Abstract
Background. Plasmodium falciparum reticulocyte-binding protein homologue 5 (PfRH5) is a blood-stage parasite protein essential for host erythrocyte invasion. PfRH5-specific antibodies raised in animals inhibit parasite growth in vitro, but the relevance of naturally acquired PfRH5-specific antibodies in humans is unclear.
Methods. We assessed pre–malaria season PfRH5-specific immunoglobulin G (IgG) levels in 357 Malian children and adults who were uninfected with Plasmodium. Subsequent P. falciparum infections were detected by polymerase chain reaction every 2 weeks and malaria episodes by weekly physical examination and self-referral for 7 months. The primary outcome was time between the first P. falciparum infection and the first febrile malaria episode. PfRH5-specific IgG was assayed for parasite growth-inhibitory activity.
Results. The presence of PfRH5-specific IgG at enrollment was associated with a longer time between the first blood-stage infection and the first malaria episode (PfRH5-seropositive median: 71 days, PfRH5-seronegative median: 18 days; P = .001). This association remained significant after adjustment for age and other factors associated with malaria risk/exposure (hazard ratio, .62; P = .02). Concentrated PfRH5-specific IgG purified from Malians inhibited P. falciparum growth in vitro.
Conclusions. Naturally acquired PfRH5-specific IgG inhibits parasite growth in vitro and predicts protection from malaria. These findings strongly support efforts to develop PfRH5 as an urgently needed blood-stage malaria vaccine.
Clinical Trials Registration NCT01322581.
Keywords: RH5, blood-stage immunity, endemic population, Plasmodium falciparum, prospective cohort study, malaria
Of the 5 Plasmodium species that infect humans, Plasmodium falciparum is the deadliest, causing 0.7–1.2 million deaths annually, mostly among African children [1, 2]. Efforts to control malaria are threatened by the emergence of drug-resistant parasites [3] and insecticide-resistant mosquitoes [4], and therefore the development of a malaria vaccine is widely viewed as a critical step toward reducing malaria morbidity and mortality. In recent years, the malaria research community has shifted focus from vaccines that would mitigate symptoms caused by blood-stage infection to those that would induce sterile immunity by targeting parasites during the pre-erythrocytic stages [5] or block transmission of the sexual stages [6]. This shift has been driven in part by the failure of blood-stage vaccine candidates to reliably confer protection from malaria in clinical trials [5]—a setback attributed to the polymorphic nature of many blood-stage antigens [7, 8] and redundant erythrocyte invasion pathways [9].
However, cautious enthusiasm for blood-stage vaccines has been rekindled by the discovery of the P. falciparum reticulocyte-binding protein homologue 5 (PfRH5). PfRH5 is essential for merozoite invasion of erythrocytes [10–12], and attempts to disrupt the gene encoding PfRH5 have failed to produce viable parasites [10, 11]. Moreover, antibodies raised in animals against either PfRH5 [13–15] or its erythrocyte receptor basigin [12] inhibit parasite invasion into erythrocytes in vitro. In contrast to other blood-stage vaccine candidates such as P. falciparum merozoite surface protein 1 (PfMSP1) and apical membrane protein 1 (PfAMA1), which are highly polymorphic and immunogenic [16–19], PfRH5 has limited genetic diversity among clinical P. falciparum isolates [20] and has demonstrated poor natural immunogenicity in Kenya [13]. Thus, the functional and clinical relevance of naturally acquired PfRH5-specific antibodies in humans still remains unclear.
In this prospective study of children and adults in Mali, we sought to determine whether naturally acquired antibodies specific for PfRH5 are associated with protection from malaria and inhibit P. falciparum growth in vitro.
MATERIALS AND METHODS
Study Design and Participants
This study was conducted in Kalifabougou, Mali, where intense P. falciparum transmission occurs from June through December [21]. We enrolled 695 healthy children and adults, aged 3 months to 25 years, in May 2011 and followed them through the ensuing malaria season until January 2012. The disproportionate sample size of age groups reflects the design of this ongoing study of malaria immunity that focuses on older children as they transition from malaria susceptibility to immunity. Exclusion criteria at enrollment included a hemoglobin level <7 g/dL, axillary temperature ≥37.5°C, acute systemic illness, underlying chronic disease, use of antimalarial or immunosuppressive medications in the past 30 days, or pregnancy.
Clinical malaria was defined as any level of parasitemia, an axillary temperature of ≥37.5°C within 24 hours, and no other cause of fever discernible by physical exam. The primary endpoint was the time between the first polymerase chain reaction (PCR)–detected P. falciparum blood-stage infection and the first or only febrile malaria episode. A secondary endpoint was recurrent malaria episodes. We also explored secondary definitions of malaria using parasite density thresholds of ≥500, ≥2500, and ≥5000 parasites/µL.
Malaria episodes were detected prospectively by self-referral to the study clinic and weekly active clinical surveillance visits. All individuals with signs and symptoms of malaria and any level of parasitemia detected by microscopy were treated according to the Malian National Malaria Control Program guidelines.
Sample Collection
Blood Smears
Thick blood smears were stained with Giemsa and counted against 300 leukocytes. Parasite densities were recorded as the number of asexual parasites per microliter of blood based on a mean leukocyte count of 7500 cells/µL.
Blood Samples
At enrollment in May 2011 and at the end of the malaria transmission season in January 2012, blood samples were drawn by venipuncture into sodium citrate–containing Vacutainer tubes (BD). Plasma was separated by centrifugation and cryopreserved. Hemoglobin typing was performed with a D-10 instrument (Bio-Rad). Baseline hemoglobin values, measured by a HemoCue analyzer, were used to determine anemia status based on World Health Organization criteria [22]. During scheduled clinic visits (2-week intervals for 7 months), blood was collected by fingerprick onto 903 filter paper (Whatman) for PCR analysis.
Detection and Quantification of P. falciparum Infections
Detailed methods for the detection and quantification of P. falciparum by PCR have been described [21]. In brief, for each participant, nested PCR was performed to amplify parasite DNA directly from filter paper blood spots in chronological order until the first P. falciparum infection was detected. Individuals who were PCR positive at enrollment were excluded from further analysis. Quantitative real-time PCR was then performed on nested PCR–positive samples (ie, the first PCR-positive sample for each individual). Calculated parasite densities were estimated from cycle thresholds as previously described [21].
Recombinant Proteins and Immunoglobulin G Antibody Responses to PfRH5 and PfAMA1
Methods for recombinant protein expression and determination of immunoglobulin G (IgG) responses by enzyme-linked immunosorbent assay (ELISA) were adapted from published protocols [12, 13, 23] and are described in the Supplementary Data. Antibody levels were expressed in arbitrary units (AU), where an AU of 1 is defined as the mean optical density (OD) value plus 3 standard deviations for 20 malaria-naive US donors. Individuals with antigen-specific AU ≤1 or AU >1 were defined as nonresponders or responders to that antigen, respectively. PfAMA1-specific IgG responses were categorized as AU ≤1, AU 1.01–100, or AU >100 for statistical analyses.
IgG Preparation and P. falciparum Growth Inhibition Assays
Methods for preparing total IgG and antigen-specific IgG from plasma and for performing the growth inhibition assays (GIAs) have been described previously [24, 25] as outlined in the Supplementary Data. GIAs were performed on P. falciparum (3D7 and FVO) with PfRH5-specific IgG, the flow-through fraction, or total IgG from US donors.
Microsatellite Analysis
To determine the multiplicity of infection, which represents the number of simultaneous P. falciparum infections in an individual, we performed microsatellite analysis using previously described methods [26] as outlined in the Supplementary Data.
Statistical Analysis
The use of specific statistical tests and methods are indicated in the Results. A detailed description of data analysis can be found in the Supplementary Data. Statistical significance was defined as a 2-tailed P value of <.05. All analyses were performed in R version 2.15.1 (http://www.R-project.org) or Prism 5.0d (GraphPad Software).
Ethical Approval
The Ethics Committee of the Faculty of Medicine, Pharmacy and Dentistry at the University of Sciences, Technique and Technology of Bamako, and the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health approved this study. Written informed consent was obtained from adult participants and from the parents or guardians of participating children. The study is registered on http://www.clinicaltrials.gov (NCT01322581).
RESULTS
Study Population and Clinical Malaria Episodes
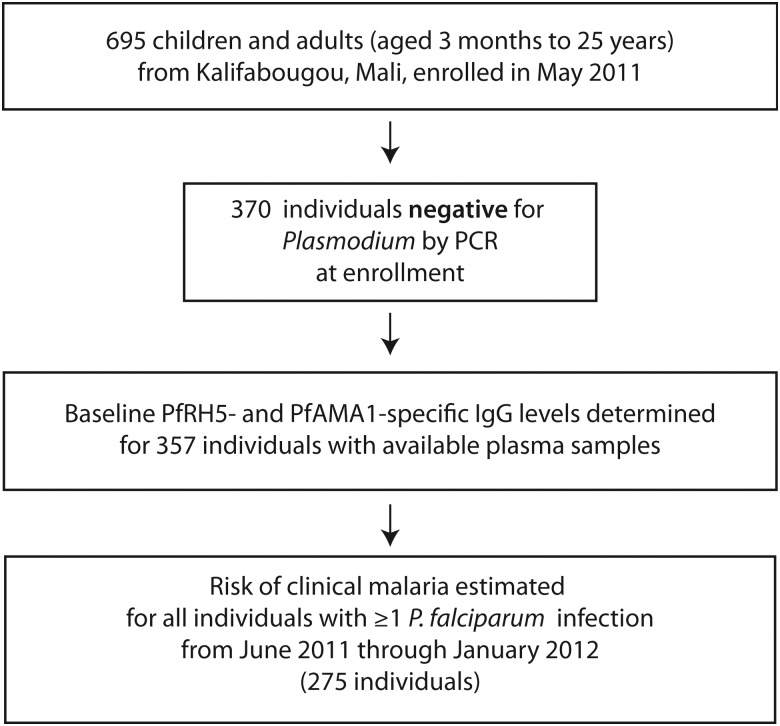
Of 695 individuals enrolled, 645 (93%) had complete follow-up, and 370 individuals (53%) were PCR-negative for Plasmodium blood-stage infection at enrollment just before the malaria season. Of these, 357 had samples available for serological analysis (Figure 1). Baseline characteristics of these individuals are shown in Table 1. During the 7-month study, 294 individuals (82%) became PCR-positive for P. falciparum infection. Of these, 19 children who were <6 months old were excluded from risk analysis to remove confounding by maternal antibodies. Among the remaining 275 individuals included in the analyses (Figure 1), 213 individuals experienced ≥1 P. falciparum malaria episode using the primary case definition, with 359 total episodes.
Figure 1.
Study participants and sampling flowchart. Abbreviations: IgG, immunoglobulin G; PCR, polymerase chain reaction; PfAMA1, Plasmodium falciparum apical membrane protein 1; PfRH5, Plasmodium falciparum reticulocyte-binding protein homologue 5.
Table 1.
Baseline Demographic and Clinical Characteristics of the 357 Participants Who Were Polymerase Chain Reaction–Negative for Plasmodium falciparum at Enrollment, by PfRH5 Immunoglobulin G Status
| Characteristic | PfRH5 IgG Statusa |
All (N = 357) | P Valueb | |
|---|---|---|---|---|
| PfRH5 IgG AU ≤1 (n = 300) | PfRH5 IgG AU >1 (n = 57) | |||
| No. (%) | No. (%) | No. (%) | ||
| Age group | ||||
| 3–5 mo | 24 (8.0) | 0 (0) | 24 (6.7) | <.001 |
| 6 mo–1 y | 45 (15) | 3 (5.3) | 48 (13) | |
| 2–3 y | 36 (12) | 4 (7.0) | 40 (11) | |
| 4–6 y | 58 (19) | 3 (5.3) | 61 (17) | |
| 7–9 y | 92 (31) | 27 (47) | 119 (33) | |
| 10–14 y | 33 (11) | 10 (18) | 43 (12) | |
| 15–25 y | 12 (4.0) | 10 (18) | 22 (6.2) | |
| Male sex | 153 (51) | 27 (47) | 180 (50) | .67 |
| Sickle cell trait | 29 (9.7) | 7 (12) | 36 (10) | .63 |
| Mild anemia at baseline | 101 (34) | 8 (14) | 109 (31) | .003 |
| PfAMA1 IgG AU | ||||
| <1 | 58 (19) | 1 (1.8) | 59 (17) | <.001 |
| 1.01–100 | 156 (52) | 30 (53) | 186 (52) | |
| >100 | 86 (29) | 26 (46) | 112 (31) | |
Abbreviations: AU, arbitrary units; IgG, immunoglobulin G; PfAMA1, Plasmodium falciparum apical membrane protein 1; PfRH5, Plasmodium falciparum reticulocyte-binding protein homologue 5.
a AU calculated by dividing the antigen-specific optical density (OD) of the test sample by the mean antigen-specific OD plus 3 standard deviations of 20 US donors.
b P values shown are for Fisher exact test between the PfRH5 IgG subgroups for each characteristic.
Baseline Characteristics of PfRH5 Nonresponders and Responders
At baseline, 57 of 357 individuals (16%) had PfRH5-specific IgG AU >1 before the malaria season and were classified as PfRH5 responders. Sex and sickle cell trait (HbAS) were similarly distributed between PfRH5 responders and nonresponders (Table 1). Fewer PfRH5 responders were anemic (14% vs 34%; P = .003). Compared to nonresponders, PfRH5 responders were significantly overrepresented in older groups (P<.001; Table 1) and among those with higher PfAMA1-specific IgG levels (P < .001; Table 1).
Acquisition of PfRH5-Specific Antibodies and Relationship to Age, P. falciparum Transmission, and Placental Transfer
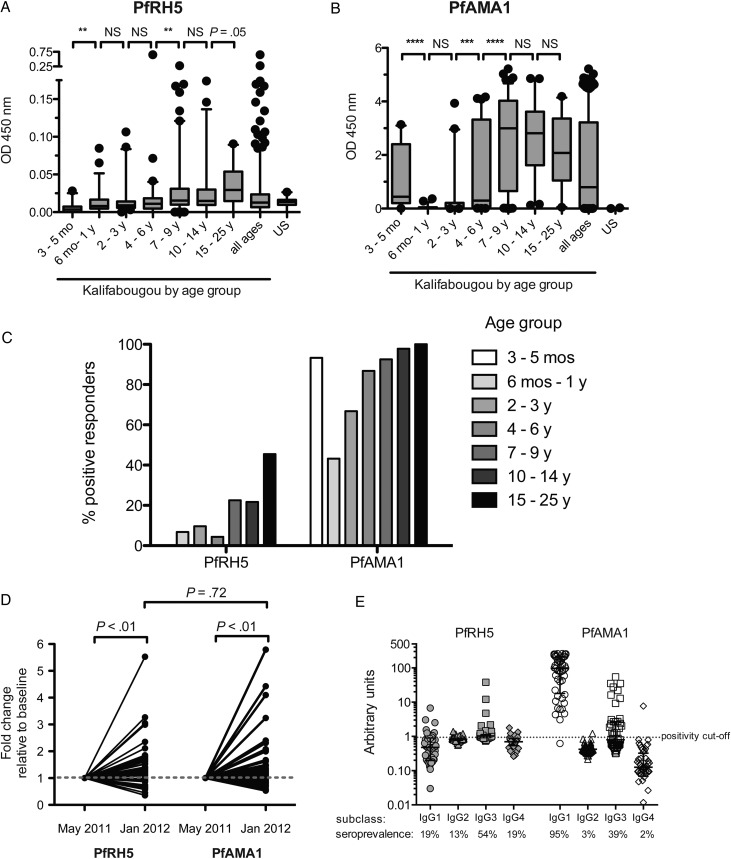
In areas of intense P. falciparum transmission such as Mali, clinical immunity to malaria and P. falciparum–specific antibodies are acquired gradually over years of repeated infections [27]. Like antibody responses to PfAMA1, PfRH5-specific IgG levels increased with age, albeit at a slower rate, with significant increases occurring by 7–9 years of age, compared with 4–6 years of age for PfAMA1 (Figure 2A and 2B). In general, PfRH5-specific IgG levels were approximately 2 orders of magnitude lower than PfAMA1-specific antibodies by semiquantitative comparison of median OD values (Figure 2A and 2B). The percentage of positive responders (seroprevalence) was lower for PfRH5 than for PfAMA1 overall (16% vs 83%, respectively) and within each age group (Figure 2C).
Figure 2.
Naturally acquired immunoglobulin G (IgG) specific for PfRH5 and PfAMA1 increases with age and is boosted by Plasmodium falciparum transmission. Semiquantitative levels of immunoglobulin G (IgG) specific for PfRH5 (A) and PfAMA1 (B) in plasma samples from 357 Malians across age groups, as indicated by background-subtracted optical density (OD 450 nm) in enzyme-linked immunosorbent assay. Boxes enclose interquartile range, central lines represent medians, whiskers indicate the 5th–95th percentile, and dots are outliers. Between-group differences were assessed by the Mann–Whitney test. C, Seroprevalence of IgG with arbitrary units (AU) >1 specific for PfRH5 or PfAMA1 across the indicated age groups, where an AU of 1 is defined as the mean OD value plus 3 standard deviations for 20 malaria-naive US donors. D, Fold-change in PfRH5-specific and PfAMA1-specific IgG levels from before to after a single malaria transmission season in 50 individuals who began the season uninfected and had ≥1 P. falciparum infection between May 2011 and January 2012. Each individual was tested for both antigens. Fold-change relative to a baseline of 1 was compared by the Wilcoxon signed-rank test. Fold-change differences between PfRH5 and PfAMA1 were compared by the Mann–Whitney test. E, PfRH5-specific IgG subclass responses in AU among PfRH5 responders (n = 48) and PfAMA1 responders (n = 64) with seroprevalence for each antigen-specific IgG subclass shown below. Central lines represent medians, and error bars indicate the interquartile range. **P < .01; ***P < .001; ****P < .0001. Abbreviations: AU, arbitrary units; IgG, immunoglobulin G; NS, not significant; OD, optical density; PfAMA1, Plasmodium falciparum apical membrane protein 1; PfRH5, Plasmodium falciparum reticulocyte-binding protein homologue 5.
To determine the extent to which vaccine-induced PfRH5-specific IgG levels might be boosted by natural infection, we compared PfRH5-specific IgG levels before and after the malaria season. PfRH5-specific IgG levels significantly increased after the malaria season, and fold-changes were similar to that of PfAMA1-specific IgG (median fold-change, 1.2 [interquartile range {IQR}, .88–1.6] and 1.1 [IQR, .91–1.5], respectively; P = .72; Figure 2D).
Whereas the seroprevalence of PfAMA1-specific IgG in infants aged 3–5 months (93%) was similar to that of adults (100%)—consistent with IgG transfer across the placenta—we observed no evidence of maternally derived PfRH5-specific IgG in the same infants (Figure 2C). Given the variable efficiency with which IgG subclasses cross the placenta (IgG1 ≈ IgG4 > IgG3 > IgG2) [28], we hypothesized that PfRH5-specific IgG would be enriched in IgG3, in contrast to PfAMA1-specific IgG1 enrichment [29, 30]. Indeed, we observed that the predominant PfRH5-specific IgG subclass is IgG3 (54% of PfRH5 responders), whereas IgG1 predominates the PfAMA-1-specific IgG response (Figure 2E).
PfRH5-Specific IgG and Protection From Febrile Malaria
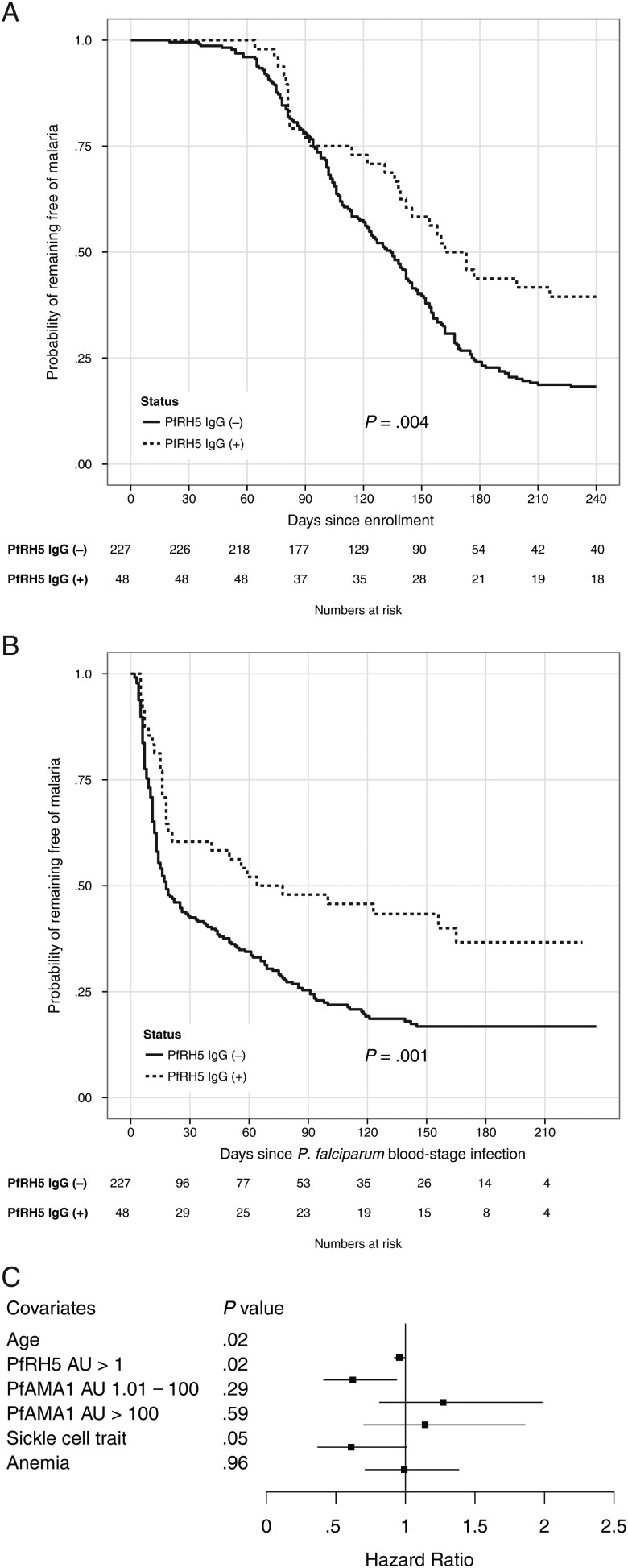
To minimize the potential confounding that results from heterogeneity in exposure to infective mosquito bites [31], we restricted the analysis of malaria risk to individuals with a documented blood-stage infection during the study period (n = 275 [77%]; Figure 1). Using the enrollment date as the start time for risk analysis, the Kaplan-Meier estimator revealed a significant delay of 34 days in median time to first malaria episode in PfRH5 responders compared to nonresponders (168 days [95% confidence interval {CI}, 142–240 days] vs 134 days [95% CI, 122–144 days], respectively; P = .004; Figure 3A). In contrast, using the time of first P. falciparum blood-stage infection as the start time, the difference in median time to first febrile malaria episode between PfRH5 responders and nonresponders increased to 53 days (71 days [95% CI, 21–229 days] vs 18 days [95% CI, 14–29 days], respectively; P = .001; Figure 3B). The presence of PfRH5-specific IgG before the malaria season was associated with a reduced prospective risk of malaria even after controlling for covariates by Cox regression (hazard ratio, .62 [95% CI, .41–.94]; P = .02; Table 2). As expected, age was also associated with a reduced risk of malaria (Table 2). Hazard ratio estimates of malaria risk using secondary definitions of malaria (ie, different parasite density thresholds) are shown in Table 2.
Figure 3.
PfRH5-specific immunoglobulin G (IgG) predicts protection from febrile malaria. Time to first febrile malaria episode using enrollment date (A) or first polymerase chain reaction (PCR)–confirmed Plasmodium falciparum blood-stage infection (B) as the start time in individuals with and without detectable PfRH5-specific IgG, as determined by Kaplan-Meier survival analysis. C, A Cox model was used to evaluate the effect of different covariates on the risk of the first febrile malaria episode using time of first PCR-detectable P. falciparum blood-stage infection as the start time for malaria risk analysis. Hazards ratios and 95% confidence intervals are represented by boxes and horizontal bars, respectively. Abbreviations: AU, arbitrary units; IgG, immunoglobulin G; PfAMA1, Plasmodium falciparum apical membrane protein 1; PfRH5, Plasmodium falciparum reticulocyte-binding protein homologue 5.
Table 2.
Effect of PfRH5 Immunoglobulin G on Risk of First or Only Malaria Episodea
| Parasite Density Threshold for Defining Malaria Episode (No. of Events) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Parasitemia (213) |
≥500 (200) |
≥2500 (196) |
≥5000 (184) |
|||||||||||||
| Variable | HR | Lower | Upper | P Value | HR | Lower | Upper | P Value | HR | Lower | Upper | P Value | HR | Lower | Upper | P Value |
| 95% CL | 95% CL | 95% CL | 95% CL | 95% CL | 95% CL | 95% CL | 95% CL | |||||||||
| Age | .96 | .92 | .99 | .02 | .96 | .93 | 1.0 | .05 | .96 | .92 | 1.0 | .04 | .96 | .92 | 1.0 | .03 |
| PfRH5 | ||||||||||||||||
| AU >1 | .62 | .41 | .94 | .02 | .59 | .38 | .90 | .02 | .59 | .38 | .91 | .02 | .65 | .41 | 1.0 | .05 |
| PfAMA1 | ||||||||||||||||
| 1.01–100 | 1.3 | .82 | 2.0 | .29 | 1.3 | .83 | 2.1 | .24 | 1.3 | .84 | 2.2 | .21 | 1.2 | .77 | 2.0 | .39 |
| >100 | 1.1 | .70 | 1.9 | .59 | 1.1 | .67 | 1.8 | .69 | 1.1 | .64 | 1.8 | .81 | .84 | .50 | 1.4 | .52 |
| Sickle cell trait | .61 | .37 | 1.0 | .05 | .57 | .34 | .96 | .03 | .51 | .30 | .88 | .02 | .42 | .24 | .75 | .004 |
| Anemia | .99 | .71 | 1.4 | .96 | .91 | .65 | 1.3 | .61 | .90 | .63 | 1.3 | .54 | .88 | .61 | 1.3 | .47 |
Abbreviations: AU, arbitrary units; CL, confidence limit; HR, hazard ratio; PfAMA1, Plasmodium falciparum apical membrane protein 1; PfRH5, Plasmodium falciparum reticulocyte-binding protein homologue 5.
a Start time for analysis was first polymerase chain reaction–confirmed Plasmodium falciparum blood-stage infection. Risk of first or only malaria episode was adjusted for age, PfAMA1 immunoglobulin G levels, sickle cell trait, and anemia status in the classic Cox model.
Using the Andersen-Gill model with a robust variance estimator [32], the presence of PfRH5-specific IgG before the malaria season was also associated with protection from malaria when all malaria episodes for each individual were taken into account. The occurrence rate ratio for all episodes of malaria in PfRH5 responders was .71 (95% CI, .52–.98; P = .03; Table 3). PfRH5-specific IgG was also significantly associated with reduced malaria incidence when a parasite density threshold of ≥500 parasites/µL was used, but not for thresholds of ≥2500 and ≥5000 parasites/µL (Table 3).
Table 3.
Effect of PfRH5 Immunoglobulin G on Risk of Recurrent Malaria Episodesa
| Parasite Density Threshold for Defining Malaria Episode (No. of Events) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Parasitemia (359) |
≥500 (334) |
≥2500 (305) |
≥5000 (279) |
|||||||||||||
| Variable | RR | Lower | Upper | P Value | RR | Lower | Upper | P Value | RR | Lower | Upper | P Value | RR | Lower | Upper | P Value |
| 95% CL | 95% CL | 95% CL | 95% CL | 95% CL | 95% CL | 95% CL | 95% CL | |||||||||
| Age | .95 | .93 | .98 | <.001 | .96 | .93 | .98 | <.001 | .96 | .93 | .98 | <.001 | .96 | .93 | .98 | .001 |
| PfRH5 | ||||||||||||||||
| AU >1 | .71 | .52 | .98 | .03 | .66 | .46 | .94 | .02 | .71 | .49 | 1.0 | .06 | .77 | .53 | 1.1 | .18 |
| PfAMA1 | ||||||||||||||||
| AU 1.01–100 | 1.3 | .94 | 1.9 | .11 | 1.4 | .99 | 2.0 | .06 | 1.3 | .93 | 1.9 | .12 | 1.2 | .86 | 1.8 | .26 |
| AU >100 | 1.3 | .83 | 2.0 | .26 | 1.3 | .83 | 2.1 | .23 | 1.3 | .80 | 2.1 | .30 | 1.1 | .65 | 1.8 | .75 |
| Sickle cell trait | .78 | .55 | 1.1 | .14 | .72 | .48 | 1.1 | .09 | .60 | .39 | .91 | .02 | .53 | .33 | .86 | .01 |
| Anemia | .89 | .72 | 1.1 | .27 | .84 | .67 | 1.1 | .13 | .89 | .70 | 1.1 | .32 | .91 | .71 | 1.2 | .48 |
Abbreviations: AU, arbitrary units; CL, confidence limit; HR, hazard ratio; PfAMA1, Plasmodium falciparum apical membrane protein 1; PfRH5, Plasmodium falciparum reticulocyte-binding protein homologue 5; RR, rate ratio.
a Start time for analysis was at study enrollment. Risk of recurrent malaria episodes was adjusted for age, PfAMA1 immunoglobulin G levels, sickle cell trait, and anemia status using the Andersen-Gill extension of the Cox model.
Effect of PfRH5-Specific IgG on Parasite Density and Multiplicity of Infection
The transfer of purified IgG from malaria-immune adults to children with febrile malaria rapidly reduces parasitemia [33]. We therefore hypothesized that individuals with PfRH5-specific IgG would have lower parasite densities at the first PCR-detectable blood-stage infection. Indeed, PfRH5 responders had lower median calculated parasite densities than age- and sex-matched nonresponders (1000 parasites/µL [IQR, 31–24 000]; 15 000 parasites/µL [IQR, 770–180 000], respectively; P = .01; Supplementary Figure 1A). However, multiple linear regression analysis identified age, HbAS, and fever at the time of detection as significant independent predictors of parasite density, but not PfRH5-specific IgG levels (Supplementary Table 1).
Because the gene encoding PfRH5 is relatively conserved across geographically disparate parasite isolates [20] and elicits broadly inhibitory antibodies [13–15], we hypothesized that PfRH5 responders would have fewer parasite clones at their first malaria episode. However, the MOI at the first malaria episode was not statistically different between PfRH5 nonresponders and responders (median MOI, 1.0 alleles [IQR, 1.0–2.5] and 1.0 alleles [IQR, 1.0–2.0], respectively; P = .87; Supplementary Figure 1B).
Inhibition of P. falciparum Growth In Vitro by Naturally Acquired PfRH5-Specific IgG
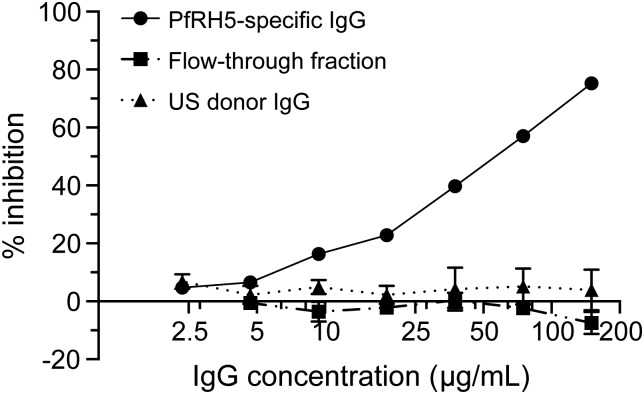
To investigate the functional activity of naturally acquired PfRH5-specific IgG, we initially performed antigen reversal GIA experiments (described in [34]) using individual plasma samples from PfRH5 responders but failed to observe reversal of GIA with recombinant PfRH5 (data not shown). We then assayed the in vitro growth-inhibitory activity of affinity-purified PfRH5-specific IgG from plasma pooled from PfRH5 responders. Pooling of plasma was necessary given the limited availability of plasma and generally low PfRH5-specific IgG reactivity. Purified PfRH5-specific IgG inhibited growth of homologous 3D7 parasites in vitro with an Ab50 (IgG concentration that demonstrates 50% inhibition in the GIA) of 55 µg/mL, whereas the flow-through fraction and IgG from non-malaria-exposed US donors demonstrated no inhibition (Figure 4). By ELISA, we determined that approximately 0.4 ng/mL of purified PfRH5-specific IgG gave an AU = 1 (Supplementary Figure 2). PfRH5-specific IgG at 150 µg/mL also demonstrated 52% growth inhibition of heterologous FVO parasites, with <3% inhibition demonstrated by flow-through and US-donor IgG at the same IgG concentration (data not shown).
Figure 4.
Affinity-purified, human PfRH5-specific IgG inhibits growth of Plasmodium falciparum parasites (3D7) in vitro. Growth inhibitory activity was assessed on P. falciparum 3D7 parasites using PfRH5-specific IgG affinity purified from uninfected, Malian donors; the IgG flow-through fraction; or IgG purified from US donors at IgG concentrations from 2.34 to 150 µg/mL. Data points represent the mean of duplicate wells from 1 experiment. Error bars represent standard deviations. Some error bars are not visible on this scale due to small standard deviations. Abbreviations: IgG, immunoglobulin G; PfRH5, Plasmodium falciparum reticulocyte-binding protein homologue 5.
DISCUSSION
The merozoite protein PfRH5 is an attractive candidate for a blood-stage malaria vaccine given its essential role in erythrocyte invasion [12], limited sequence polymorphisms [20], and ability to induce broadly inhibitory antibodies after animal immunization [13–15]. Here, we show that despite the relatively low immunogenicity of PfRH5, the presence of PfRH5-specific IgG immediately before the malaria-transmission season correlated with a delayed time to the first febrile malaria episode and reduced the overall prospective risk of febrile malaria during the ensuing malaria season. In doing so, we applied a novel strategy for assessing the prospective risk of malaria that improves the statistical power to detect associations between immune responses to blood-stage infection and clinical protection from malaria. Importantly, we also provide evidence that naturally acquired PfRH5-specific antibodies are biologically active against P. falciparum in vitro.
Consistent with a recent study in Kenya [13], we observed that IgG reactivity to PfRH5 is approximately 2 orders of magnitude lower than IgG reactivity to PfAMA1. Moreover, only 16% of individuals in this study had PfRH5-specific IgG levels above the positivity threshold. Despite low immunogenicity, PfRH5-specific IgG levels increased in an age- and exposure-dependent manner and were boosted by natural P. falciparum infections in a manner similar to antibody responses to other blood-stage antigens [35–37]. Taken together, this implies that PfRH5-specific antibody responses are short-lived and rapidly decrease during the dry season in the absence of parasite exposure. Seasonal malaria transmission may partially explain why PfRH5 seroprevalence in our study (16%) is lower than that observed in a recent study conducted in Papua New Guinea (53%), where antibody levels were assessed at the end of the high-transmission season [38]. Curiously, the absence of detectable PfRH5-specific IgG in young infants suggests that maternally derived PfRH5-specific IgG may either be short-lived and/or less efficiently transferred across the placenta relative to PfAMA1-specific IgG. In support of the latter hypothesis, we observed that PfRH5-specific IgG responses were enriched in IgG3. Relative to IgG1, which characterizes the PfAMA1-specific IgG response (Figure 2E) [29, 30], IgG3 is transferred across the placenta less efficiently [28].
Despite the low immunogenicity of PfRH5, detectable PfRH5-specific IgG was associated with an increased time between the first PCR-detectable P. falciparum blood-stage infection and the first or only febrile malaria episode, corroborating the recent findings in Papua New Guinea [38]. We further show that PfRH5 responders had reduced risk of recurrent malaria episodes during the study period. The association between PfRH5-specific IgG and reduced malaria risk was independent of factors associated with malaria risk and cumulative P. falciparum exposure. In contrast to a recent meta-analysis [39], we did not observe a significant association between PfAMA1-specific IgG and protection from malaria, whether levels were analyzed in multivariate analysis as a categorical (Tables 2 and 3) or continuous variable (data not shown). One possible explanation for this discrepancy is that the protective effect of AMA1-specific IgG in the meta-analysis was only observed in those with asymptomatic P. falciparum infection at the time of serological sampling [40], whereas in the present study individuals with asymptomatic infection at baseline were excluded, as this is a known predictor of decreased malaria risk within this population [41]. Alternatively, IgG specific for the more conserved PfRH5 protein may neutralize erythrocyte invasion by diverse parasite clones at lower IgG levels, whereas higher IgG levels may be required to broadly neutralize the more polymorphic PfAMA1 protein [19].
Importantly, individuals who did not have a documented P. falciparum blood-stage infection during the study period were excluded from the analysis of malaria risk. This strategy ensured that individuals who lacked proof of exposure to blood-stage parasites were not misclassified as being clinically immune to malaria. In this study we also applied an approach for assessing malaria risk in which the “at-risk” period began at the point of the first PCR-detectable blood-stage infection. This strategy is conceptually similar to an approach applied in Uganda, in which malaria risk was assessed after smear-confirmed parasitemia [42]. Both approaches differ from the more common method of assessing malaria risk from the time of study enrollment. Although more resource intensive, our strategy increases statistical power by decreasing the variability in the pre-infection interval (Figure 3B).
Clinical malaria episodes have been linked to the emergence of novel parasite clones capable of escaping host blood-stage immunity [43]. Because PfRH5 is relatively conserved, one might expect a restricted number of circulating “escape” clones in PfRH5 responders who experience clinical malaria. However, we failed to see any difference in MOI at the first malaria episode between responders and nonresponders, possibly due to lack of statistical power. Other possible explanations include the inability of PfRH5-specific antibodies to restrict the number of escape clones during a clinical episode, rapid waning of PfRH5-specific IgG to subprotective levels prior to the clinical episode, and/or limited genetic diversity among parasites circulating at the study site. The first explanation is not supported by previous work, which showed that a single PfRH5 allelic variant can elicit broadly neutralizing antibodies [13–15].
Adequate titers of blood-stage antibodies should theoretically reduce parasite densities to subclinical levels. Although PfRH5 responders had lower parasite densities during the first malaria episode, this effect did not remain statistically significant in multivariate analysis, possibly due to lack of statistical power. Although speculative, it is also possible that PfRH5-specific IgG exerts a stronger anti-disease than anti-parasite effect. The PfRH5 receptor basigin is expressed on many cells of the human immune system [12], and it is possible that antibodies targeting PfRH5 disrupt immunomodulatory PfRH5-basigin interactions.
This is one of the first studies to demonstrate that human PfRH5-specific antibodies induced by natural infection have parasite-inhibitory activity in vitro and is consistent with a recent study showing parasite-inhibitory activity of PfRH5-specific IgG purified from Senegalese adults [44]. Human PfRH5-specific IgG, with a GIA Ab50 of approximately 55 µg/mL here, appears to be more potent than PfAMA1- and PfMSP1-specific IgG, which have published GIA Ab50 values of approximately 100 µg/mL and approximately 620 µg/mL, respectively [45]. We also demonstrated GIA activity of PfRH5-specific IgG against the heterologous FVO parasite, which differs from 3D7 by 4 amino acid residues within PfRH5 [10]. Although the percentage of inhibition by PfRH5-specific IgG at 150 µg/mL was lower for FVO than for 3D7 parasites (52% and 75%, respectively), this may reflect degradation of the PfRH5-specific IgG preparation rather than a strain-specific effect, as the FVO experiments were performed subsequent to the 3D7 experiments using frozen-thawed IgG. Of note, the in vitro PfRH5-specific IgG Ab50 is approximately 4 orders of magnitude greater than the concentration of PfRH5-specific IgG we observed in plasma samples from study volunteers. The relationship between clinically significant levels of RH5-specific IgG in vivo and the Ab50 measured in vitro remains to be determined.
Naturally acquired IgGs specific for several P. falciparum blood-stage antigens have been variably associated with protection from malaria in prospective cohort studies [39], but none have demonstrated broad protective efficacy as vaccine candidates in phase 2b trials [7, 46, 47]. Thus, even in this rigorously conducted prospective study, the association between PfRH5-specific IgG and reduced malaria risk must be interpreted cautiously. Importantly, in this study, several individuals with detectable PfRH5-specific IgG experienced clinical malaria, and not all protected individuals had detectable PfRH5-specific IgG (Figure 3), indicating that PfRH5-specific IgG is not necessary or sufficient to protect from malaria. Thus, further studies are needed to address the quality and kinetics of naturally acquired PfRH5-specific antibodies and to determine if other protective factors coassociate with PfRH5-specific antibodies. Because PfRH5 is believed to form a complex with at least 1 other merozoite protein [48], coevaluation of antibody responses specific for PfRH5-interacting proteins, as was conducted in Papua New Guinea [38], may provide important insights.
In summary, this study demonstrates that naturally acquired PfRH5-specific IgG inhibits parasite growth in vitro and is associated with protection from malaria. These findings strongly support the development of PfRH5 as an urgently needed blood-stage vaccine candidate, possibly as a component of a multiantigen, multistage malaria vaccine.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the study participants and research support staff in Kalifabougou and Bamako, Mali. We also thank Stacy Ricklefs, Amed Ouattara, Sudhaunshu Joshi, Christopher Jacobs, and Christopher Plowe for helpful advice on microsatellite analysis.
Financial support. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health; and the Wellcome Trust (grant number 098051 to G. J. W.). The PATH Malaria Vaccine Initiative provided support for the GIA Reference Center.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Murray CJ, Rosenfeld LC, Lim SS, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–31. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. World malaria report 2012. Geneva, Switzerland: WHO; 2012. [Google Scholar]
- 3.Miotto O, Almagro-Garcia J, Manske M, et al. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet. 2013;45:648–55. doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranson H, N'Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–8. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Butler NS, Vaughan AM, Harty JT, Kappe SH. Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol. 2012;33:247–54. doi: 10.1016/j.it.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 6.maIERA Consultative Group on Vaccines. A research agenda for malaria eradication: vaccines. PLoS Med. 2011;8:e1000398. doi: 10.1371/journal.pmed.1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thera MA, Doumbo OK, Coulibaly D, et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med. 2011;365:1004–13. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takala SL, Plowe CV. Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming ‘vaccine resistant malaria. Parasite Immunol. 2009;31:560–73. doi: 10.1111/j.1365-3024.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–66. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Hayton K, Gaur D, Liu A, et al. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe. 2008;4:40–51. doi: 10.1016/j.chom.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baum J, Chen L, Healer J, et al. Reticulocyte-binding protein homologue 5—an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int J Parasitol. 2009;39:371–80. doi: 10.1016/j.ijpara.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Crosnier C, Bustamante LY, Bartholdson SJ, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–7. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas AD, Williams AR, Illingworth JJ, et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Commun. 2011;2:601. doi: 10.1038/ncomms1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams AR, Douglas AD, Miura K, et al. Enhancing blockade of Plasmodium falciparum erythrocyte invasion: assessing combinations of antibodies against PfRH5 and other merozoite antigens. PLoS Pathog. 2012;8:e1002991. doi: 10.1371/journal.ppat.1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bustamante LY, Bartholdson SJ, Crosnier C, et al. A full-length recombinant Plasmodium falciparum PfRH5 protein induces inhibitory antibodies that are effective across common PfRH5 genetic variants. Vaccine. 2013;31:373–9. doi: 10.1016/j.vaccine.2012.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller LH, Roberts T, Shahabuddin M, McCutchan TF. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1) Mol Biochem Parasitol. 1993;59:1–14. doi: 10.1016/0166-6851(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira MU, Ribeiro WL, Tonon AP, Kawamoto F, Rich SM. Sequence diversity and evolution of the malaria vaccine candidate merozoite surface protein-1 (MSP-1) of Plasmodium falciparum. Gene. 2003;304:65–75. doi: 10.1016/s0378-1119(02)01180-0. [DOI] [PubMed] [Google Scholar]
- 18.Duan J, Mu J, Thera MA, et al. Population structure of the genes encoding the polymorphic Plasmodium falciparum apical membrane antigen 1: implications for vaccine design. Proc Natl Acad Sci U S A. 2008;105:7857–62. doi: 10.1073/pnas.0802328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takala SL, Coulibaly D, Thera MA, et al. Extreme polymorphism in a vaccine antigen and risk of clinical malaria: implications for vaccine development. Sci Transl Med. 2009;1:2ra5. doi: 10.1126/scitranslmed.3000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manske M, Miotto O, Campino S, et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487:375–9. doi: 10.1038/nature11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran TM, Li S, Doumbo S, et al. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis. 2013;57:40–7. doi: 10.1093/cid/cit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12:444–54. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 23.Bushell KM, Sollner C, Schuster-Boeckler B, Bateman A, Wright GJ. Large-scale screening for novel low-affinity extracellular protein interactions. Genome Res. 2008;18:622–30. doi: 10.1101/gr.7187808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miura K, Zhou H, Moretz SE, et al. Comparison of biological activity of human anti-apical membrane antigen-1 antibodies induced by natural infection and vaccination. J Immunol. 2008;181:8776–83. doi: 10.4049/jimmunol.181.12.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malkin EM, Diemert DJ, McArthur JH, et al. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73:3677–85. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaukat AM, Gilliams EA, Kenefic LJ, et al. Clinical manifestations of new versus recrudescent malaria infections following anti-malarial drug treatment. Malar J. 2012;11:207. doi: 10.1186/1475-2875-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crompton PD, Kayala MA, Traore B, et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A. 2010;107:6958–63. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol. 1994;1:667–9. doi: 10.1128/cdli.1.6.667-669.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanisic DI, Richards JS, McCallum FJ, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77:1165–74. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tongren JE, Drakeley CJ, McDonald SL, et al. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect Immun. 2006;74:257–64. doi: 10.1128/IAI.74.1.257-264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bousema T, Kreuels B, Gosling R. Adjusting for heterogeneity of malaria transmission in longitudinal studies. J Infect Dis. 2011;204:1–3. doi: 10.1093/infdis/jir225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2000. (Statistics for biology and health) [Google Scholar]
- 33.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–7. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 34.Miura K, Zhou H, Muratova OV, et al. In immunization with Plasmodium falciparum apical membrane antigen 1, the specificity of antibodies depends on the species immunized. Infect Immun. 2007;75:5827–36. doi: 10.1128/IAI.00593-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persson KE, McCallum FJ, Reiling L, et al. Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J Clin Invest. 2008;118:342–51. doi: 10.1172/JCI32138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osier FH, Fegan G, Polley SD, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun. 2008;76:2240–8. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards JS, Stanisic DI, Fowkes FJ, et al. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis. 2010;51:e50–60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- 38.Richards JS, Arumugam TU, Reiling L, et al. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol. 2013;191:795–809. doi: 10.4049/jimmunol.1300778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fowkes FJ, Richards JS, Simpson JA, Beeson JG. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. PLoS Med. 2010;7:e1000218. doi: 10.1371/journal.pmed.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polley SD, Mwangi T, Kocken CH, et al. Human antibodies to recombinant protein constructs of Plasmodium falciparum apical membrane antigen 1 (AMA1) and their associations with protection from malaria. Vaccine. 2004;23:718–28. doi: 10.1016/j.vaccine.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 41.Crompton PD, Traore B, Kayentao K, et al. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis. 2008;198:1265–75. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenhouse B, Ho B, Hubbard A, et al. Antibodies to Plasmodium falciparum antigens predict a higher risk of malaria but protection from symptoms once parasitemic. J Infect Dis. 2011;204:19–26. doi: 10.1093/infdis/jir223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kun JF, Missinou MA, Lell B, et al. New emerging Plasmodium falciparum genotypes in children during the transition phase from asymptomatic parasitemia to malaria. Am J Trop Med Hyg. 2002;66:653–8. doi: 10.4269/ajtmh.2002.66.653. [DOI] [PubMed] [Google Scholar]
- 44.Patel SD, Ahouidi AD, Bei AK, et al. Plasmodium falciparum merozoite surface antigen, PfRH5, elicits detectable levels of invasion-inhibiting antibodies in humans. J Infect Dis. 2013;208:1679–87. doi: 10.1093/infdis/jit385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miura K, Zhou H, Diouf A, et al. Anti-apical-membrane-antigen-1 antibody is more effective than anti-42-kilodalton-merozoite-surface-protein-1 antibody in inhibiting Plasmodium falciparum growth, as determined by the in vitro growth inhibition assay. Clin Vaccine Immunol. 2009;16:963–8. doi: 10.1128/CVI.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genton B, Betuela I, Felger I, et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1–2b trial in Papua New Guinea. J Infect Dis. 2002;185:820–7. doi: 10.1086/339342. [DOI] [PubMed] [Google Scholar]
- 47.Ogutu BR, Apollo OJ, McKinney D, et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in western Kenya. PLoS One. 2009;4:e4708. doi: 10.1371/journal.pone.0004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L, Lopaticki S, Riglar DT, et al. An EGF-like protein forms a complex with PfRh5 and is required for invasion of human erythrocytes by Plasmodium falciparum. PLoS Pathog. 2011;7:e1002199. doi: 10.1371/journal.ppat.1002199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.