Abstract
Legionella pneumophila, the causative agent of a severe pneumonia named Legionnaires' disease, is an important human pathogen that infects and replicates within alveolar macrophages. Its virulence depends on the Dot/Icm type IV secretion system (T4SS), which is essential to establish a replication permissive vacuole known as the Legionella containing vacuole (LCV). L. pneumophila infection can be modeled in mice however most mouse strains are not permissive, leading to the search for novel infection models. We have recently shown that the larvae of the wax moth Galleria mellonella are suitable for investigation of L. pneumophila infection. G. mellonella is increasingly used as an infection model for human pathogens and a good correlation exists between virulence of several bacterial species in the insect and in mammalian models. A key component of the larvae's immune defenses are hemocytes, professional phagocytes, which take up and destroy invaders. L. pneumophila is able to infect, form a LCV and replicate within these cells. Here we demonstrate protocols for analyzing L. pneumophila virulence in the G. mellonella model, including how to grow infectious L. pneumophila, pretreat the larvae with inhibitors, infect the larvae and how to extract infected cells for quantification and immunofluorescence microscopy. We also describe how to quantify bacterial replication and fitness in competition assays. These approaches allow for the rapid screening of mutants to determine factors important in L. pneumophila virulence, describing a new tool to aid our understanding of this complex pathogen.
Keywords: Infection, Issue 81, Bacterial Infections, Infection, Disease Models, Animal, Bacterial Infections and Mycoses, Galleria mellonella, Legionella pneumophila, insect model, bacterial infection, Legionnaires' disease, haemocytes
Introduction
Animal models of infection have proved invaluable in the determination of bacterial virulence factors. However, invertebrate models have gained increased attention as a viable alternative to traditional mammalian models of infection. The larvae of the wax moth, Galleria mellonella is increasingly being used to study a number of important human pathogens, including Gram-positive1 and Gram-negative bacteria2,3 and several pathogenic fungi4,5. Using an insect model has a number of advantages over traditional mammalian models, as an invertebrate, G. mellonella is not subject to the ethical limitations of mammalian models. In addition, the larvae can be easily maintained, infected by injection without anesthesia, undergo pretreatment with chemical inhibitors6 and sustain incubation at 37 °C7. Interestingly, a good correlation between the pathogenicity of several microorganisms in G. mellonella and mammalian models of infection has been established2,8. The increased understanding of the immune system of G. mellonella has also assisted in the characterization of this model organism. Although insects do not have an adaptive immune system as found in mammals, they do have sophisticated cellular and humeral defenses including the production of antimicrobial peptides9. Hemocytes are the major mediator of cellular defenses and are the most numerous cell type found in the hemolymph (or blood) of G. mellonella10, These cells are professional phagocytes and perform similar functions to human macrophages and neutrophils by both taking up and degrading bacteria in a phago-lysosomal compartment10,11 and forming nodules around invading bacteria, physically restricting bacterial replication12.
Legionella pneumophila is a respiratory pathogen that causes severe pneumonia (Legionnaires' disease) in susceptible populations such as the elderly or immunocompromised13. Legionella is found ubiquitously in both environmental and man-made water sources, where it is a pathogen of various species of fresh water amoebae14,15. Legionella survives and replicates within these professional phagocytes by utilizing a multi-protein complex known as the Dot/Icm (defective in organelle trafficking/intracellular multiplication) type 4 secretion system (T4SS) to translocate over 275 effector proteins into the host cell16-20. These proteins serve to subvert the normal host cell phagocytic pathways, leading to the creation of the Legionella containing vacuole (LCV). The LCV avoids fusion with lysosomes and instead recruits endoplasmic reticulum (ER)-derived vesicles, resulting in a specialized compartment that resembles the rough ER21,22. L. pneumophila is considered an accidental human pathogen; the same strategies that allow it to replicate within amoebae, also allow replication in human alveolar macrophages23.
Mammalian hosts have been characterized as models for human Legionella infection including mice and guinea pigs24,25. However, the majority of mouse strains are resistant to Legionella infection26 with the exception of the inbred albino A/J mouse, which develops a mild, self-limiting infection24. Although the guinea pig model more closely resembles human disease25, the lack of mutants and increased cost discourages their use27. In addition, several invertebrate models have been developed for Legionella pneumophila infection including Caenorhabditis elegans28, Drosophila melanogaster29 and several species of amoebae30-32. However, these models have weaknesses, virulence in the C. elegans system is not Dot/Icm-dependent28, limiting the utility of this model. The Drosophila model has proved effective in investigating bacterial virulence factors29 and appears to be promising however, this model has not been fully characterized. Single celled amoebae are the environmental hosts of L. pneumophila and are ideal for investigating the action of virulence factors at a molecular level33 however lack several important mediators of the mammalian host cell response to infection such as caspases34. The weaknesses of the existing models, along with the high cost and ethical concerns related to mammalian experimentation, has led to the search for other appropriate model organisms29,35.
We have recently demonstrated that G. mellonella is a suitable model for L. pneumophila pathogenesis36,37. This protocol details the experimental techniques used for infecting G. mellonella larvae, analyzing larval morality, extracting hemocytes for counting and immunofluorescence and determining replication by viable CFU counts from infected larvae.
Protocol
1. Preparation of L. pneumophila for Infection
- Prepare charcoal yeast extract (CYE) plates (2 g/L activated charcoal, 10 g/L yeast extract, 13 g/L agar, 10 g/L N-(2-acetamido)-2-aminothanesulfonic acid (ACES), 1 g/L α-ketoglutarate, 0.4 g/L L-cysteine HCl and 0.25 g/L ferric pyrophosphate, pH 6.9). Note: If required, add kanamycin (25 μg/ml) and/or chloramphenicol (6 μg/ml) to CYE plates.
- Carry out all L. pneumophila work at biosafety containment level 2 (BSL-2) in a microbial safety cabinet (MSC) in compliance with local rules.
Streak L. pneumophila from -80 °C glycerol stocks onto CYE plates
Incubate plates for 4 days at 37 °C. Note: Incubation of plates for 4 days significantly increases the virulence of L. pneumophila in G. mellonella over incubation for 3 days.
One day before infection, resuspend one loop full of bacteria (containing several colonies) in 1 ml of prewarmed (37 °C) ACES yeast extract (AYE) broth and measure the absorbance at 600 nm (OD600) using a spectrophotometer.
- Inoculate a fresh 3 ml AYE culture (with antibiotics if required) to a final OD600 of 0.1.
- Include a media only control to ensure sterility of the media.
Incubate at 37 °C in a shaking incubator at 200 rpm for 21 hr. Note: Bacteria should be grown to post-exponential phase for infection38. Growing for 21 hr allows experiments to be standardized. Note: If required for protein induction, add 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) overnight.
Measure the OD600 (bacteria should be in post-exponential growth phase, OD600 2.5-3).
Dilute bacterial culture to give 1 x 109 CFU/ml in sterile Dulbecco's phosphate buffered saline (D-PBS). Note: Based on previous results, an OD600 of 1 corresponds to 1 x 109 CFU/ml, however this should be confirmed for different L. pneumophila strains. Note: if induction of a protein from a plasmid is required, add 1 mM of IPTG to the inoculum.
Plate the inoculum as described in section 9 as a control to ensure the expected CFU is present in the inoculum.
2. Preparation of Larvae
Purchase sufficient G. mellonella larvae from a commercial supplier. Larvae are shipped at 5th or 6th instar stage (approximately between 2-3 cm in length) and are suitable for use immediately. Note: A method describing how to rear larvae has been described previously39 . Note: Larvae can be stored at room temperature for up to two weeks and do not require food. Immediately discard any larvae showing signs of pupation.
Prepare the container for larva by placing a circle of 10 cm filter paper in the bottom of a 10 cm Petri dish.
Using blunt tipped tweezers, place ten healthy larvae of approximately similar size into the Petri dish. Note: Discard unhealthy brown colored or blotchy looking insects. Healthy larvae are uniformly creamy colored with no areas of dark discoloration and are able to right themselves quickly if turned over.
3. Infection of G. mellonella Larvae
Prepare the injection platform by taping a circle of filter paper to the surface.
Securely tape a P1000 tip horizontally to the filter paper to create an injection platform. This does not need to be sterile.
- Sterilize a 20 μl microtiter syringe by aspirating 70% ethanol and incubating for at least 10 min.
- Wear puncture proof gloves while injecting,
Remove any residual ethanol by aspirating and expelling sterile water several times.
Using the syringe, aspirate 10 μl of the L. pneumophila 1 x 109 CFU/ml suspension.
Take one larva and gently but firmly turn it on its back, bent over the P1000 tip.
Place the tip of the syringe over the front, right proleg of the larvae.
- Gently, insert the tip of the needle into the proleg, making sure that it is inside the larvae, and smoothly inject all of the syringe contents. Note: If the syringe is inserted correctly, it should be possible to pick up the larvae using only the syringe to place it into the chamber.
- After injection, observe the larvae for a few seconds. The larvae will start to crawl after a couple of seconds but should not excrete fluid.
Briefly observe the larvae a few hours post infection; infection with 107 CFU L. pneumophila 130b does not cause any symptoms within the first 5-8 hr p.i. Therefore if larvae are turning grey/black before this point, the experiment should be discontinued. Note: Inoculation of larvae with 1 mM IPTG does not affect larval viability over the course of the experiment.
In the same manner, inject a total of 10 larvae per condition including 10 larvae injected with D-PBS to serve as a control to analyze larval mortality.
Tape Petri dishes closed and place in secondary containment.
Incubate in a standard bacterial incubator at 37 °C, for the duration of the experiment.
4. Pretreatment of Larvae with a Chemical Inhibitor
Prior to infection, prepare larvae for injection as described in section 3.1-3.6.
Inject 10 μl of a 100 μM solution of Cytochalasin D into the front, left proleg of 10 larvae. Note: Inhibitor is injected into a different proleg from bacterial suspension to reduce injury to the larvae.
Inject 10 μl of DMSO into 10 larvae as a control.
Incubate larvae at 37 °C for 4 hr.
Inject pretreated larvae as described in section 3 into the front, right proleg with either 1 x 107 CFU of WT L. pneumophila or a PBS control.
5. Analysis of Larval Mortality
At 18 hr post infection (p.i.), examine all infected larvae for mortality.
To check mortality, use blunt tipped tweezers to turn over the larvae and look for movement of the legs, healthy larvae should right themselves quickly. Pigmentation indicates a strong immune reaction to infection. If there is any movement, count as alive.
Record number of dead and alive larvae.
Repeat this process at all other time points chosen. Note: If incubation is continued for more than three days, pupae may be seen. Remove any pupae and euthanize by freezing at - 20 °C before metamorphosis can occur.
6. Extraction of Hemolymph
Randomly select three larvae and place into a 14 ml tube at selected time points such as 5 and 18 hr p.i.,
Place this tube on ice for 5-10 min, until no movement of the legs of the larvae can be observed.
Place the anesthetized larvae onto a Petri dish and, using a scalpel, make an incision between two segments near the tail of the larvae.
- Squeeze the larvae into a sterile 1.5 ml centrifuge tube to collect the hemolymph.
- Pool hemolymph from at least three individuals. One larvae gives between 15-50 μl of hemolymph depending on size. Note: During hemolymph extraction it is very easy to disrupt the gut, resulting in potential contamination of the samples. Reduce contamination by cutting the larvae near the tail (away from the gut) however, antibiotic selection will always be required when plating out the bacteria. Note: To prevent hemolymph from turning brown and coagulating, process the hemolymph within 10 min after collection.
Discard the larval body into a new 14 ml Falcon tube, seal and place at - 20 °C overnight to ensure the larvae are dead.
Autoclave dead larvae and dispose according to local rules.
7. Determination of Hemocyte Viability
Extract hemolymph as described above.
Mix 20 μl of extracted hemolymph with 20 μl of 0.02% (v/v) Trypan blue in PBS in one well of a 96 well plate.
Incubate for 5 min at RT.
Load 10 μl of hemolymph onto a hemocytometer and count viable (not blue) cells.
Count each sample in triplicate to reduce error.
8. Processing of Extracted Hemocytes for Immunofluorescence Microscopy
Mix the pooled hemolymph extracted from at least three larvae and pipette onto a 10-15 mm glass coverslip in a 24-well plate. Note: Coverslips do not require treatment as hemocytes can adhere to glass .
Add 0.5 ml of D-PBS and mix well by pipetting up and down.
Centrifuge the plate for 10 min at 500 x g at room temperature (RT) using an aerosol-tight centrifuge plate holder.
Examine each well using an inverted microscope to check that the hemocytes have adhered.
Remove the supernatant and carefully wash the cells three times by adding 0.5 ml D-PBS to the wall of the well, rocking the plate 2-3x and removing the D-PBS with a pipette.
Fix the cells by addition of 0.5 ml of 4% (v/v) paraformaldehyde (PFA) in PBS.
Incubate the cells for between 20-30 min at RT.
Wash the cells three times with D-PBS, as before.
Add 0.5 ml 15 mM NH4Cl in PBS to quench residual PFA and incubate at RT for 15 min.
Wash the cells three times with D-PBS. Note: At this stage, the coverslips can be stored overnight at 4 °C.
Add 0.5 ml 0.1% Triton X-100 in PBS and incubate for 5 min at RT to permeabilize cells.
Block for 1 hr with blocking solution (2% (w/v) BSA in PBS).
Incubate for 1 hr at RT in the dark with the primary antibody diluted in blocking solution at the dilution specified by the manufacturer.
Wash 3x with PBS.
Incubate for 1 hr with the secondary antibody and DAPI for visualization of bacteria as above.
Wash 3x with PBS.
Mount the coverslips using one drop of mounting reagent onto glass slides.
Incubate overnight in the dark at RT to fully dry the mounting solution.
Image slides on a fluorescence microscope.
9. Quantification of Bacterial CFU
Add 100 μg/ml spectinomycin to CYE plates to avoid contamination by gut flora. L. pneumophila strain 130b is naturally resistant to spectinomycin40.
Before hemolymph extraction, weigh 1.5 ml centrifuge tubes.
Extract hemolymph as described in section 6 and place in weighed tubes, add 1 μl of 5 mg/ml digitonin, mix well and incubate for 5 min at RT to lyse hemocytes.
Reweigh the tube with hemolymph and determine the weight of hemolymph extracted.
Perform ten fold serial dilutions of the hemolymph in sterile AYE media.
Using a pen, divide the base of a CYE plate into six equal sectors and label.
Plate three drops of 25 μl of each dilution (starting with the most dilute) in each section of the plate.
Incubate the plates with the lids upmost overnight at 37 °C.
Once the drops have dried fully, turn the plate over and incubate at 37 °C for at least a further two days.
Quantify the bacteria extracted by counting the colonies at each dilution and normalize to the weight of hemolymph extracted.
10. Determination of the Competitive Index (CI)
Confirm that both strains grow equally well in broth culture and on CYE agar plates prior to attempting the competitive index.
- Prepare WT or kanamycin resistant mutant bacterial suspensions as described in section 1 and mix in a 1:1 ratio.
- Plate serial dilutions of the inoculum onto CYE spectinomycin (100 μg/ml) and CYE spectinomycin /kanamycin
Infect larvae and extract hemolymph at suitable time points as described above.
Determine viable counts by extracting hemolymph and plating serial dilutions onto CYE spectinomycin and CYE spectinomycin/kanamycin.
Calculate the competitive index (CI) as follows: CI = (mutant output/WT output)/(mutant inoculum/WT inoculum).
Representative Results
Here it is demonstrated that G. mellonella is an appropriate, easy to use model to study L. pneumophila infection. Previously it has been shown that L. pneumophila virulence in macrophages, amoebae and mammalian models is dependent on the presence of the Dot/Icm secretion system 41-43. G. mellonella larvae were infected as described above and the virulence of the wild type (WT) and a Dot/Icm-deficient strain compared. Infection with 107 CFU of L. pneumophila strain 130b resulted in 100% mortality within 24 hr post infection (p.i.). However, the L. pneumophila ΔdotA strain, which does not have a functional Dot/Icm T4SS secretion system, was avirulent in this assay (Figure 1). This demonstrates that L. pneumophila virulence in G. mellonella depends on the translocation of Dot/Icm effectors, making this model suitable for characterization of the function of these proteins.
Recently, it was shown that inhibition of phagocytosis by cytochalasin treatment increased the susceptibly of the larvae to infection by the yeast Candida albicans6. As L. pneumophila is an intracellular pathogen, it was decided to determine if uptake of the bacteria is crucial in its pathogenesis in this model. Larvae were pretreated with 10 μl of 100 μM Cytochalasin D (CyD) for 4 hr at 37 °C, then infected with 107 CFU of WT L. pneumophila 130b and mortality monitored at 24 hr p.i. Treatment with the inhibitor alone did not affect larval survival. However, pretreated, infected larvae displayed significantly greater survival (P = 0.0066, unpaired T-test) compared to DMSO-treated, infected insects (Figure 2). The effect of CyD treatment was abolished by 48 hr p.i. (results not shown); this may be due to the half-life of the drug in G. mellonella. This demonstrates that uptake of L. pneumophila into G. mellonella hemocytes is a crucial aspect of bacterial virulence.
In order to validate expression and determine the subcellular localization of an effector protein in G. mellonella, hemocytes were extracted and processed for immunofluorescence microscopy. Larvae were infected with WT and ΔdotA L. pneumophila 130b expressing a fragment of the well-defined T4SS effector, SidC41-918, fused to 4 N-terminal HA tags. This effector was demonstrated to bind the LCV via a phosphoinositide-4-phosphate-binding domain44. Using anti-HA (red) and anti-Legionella (green) antibodies, 4HA-SidC41-918 localized to the LCV in infected hemocytes (Figure 3). This localization has previously been shown in the amoebae Dictyostelium discoideum and in mammalian macrophages44,45 confirming the comparability of this model.
The importance of proteins for virulence is usually determined by comparing the growth kinetics of wild type and mutant bacteria. In order to follow the bacterial replication kinetics over the course of the infection, three larvae were sacrificed at each time point (0, 5, 18, and 24 hr p.i.), the hemolymph collected and pooled and the CFU/0.1g of extracted hemolymph determined. After an initial dip at 5 hr p.i., the CFU of the WT bacteria increases up to 24 hr p.i. however, the ΔdotA strain undergoes no replication and is cleared at 18 hr p.i. (Figure 4).
The ability of L. pneumophila to cause lysis of macrophages in a T4SS-dependent manner has long been documented46, however no similar studies have been performed in vivo. The concentration of circulating hemocytes was determined at 5, 18, and 24 hr p.i. Larvae were infected with WT or ΔdotA L. pneumophila 130b, hemocytes extracted from infected insects and viable cells counted using the trypan blue exclusion method. At 5 hr p.i. no difference in hemocyte counts between the strains could be seen (Figure 5). However, at 18 hr p.i. there was a significant drop in hemocyte concentration in WT, but not ΔdotA, infected larvae. This difference persisted at 24 hr p.i. The drop in hemocyte number, combined with the presence of intracellular bacteria as seen by immunofluorescence, suggests that L. pneumophila replicates within hemocytes then lyses them, allowing the bacteria to undergo several rounds of replication.
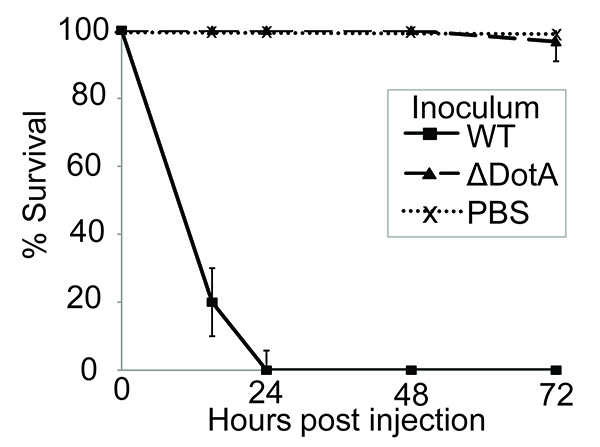 Figure 1. Infection with L. pneumophila induces Dot/Icm-dependent larval mortality. 10 larvae were infected with PBS alone or 107 CFU of wild type (WT) or ΔdotA L. pneumophila 130b, incubated at 37 °C for 72 hr and the time of death of the larvae recorded. All larvae infected with the WT succumbed to infection within 24 hr post infection (p.i.), however no mortality was seen in larvae inoculated with PBS alone or the ΔdotA strain. Results are the mean of three separate experiments, ± standard deviation.
Figure 1. Infection with L. pneumophila induces Dot/Icm-dependent larval mortality. 10 larvae were infected with PBS alone or 107 CFU of wild type (WT) or ΔdotA L. pneumophila 130b, incubated at 37 °C for 72 hr and the time of death of the larvae recorded. All larvae infected with the WT succumbed to infection within 24 hr post infection (p.i.), however no mortality was seen in larvae inoculated with PBS alone or the ΔdotA strain. Results are the mean of three separate experiments, ± standard deviation.
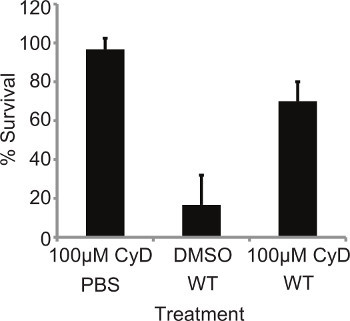 Figure 2. Mortality is dependent on bacterial internalization. 10 L. pneumophila larvae were pretreated with 10 μl of 100 μM Cytochalasin D (CyD) for 4 hr at 37 °C then infected with 107 WT and mortality monitored at 24 hr p.i. Pretreated larvae demonstrated significantly (P = 0.0066, unpaired T-test) reduced mortality. Results represent the mean of at four independent experiments ± standard deviations with 10 larvae per condition.
Figure 2. Mortality is dependent on bacterial internalization. 10 L. pneumophila larvae were pretreated with 10 μl of 100 μM Cytochalasin D (CyD) for 4 hr at 37 °C then infected with 107 WT and mortality monitored at 24 hr p.i. Pretreated larvae demonstrated significantly (P = 0.0066, unpaired T-test) reduced mortality. Results represent the mean of at four independent experiments ± standard deviations with 10 larvae per condition.
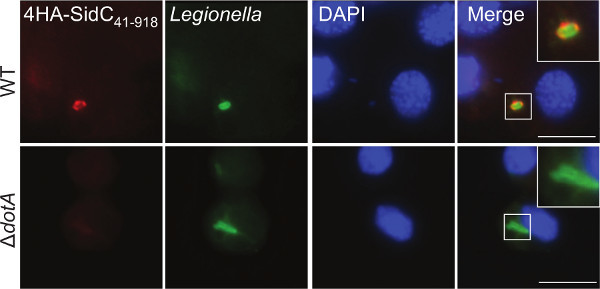 Figure 3. Immunofluorescence imaging of effector proteins in extracted hemocytes. Hemocytes were extracted from larvae infected with L. pneumophila 130b WT or ΔdotA expressing 4HA-SidC41-918 at 5 hr p.i. Cells were stained using anti-HA (red) and anti-Legionella (green) antibodies and DAPI DNA stain (blue) to visualize the nuclei. 4HA-SidC41-918 was observed surrounding WT, but not ΔdotA, bacteria. Scale bar 5 μm.
Figure 3. Immunofluorescence imaging of effector proteins in extracted hemocytes. Hemocytes were extracted from larvae infected with L. pneumophila 130b WT or ΔdotA expressing 4HA-SidC41-918 at 5 hr p.i. Cells were stained using anti-HA (red) and anti-Legionella (green) antibodies and DAPI DNA stain (blue) to visualize the nuclei. 4HA-SidC41-918 was observed surrounding WT, but not ΔdotA, bacteria. Scale bar 5 μm.
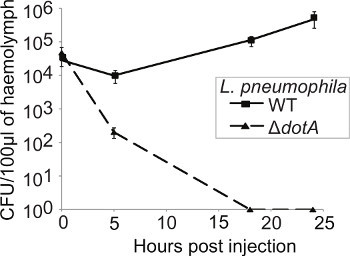 Figure 4. L. pneumophila replicates within G. mellonella in a Dot/Icm-dependent manner. Larvae were infected with WT or ΔdotA L. pneumophila and at 0, 5, 18, and 24 hr p.i. the hemolymph from three infected insects pooled, plated onto CYE plates and the CFU determined and normalized to the inoculum and to the weight of hemolymph extracted. WT L. pneumophila replicated over the course of the experiment while the ΔdotA strain was cleared within 18 hr p.i. Results are the mean of three separate experiments ± standard deviation.
Figure 4. L. pneumophila replicates within G. mellonella in a Dot/Icm-dependent manner. Larvae were infected with WT or ΔdotA L. pneumophila and at 0, 5, 18, and 24 hr p.i. the hemolymph from three infected insects pooled, plated onto CYE plates and the CFU determined and normalized to the inoculum and to the weight of hemolymph extracted. WT L. pneumophila replicated over the course of the experiment while the ΔdotA strain was cleared within 18 hr p.i. Results are the mean of three separate experiments ± standard deviation.
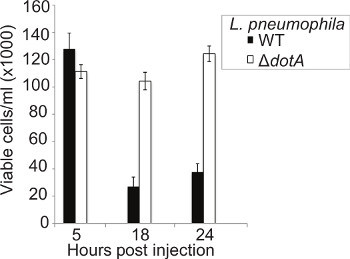 Figure 5. Infection with WT L. pneumophila results in significant hemocyte destruction. Hemocytes were extracted at 5, 18, and 24 hr p.i. from larvae infected with WT or ΔdotA L. pneumophila and viable cells counted using a hemocytometer. No difference in the number of cells was seen at 5 hr p.i. between the strains however at 18 hr p.i. only approximately 15% of hemocytes remain in larvae infected with the WT strain compared to the ΔdotA strain. Results are the mean of three separate experiments, ± standard deviation.
Figure 5. Infection with WT L. pneumophila results in significant hemocyte destruction. Hemocytes were extracted at 5, 18, and 24 hr p.i. from larvae infected with WT or ΔdotA L. pneumophila and viable cells counted using a hemocytometer. No difference in the number of cells was seen at 5 hr p.i. between the strains however at 18 hr p.i. only approximately 15% of hemocytes remain in larvae infected with the WT strain compared to the ΔdotA strain. Results are the mean of three separate experiments, ± standard deviation.
Discussion
The Galleria mellonella larval model for Legionella pneumophila infection is a useful tool for in vivo studies of pathogenesis. Here it is shown that a number of aspects of macrophage infection can be recapitulated in the G. mellonella model, including the role of the Dot/Icm T4BSS in virulence and bacterial replication and the localization of the Dot/Icm-effector SidC. Additionally, we demonstrate that a chemical inhibitor of actin polymerization significantly reduces larval mortality, mimicking results obtained in macophages47 and supporting evidence that internalization of the bacteria is required to cause larval mortality. Previously, it has been demonstrated that the variations in virulence between L. pneumophila strains seen in other infection models can be verified in G. mellonella and that induction of virulence factors in post-exponential growth phase is required for bacterial virulence36, confirming that G. mellonella is a suitable model for L. pneumophila infection.
Determining the CFU of L. pneumophila from larvae infected either singly or in mixed infections greatly increases the utility of the model. Previously, several factors have been discovered that have subtle effects on bacterial replication in one or more models of infection29,48-51. Although the larvae do not possess an adaptive immune system, the presence of the innate immune response provides stronger selection compared to macrophages alone, which may serve to amplify subtle phenotypes. Therefore, it is possible that, while these strains will probably not significantly affect larval mortality, they may demonstrate decreased bacterial replication or fitness in the G. mellonella model. As well as replication of L. pneumophila in the larvae, we have shown significant hemocyte depletion late in infection. As L. pneumophila is expected to lyse host cells at the end of its replication cycle, measuring hemocyte depletion may also serve as an indirect measurement of bacterial replication. Hemocyte depletion has previously been correlated with insect mortality in infection3,52, although recent results suggest that this picture is more complex than first belived37. Recently, it has been shown that starvation of larvae leads to increased susceptibility to infection through a suppression of immune responses53. In the assays described here, larvae were not fed for the duration of the study and it is not known how well fed larvae would respond to L. pneumophila infection.
One of the advantages of G. mellonella as a model organism is the ease of extraction and quantification of hemocytes from infected larvae. Previous videos have shown various methods for extracting hemocytes from insects54,55 however, the method presented here is simple and suitable for immediate processing. Once extracted, hemocytes can be easily quantified, used for immunofluorescence, transmission electron microscopy36 or flow cytometry56 or cultured and infected ex vivo3 allowing the response of the cells to infection to be investigated in detail. This significantly increases the flexibility of the model. One caveat to immunofluorescence in G. mellonella is the limited supply of antibodies validated against G. mellonella proteins. However, studies have demonstrated the creation of antibodies against larval proteins57 and antibodies against human immune related proteins were found to recognize G. mellonella proteins11 demonstrating the potential for immunofluorescence on G. mellonella hemocytes.
The ease of G. mellonella infection allows for rapid, medium throughput screens that could be used to compare the virulence of various Legionella species and strains and could be used to further analyze previously identified virulence factors such as adhesion molecules58 or the type 2 secretion system59 which are required for virulence in other models. In addition, use of this model will allow the identification and further characterization of novel virulence factors including secreted and translocated effector proteins. Recently, it has been shown that phospholipase C activity of L. pneumophila has a role in G. mellonella virulence60 and that the Dot/Icm effector protein SdhA is required for virulence37. In addition, we have recently demonstrated that there is a correlation between the phenotypes observed in G. mellonella and in the A/J mouse strain37.
This underlines the value of this tool to complement environmental protozoan and unicellular host and murine infection models. The G. mellonella model will become even more valuable in the future, once the larval genomic sequence will be available and more genetic tools are established. Steps in this direction include the recent publication detailing the immune-related transcriptome61 and the formation of an initiative to advance gene silencing in Lepidoptera spp62.
By using G. mellonella larvae, we have a number of simple, rapid readouts of bacterial virulence that can be used to investigate the pathogenesis of L. pneumophila. Establishment of these assays and wider screening of L. pneumophila strains and serogroups will increase the utility of this new tool and will contribute to our understanding of L. pneumophila pathogenesis.
Disclosures
The authors have nothing to disclose.
Acknowledgments
C.R. Harding was supported by the Wellcome Trust studentship WT086724.
References
- Olsen RJ, Watkins ME, Cantu CC, Beres SB, Musser JM. Virulence of serotype M3 Group A Streptococcus strains in wax worms (Galleria mellonella larvae. Virulence. 2011;2:111–119. doi: 10.4161/viru.2.2.14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander G, Rahme LG, Ausubel FM. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol. 2000;182:3843–3845. doi: 10.1128/jb.182.13.3843-3845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K, et al. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl. Environ. Microbiol. 2010;76:310–317. doi: 10.1128/AEM.01301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowlds P, Barron A, Kavanagh K. Physical stress primes the immune response of Galleria mellonella larvae to infection by Candida albicans. Microbes Infect. 2008;10:628–634. doi: 10.1016/j.micinf.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Renwick J, Daly P, Reeves EP, Kavanagh K. Susceptibility of larvae of Galleria mellonella to infection by Aspergillus fumigatus is dependent upon stage of conidial germination. Mycopathologia. 2006;161:377–384. doi: 10.1007/s11046-006-0021-1. [DOI] [PubMed] [Google Scholar]
- Banville N, Fallon J, McLoughlin K, Kavanagh K. Disruption of haemocyte function by exposure to cytochalasin b or nocodazole increases the susceptibility of Galleria mellonella larvae to infection. Microbes Infect. 2012;13:1191–1198. doi: 10.1016/j.micinf.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Mowlds P, Kavanagh K. Effect of pre-incubation temperature on susceptibility of Galleria mellonella larvae to infection by Candida albicans. Mycopathologia. 2008;165:5–12. doi: 10.1007/s11046-007-9069-9. [DOI] [PubMed] [Google Scholar]
- Joyce SA, Gahan CG. Molecular pathogenesis of Listeria monocytogenes in the alternative model host Galleria mellonella. Microbiology. 2010;156:3456–3468. doi: 10.1099/mic.0.040782-0. [DOI] [PubMed] [Google Scholar]
- Mak P, Zdybicka-Barabas A, Cytrynska M. A different repertoire of Galleria mellonella antimicrobial peptides in larvae challenged with bacteria and fungi. Dev. Comp. Immunol. 2010;34:1129–1136. doi: 10.1016/j.dci.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002;32:1295–1309. doi: 10.1016/s0965-1748(02)00092-9. [DOI] [PubMed] [Google Scholar]
- Bergin D, Reeves EP, Renwick J, Wientjes FB, Kavanagh K. Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect. Immun. 2005;73:4161–4170. doi: 10.1128/IAI.73.7.4161-4170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe NA, Gagen SJ. Studies on the in vivo cellular reactions of insects: an ultrastructural analysis of nodule formation in Galleria mellonella. Tissue Cell. 1977;9:73–85. doi: 10.1016/0040-8166(77)90050-7. [DOI] [PubMed] [Google Scholar]
- Fraser DW, et al. Legionnaires' disease: description of an epidemic of pneumonia. N. Engl. J. Med. 1977;297:1189–1197. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- Rowbotham TJ. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 1980;33:1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields BS. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- Berger KH, Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Segal G, Purcell M, Shuman HA. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Andrews HL, Wong SK, Isberg RR. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- Zhu W, et al. Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PLoS One. 2011;6:e17638. doi: 10.1371/journal.pone.0017638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein D, et al. Genome-scale identification of Legionella pneumophila effectors using a machine learning approach. PLoS Pathog. 2009;5:e1000508. doi: 10.1371/journal.ppat.1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MA. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal G, Shuman HA. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun. 1999;67:2117–2124. doi: 10.1128/iai.67.5.2117-2124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieland J, et al. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice. A murine model of human Legionnaires' disease. Am. J. Pathol. 1994;145:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- Baskerville A, Fitzgeorge RB, Broster M, Hambleton P, Dennis PJ. Experimental transmission of legionnaires' disease by exposure to aerosols of Legionella pneumophila. Lancet. 1981;2:1389–1390. doi: 10.1016/s0140-6736(81)92803-8. [DOI] [PubMed] [Google Scholar]
- Wright EK, et al. Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr. Biol. 2003;13:27–36. doi: 10.1016/s0960-9822(02)01359-3. [DOI] [PubMed] [Google Scholar]
- Padilla-Carlin DJ, McMurray DN, Hickey AJ. The guinea pig as a model of infectious diseases. Comp. Med. 2008;58:324–340. [PMC free article] [PubMed] [Google Scholar]
- Komura T, Yasui C, Miyamoto H, Nishikawa Y. Caenorhabditis elegans as an alternative model host for Legionella pneumophila, and protective effects of Bifidobacterium infantis. Appl. Environ. Microbiol. 2011;76:4105–4108. doi: 10.1128/AEM.03021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubori T, Shinzawa N, Kanuka H, Nagai H. Legionella metaeffector exploits host proteasome to temporally regulate cognate effector. PLoS Pathog. 2010;6:e1001216. doi: 10.1371/journal.ppat.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Kwaik Y. The phagosome containing Legionella pneumophila within the protozoan Hartmannella vermiformis is surrounded by the rough endoplasmic reticulum. Appl. Environ. Microbiol. 1996;62:2022–2028. doi: 10.1128/aem.62.6.2022-2028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molmeret M, Horn M, Wagner M, Santic M, Abu Kwaik Y. Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 2005;71:10–1128. doi: 10.1128/AEM.71.1.20-28.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon JM, Rupper A, Cardelli JA, Isberg RR. Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions. Infect. Immun. 2000;68:2939–2947. doi: 10.1128/iai.68.5.2939-2947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbi H, Weber SS, Ragaz C, Nyfeler Y, Urwyler S. Environmental predators as models for bacterial pathogenesis. Environ. Microbiol. 2007;9:563–575. doi: 10.1111/j.1462-2920.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- Khoa DB, Trang LT, Takeda M. Expression analyses of caspase-1 and related activities in the midgut of Galleria mellonella during metamorphosis. Insect Mol Biol. 2012;21:247–256. doi: 10.1111/j.1365-2583.2011.01131.x. [DOI] [PubMed] [Google Scholar]
- Brassinga AK, et al. Caenorhabditis is a metazoan host for Legionella. Cell Microbiol. 2011;12:343–361. doi: 10.1111/j.1462-5822.2009.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CR, et al. Legionella pneumophila pathogenesis in the Galleria mellonella infection model. Infect. Immun. 2012;80:2780–2790. doi: 10.1128/IAI.00510-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CR, et al. The Dot/Icm effector SdhA is necessary for virulence of Legionella pneumophila in Galleria mellonella and A/J mice. Infect. Immun. 2013;81:10–1128. doi: 10.1128/IAI.00296-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B, Swanson MS. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramarao N, Nielsen-Leroux C, Lereclus D. The insect Galleria mellonella as a Powerful Infection Model to Investigate Bacterial Pathogenesis. J. Vis. Exp. 2012. p. e4392. [DOI] [PMC free article] [PubMed]
- Suter TM, Viswanathan VK, Cianciotto NP. Isolation of a gene encoding a novel spectinomycin phosphotransferase from Legionella pneumophila. Antimicrob. Agents Chemother. 1997;41:1385–1388. doi: 10.1128/aac.41.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbi H, Segal G, Shuman HA. Icm/dot-dependent upregulation of phagocytosis by Legionella pneumophila. Mol. Microbiol. 2001;42:603–617. doi: 10.1046/j.1365-2958.2001.02645.x. [DOI] [PubMed] [Google Scholar]
- Watarai M, et al. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J. Exp. Med. 2001;194:1081–1096. doi: 10.1084/jem.194.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santic M, Asare R, Doric M, Abu Kwaik Y. Host-dependent trigger of caspases and apoptosis by Legionella pneumophila. Infect. Immun. 2007;75:2903–2913. doi: 10.1128/IAI.00147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber SS, Ragaz C, Reus K, Nyfeler Y, Hilbi H. Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog. 2006;2(4):e46. doi: 10.1371/journal.ppat.0020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZQ, Isberg RR. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. U.S.A. 2004;101:841–846. doi: 10.1073/pnas.0304916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alli OA, et al. Temporal pore formation-mediated egress from macrophages and alveolar epithelial cells by Legionella pneumophila. Infect. Immun. 2000;68:6431–6440. doi: 10.1128/iai.68.11.6431-6440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JA, Winn WC. Treatment of alveolar macrophages with cytochalasin D inhibits uptake and subsequent growth of Legionella pneumophila. Infect. Immun. 1986;51:31–36. doi: 10.1128/iai.51.1.31-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo SL, Yan L, Littman M, Samrakandi MM, Cirillo JD. Role of the Legionella pneumophila rtxA gene in amoebae. Microbiology. 2002;148:1667–1677. doi: 10.1099/00221287-148-6-1667. [DOI] [PubMed] [Google Scholar]
- Ridenour DA, Cirillo SL, Feng S, Samrakandi MM, Cirillo JD. Identification of a gene that affects the efficiency of host cell infection by Legionella pneumophila in a temperature-dependent fashion. Infect. Immun. 2003;71:6256–6263. doi: 10.1128/IAI.71.11.6256-6263.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samrakandi MM, Cirillo SL, Ridenour DA, Bermudez LE, Cirillo JD. Genetic and phenotypic differences between Legionella pneumophila strains. J. Clin. Microbiol. 2002;40:1352–1362. doi: 10.1128/JCM.40.4.1352-1362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomma M, et al. The Legionella pneumophila F-box protein Lpp2082 (AnkB) modulates ubiquitination of the host protein parvin B and promotes intracellular replication. Cell. Microbiol. 2010. [DOI] [PubMed]
- Champion OL, et al. Galleria mellonella as an alternative infection model for Yersinia pseudotuberculosis. Microbiology. 2009;155:1516–1522. doi: 10.1099/mic.0.026823-0. [DOI] [PubMed] [Google Scholar]
- Banville N, Browne N, Kavanagh K. Effect of nutrient deprivation on the susceptibility of Galleria mellonella larvae to infection. Virulence. 2012;3:497–503. doi: 10.4161/viru.21972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qayum AA, Telang A. A protocol for collecting and staining hemocytes from the yellow fever mosquito Aedes aegypti. J. Vis. Exp. 2011. p. e2772. [DOI] [PMC free article] [PubMed]
- Stoepler TM, Castillo JC, Lill JT, Eleftherianos I. A simple protocol for extracting hemocytes from wild caterpillars. J. Vis. Exp. 2012. p. e4173. [DOI] [PMC free article] [PubMed]
- Garcia-Garcia E, Garcia-Garcia PL, Rosales C. An fMLP receptor is involved in activation of phagocytosis by hemocytes from specific insect species. Dev. Comp. Immunol. 2009;33:728–739. doi: 10.1016/j.dci.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Bogus M, Scheller K. Allatotropin released by the brain controls larval molting in Galleria mellonella by affecting juvenile hormone synthesis. Int. J. Dev. Biol. 1996;40:205–210. [PubMed] [Google Scholar]
- Chang B, Kura F, Amemura-Maekawa J, Koizumi N, Watanabe H. Identification of a novel adhesion molecule involved in the virulence of Legionella pneumophila. Infect. 2005;73:4272–4280. doi: 10.1128/IAI.73.7.4272-4280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciotto NP. Many substrates and functions of type II secretion: lessons learned from Legionella pneumophila. Future Microbiol. 2009;4:797–805. doi: 10.2217/FMB.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurass P, et al. The Legionella pneumophila Dot/Icm-secreted effector PlcC/CegC1 together with PlcA and PlcB promotes virulence and belongs to a novel zinc metallophospholipase C family present in bacteria and fungi. J. Biol. Chem. 2013. [DOI] [PMC free article] [PubMed]
- Vogel H, Altincicek B, Glockner G, Vilcinskas A. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomics. 2011;12:1471–2164. doi: 10.1186/1471-2164-12-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenius O, et al. RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J. Insect. Physiol. 2011;57:231–245. doi: 10.1016/j.jinsphys.2010.11.006. [DOI] [PubMed] [Google Scholar]


