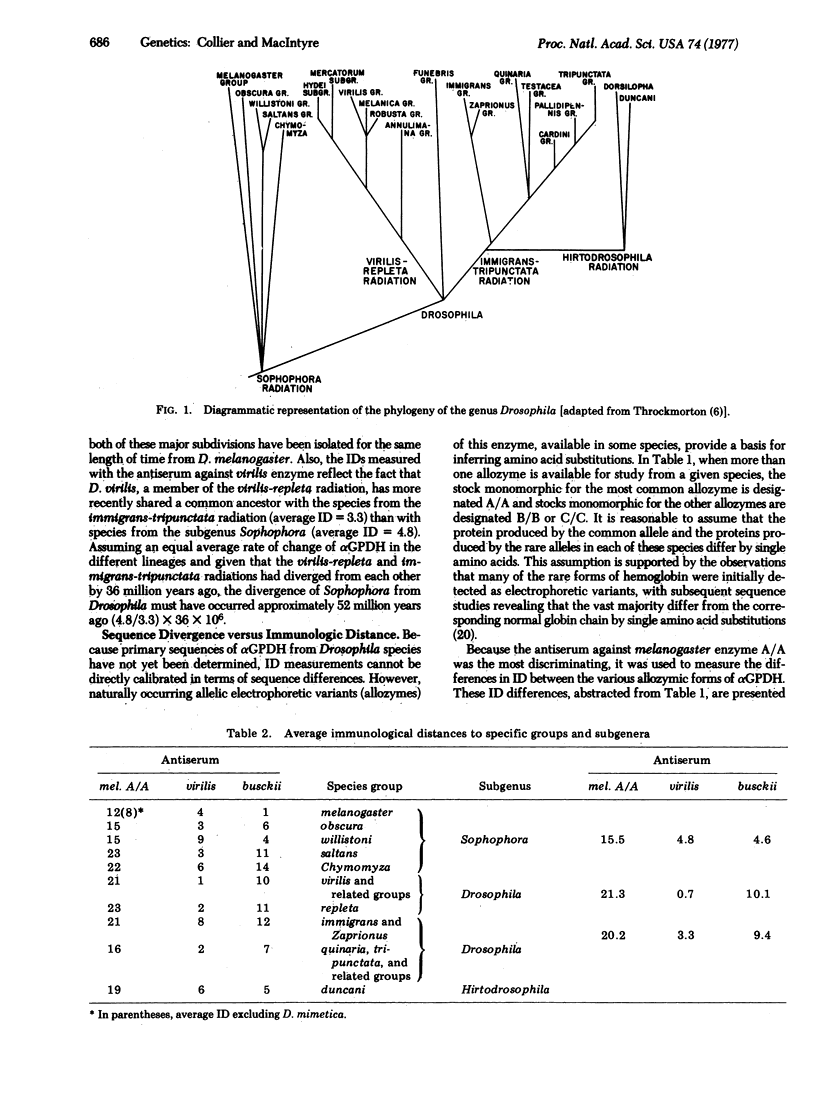
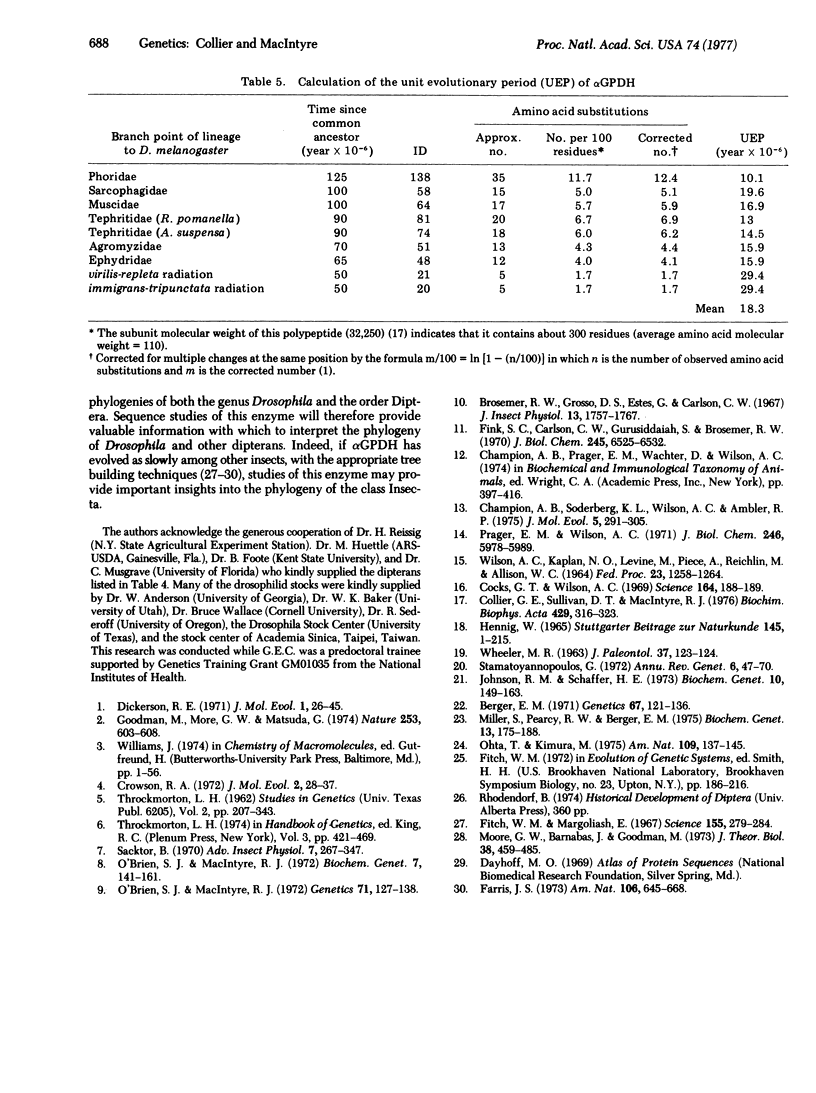
Abstract
Antisera were prepared against purified alpha-glycerophosphate dehydrogenase (EC 1.1.1.8) (alphaGPDH) from Drosophila melanogaster, D. virilis, and D. busckii. The immunological distances between the enzymes from the 3 species and those from 31 additional drosophilid species agree in general with the accepted phylogeny of the genus. These data permit an estimate that the subgenus Sophophora diverged 52 million years ago from the line leading to the subgenus Drosophila. The antiserum against melanogaster alphaGPDH was capable of distinguishing allelic variants of alphaGPDH. On the basis of presumed single amino acid substitutions, no drosophilid alphaGPDH tested differed from the melanogaster enzyme by more than eight or nine substitutions. The study was extended to include representatives of six other dipteran families. The immunological distances between alphaGPDH from Drosophila and alphaGPDH from these dipterans were reasonably consistent with a phylogeny of the order Diptera established by more conventional means. The unit evolutionary period of this enzyme was estimated to be 18 million years.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger E. M. A temporal survey of allelic variation in natural and laboratory populations of Drosophila melanogaster. Genetics. 1971 Jan;67(1):121–136. doi: 10.1093/genetics/67.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion A. B., Soderberg K. L., Wilson A. C. Immunological comparison of azurins of known amino acid sequence. Dependence of cross-reactivity upon sequence resemblance. J Mol Evol. 1975 Sep 8;5(4):291–305. doi: 10.1007/BF01732216. [DOI] [PubMed] [Google Scholar]
- Cocks G. T., Wilson A. C. Immunological detection of single amino acid substitutions in alkaline phosphatase. Science. 1969 Apr 11;164(3876):188–189. doi: 10.1126/science.164.3876.188. [DOI] [PubMed] [Google Scholar]
- Crowson R. A. A systematist looks at cytochrome c. J Mol Evol. 1972 Dec 29;2(1):28–37. doi: 10.1007/BF01653940. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E. The structures of cytochrome c and the rates of molecular evolution. J Mol Evol. 1971;1(1):26–45. doi: 10.1007/BF01659392. [DOI] [PubMed] [Google Scholar]
- Fink S. C., Carlson C. W., Gurusiddaiah S., Brosemer R. W. Glycerol 3-phosphate dehydrogenases in social bees. J Biol Chem. 1970 Dec 25;245(24):6525–6532. [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Goodman M., Moore G. W., Matsuda G. Darwinian evolution in the genealogy of haemoglobin. Nature. 1975 Feb 20;253(5493):603–608. doi: 10.1038/253603a0. [DOI] [PubMed] [Google Scholar]
- Johnson F. M., Schaffer H. E. Isozyme variability in species of the genus Drosophila. VII. Genotype-environment relationships in populations of D. melanogaster from the Eastern United States. Biochem Genet. 1973 Oct;10(2):149–163. doi: 10.1007/BF00485762. [DOI] [PubMed] [Google Scholar]
- Miller S., Pearcy R. W., Berger E. Polymorphism at the alpha-glycerophosphate dehydrogenase locus in Drosophila melanogaster. I. Properties of adult allozymes. Biochem Genet. 1975 Apr;13(3-4):175–188. doi: 10.1007/BF00486013. [DOI] [PubMed] [Google Scholar]
- Moore G. W., Barnabas J., Goodman M. A method for constructing maximum parsimony ancestral amino acid sequences on a given network. J Theor Biol. 1973 Mar;38(3):459–485. doi: 10.1016/0022-5193(73)90252-x. [DOI] [PubMed] [Google Scholar]
- O'Brien S. J., MacIntyre R. J. The -glycerophosphate cycle in Drosophila melanogaster. I. Biochemical and developmental aspects. Biochem Genet. 1972 Oct;7(2):141–161. doi: 10.1007/BF00486085. [DOI] [PubMed] [Google Scholar]
- O'Brien S. J., Macintyre R. J. The -glycerophosphate in Drosophila melanogaster. II. Genetic aspects. Genetics. 1972 May;71(1):127–138. doi: 10.1093/genetics/71.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. I. Micro-complement fixation studies. J Biol Chem. 1971 Oct 10;246(19):5978–5989. [PubMed] [Google Scholar]
- Stamatoyannopoulos G. The molecular basis of hemoglobin disease. Annu Rev Genet. 1972;6:47–70. doi: 10.1146/annurev.ge.06.120172.000403. [DOI] [PubMed] [Google Scholar]
- WILSON A. C., KAPLAN N. O., LEVINE L., PESCE A., REICHLIN M., ALLISON W. S. EVOLUTION OF LACTIC DEHYDROGENASES. Fed Proc. 1964 Nov-Dec;23:1258–1266. [PubMed] [Google Scholar]


