The decision algorithm for additional cohorts was similar with the exception of cohort C150 (150 mg for 28 days duration). If antiviral activity was not observed in the C150 cohort, no additional cohorts were to be enrolled (Figure 1).
Figure 1.
Study design. Safety and virologic results were reviewed after each cohort was complete and prior to the selection of the next cohort. If predefined safety criteria were met, the next cohort to enroll was based on the observed virologic activity. Each cohort enrolled 10 new subjects: 8 active, 2 placebo. The cohort sequence enrolled in the study was A150, followed by B150, which was followed by C150 which was final cohort. Following cohort C150, enrollment was terminated according to predefined protocolcriteria for observed antiviral activity. V, predefined criteria for virologic activity was observed in the prior cohort. The next cohort enrolled would receive a lower dose of ITX5061. NV, predefined criteria for virologic activity was not observed in the prior cohort. For A150 and B150, the next cohort enrolled would receive the same ITX5061 dose for longer duration. For C150, A75, B75, and C75, the study would end if antiviral activity was not observed.
Population
The study enrolled men and women between the ages of 18 and 65 years with previously untreated, chronic HCV genotype 1 infection and no significant hepatic fibrosis. Liver fibrosis ≤METAVIR stage 2 was confirmed by biopsy (within 2 years) or HCV FibroSURE (within 1 year) [17]. Individuals with decompensated liver disease, hepatocellular carcinoma, Gilbert's and coinfection with human immunodeficiency virus type 1 (HIV-1) and/or hepatitis B virus were excluded. Subjects were also required to have laboratory parameters within the following ranges: Absolute neutrophil count (ANC) ≥1000/mm3; hemoglobin ≥12 g/dL for men and ≥11 g/dL for women; platelet count ≥120 000/mm3; alanine aminotransferase (ALT) ≤5 × upper limit of normal (ULN); International normalized ratio (INR) <1.5; total bilirubin ≤ULN; calculated creatinine clearance (CrCl) ≥80 mL/min, by the Cockcroft-Gault equation. Subjects who participated in an earlier cohort were not eligible for subsequent cohorts.
Study Drug
Both ITX5061 and placebo were formulated as a 25 mL oral solution and stored under refrigerated conditions. ITX5061 was prepared as 150 mg [6 mg/mL], 75 mg [3 mg/mL], and 25 mg [1 mg/mL]) in a vehicle consisting of 20% (w/w) hydroxypropyl-β-cyclodextrin in 10 mM aqueous citric acid.
HCV Virology
Serum HCV RNA levels were assessed prior to, during, and following treatment with ITX5061 or placebo. For the 3-day cohorts, HCV RNA was scheduled on treatment days 0, 1, 2 and on posttreatment days 3, 9, and 16. For the 14-day cohorts: HCV RNA was scheduled on treatment days 0, 1, 2, 3, 7, 10, 13, and on posttreatment days 14, 20, and 27. For the 28-day cohorts, HCV RNA was scheduled on treatment days 0, 1, 2, 3, 7, 10, 14, 21, 27, and on post-treatment days 28, 34, and 41. Serum HCV RNA quantification was performed at the ACTG Virology Specialty Laboratory using the COBAS TaqMan HCV Test, v1.0 (Roche Molecular Systems, Pleasanton, California). For each cohort, HCV RNA measurement was performed simultaneously in batch on all specimens.
Pharmacology
ITX5061 was taken with food. In part A, predose samples were collected at day 0 and day 1 and an intensive pharmacokinetic study was completed on day 2. In part B, predose samples were collected on days 0, 1, 2, 3, 7, and 10 and an intensive pharmacokinetic study was completed on day 13. In part C, predose samples were collected on days 0, 7, 14, and 21 and an intensive pharmacokinetic study was completed on day 27. The intensive pharmacokinetic study included sampling over an 8-hour period (0 [predose], 15 minutes, 30 minutes, 1, 2, 3, 4, 6, 8 hours) and a 24-hour sample collected when the subject returned on the next day. Samples were obtained by venipuncture in EDTA anticoagulant. The whole blood samples were centrifuged at 800 × g. The resulting plasma was transferred into cryovials and stored at −70°C. Shipment of samples was completed overnight on dry ice and was stored at −70°C. Plasma ITX5061 concentrations were determined with a previously reported liquid chromatography/mass spectrometry assay [18]. Mass spectrometric conditions were determined after optimization of both ITX5061 and the deuterated internal standard in a 1:1 mixture of acetonitrile in 0.1% formic acid solution. Precursor ions of ITX5061 and the internal standard were confirmed from the spectra obtained from infusion of aqueous solutions of the analytes. Acceptance criteria for the lower limit of quantification (LLOQ) required a coefficient of variation (CV%) value of ≤20%, and the remaining quality controls required a CV% value of ≤15%. Accuracy (% deviation) for the quality controls was within ±15%. The data were analyzed by noncompartmental analysis using Phoenix 64, WinNonlin 6.3. Linear trapezoidal method was used for computation of area under the concentration-time curve (AUC).
Statistical Methods
The primary endpoint on antiviral activity was binary: HCV RNA decrease ≥1 log10 IU/mL from baseline (day 0) at the end of treatment. With the study antiviral activity criteria of ≥4 subjects with HCV RNA ≥1 log10 IU/mL decrease in each cohort of 8 active subjects, the probability of concluding activity within cohort was 89% assuming 65% true proportion with such HCV RNA reduction. The confidence intervals (CI) around proportions were calculated using the Blyth-Still-Casella method. The continuous measures are summarized using median, Q1 (first quartile) and Q3 (third quartile); mean and standard deviation (SD) are also provided for the pharmacokinetic parameters. Wilcoxon signed rank tests were used to assess significant changes from baseline in continuous measures, and the relationships between treatment duration and response in continuous measures were assessed using Jonckheere-Terpstra tests. Spearman rank correlation was used to examine association between continuous measures. Statistical tests were 2-sided, and P-value < .05 was considered significant in each test without adjustment for multiple tests in this exploratory study. Analyses were conducted using SAS version 9.2.
RESULTS
Study Population
The study enrolled 30 subjects (24 active; 6 placebo) sequentially in 3 cohorts with the administration of ITX5061 150 mg daily for 3 days (A150, December 2010–March 2011), 14 days (B150, May 2011–July 2011), and 28 days (C150, October 2011–January 2012) at 10 US sites (Table 1). Of the 30 subjects, 16 were female (53%); 15 were non-Hispanic black (50%), 9 were non-Hispanic white (30%), and 6 (20%) were Hispanic regardless of race. The median age was 50 years, and the median body weight was 88.2 kilograms. At baseline, the median HCV RNA level was 6.21 log10 IU/mL (Q1 = 5.72, Q2 = 6.87). The majority (97%) of subjects completed study treatment and follow-up. One subject on active treatment enrolled in C150 stopped ITX5061 after 2 days due to personal reason (not side effects) and discontinued the protocol at day 6. This subject was excluded from antiviral activity and pharmacokinetic analyses.
Table 1.
Baseline Study Population Characteristics
| Characteristic | Cohort A150 |
Cohort B150 |
Cohort C150 |
Total | |||
|---|---|---|---|---|---|---|---|
| ITX5061 | Placebo | ITX5061 | Placebo | ITX5061 | Placebo | ||
| N | 8 | 2 | 8 | 2 | 8 | 2 | 30 |
| Age, median years (Q1, Q3) | 54 (50, 55) | 56 | 46 (37,51) | 49 | 51 (47,54) | 47 | 50 (46, 54) |
| Female sex | 5 (63%) | 1 (50%) | 5 (63%) | 1 (50%) | 4 (50%) | 0 (0%) | 16 (53%) |
| Race/ethnicity | |||||||
| White | 3 (38%) | 1 (50%) | 3 (38%) | 1 (50%) | 1 (13%) | 0 (0%) | 9 (30%) |
| Black | 4 (50%) | 1 (50%) | 2 (25%) | 1 (50%) | 6 (75%) | 1 (50%) | 15 (50%) |
| Hispanic | 1 (13%) | 0 (0%) | 3 (38%) | 0 (0%) | 1 (13%) | 1 (50%) | 6 (20%) |
| History of injection drug use | 3 (38%) | 2 (100%) | 6 (75%) | 1 (50%) | 5 (63%) | 1 (50%) | 18 (60%) |
| Weight, median kilograms (Q1, Q3) | 88.6 (84.7,98.7) | 94.0 | 94.8 (85.7,104.1) | 79.3 | 75.3 (65.0, 87.0) | 80.6 | 88.2 (80.2, 100.5) |
| Log10 HCV RNA, median IU/mL, (Q1, Q3) | 7.03 (6.39, 7.32) | 6.18 | 5.70 (5.31, 6.28) | 5.95 | 6.26 (5.99, 6.93) | 6.41 | 6.21 (5.72, 6.87) |
| Serum alanine aminotranferase/ULNa, median Q1, Q3) | 1.06 (0.62, 2.35) | 0.84 | 1.20 (0.79, 2.02) | 1.06 | 1.04 (0.70, 1.38) | 0.80 | 1.06 (0.65, 1.42) |
| Direct bilirubin/ULN, median (Q1, Q3) | 0.58 (0.08, 0.97) | 0.75 | 0.50 (0.25 0.83) | 0.50 | 0.50 (0.38, 0.71) | 0.75 | 0.50 (0.33, 0.93) |
| Indirect bilirubin, median mg/dL (Q1, Q3) | 0.30 (0.30, 0.60) | 0.45 | 0.50 (0.25, 0.75) | 0.20 | 0.35 (0.25, 0.65) | 0.25 | 0.30 (0.20, 0.60) |
| Total cholesterola, median mg/dL(Q1, Q3) | 173 (143, 228) | 163 | 177 (147, 192) | 180 | 176 (152, 205) | 147 | 174 (143. 196) |
| HDL cholesterola, median mg/dL(Q1, Q3) | 50 (46, 60) | 49 | 53 (47, 65) | 51 | 62 (54, 68) | 57 | 53 (48, 63) |
| LDL cholesterola, median mg/dL(Q1, Q3) | 88 (80, 131) | 100 | 99 (85, 116) | 100 | 102 (80, 109) | 79 | 99 (79, 115) |
Abbreviations: HCV, hepatitis C virus; HDL, high-density lipoprotein; LDL, low-density lipoprotein; N, number; ULN, upper limit of normal.
a Two subjects had nonfasting, baseline lipid profiles.
Safety
Of the 30 enrolled subjects, 6 (A150, n = 3; B150, n = 2; C150, n = 1) were reported to have grade 3 or higher adverse events at or after study initiation; all events were either not treatment related or existed prior to drug/placebo dosing (Supplementary Table 1). One subject who received placebo experienced alcohol withdrawal with an elevated AST level. Four subjects who received ITX5061 had grade 3 conditions prior to dosing: polysubstance abuse (n = 2), depression/bipolar disorder (n = 1), chronic back pain (n = 1), elevated serum ALT, and leg pain (n = 1). New signs/symptoms and laboratory events were of mild to moderate severity (grades 1 and 2, see Supplementary Table 2), and no significant changes in liver enzymes, total bilirubin, and ALT and AST levels were observed.
Virologic Activity and Serum HDL Levels
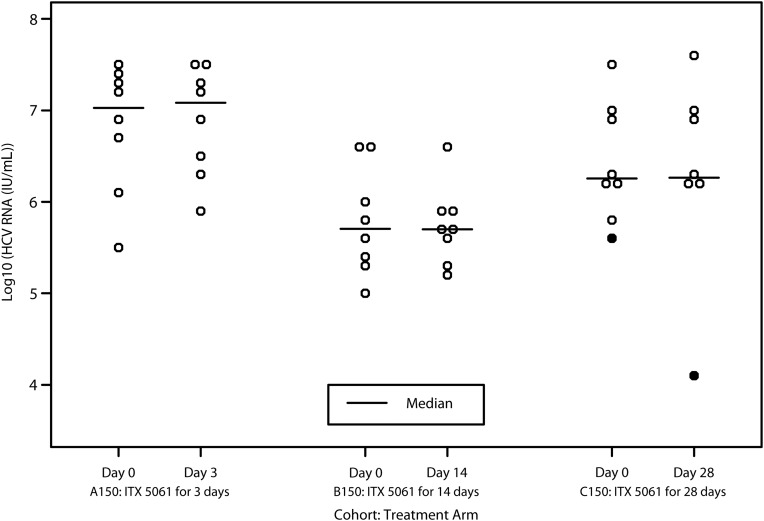
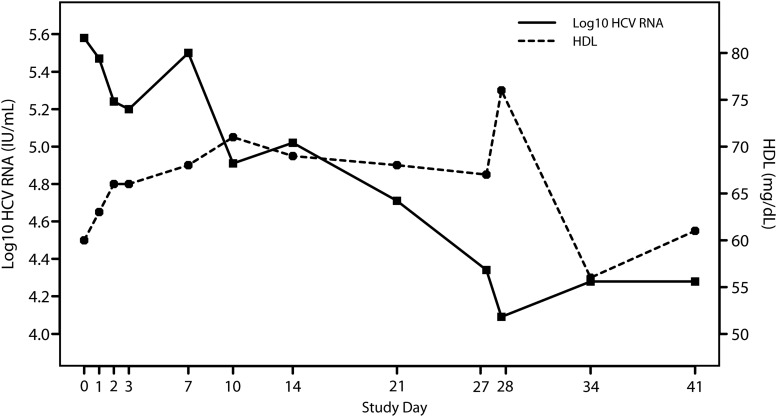
None of the 8 subjects who received 150 mg of ITX5061 for 3 days (A150) and 14 days (B150) had a ≥1 log10 IU/mL decrease in HCV RNA level at the end of dosing compared to baseline: 1-sided 97.5% CI is (0%,34.9%) within cohort. In the 28-day cohort (C150), one of 7 subjects who completed ITX5061 dosing had a decrease in HCV RNA >1 log10 IU/mL from baseline to the end of dosing (1.49 log10 IU/mL) (Figure 2). This subject was a 63-year-old non-Hispanic, black female with baseline HCV RNA level of 5.58 log10 IU/mL and a serum alanine aminotransferase level that was 2.7 times the upper limit of normal; the subject's HCV RNA and serum HDL changes over time are displayed in Figure 3. The estimated proportion of subjects with antiviral activity in C150 was 14.3% (2-sided 95% CI, .7%–55.4%). None of the 6 subjects treated with placebo demonstrated antiviral activity. Similarly, none of the actively treated ITX5061 arms demonstrated statistically significant changes in log10 HCV RNA level at the end of treatment compared to baseline (all P values > .05).
Figure 2.
Change in serum log10 HCV RNA level during the dosing interval (•represents the single subject with >1 log10 decline). Abbreviation: HCV, hepatitis C virus.
Figure 3.
Change in serum log10HCV RNA and HDL levels over time for the subject dosed with ITX5061 for 28 days (C150) in whom aprotocol-defined HCV RNA response was observed. Abbreviations: HCV, hepatitis C virus; HDL, high-density lipoprotein.
The median change in log10 HCV RNA from baseline to the end of treatment among actively treated subjects was 3-day cohort, 0.06 log10 IU/mL (Q1 = −0.06, Q3 = 0.17; P = .46); 14-day cohort, −0.04 log10 IU/mL (Q1 = −0.11,Q3 = 0.08; P = .64); and 28-day cohort, 0.03 log10 IU/mL (Q1 = −0.14, Q3 = 0.10; P = .94; Table 2). There was no suggestion of increased viral reduction with longer duration (P = .43). As a surrogate for SR-B1 blockade, serum total and HDL levels at baseline and at the end of treatment were also assessed (Supplementary Table 3). The median change in HDL level was 3-day cohort, 9 mg/dL (Q1 = 6, Q3 = 14, P = .008); 14-day cohort, 2 mg/dL (Q1 = −4, Q3 = 9, P = .74); and 28-day cohort, 10 mg/d (Q1 = −1, Q3 = 25, P = .125). Overall, the change in HDL level was not significantly correlated with the change in HCV RNA level, and no statistically significant dose-response relationship was detected in the end of treatment change from baseline in HCV RNA and any lipid level (total and HDL cholesterol).
Table 2.
HCV RNA Levels Prior to (Day 0), at the End of Treatment and the Change in HCV RNA Levels Change for Subjects Dosed With ITX5061 150 mg Daily for 3 (A150), 14 (B150), and 28 (C150) Days
| Log10 HCV RNA, IU/mL | Cohort A150 | Cohort B150 | Cohort C150 |
|---|---|---|---|
| Day 0 | |||
| N | 8 | 8 | 8 |
| Median | 7.03 | 5.70 | 6.26 |
| Q1, Q3 | 6.39, 7.32 | 5.31, 6.28 | 5.99, 6.93 |
| End of Treatment | |||
| N | 8 | 8 | 7 |
| Median | 7.08 | 5.70 | 6.26 |
| Q1, Q3 | 6.36, 7.36 | 5.49, 5.86 | 6.20, 6.98 |
| Change in log10 HCV RNA from Day 0 at the End of Treatment* | |||
| N | 8 | 8 | 7 |
| Median | 0.06 | −0.04 | 0.03 |
| Q1, Q3 | −0.06, 0.17 | −0.11, 0.08 | −0.14, 0.10 |
| P Valuea | 0.46 | 0.64 | 0.94 |
*P-value=.43 by Jonckheere-Terpstra Test for trend across cohorts. Abbreviations: HCV, hepatitis C virus; N, number.
a Wilcoxon Signed Rank test.
Pharmacology
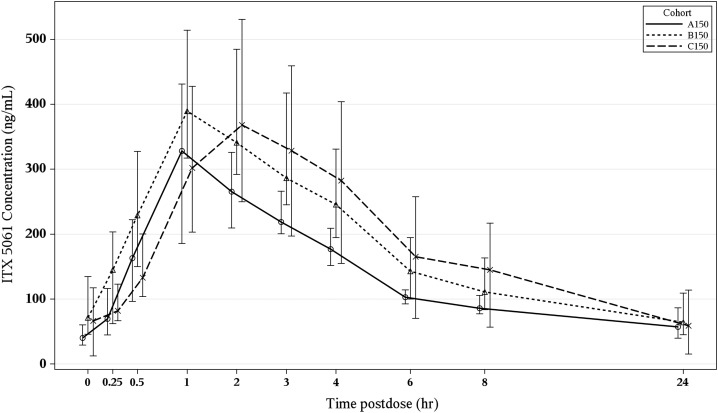
As shown in Figure 4, the plasma concentration profiles in A150, B150, and C150 yielded similar profiles with a rapid absorption phase achieving a peak concentration within the first 1–2 hours. The peak was then followed by a biexponential pattern with an initial decline over the next 8 hours and a more prolonged terminal phase between 12 and 24 hours. Trough concentrations and pharmacokinetic parameters demonstrated a pattern that reflected the long plasma half-life requiring prolonged dosing to reach a steady state. Table 3 summarizes the plasma concentrations and pharmacokinetic parameters of interest. There was statistically significant trend of shorter half-life and longer treatment duration (P = .02). No statistically significant associations were observed between pharmacokinetic parameters (AUC, Cmin, and Cmax) and changes from baseline at the end of treatment in (a) HCV RNA and (b) HDL. No trends over time were noted to suggest relationship between HCV RNA change and trough concentration, and between HDL change and trough concentration.
Figure 4.
Plasma concentration vs time curves for ITX5061 after 3 (cohort A150), 14 (cohort B150) and 28 (cohort C150) days of dosing at 150 mg by mouth daily (bars represent interquartile ranges).
Table 3.
Plasma Concentrations and Pharmacokinetic Parameters for ITX 5061 After 3 (Cohort A150), 14 (Cohort B150) and 28 (Cohort C150) Days of Dosing at 150 mg Daily*
| Cohort A150 (N = 8) | Cohort B150 (N = 8) | Cohort C150 (N = 7) | P Value† | |
|---|---|---|---|---|
| Half Life (hr) | .02 | |||
| Mean (SD) | 29.2 (15.5) | 19.1 (5.3) | 16.7 (11.4) | |
| Median (Q1,Q3) | 26.7 (18.4, 40.0) | 18.5 (17.5, 19.7) | 13.4 (8.4, 23.6) | |
| Tmax (hr) | .31 | |||
| Mean (SD) | 1.8 (1.0) | 1.5 (0.7) | 2.0 (0.5) | |
| Median (Q1,Q3) | 1 (1, 3) | 1.1 (1, 2) | 2 (2, 2) | |
| Cmin (ng/mL) | ||||
| Mean (SD) | 44.3 (19.3) | 78.3 (44.5) | 66.4 (46.4) | .22 |
| Median (Q1,Q3) | 40 (28.4, 60.3) | 61.3 (44.4, 121.7) | 66.4 (12.5, 113.3) | |
| Cmax (ng/mL) | .28 | |||
| Mean (SD) | 346.6 (116.7) | 435.4 (142.4) | 419.0 (170.7) | |
| Median (Q1,Q3) | 363.3 (225.6, 431.4) | 434.2 (348.4, 515.8) | 427.9 (249.7, 531.0) | |
| C0 (ng/mL) | .22 | |||
| Mean (SD) | 44.5 (19.1) | 84.4 (46.6) | 68.4 (49.5) | |
| Median (Q1,Q3) | 40.0 (29.1, 60.3) | 71.3 (45.6, 134.8) | 66.4 (12.5, 117.2) | |
| C24 (ng/mL) | ||||
| Mean (SD) | 63.2 (30.2) | 74.4 (34.8) | 66.6 (44.3) | .65 |
| Median (Q1,Q3) | 57.1 (39.6, 86.6) | 64.1 (45.2, 109.2) | 59 (15.4, 113.8) | |
| Clearance (L/hr) | .18 | |||
| Mean (SD) | 60.2 (16.0) | 47.3 (17.3) | 53.6 (34.2) | |
| Median (Q1,Q3) | 59.5 (45.7, 70.7) | 46.1 (34.8, 56.4) | 38.2 (28.2, 90.0) | |
| AUC (ng*hr/mL) | .18 | |||
| Mean (SD) | 2644.4 (675.7) | 3585.1 (1394.3) | 3659.1 (1668.4) | |
| Median (Q1,Q3) | 2527.5 (2133.0, 3294.5) | 3268.0 (2665.0, 4486.0) | 3929.0 (1667.0, 5320.0) |
Abbreviations: AUC, area under the concentration-time curve over 24 hours. Cmax, maximum concentration over 24 hours; Cmin, minimum concentration over 24 hours; C0, pre-dose concentration; C24, concentration at 24 hours; Tmax, time at maximum concentration.
DISCUSSION
Advances in the understanding of the biology of HCV has led to the development of very promising antiviral drugs targeted to nonstructural proteins with enzymatic function (eg, NS3/4A protease) or other nonstructural proteins involved in replication (eg, NS5A). Similar advances have been made with respect to deciphering HCV entry into hepatocytes using multiple cellular receptors including scavenger receptor class B, type1 (SRB1). Although antiviral strategies designed to inhibit entry including blocking antibodies and small molecules are under investigation, our study is one of the first clinical trials of a putative HCV entry inhibitor in adults with chronic HCV infection. As such, several of our findings are noteworthy in this study evaluating the potential effect of viral entry inhibition in chronically infected patients.
The effect, if any, that inhibition of viral entry has on levels of viremia is unknown. In contrast, the antiviral activity of drugs that effectively inhibit HCV nonstructural proteins can be readily detected by monitoring HCV RNA levels over a few days. We evaluated the SRB1 entry inhibitor for up to 28 days at a dose previously shown to raise HDL cholesterol levels in patients. Nonetheless, only one of 23 subjects dosed with ITX5061 for 3, 14, or 28 days demonstrated a >1 log10 reduction in HCV RNA from baseline, and the median HCV RNA level in all cohorts was similar at pretreatment and the end of dosing. The subject with a significant HCV response in the 28-day cohort had a relatively low HCV RNA at baseline and had an increase in HDL cholesterol that was consistent with SRB1 inhibition. However, this individual was similar to other subjects who did not demonstrate viral suppression with respect to ITX5061 exposure, and overall, there was no significant correlation between changes in HDL and HCV RNA levels across the entire study population. Taken together, ITX5061 alone is unlikely to have a significant effect on viremia in chronically infected patients over the dose (150 mg) and durations (up to 28 days) of exposure studied.
One potential explanation for the lack of observed antiviral effect could be that the magnitude of drug concentration achieved at 150 mg daily was inadequate to inhibit HCV entry. However, the plasma pharmacokinetics of ITX5061 were similar to those observed in prior studies and appeared to achieve concentrations that were in excess of the in vitro inhibitory values that had been used to design the dosing regimens. However, ITX5061 is highly bound to plasma proteins in vitro (approximately 99%); thus, it is possible that the unbound concentrations that are available for SRB1 receptor blockade may have been inadequate to prevent HCV entry. Another aspect of ITX5061 pharmacokinetics that could have contributed to our findings was the plasma concentration profile exhibited after oral dosing. Although a rapid Cmax was achieved, the decline in plasma concentrations occurred rapidly with a fairly flat concentration profile for the 12–24 hour segment of the dosing period. It is possible that these lower concentrations may have been inadequate to achieve SRB1 receptor blockade throughout the dosing interval and that more frequent dosing (twice or thrice daily) may have been more effective.
Nonetheless, although not directly measured, the expected liver concentration of ITX5061 was expected to be roughly 10-fold higher than plasma, suggesting that the 150 mg daily dose evaluated should have provided more than adequate ITX5061 exposure to assess for anti-HCV activity. As such, insufficient ITX5061 concentration at the 150 mg dose studied is unlikely to completely explain our findings. Despite achieving adequate drug concentration, inadequate duration of exposure represents another consideration to explain our findings. The rate of turnover of the pool of infected hepatocytes is not known, and it is possible that exposures longer than 28 days may be required to meaningfully impact the pool of infected cells.
Another potential explanation for our findings may be that entry inhibition by blocking SR-B1 may be ineffective in the setting of an established chronic infection. In a humanized mouse model, Ploss and colleagues demonstrated markedly reduced infectivity with a bicistronic HCV genome expressing CRE recombinase in SR-B1 deficient mice, validating the role of this receptor for HCV uptake [19]. However, it is possible that in the context of an established infection, HCV may use other hepatocyte receptors to gain entry or that SR-B1 independent cell-to-cell spread of HCV may be an important mechanism for the maintenance of chronic infection, and thus viral replication may not affected by SR-B1 blockade. Indeed, Catanese and coworkers demonstrated that while SR-B1 was directly involved in cell to cell transmission, the virus was able to lose SR-BI dependence for cell-to-cell spread [20].
Alternatively, the presence of preexisting polymorphisms in the HCV E2 glycoprotein that confer resistance to ITX5061 may have led to ineffective blockade of SR-B1. In cell culture-adapted JFH-1 mutants, an amino acid change in E2 at position 415 (N415D) or position 451 (G451R) have been shown to confer resistance to ITX5061 as the result of reduced dependency of the virus on SR-B1 for entry and increased affinity for CD81 [16, 21, 22]. However, these mutations are found in a highly conserved region of E2 downstream of HVR1 suggesting that the presence of high levels of preexisting variants with reduced SR-B1 dependency is unlikely in our study population [23]. Alternatively, it is possible that exposure to ITX5061monotherapy may have selection for low-level preexisting variants; however, the lack of initial HCV suppression followed by viral rebound argues against the selection of such variants during treatment as a likely explanation for our findings [16]. Interestingly, in a genotype 2a infectious virus system, Zhu and colleague found that ITX5061 was additive to synergistic when given in combination with interferon-α, ribavirin, and HCV protease and a nucleoside analogue polymerase inhibitors. As such, in chronically infected patients, ITX5061 in combination with direct acting antivirals may be more effective than ITX5061monotherapy.
In conclusion, in this proof of concept trial, ITX5061 was safe and well tolerated over 28 days of dosing in noncirrhotic patients with chronic HCV infection but did not meet predefined criteria for virologic activity. The intriguing finding in one subject of a >1 log10 decline in HCV viremia coupled with ongoing uncertainty regarding the role, if any, of HCV entry inhibition in chronic infection suggests that additional strategies may warrant further investigation including the combination of putative entry inhibitors with oral HCV direct acting antivirals and their potential use to prevent new infection following HCV exposure (postexposure prophylaxis) or recurrent HCV infection following liver transplantation, which is currently under investigation (NCT01560468).
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the study participants and their families. The A5277 study team also acknowledges the expert guidance of the study by Katharine Bergstrom, MS, CCRP, and active participation of site principal investigators and research coordinators: Pablo Tebas, MD, Principal Investigator; Deborah Kim, RPh, Site Pharmacist; and Kathryn Maffei, BSN, Study Nurse/Coordinator—University of Pennsylvania (Site 6201), ACTG CTU grant AI-069467-07; CFAR grant 5-P30-AI-045008-15. Andi Weiss, B Pharm, and Ilene Wiggins, RN—Johns Hopkins University AIDS Clinical Trials Unit (Site 201), ACTG CTU grant AI69465; CTSA grant NIH UL1 RR025005 awarded to Johns Hopkins University. Dr Kara Chew and Maria Palmer PA—UCLA CARE Center (Site 601), ACTG CTU grant UM1 AI069424; CTSI grant UL1TR000124 citation for Publications. Amneris Luque, MD, and Mary Adams, RN—University of Rochester (Site 1101), ACTG CTU grant UMI AI069511; CRC grant UL1 RR024160. Susanna Naggie, MD, and Cara Johnson, RN—Duke University Medical Center CRS (Site 1601), ACTG CTU grant 5UM1-AI069484-07.Kerry Upton and Dana Green, Alabama Therapeutics CRS (Site 5801), ACTG CTU grant U01 AI069452; grant UL1TR00165. Julie Hoffman, RN, and Linda Mexiner, RN—University of California, San Diego (Site 0701), ACTG CTU grant AI69432. Annie Luetkemeyer, MD, and Anna Smith, RN—UCSF AIDS CRS (Site 801), ACTG CTU grant 5UO1 AI069502. Kenneth Sherman, MD, PhD, and Michelle Saemann, RN—University of Cincinnati (Site 2401), ACTG CTU grant AI-069513. Jorge L. Santana Bagur, MD, and Santiago Marrero de León, MD—Puerto Rico-AIDS Clinical Trials Unit (Site 5401), ACTG CTU grant 5UM1AI069415-07. The technical support for HCV RNA and HCV genotyping efforts by J. Darren Hazelwood, UAB Virology Specialty Laboratory 54 (Dr Victoria Johnson) is greatly appreciated. Birmingham Veterans Affairs Medical Center core laboratory facilities, UAB VSL (NIH/NIAID 7UM1AI068636), and UAB Center for AIDS Research laboratory facilities (UAB CFAR P30AI27767-24) are acknowledged. The technical support of the ACTG Pharmacology Specialty Laboratory and the Translational Pharmacology Research Core, School of Pharmacy and Pharmaceutical Sciences at the University at Buffalo, NYS Center of Excellence in Bioinformatics and Life Sciences is greatly appreciated. ITX5061 and internal standard were obtained by the University at Buffalo through a material transfer agreement with iTherX Pharmaceuticals, Inc, San Diego, California.
Financial support. This work was supported by award number U01AI068636 from the National Institute of Allergy and Infectious Diseases (NIAID) and supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR). Additional financial support for this study came from UM1 AI068634 and K24 AI078884 (M. J. G.) from the National Institute of Allergy and Infectious Diseases with additional support from K24DA00432 (M. S. S.) from the National Institute on Drug Abuse and by grant number UL1 RR 025005 from the National Center for Research Resources (N. C. R. R.), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Potential conflicts of interest. F. W.-S. is an employee of TherX Pharmaceuticals, Inc., San Diego, CA. All other authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 2.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–44. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poordad F, McCone J, Bacon BR, et al. Boceprevir for Untreated Chronic HCV Genotype 1 Infection. N Engl J Med. 2011;364:1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 5.Calle SB, Manns MP. HCV's days are numbered: next-generation direct-acting antivirals and host-targeting agents. Antivir Ther. 2012;17:1133–46. doi: 10.3851/IMP2425. [DOI] [PubMed] [Google Scholar]
- 6.Zeisel MB, Fofana I, Fafi-Kremer S, Baumert TF. Hepatitis C virus entry into hepatocytes: molecular mechanisms and targets for antiviral therapies. J Hepatol. 2011;54:566–76. doi: 10.1016/j.jhep.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Evans MJ, von Hahn T, Tscherne DM, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–5. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 8.Ploss A, Evans MJ, Gaysinskaya VA, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–6. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris HJ, Davis C, Mullins JG, et al. Claudin association with CD81 defines hepatitis C virus entry. J Biol Chem. 2010;285:21092–102. doi: 10.1074/jbc.M110.104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartosch B, Vitelli A, Granier C, et al. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem. 2003;278:41624–30. doi: 10.1074/jbc.M305289200. [DOI] [PubMed] [Google Scholar]
- 11.Syder AJ, Lee H, Zeisel MB, et al. Small molecule scavenger receptor BI antagonists are potent HCV entry inhibitors. J Hepatol. 2011;54:48–55. doi: 10.1016/j.jhep.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–20. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 13.Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest. 2001;108:793–7. doi: 10.1172/JCI14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieland TJ, Penman M, Dori L, Krieger M, Kirchhausen T. Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI. Proc Natl Acad Sci U S A. 2002;99:15422–7. doi: 10.1073/pnas.222421399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masson D, Koseki M, Ishibashi M, et al. Increased HDL cholesterol and apoA-I in humans and mice treated with a novel SR-BI inhibitor. Arterioscler Thromb Vasc Biol. 2009;29:2054–60. doi: 10.1161/ATVBAHA.109.191320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu H, Wong-Staal F, Lee H, et al. Evaluation of ITX5061, a scavenger receptor B1 antagonist: resistance selection and activity in combination with other hepatitis C virus antivirals. J Infect Dis. 2012;205:656–62. doi: 10.1093/infdis/jir802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.mbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–75. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 18.Hochreiter J, Lapham J, Wong-Staal F, et al. ITX5061 quantitation in human plasma with reverse phase liquid chromatography and mass spectrometry detection. Antivir Ther. 2013;18:329–36. doi: 10.3851/IMP2354. [DOI] [PubMed] [Google Scholar]
- 19.Dorner M, Horwitz JA, Robbins JB, et al. A genetically humanized mouse model for hepatitis C virus infection. Nature. 2011;474:208–11. doi: 10.1038/nature10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catanese MT, Loureiro J, Jones CT, Dorner M, von HT, Rice CM. Different requirements for SR-BI in hepatitis C virus cell-free versus cell-to-cell transmission. J Virol. 2013;87:8282–93. doi: 10.1128/JVI.01102-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong J, Gastaminza P, Chung J, et al. Persistent hepatitis C virus infection in vitro: Coevolution of virus and host. J Virol. 2006;80:11082–93. doi: 10.1128/JVI.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grove J, Nielsen S, Zhong J, et al. Identification of a residue in hepatitis C virus E2 glycoprotein that determines scavenger receptor BI and CD81 receptor dependency and sensitivity to neutralizing antibodies. J Virol. 2008;82:12020–9. doi: 10.1128/JVI.01569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhillon S, Witteveldt J, Gatherer D, et al. Mutations within a conserved region of the hepatitis C virus E2 glycoprotein that influence virus-receptor interactions and sensitivity to neutralizing antibodies. J Virol. 2010;84:5494–507. doi: 10.1128/JVI.02153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.