Abstract
Activation of the NLRP3 inflammasome and subsequent generation of interleukin 1β is initiated in macrophages upon recognition of several stimuli. In the present work, we show that gain-of-function gene variants of inflammasome components known to predispose individuals to inflammatory disorders have a host-protective role during infection with Mycobacterium tuberculosis. By isolation of macrophages from patients and healthy blood donors with genetic variants in NLRP3 and CARD8 and subsequent infection of the cells with virulent M. tuberculosis, we show that these gene variants, combined, are associated with increased control of bacterial growth in human macrophages.
Keywords: NLRP3, CARD8, inflammasome, IL-1β, human macrophage, C10X, Q705K, tuberculosis, mycobacteria
Tuberculosis, caused by Mycobacterium tuberculosis, is a global health threat. However, most people who are exposed to M. tuberculosis never develop active disease, suggesting that individual host factors influence the susceptibility to the bacterium. M. tuberculosis resides in human macrophages, where it activates several receptors, including NLRP3 [1]. NLRP3 activation leads to recruitment of the adaptor ASC, which, together with NLRP3, forms the NLRP3 inflammasome. This inflammasome in turn activates caspase-1, cleaving pro–interleukin 1β (IL-1β) to active IL-1β [2]. IL-1β secretion is further regulated by the adaptor protein CARD8 via interaction with caspase-1 and/or by inhibiting NFκB-mediated proIL-1β synthesis [3]. The mechanisms behind IL-1β release remain elusive, but production of this cytokine plays a pivotal role in the control of tuberculosis [4]. On the other hand, upregulated IL-1β production, as observed in individuals carrying gain-of-function variants of the NLRP3 gene, predisposes for inflammatory disorders [5]. We have previously described 2 such variants in the NLRP3 gene linked to inflammatory disorders: a unique M299V mutation in 2 siblings [6] and a combination of a common Q705K polymorphism in NLRP3 and a C10X polymorphism in CARD8 in a patient [7]. The C10X polymorphism introduces an early stop codon in CARD8, resulting in decreased expression levels of CARD8 [8]. These polymorphisms are common, with allele frequencies of 6.5% (Q705K) and 34% (C10X) in a Swedish cohort and approximately 4% for both polymorphisms combined [7]. Here, we studied whether gene variants in inflammasome genes, besides predisposing for inflammatory disorders, could have a protective role during intracellular infection with M. tuberculosis. We show that macrophages from individuals carrying a gain-of-function variant in the NLRP3 gene (ie, M299V or Q705K) in combination with C10X in the CARD8 gene are superior in controlling M. tuberculosis growth.
MATERIALS AND METHODS
A detailed description is presented in the Supplementary Materials.
Patients and Blood Donors
The first study subject (patient 1, a male with the Q705K and C10X polymorphisms) has a history of inflammatory disease, as previously described [7]. The second subject (patient 2, a male with the M299V and C10X polymorphisms) and the third subject (patient 3, a female with the M299V polymorphism) are siblings with previous symptoms of inflammation, as described in more detail elsewhere [6]. Human monocyte–derived macrophages (hMDMs) from sex-matched individuals carrying wild-type NLRP3 and CARD8 served as controls. Whole-blood specimens from healthy donors was collected at the blood bank at Linköping University Hospital.
Preparation of Cells
hMDMs from whole-blood specimens (from healthy blood donors, patients, or healthy controls) were prepared as described elsewhere [9].
Preparation of Bacteria and Experimental Infection
The M. tuberculosis strains (luciferase-expressing H37Rv-lux, H37Rv-GFP, and H37Ra-GFP) were grown in Middlebrook 7H9 broth (Difco) supplemented with albumin-dextrose-catalase (Becton Dickinson) and Tween-80.
Evaluation of Bacterial Growth
The number of bacteria in infected samples was evaluated by luminescence, in which the luminescence correlates to the number of colony-forming units, as described in the Supplementary Materials.
Evaluation of CD63 Colocalization or Lysotracker Staining
For CD63 staining, fixed cells were stained with anti-CD63 antibody (Sanquin) and Alexa 594–conjugated goat anti-mouse antibody (Invitrogen). For Lysotracker (Invitrogen) staining, Lysotracker Red DND-99 was added during the last 2 hours of incubation. The H37Ra-GFP used has earlier been shown to inhibit phagolysosomal fusion to the same extent as H37Rv [10].
Genotyping Assay
DNA was isolated from whole blood, using the Maxwell Blood DNA Purification Kit (Promega). The Q705K polymorphism of NLRP3 (rs35829419) and the C10X polymorphism of CARD8 (rs2043211) were genotyped using the Taqman SNP Genotyping assay (Applied Biosystems).
Statistical Analyses
The distribution of bacterial growth data in macrophages from 43 healthy donors included in the study was tested with the D'Agostino and Pearson test. On the basis of results of that test, the Student's t test (for comparison between 2 groups) and 1-way analysis of variance with the Tukey post hoc test (for comparison between multiple groups) were performed using Graph Pad Prism 5. Data are means ± standard error of the mean. Statistical significance is defined as a P value of <.05.
Study Approval
Written informed consent was obtained from all subjects, and the study was approved by the regional ethics committee in Linköping (M177-07). Peripheral blood mononuclear cells were isolated from blood specimens obtained from these subjects and from anonymous, healthy blood donors in accordance with the guidelines of the local ethics committee and the declaration of Helsinki.
RESULTS
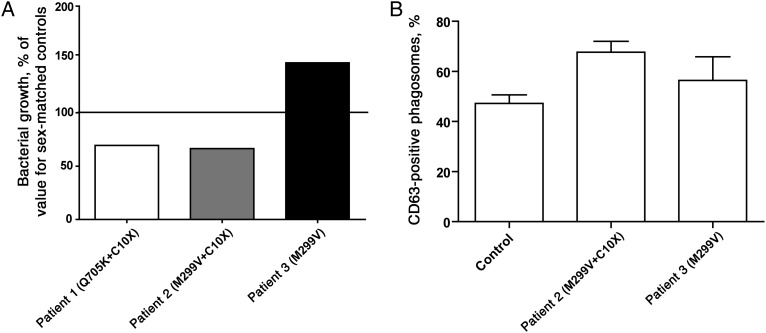
As a part of previous studies of patients with genetic variants in inflammasome-related genes linked to increased IL-1β production [6, 7], we tested the ability of hMDMs from these patients to handle M. tuberculosis infection. Cells were infected with H37Rv-lux, and bacterial growth was evaluated after 48 hours (day 2) and compared to findings for healthy, matched controls. hMDMs from patient 1 (who had the Q705K and C10X polymorphisms) and patient 2 (who had the M299V and C10X polymorphisms) displayed increased control of M. tuberculosis, compared with those from wild-type control subjects, whereas hMDMs from patient 3 (who had the M299V polymorphism only) showed decreased growth control (Figure 1A). On the basis of these observations, we hypothesized that combined genetic variants in NLRP3 and CARD8 conferred the elevation of IL-1β levels and increased the M. tuberculosis–restricting capacity of the cells. An increased IL-1β level during mycobacterial infection has also been shown to enhance phagolysosomal fusion [11]. To investigate whether phagosomal maturation was altered in hMDMs carrying genetic variants of NLRP3, patient-derived macrophages were infected with GFP-expressing H37Rv, and the percentage of CD63-positive phagosomes was analyzed. hMDMs from patient 2 (who had NLRP3 M299V and CARD8 C10X) showed an increase in the percentage of CD63-positive phagosomes as compared to healthy donors and patient 3 (who had NLRP3 M299V and wild-type CARD8; Figure 1B).
Figure 1.
Macrophages from patients with inflammatory disease display increased growth restriction of H37Rv and increased phagsomal fusion. Human monocyte–derived macrophages (hMDMs) from patients carrying different genetic variants in NLRP3 and/or CARD8 were infected with H37Rv at multiplicity of infection of 10. A, Luminescence was evaluated as a measure of phagocytosis on day 0. At 48 hours (ie, on day 2), luminescence was evaluated again, and the fold-change in growth was calculated as the day 2 value divided by the day 0 value. The figure shows the total fold-change from both the lysate and supernatant fraction, where the fold-change of H37Rv in cells from individuals with the respective gene variant are compared to that in cells from sex-matched control individuals with no polymorphisms. B, hMDMs from patient 2 and patient 3 were seeded on glass coverslips and stained for CD63 at 48 hours after infection. The percentage of CD63-positive phagosomes was evaluated by confocal microscopy in a blinded fashion (n = 2). Cells from healthy blood donors were included as controls (n = 18).
Previous studies have shown that NLRP3 Q705K in combination with CARD8 C10X results in constitutive secretion of IL-1β (20–200 pg/mL) in nonstimulated cells [5, 7]. The enhanced growth control and phagolysosomal fusion seen in patients in the present study could be because their hMDMs are being differentiated in a more proinflammatory milieu in vivo and/or are preactivated. To mimic this scenario, hMDMs were differentiated ex vivo in the presence of IL-1β for 7 days before cells were infected and CD63-positive phagosomes or acidified phagosomes were evaluated. However, neither CD63 translocation (Supplementary Figure 1A) nor acidification (Supplementary Figure 1B) was affected by differentiation in the presence of IL-1β. Furthermore, differentiation in the presence of IL-1β not only failed to stimulate increased phagosomal functionality, but also did not affect growth control (Supplementary Figure 1C). Increasing the IL-1β concentration (250 pg/mL, 2.5 ng/mL, and 25 ng/mL) during differentiation did not affect the outcome (data not shown), nor did preactivation of cells with IL-1β shortly before infection (Supplementary Figure 1D). Recently, a case report showed that the IL-1 receptor antagonist anakinra caused reactivation of latent tuberculosis [4]. To mimic latent tuberculosis, hMDMs were infected with a lower multiplicity of infection of 1 before either generation of IL-1β or signaling by IL-1β was blocked, and bacterial growth was analyzed. Although only low amounts of IL-1β are seen during low-burden infection in hMDMs, blocking of IL-1β signaling by the addition of anakinra significantly increased the growth of M. tuberculosis (Supplementary Figure 1E), whereas no effect could be seen during inhibition of caspase-1 by YVAD (Supplementary Figure 1F).
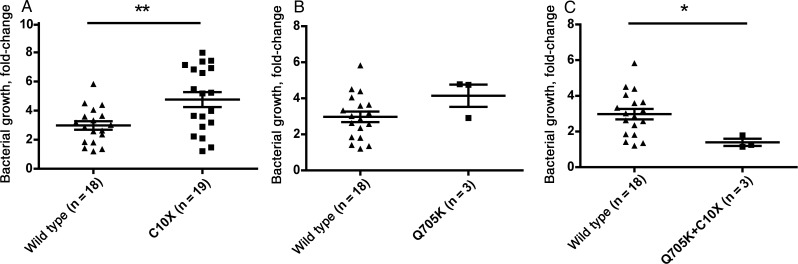
Because it was not possible to generate hMDMs resembling the cells from the patients by adding exogenous IL-1β during incubation ex vivo, we decided to take advantage of the fact that NLRP3 Q705K and CARD8 C10X are both relatively common polymorphisms. To this end, blood specimens from 43 anonymous blood donors were used to isolate hMDMs, which were infected with H37Rv-lux. The blood sample from each donor was subjected to gene analysis, and the allele frequencies are shown in Supplementary Table 1. Because the data presented in Figure 1 suggest that CARD8 C10X might be the sole determinant and that polymorphisms in NLRP3 are redundant for increased M. tuberculosis control, we first compared the M. tuberculosis–restricting ability of hMDMs from subjects who carried CARD8 C10X (i.e., C10X/Q705K individuals were excluded) to that in hMDMs from wild-type individuals. hMDMs from 19 donors harboring CARD8 C10X were less able to control growth (P = .005; Figure 2A). Further comparison of the 3 individuals carrying NLRP3 Q705K but lacking the CARD8 C10X polymorphism against the wild-type population did not reveal any significant differences (Figure 2B). However, comparison of bacterial growth between the wild-type population and the 3 individuals heterozygous for C10X plus Q705K (Figure 2C) revealed significantly reduced bacterial growth (P = .04).
Figure 2.
Macrophages harboring both CARD8 C10X and NLRP3 Q705K polymorphisms display increased growth control of M. tuberculosis. Human monocyte–derived macrophages from healthy blood donors (n = 43) were infected with H37Rv-lux at multiplicity of infection 10 for 1 hour before luminescence was measured (ie, on day 0). Forty-eight hours after infection (ie, on day 2), luminescence was measured again. Bacterial growth was calculated as the day 2 value divided by the day 0 value. The same donors were genotyped for 2 polymorphisms (Q705K and C10X), and the mean bacterial growth was compared between different groups. The figure shows the comparison between both heterozygous and homozygous individuals for CARD8 C10X (n = 19) and individuals with none of the studied polymorphisms (wild type; n = 18; A), individuals heterozygous for NLRP3 Q705K (n = 3) and wild-type individuals (B), and individuals heterozygous for CARD8 C10X plus NLRP3 Q705K (n = 3) and the wild-type population (C). The Student's t test was used for comparison in all panels. *P < .05, **P < .01.
DISCUSSION
Carriage of a combination of NLRP3 Q705K and CARD8 C10X has been linked to increased risk or severity of several inflammatory diseases [12] and to spontaneous production of IL-1β in plasma in patients and asymptomatic carriers [7]. Asymptomatic individuals carrying only one of the polymorphisms display the same low basal level of IL-1β as healthy wild-type individuals, whereas asymptomatic double carriers have slightly higher plasma levels of IL-1β than healthy wild-type individuals [13], which further underlines the synergistic relationship between these polymorphisms. Although the current study includes a limited number of individuals and caution should be taken when drawing conclusions, the data suggest that this synergy extends not only to increasing the risk of inflammatory diseases, but also confers protection against intracellular infections. Increased generation of IL-1β constitutes an attractive explanation of the decreased bacterial growth, but adding exogenous IL-1β did not affect bacterial growth. We addressed this by inhibiting the generation of IL-1β by YVAD. However, the lack of effect could be explained by incomplete inhibition of caspase-1 by YVAD [9]. In contrast, blocking of the IL-1 receptor by using anakinra increased bacterial growth in macrophages infected at multiplicity of infection of 1. Blocking of the IL-1 receptor would not only serve to decrease the direct effects of IL-1β, it would also inhibit the IL-1 receptor–driven transcription of proIL-1β. However, the IL-1 receptor also binds IL-1α, which can be released upon inflammasome activation, and a role for IL-1α during infection with M. tuberculosis cannot be ruled out. These data collectively show that although IL-1 receptor signaling is a prerequisite for the control of bacterial growth, the lack of correlation between the absolute levels of IL-1β and increased growth control in macrophages suggests that inflammasome-dependent mechanisms other than IL-1β production are involved in the beneficial effects on bacterial growth in macrophages of individuals carrying the studied genes. Whether IL-1β plays a role in the beneficial effects remains to be elucidated, but irrespective of the mediator, we show that combined gene variants in NLRP3 and CARD8 result in increased phagolysosomal fusion in hMDMs, giving an explanation for the differences in bacterial growth.
The CARD8 C10X polymorphism leads to decreased expression of functional CARD8 [8] and thereby to the loss of CARD8-mediated inhibition of caspase-1 and NFκB, leading to increased levels of IL-1β [3], as well as to caspase-1–dependent cell death, termed pyroptosis, in Salmonella-infected cells [8]. Pyroptosis is accompanied by increased IL-1β levels and might be beneficial to the host during Salmonella infection, but the necrotic nature of pyroptosis favors bacterial spreading to neighboring cells and is detrimental for the M. tuberculosis–infected host [14]. A recent study showed that autoproteolyzed caspase-1 generated by an ASC-containing inflammasome (ie, NLRP3 inflammasome) is responsible for the generation of IL-1β, whereas alternatively activated caspase-1 interacts with other inflammasomes to cause pyroptosis [15]. This might explain why CARD8 C10X alone contributes to increased growth of M. tuberculosis by increasing the levels of alternatively activated caspase-1 and pyroptosis, whereas in conjunction with gain-of-function variants in NLRP3, caspase-1 is autoproteolyzed and redirected from causing pyroptosis to other effects, including generation of IL-1β.
In summary, although the role of NLRP3 and inflammasome activation during in vivo infection has been questioned, our data suggest that enhanced inflammasome activation (eg, by activating polymorphisms/mutations) leads to increased anti–M. tuberculosis activity in macrophages from both patients with inflammatory disorders and healthy individuals. Activation of NLRP3 by a small peptide, acALY18, has been suggested as a broad-spectrum treatment against other intracellular pathogens, strengthening the idea of increased NLRP3 activity as a protective player in infected macrophages [16]. Although we were able to identify only a small number of healthy double carriers of Q705K and C10X, it is tempting to speculate that these genetic variants predispose for increased inflammatory dysfunction but simultaneously lead to decreased capacity of intracellular bacteria such as M. tuberculosis to establish infection. The enormous selective pressure posed by M. tuberculosis through history might explain the high frequency of the studied polymorphisms and suggests a role for NLRP3 inflammasome variants in protection against tuberculosis. Therefore, extending our studies to include patients with tuberculosis and their healthy household contacts in tuberculosis-endemic settings would be of great interest.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgment. We thank Dr Per Eriksson for patient inclusion and critical comments on the manuscript.
Financial support. This work was supported by the Swedish Research Council (grants 2012-3349 and 2009-3821), the Bill and Melinda Gates Foundation, the Swedish Heart-Lung Foundation, and the Carl Trygger Foundation.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mishra BB, Moura-Alves P, Sonawane A, et al. Mycobacterium tuberculosis Protein ESAT-6 is a Potent Activator of the NLRP3/ASC Inflammasome. Cell Microbiol. 2010;12:1046–63. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 2.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–25. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 3.Razmara M, Srinivasula SM, Wang L, et al. CARD-8 protein, a new CARD family member that regulates caspase-1 activation and apoptosis. J Biol Chem. 2002;277:13952–8. doi: 10.1074/jbc.M107811200. [DOI] [PubMed] [Google Scholar]
- 4.Settas LD, Tsimirikas G, Vosvotekas G, Triantafyllidou E, Nicolaides P. Reactivation of pulmonary tuberculosis in a patient with rheumatoid arthritis during treatment with IL-1 receptor antagonists (anakinra) J Clin Rheumatol. 2007;13:219–20. doi: 10.1097/RHU.0b013e31812e00a1. [DOI] [PubMed] [Google Scholar]
- 5.Verma D, Sarndahl E, Andersson H, et al. The Q705 K polymorphism in NLRP3 is a gain-of-function alteration leading to excessive interleukin-1beta and IL-18 production. PLoS One. 2012;7:e34977. doi: 10.1371/journal.pone.0034977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma D, Eriksson P, Sahdo B, et al. Two adult siblings with atypical cryopyrin-associated periodic syndrome due to a novel M299 V mutation in NLRP3. Arthritis Rheum. 2010;62:2138–43. doi: 10.1002/art.27489. [DOI] [PubMed] [Google Scholar]
- 7.Verma D, Lerm M, Blomgran Julinder R, Eriksson P, Soderkvist P, Sarndahl E. Gene polymorphisms in the NALP3 inflammasome are associated with interleukin-1 production and severe inflammation: relation to common inflammatory diseases? Arthritis Rheum. 2008;58:888–94. doi: 10.1002/art.23286. [DOI] [PubMed] [Google Scholar]
- 8.Ko DC, Shukla KP, Fong C, et al. A genome-wide in vitro bacterial-infection screen reveals human variation in the host response associated with inflammatory disease. Am J Hum Genet. 2009;85:214–27. doi: 10.1016/j.ajhg.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welin A, Eklund D, Stendahl O, Lerm M. Human macrophages infected with a high burden of ESAT-6-expressing M. tuberculosis undergo caspase-1- and cathepsin B-independent necrosis. PLoS One. 2011;6:e20302. doi: 10.1371/journal.pone.0020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welin A, Raffetseder J, Eklund D, Stendahl O, Lerm M. Importance of phagosomal functionality for growth restriction of Mycobacterium tuberculosis in primary human macrophages. J Innate Immun. 2011;3:508–18. doi: 10.1159/000325297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Master SS, Rampini SK, Davis AS, et al. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008;3:224–32. doi: 10.1016/j.chom.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoultz I, Verma D, Halfvarsson J, et al. Combined polymorphisms in genes encoding the inflammasome components NALP3 and CARD8 confer susceptibility to Crohn's disease in Swedish men. Am J Gastroenterol. 2009;104:1180–8. doi: 10.1038/ajg.2009.29. [DOI] [PubMed] [Google Scholar]
- 13.Sahdo B, Fransén K, Asfaw Idosa B, et al. Cytokine profile in a cohort of healthy blood donors carrying polymorphisms in genes encoding the NLRP3 inflammasome. PLoS ONE. 2013;8:e75457. doi: 10.1371/journal.pone.0075457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao LY, Guo S, McLaughlin B, Morisaki H, Engel JN, Brown EJ. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol Microbiol. 2004;53:1677–93. doi: 10.1111/j.1365-2958.2004.04261.x. [DOI] [PubMed] [Google Scholar]
- 15.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8:471–83. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thacker JD, Balin BJ, Appelt DM, et al. NLRP3 inflammasome is a target for development of broad-spectrum anti-infective drugs. Antimicrob Agents Chemother. 2012;56:1921–30. doi: 10.1128/AAC.06372-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.