Abstract
Background
Regular exercise increases exercise self-efficacy and health-related quality of life (HRQOL); however, the mechanisms are unknown. We examined the associations of exercise adherence and physiological improvements with changes in exercise self-efficacy and HRQOL.
Methods
Middle-aged adults (N=202) were randomized to 12 months aerobic exercise (360 minutes/week) or control. Weight, waist circumference, percent body fat, cardiopulmonary fitness, HRQOL (SF-36), and exercise self-efficacy were assessed at baseline and 12 months. Adherence was measured in minutes/day from activity logs.
Results
Exercise adherence was associated with reduced bodily pain, improved general health and vitality, and reduced role-emotional scores (Ptrend≤0.05). Increased fitness was associated with improved physical functioning, bodily pain and general health scores (Ptrend≤0.04). Reduced weight and percent body fat were associated with improved physical functioning, general health, and bodily pain scores (Ptrend<0.05). Decreased waist circumference was associated with improved bodily pain and general health but with reduced role-emotional scores (Ptrend≤0.05). High exercise adherence, increased cardiopulmonary fitness and reduced weight, waist circumference and percent body fat were associated with increased exercise self-efficacy (Ptrend<0.02).
Conclusions
Monitoring adherence and tailoring exercise programs to induce changes in cardiopulmonary fitness and body composition may lead to greater improvements in HRQOL and self-efficacy that could promote exercise maintenance.
Keywords: physical activity, intervention study, aerobic, physical fitness
Background
Social cognitive theory suggests that positive experiences with exercise such as improved exercise self-efficacy and health-related quality of life (HRQOL) may reinforce behavior change and improve future exercise adherence.1–5 Identifying correlates of improved exercise self-efficacy and HRQOL during an exercise intervention could help in identifying ways to improve exercise self-efficacy and HRQOL, and ultimately, adherence to exercise recommendations.
Previous research has shown that exercise improves quality of life (QOL) through its effect on self-efficacy. A cross-sectional analysis of 249 older women found that exercise affected mental and physical health status through its effects on self-efficacy.6 A longitudinal analysis of this sample also found associations of amount of exercise with self-efficacy and of exercise self-efficacy with mental and physical health status.7 Further, Sonstroem et al. proposed a model suggesting that exercise-induced physical changes affect self-efficacy which then affect self-esteem, a focal aspect of QOL.8,9 McAuley and colleagues tested this model in older adults (N=174) and reported that exercise frequency, weight loss, and improved fitness were directly associated with different aspects of self-esteem.10
Several intervention studies examined associations of exercise-induced physiological changes and exercise adherence with HRQOL. In a 12-month exercise trial in 173 postmenopausal women, improved cardiopulmonary fitness (VO2max) was associated with increased HRQOL (physical functioning).11 In a 15-week exercise trial among 53 postmenopausal breast cancer survivors, there were positive relationships between changes in cardiopulmonary fitness and HRQOL measures [Functional Assessment of Cancer Therapy (FACT)-Breast, FACT-General].12 A 15-week exercise trial in colon cancer survivors found a greater increase in HRQOL (FACT-Colorectal) among participants who increased cardiopulmonary fitness compared with those who declined.13 Change in peak cardiovascular fitness also mediated the change in patient-rated physical functioning in a study of 122 lymphoma patients.14
Some studies have examined the effect of intervention adherence on HRQOL in exercise trials. 11,15,16 Although these studies have reported the relationships between adherence, physiological changes and HRQOL, few studies have focused on different aspects of HRQOL. Further, few studies have simultaneously examined the relationships of adherence, physiological changes and exercise self-efficacy with various aspects of HRQOL to understand how these factors affect HRQOL.
The purpose of this study was to examine associations of exercise adherence and physiological changes (i.e., changes in cardiopulmonary fitness, weight, waist circumference, and percent body fat) with changes in exercise self-efficacy and eight aspects of HRQOL in a 12-month exercise trial. We hypothesized that high adherence and favorable physiological changes would be associated with increased exercise self-efficacy and HRQOL comparing baseline to 12 months’ data. Based on previous studies reporting a mediating role of self-efficacy on the relationship between exercise and quality of life or self-esteem,6,7,9 we also tested a potential confounding effect of exercise self-efficacy on the associations between adherence, physiological changes and HRQOL. Given the exploratory nature of this secondary analysis, no hypothesis was developed.
Methods
Study design and Participants
The study participants were middle-aged to older men (n=102) and women (n=100), recruited to an exercise trial that tested the effects of exercise on colon cancer biomarkers.17 Eligibility criteria included: age 40 to 75 years old; a colonoscopy within the previous 3 years; less than 90 minutes per week of moderate-to-vigorous intensity exercise during the previous 3 months [or had maximal oxygen consumption (VO2max) classified in low fitness level (i.e., fair or poor according to the American College of Sports Medicine guidelines 18); less than 2 alcohol drinks per day; no history of invasive cancer, colorectal diseases associated with increased colon cancer risk (e.g., familial polyposis and ulcerative colitis) or other serious medical conditions; normal response to a maximal exercise tolerance test; and normal blood chemistries.
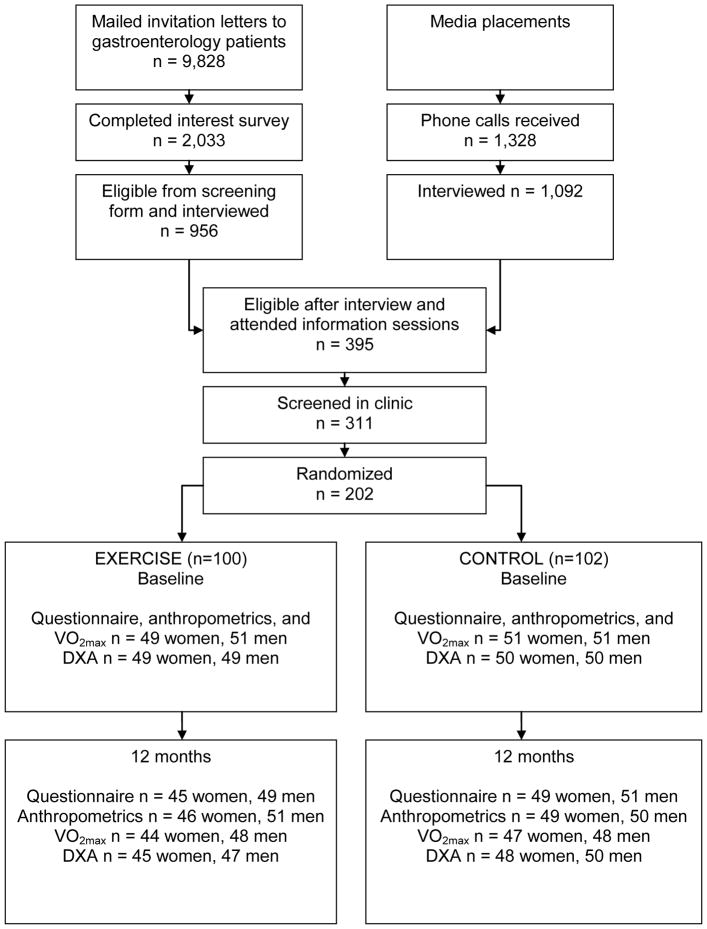
Participants were recruited though gastroenterology practices, media placement, flyers, a study web site, and referrals (Figure 1).19 Of the invitation letters sent to 9,828 gastroenterology clinic patients, 2,033 (21%) responded and 956 were interviewed. A total of 1,328 people responded to media placement, and 1,092 were interviewed. Three primary reasons for ineligibility at interview were: 1) unwilling to be randomized (n=297), 2) not sedentary (n=339), and 3) insufficient time for study participation (n=48). Of those who were eligible after interview, 395 attended information sessions, 311 were screened in clinic, and 202 were enrolled.
Figure 1.
Recruitment and flow of participants in the study
Participants were randomized to exercise or control groups by the study coordinator using a computerized program developed by the study biostatistician. We used a stratified blocked randomization with a block size of 4. The randomization program stratified participants by gender, use of non-steroidal anti-inflammatory medications (more than 2 times per week or less), current smoking status, and among women, menopausal status and current use of postmenopausal hormone therapy and assigned them to study groups within the strata. This procedure was used to ensure a balance of these characteristics between the two study groups. All participants signed informed consent approved by the Fred Hutchinson Cancer Research Center Institutional Review Board.
Intervention
The intervention goal was 60 minutes per day of moderate-to-vigorous intensity aerobic exercise, 6 days per week, for 12 months. Participants exercised three times per week with exercise specialists at one of four facilities. Participants were provided with Polar heart rate monitors (Polar Electro Inc., Lake Success, NY) and advised to exercise at 60% to 85% of their maximal heart rate based on their baseline VO2max test. Participants also were asked to exercise at home or a gym an additional 3 days per week. They were asked not to change their diet habits.
The intervention was not based on specific behavioral theories, but used various strategies that were shown to be effective in promoting behavioral change such as monitoring, feedback, and self-monitoring. Over the first 12 weeks, participants gradually increased their intensity and duration of physical activity, which included facility (3/week) and home (3/week) sessions. Participants completed daily activity logs on which they recorded type of exercise, length of sessions, and maximal heart rate. Behaviorally-trained interventionists reinforced participants’ success in intervention as participants progressed in behavior change. The exercise specialists met individually with participants every month to review their progress, to address any adherence-related problems and to discuss strategies to manage the problems. Graphs showing their study goals and current performance from the exercise logs and facility attendance were created and the discrepancies between the two were discussed. Quarterly newsletters including exercise tips and study progress were sent to participants. We provided small incentives (e.g., water bottles) to participants after achieving certain goals. Group social events such as hiking were arranged.
Controls were asked not to change their exercise and diet habits during the trial. After completion of the trial, controls were given the opportunity to participate in exercise classes for 2 months.
Measurements
Both exercisers and controls completed assessments at baseline and 12 months. Anthropometric information was also collected at 6 months. Study staff members involved in the assessment were blinded to randomization status. Demographic and health information was collected by self-administered questionnaire. Demographic information consisted of gender, age, ethnicity, and education. Health information included history of serious health conditions or cancer risk factors, menopausal status, current smoking habit, and use of medications. Participants in the exercise group completed daily facility and home exercise logs for the entire intervention. Adherence was calculated as mean number of self-reported minutes of moderate-to-vigorous exercise per week.
Cardiopulmonary fitness (VO2max) was measured using a modified branching treadmill protocol20 by maximal-graded treadmill test and expressed as ml/kg per minute.18 Heart rate and oxygen consumption were monitored by a MedGraphics automated cart during the test (MedGraphics, St. Paul, MN). Height and weight were assessed to the nearest 0.1cm and 0.1kg, respectively using a stadiometer and balance-beam scale. Each participant was measured twice, and the average was recorded. Waist circumference was measured at the end of normal expiration over non-binding undergarments at the minimal location on the torso (natural waist) to the nearest 0.1cm. The percent body fat was assessed using a dual energy X-ray absorptiometry (DXA) whole-body scanner (GE Lunar, Madison, WI).
Exercise self-efficacy was assessed using a previously validated 5-item exercise self-efficacy scale [internal consistency of 0.76, test-retest reliability (product moment) over 2-week period of 0.90].21 The participants rated their confidence in performing regular exercise in challenging situations using an 11-point scale. The scale consisted of items such as “I am confident I can participate in regular exercise when I am on vacation.” This scale has been used to measure self-efficacy in exercise intervention studies.22,23 Scores range from 0 to 55 with higher scores indicating better exercise self-efficacy.
The 36 Item Health Status Survey Short Form (SF-36) was used to assess HRQOL.24 Eight subscale scores (i.e., physical functioning, role-physical, bodily pain, vitality, general health, social functioning, role-emotional, and mental health) were used as indicators of HRQOL.24 The eight subscales were reported to have an internal consistency of 0.38 to 0.81, discriminant validity of 0.09 to 0.62 and reliability coefficient of 0.85 (median) in 3445 patients with chronic and psychiatric conditions.25 The physical functioning scale consists of items assessing the degree of limitations when climbing several flights of stairs or walking several blocks. 24 Scores range from 0 to 100 on each scale with higher scores indicating better HRQOL.
Statistical analysis
We used t-tests, Fisher’s tests and Chi-square tests to compare the baseline characteristics between exercisers and controls as well as completers and non-completers. Effects of adherence and physiological changes during intervention on exercise self-efficacy and 8 aspects of HRQOL were examined by testing trends across subgroups. Subgroups of exercisers were created based on tertiles of: 1) exercise adherence (weekly physical activity of <295 min/wk, 295–331 min/wk, or ≥331 min/wk); 2) changes in cardiopulmonary fitness (increased Vo2max by ≤2.7%, 2.7–14.5%, or ≥14.5%); 3) percent weight change (lost <0.11%, 0.11–3.3%, or ≥3.3% of the initial body weight); 4) change in waist circumference (reduced waist circumference by <1.0cm, 1.0–3.7cm, or ≥3.7cm); and 5) change in percent body fat (decreased percent body fat by <0.49%, 0.49–2.91%, or ≥2.91%). Controls were not subclassified; as previously reported there was little change in any of these variables in the controls.19 Tests for trend (Ptrend) across the control and the subgroups in the exercise group were performed by linear regression with the covariate being subgroup categories. The differences between controls and the subgroups of exercisers were also compared. The models were further adjusted for exercise self-efficacy to determine its confounding effects. A change in the beta coefficient of more than 10% was used as the criteria for a confounder. The generalized estimating equation (GEE) model was employed to account for repeated assessments on the same subjects. Missing data were imputed using the last observation carried forward. We also performed analyses using available data. All analyses were performed by SAS program version 9.1 (SAS Institute, Cary, NC).
Results
Sample characteristics
Baseline characteristics (Table 1) of participants were not significantly different between exercise and control groups except for anti-depressant use (p=0.07). We used the cut point of p-value=0.20 for the baseline differences between exercisers and controls to determine inclusion as covariates in the GEE model. Using this criterion, anti-depressant use at baseline and 12 months were included as covariates in the GEE models. Age and gender were also included as covariates based on a previous report suggesting age and gender differences in SF-36 scores.24
Table 1.
Baseline characteristics of the study participants
| Exercise n=100 | Control n=102 | P-value | |
|---|---|---|---|
| Demographic and medical factors | |||
| Age (years), Mean(SD) | 55.4 (6.89) | 55.2 (6.84) | 0.85 |
| Female, n(%) | 49 (49.0) | 51 (50.0) | 0.89 |
| Race (non-Hispanic white), n(%) | 90 (90.0) | 95 (93.1) | 0.42 |
| College degree, n(%) | 61 (61.0) | 62 (60.8) | 0.97 |
| Post-menopause, n(%) | 30 (61.2) | 33 (64.7) | 0.72 |
| Colon polyp Hx, n(%) | 57 (57.0) | 58 (56.9) | 0.98 |
| Medication use | |||
| Antidepressant, n(%) | 12 (12.0) | 22 (21.6) | 0.07 |
| Anxiolytic n(%)* | 1 (1.0) | 2 (2.0) | 1.00 |
| Anthropometrics and Lifestyle factors | |||
| Smoker, n(%)* | 6 (6.0) | 6 (5.9) | 0.97 |
| BMI (kg/m2), Mean(SD) | 29.3 (4.66) | 29.3 (4.86) | 0.98 |
| Pedometer count (steps/day), Mean(SD) | 5963 (2662) | 6425 (3069) | 0.26 |
| Moderate-to-vigorous physical activity (min/week), Mean(SD) | 55.5 (92.1) | 58.4 (83.5) | 0.81 |
| VO2max (ml/kg/min), Mean(SD) | 27.1 (3.3) | 27.5 (6.3) | 0.58 |
Fisher’s exact test
There were no differences between completers and non-completers of the 12-month questionnaire (i.e., HRQOL and exercise self-efficacy) in baseline characteristics, exercise self-efficacy, and 8 subscales of HRQOL except for pedometer counts (See supplement table 1). All analyses were repeated using available data. Because there were no substantial differences between the analysis results of last observation carried forward and available data, we present the results of last observation carried forward.
The overall intervention effects on exercise adherence and physiological changes were reported elsewhere.19 In brief, exercisers significantly increased the amount of exercise compared with controls. Over the 12-month intervention period, female and male exercisers performed a mean [standard deviation (SD)] of 295 (102) minutes/week and 370 (86) minutes/week of moderate-to-vigorous physical activity, respectively. Cardiopulmonary fitness (VO2max) significantly increased in female [mean 2.5 ml/kg/min (10.5% increase)] and male exercisers [3.3 ml/kg/min (11% increase)], and decreased in controls (P<0.001, exercisers vs. controls). Body weight (women: −1.4 kg vs. +0.7 kg in controls, P=0.008; men: −1.8 kg vs. −0.1 kg in controls, P=0.03), waist circumference (women: −1.4 cm. vs. +2.2 cm in controls, P <0.001; men: −3.3 cm vs. −0.4 cm in controls, P=0.003), and total fat mass (women: −1.9 kg vs. +0.2 kg in controls, P=0.001; men −3.0 kg vs. +0.2 kg in controls, P<0.001) significantly reduced in exercisers compared with controls. We did not observe changes or differences between exercisers and controls for mean total daily caloric intake (results not shown).
As we previously reported,26 we did not observe any differences in 8 aspects of HRQOL at 12 months between exercise and control groups. The exercise self-efficacy score at 12 months was significantly higher among exercisers compared with controls (Exercise Δ12month-baseline = −2.9, Control Δ12month-baseline = −6.5, p=0.01).
At the baseline assessment, 4 participants (1 female control, 1 male control, and 2 male exercisers) did not complete a DXA scan. These cases were excluded from the analyses on change in percent body fat. At 12 months, some data were missing for: SF-36 (HRQOL) and exercise self-efficacy (N=8); body weight (N=6); waist circumference (N=5); cardiopulmonary fitness (N=15); and DXA (N=12).
Adherence and 12-month changes in exercise self-efficacy and HRQOL
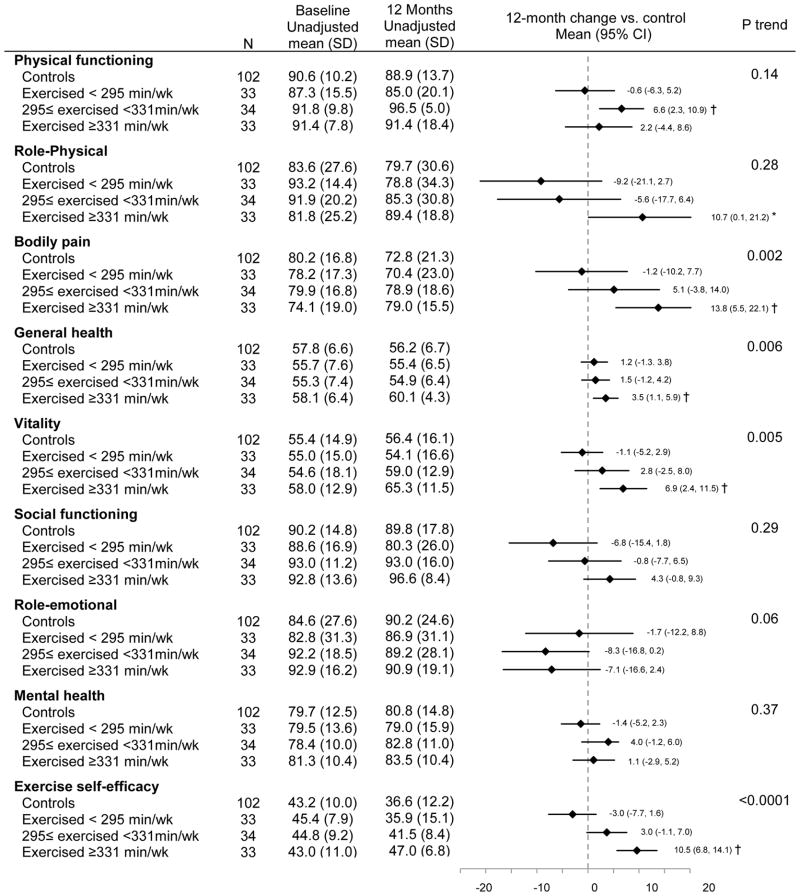
Significant linear trends were observed between increased exercise intervention adherence (mean minutes/week over the 12 months) and reduced bodily pain (Ptrend = 0.002), improved general health (Ptrend = 0.006), increased vitality (Ptrend = 0.005), and increased exercise self-efficacy (Ptrend <0.0001; Figure 2). The associations of adherence with bodily pain and general health were attenuated but remained statistically significant after adjusting for changes in exercise self-efficacy (bodily pain: Δβ coefficient = 25.8%, Ptrend =0.03, general health: Δ β coefficient = 16.1%, Ptrend =0.03). The relationship between adherence and vitality became non-significant after adjusting for changes in exercise self-efficacy (Δβ coefficient = 42.5%, Ptrend =0.11). Higher adherence was associated with reduced role-emotional score (Ptrend = 0.05). Compared with controls, exercisers in the highest tertile of adherence (exercised ≥331 minutes/week) showed significant improvements in bodily pain [Δ12month-baseline = 13.8 (95%CI: 5.5 to 22.1), P = 0.0007], general health [Δ12month-baseline = 3.5 (95% CI: 1.1 to 5.9), P = 0.005], vitality [Δ12month-baseline = 6.9 (95% CI: 2.4 to 11.5), P = 0.001], and exercise self-efficacy [Δ12month-baseline= 10.5 (95% CI: 6.8 to 14.1), P <0.0001].
Figure 2.
Baseline and 12-month HRQOL and exercise self-efficacy scores stratified by adherence
Ptrend testing a trend in change from baseline to 12 months across subgroups, adjusting for age, gender, and antidepressant use
*: p<0.05, †: p<0.01. P value comparing changes in HRQOL and exercise self-efficacy scores from baseline to 12 months between each adherence subgroup vs controls, adjusting for age, gender, and antidepressant use
Cardiopulmonary fitness and 12-month changes in exercise self-efficacy and HRQOL
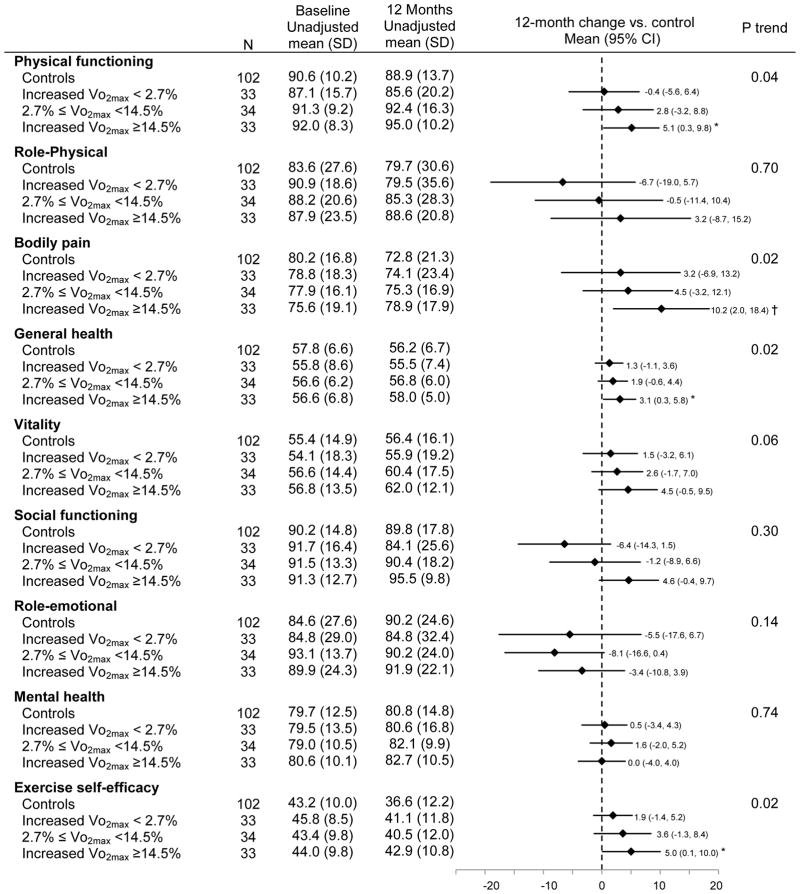
Greater increases in cardiopulmonary fitness (VO2max) were associated with larger increases in physical functioning (Ptrend = 0.04), general health (Ptrend = 0.02) and exercise self-efficacy (Ptrend = 0.02), and greater reductions in bodily pain (Ptrend = 0.02) (Figure 3). Adjusting for exercise self-efficacy had negligible effect on the relationship between cardiopulmonary fitness and physical functioning (Δβ coefficient = 8.7%, Ptrend=0.06), while exercise self-efficacy partially confounded the association of fitness with bodily pain (Δβ coefficient = 21.9%, Ptrend=0.06) and general health (Δβ coefficient = 11.9%, Ptrend=0.03). Individuals who increased VO2max by ≥14.5% increased physical functioning [Δ12month-baseline = 5.1 (95% CI: 0.3 to 9.8), P = 0.04], general health [Δ12month-baseline = 3.1 (0.3 to 5.8), P = 0.03] and exercise self-efficacy [Δ12month-baseline = 5.0 (95% CI: 0.1 to 10.0), P=0.04], and reduced bodily pain [Δ12month-baseline =10.2 (95% CI: 2.0 to 18.4), P = 0.01] compared with controls.
Figure 3.
Baseline and 12-month HRQOL and exercise self-efficacy scores stratified by percent changes in cardiopulmonary fitness (Vo2max)
Ptrend testing a trend in change from baseline to 12 months across subgroups, adjusting for age, gender, and antidepressant use
*: p<0.05, †: p<0.01. P value comparing changes in HRQOL and exercise self-efficacy scores from baseline to 12 months between each adherence subgroup vs controls, adjusting for age, gender, and antidepressant use
Body weight and 12-month changes in exercise self-efficacy and HRQOL
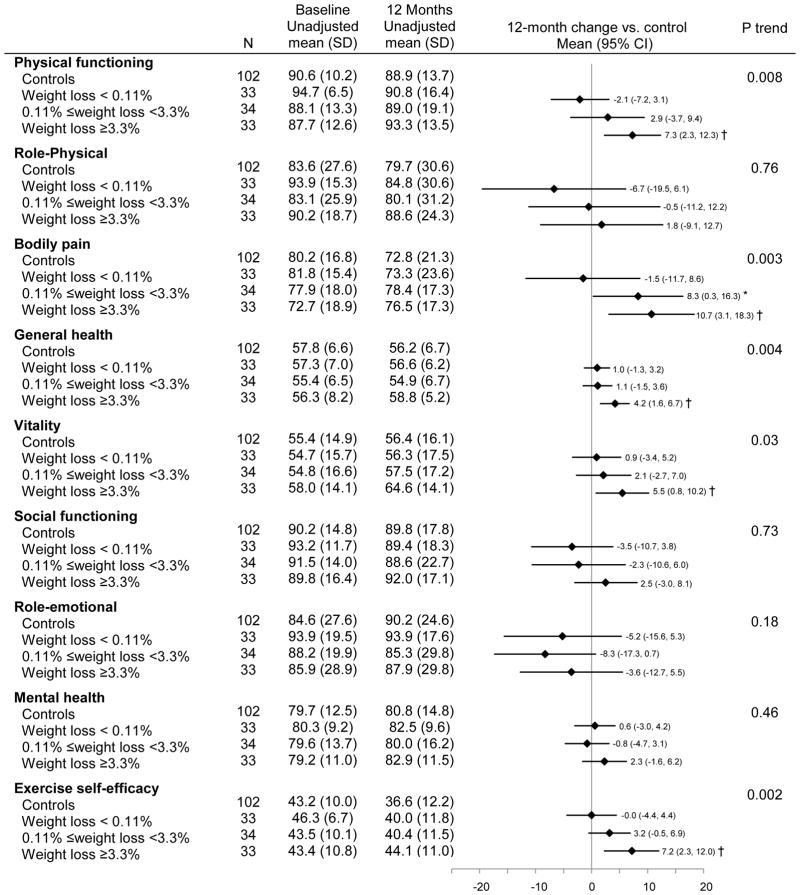
Greater reductions in body weight were associated with larger increases in physical functioning (Ptrend = 0.008), general health (Ptrend = 0.004), vitality (Ptrend = 0.003) and exercise self-efficacy (Ptrend = 0.002), and greater reductions in bodily pain (Ptrend = 0.03; Figure 4). Adjusting for exercise self-efficacy had negligible effect on the relationship between body weight and physical functioning (Δβ coefficient =7.5%, Ptrend=0.01), while exercise self-efficacy partially confounded the association of fitness with bodily pain (Δβ coefficient = 22.3%, Ptrend=0.02) and general health (Δβ coefficient =12.1%, Ptrend=0.01). The relationship between weight loss and vitality became non-significant after adjusting for exercise self-efficacy (Δβ coefficient = 41.5%, Ptrend=0.19). Compared with controls, exercisers who lost ≥3.3% of baseline body weight significantly increased physical functioning [Δ12month-baseline = 7.3 (95% CI: 2.3 to 12.3), P = 0.004], general health [Δ12month-baseline = 4.2 (95% CI: 1.6 to 6.7), P = 0.001], vitality [Δ12month-baseline = 5.5 (95% CI: 0.8 to 10.2), P = 0.02] and exercise self-efficacy score [Δ12month-baseline = 7.2 (95% CI: 2.3 to 12.0), P=0.004], and reduced bodily pain [Δ12month-baseline = 10.7 (95% CI: 3.1 to 18.3), P=0.006] at 12 months.
Figure 4.
Baseline and 12-month HRQOL and exercise self-efficacy scores stratified by percent changes in body weight
Ptrend tested a trend in change from baseline to 12 months across subgroups, adjusting for age, gender, and antidepressant use
*: p<0.05, †: p<0.01. P value compared changes in HRQOL and exercise self-efficacy scores from baseline to 12 months between each adherence subgroup vs controls, adjusting for age, gender, and antidepressant use
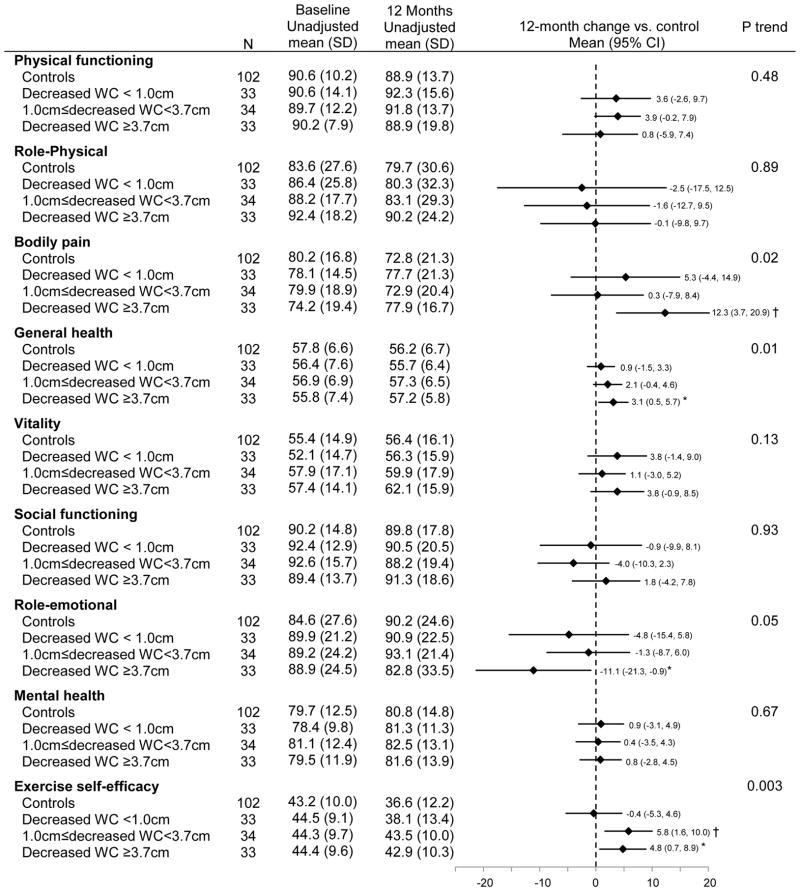
Waist circumference and 12-month changes in exercise self-efficacy and HRQOL
There were significant linear trends for greater decreases in waist circumference associated with increases in general health (Ptrend = 0.01) and exercise self-efficacy (Ptrend = 0.003), as well as decreases in bodily pain (Ptrend= 0.02; Figure 5). A greater reduction in waist circumference was associated with reduced role-emotional score (Ptrend = 0.05). Adjusting for exercise self-efficacy attenuated the associations of reduced waist circumference with bodily pain (Δβ coefficient = 26.5%, Ptrend=0.10) and general health (Δβ coefficient = 12.8%, Ptrend=0.03). Exercisers who reduced waist circumference by ≥3.7 cm decreased bodily pain [Δ12month-baseline = 12.3 (95% CI: 3.7 to 20.9), P = 0.005] and increased general health [Δ12month-baseline = 3.1 (95% CI: 0.5 to 5.7), P = 0.02], while role-emotional scores declined [Δ12month-baseline = −11.1 (95% CI: −21.3 to −0.9), P = 0.03] compared with controls.
Figure 5.
Baseline and 12-month HRQOL and exercise self-efficacy scores stratified by percent changes in waist circumference (WC)
Ptrend testing a trend in change from baseline to 12 months across subgroups, adjusting for age, gender, and antidepressant use
*: p<0.05, †: p<0.01. P value comparing changes in HRQOL and exercise self-efficacy scores from baseline to 12 months between each adherence subgroup vs controls, adjusting for age, gender, and antidepressant use
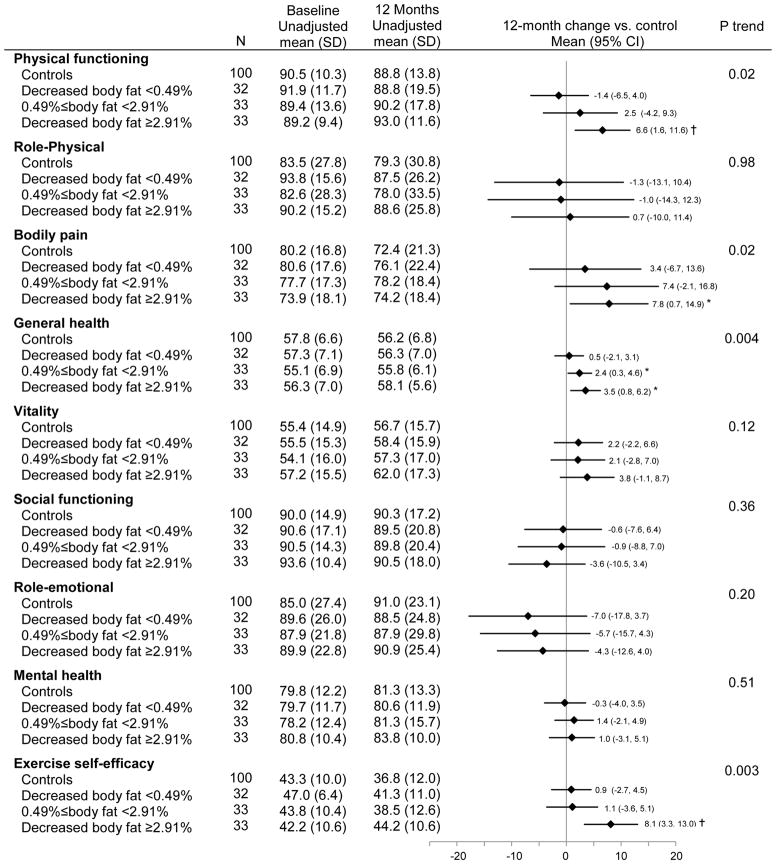
Percent body fat and 12-month changes in exercise self-efficacy and HRQOL
Greater reductions in percent body fat were associated with larger improvements in physical functioning (Ptrend = 0.02), bodily pain (Ptrend = 0.02), general health (Ptrend = 0.004) and exercise self-efficacy (Ptrend = 0.003). Adjusting for exercise self-efficacy had minimal effect on the relationship between percent body fat and physical functioning (Δβ coefficient = 10.0%, Ptrend=0.04), while exercise self-efficacy partially confounded the association of fitness with bodily pain (Δβ coefficient = 28.7%, Ptrend=0.10) and general health (Δβ coefficient =12.2%, Ptrend=0.01). When compared with controls, exercisers who reduced ≥2.91% of percent body fat significantly increased physical functioning [Δ12month-baseline 6.6 (95% CI: 1.6 to 11.6), P = 0.01], general health [Δ12month-baseline = 3.5 (95% CI: 0.8 to 6.2), P = 0.03] and exercise self-efficacy [Δ12month-baseline= 8.1 (95% CI: 3.3 to 13.0), P = 0.0004], and reduced bodily pain [Δ12month-baseline= 7.8 (95% CI: 0.7 to 14.9), P = 0.02].
Discussion
Higher adherence to the exercise prescription and greater improvements in cardiopulmonary fitness, body weight, fat mass, and waist circumference during a 12-month exercise intervention were associated with increased HRQOL and exercise self-efficacy. Our findings add to the current literature on the mechanisms of how exercise could improve HRQOL and exercise self-efficacy in exercise trials.
Our findings on adherence are consistent with those of other studies showing greater adherence to an exercise program may improve HRQOL. In a study of advanced heart failure patients, patients who successfully increased exercise ≥18% showed greater improvements in physical function and HRQOL compared with those who increased less than 18%.15 In a 12-week walking intervention, women who achieved ≥80% of exercise prescription showed significantly larger increases in vitality (HRQOL) compared with those who achieved <80% of the prescription.16
Higher adherence in this study was associated with a greater increase in exercise self-efficacy, which was consistent with previous studies.27,28 While previous exercise trials have found increases in exercise self-efficacy with a lower exercise dose,27,28 we observed a significant increase in exercise self-efficacy only among participants who exercised for ≥331 minutes/week compared to controls. The 5-item exercise self-efficacy questionnaire we used in this trial does not specify the amount of exercise in each question.21 It is possible that participants evaluated their exercise self-efficacy according to how closely they were meeting the currently prescribed exercise dose (i.e., 360 minutes/week), resulting in significant exercise self-efficacy increments only among participants exercising for ≥331 minutes/week (vs. controls).
The current study also found that positive changes in body composition parameters and cardiopulmonary fitness were related to exercise self-efficacy and HRQOL. However, the relationships between physiological parameters and HRQOL in prior weight loss and exercise studies have been mixed. In a weight loss program involving exercise and dietary weight loss, changes in cardiopulmonary fitness and weight loss were associated with favorable changes in physical composite scores (SF-36) in 5145 adults with type 2 diabetes.29 Similarly, other intervention studies using combined diet and exercise programs have shown that weight loss was associated with improved HRQOL.30–33 In contrast, exercise intervention studies have reported improved HRQOL without significant physiological changes. A 6-month exercise trial among 430 sedentary postmenopausal women found that HRQOL changes were independent of weight loss.34 In an exercise trial among 155 prostate cancer patients, participants improved HRQOL without any significant changes in body weight, BMI, waist circumference, or subcutaneous skinfolds.35
It is possible that the magnitude of weight change achieved through an exercise intervention may account for the conflicting findings on the association of weight loss with improved HRQOL. Compared with weight loss programs using dietary interventions, exercise generally produces minimal weight loss.36,37 The previously described exercise trials that did not find significant effects of weight loss on HRQOL observed small weight changes during the intervention which were not significantly different between the exercise and control groups.34,35 In our sample, only exercisers who lost more than 3.3% of baseline body weight showed significantly higher HRQOL compared to controls. Burns et al. showed that perceived weight is independently associated with HRQOL adjusting for objective weight in men.38 Thus, minimal weight change achieved through exercise interventions may not be perceived by participants and may therefore have limited effects on HRQOL.
Although prior exercise trials have found improvements in mental health aspects of HRQOL,11,34,39 we did not find any positive effect of adherence or physical changes on these subscales. Rather, we found reduced role-emotional scores among exercisers with higher adherence and greater reductions in waist circumference. It is possible that participants may have limited other recreational and social activities in order to adhere to 60 minutes/day, 6 days/ week of exercise, which may have negatively impacted their role-emotional and other mental health aspects of HRQOL. If this is confirmed in other studies, future efforts to increase exercise by a significant dose may consider promoting exercise using strategies that emphasize group aspects of the intervention or additional means of encouraging social interaction to promote social and emotional well-being.
Based on previous studies reporting a mediating role of self-efficacy on the relationships between exercise and QOL or self-esteem,6,7,9 we further tested confounding effects of exercise self-efficacy on the associations between adherence, physiological changes and HRQOL. Physical functioning was associated with body weight, percent body fat and cardiopulmonary fitness, and adjusting for exercise self-efficacy had minimum effects on the associations. Vitality was associated with adherence and weight loss, which became non-significant after adjusting for exercise self-efficacy. McAuley and colleagues showed that body fat, cardiopulmonary fitness, and exercise frequency affected different aspects of self-esteem and that these physiological parameter and exercise frequency directly affected self-esteem.10 The mechanisms behind improved HRQOL could be specific to each dimension of HRQOL, and the role of self-efficacy may differ between dimensions of HRQOL. Future studies are encouraged to test the mechanisms of how exercise and physiological changes affect HRQOL in various dimensions of HRQOL.
Although we did not test for the direction of the relationships including mediation effects, previous intervention studies have shown that weight loss 29,31 and improved cardiopulmonary fitness 14,29 mediated intervention effects on HRQOL while changes in HRQOL (weight-related quality of life) did not mediate weight loss.31 In addition, studies have shown that self-efficacy mediates exercise effects on HRQOL.6,7 Based on these findings, we speculate that adherence and physiological changes affected bodily pain and general health partially through their effects on exercise self-efficacy; vitality through exercise self-efficacy and physical functioning directly. However, the social cognitive theory states a reciprocal association of exercise with self-efficacy.1 Directions of the relationships between exercise adherence and physiological improvements with changes in exercise self-efficacy and HRQOL need to be investigated in future studies.
This study has several strengths including the use of validated measures to assess multiple physiological and psychosocial factors, a randomized controlled study design, a relatively large sample size (n=202), inclusion of both women and men, and a study duration of 12 months. Despite these strengths, there are several limitations. First, although the self-reported exercise adherence measure was associated with weight loss and fat reduction,19 there is a possibility of bias related to self-reporting. Second, the study generalizability is a limitation given that our study participants were highly selected because of the methods used for recruitment (e.g., physicians’ referral), inclusion criteria, and intervention. Future studies are needed to confirm our findings in more diverse populations. Finally, directions of the relationship between exercise adherence and physiological improvements with changes in exercise self-efficacy and HRQOL were not tested in this study.
In summary, this study examined the associations between adherence and physiological changes with HRQOL and exercise self-efficacy. We found that high adherence and favorable physiological changes (improved cardiopulmonary fitness, weight loss, and reductions in waist circumference and percent body fat) were associated with improvements in physical aspects of HRQOL and exercise self-efficacy, suggesting the importance of exercise adherence and physiological improvements for achieving positive effects on HRQOL and exercise self-efficacy through exercise. Monitoring adherence and tailoring exercise programs to induce changes in cardiopulmonary fitness and body composition may lead to greater improvements in HRQOL and self-efficacy that could influence the maintenance of exercise behaviors over time.
Supplementary Material
Figure 6.
Baseline and 12-month HRQOL and exercise self-efficacy scores stratified by changes in percent body fat
4 cases missing the baseline DXA assessment were excluded from the analysis (N=198).
Ptrend testing a trend in change from baseline to 12 months across subgroups, adjusting for age, gender, and antidepressant use
*: p<0.05, †: p<0.01. P value comparing changes in HRQOL and exercise self-efficacy scores from baseline to 12 months between each adherence subgroup vs controls, adjusting for age, gender, and antidepressant use
Acknowledgments
We wish to thank the study participants for their time and dedication to the study.
Funding source
This study was funded by R01 CA77572 and U54 CA116847 (Transdisciplinary Research on Energetics and Cancer) from the National Cancer Institute (NCI). A proportion of this work was conducted at the Clinical Research Center Facility, University of Washington funded by the National Institute of Health (M01-RR-00037) and the National Institute on Aging (AG1094). CEM received a fellowship from Canadian Institute of Health Research (CIHR). KFS received support from National Institutes of Health (NIH) 5KL2RR025015-03.
Footnotes
Trial registration:
This study is registered at www.clinicaltrials.gov (No. NCT00668161).
Contributor Information
Ikuyo Imayama, Email: iimayama@fhcrc.org, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center.
Catherine M. Alfano, Email: alfanoc@mail.nih.gov, Office of Cancer Survivorship, National Cancer Institute/National Institutes of Health
Caitlin E. Mason, Email: cmason@fhcrc.org, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center
Chiachi Wang, Email: cwan2@fhcrc.org, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center.
Liren Xiao, Email: lxiao@fhcrc.org, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center.
Catherine Duggan, Email: cduggan@fhcrc.org, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center.
Kristin L. Campbell, Email: kristin.campbell@ubc.ca, Faculty of Medicine, University of British Columbia
Karen E. Foster-Schubert, Email: kfoster@u.washington.edu, School of Medicine, University of Washington
Anne McTiernan, Email: amctiern@fhcrc.org, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center; School of Medicine & School of Public Health, University of Washington.
References
- 1.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 2.Rejeski WJ, Shelton B, Miller M, Dunn AL, King AC, Sallis JF. Mediators of increased physical activity and change in subjective well-being: results from the Activity Counseling Trial (ACT) J Health Psychol. 2001;6:159–168. doi: 10.1177/135910530100600206. [DOI] [PubMed] [Google Scholar]
- 3.McAuley E, Jerome GJ, Elavsky S, Marquez DX, Ramsey SN. Predicting long-term maintenance of physical activity in older adults. Prev Med. 2003;37:110–118. doi: 10.1016/s0091-7435(03)00089-6. [DOI] [PubMed] [Google Scholar]
- 4.McAuley E, Lox C, Duncan TE. Long-term maintenance of exercise, self-efficacy, and physiological change in older adults. J Gerontol. 1993;48:218–224. doi: 10.1093/geronj/48.4.p218. [DOI] [PubMed] [Google Scholar]
- 5.Jones F, Harris P, Waller H, Coggins A. Adherence to an exercise prescription scheme: the role of expectations, self-efficacy, stage of change and psychological well-being. Br J Health Psychol. 2005;10:359–378. doi: 10.1348/135910704X24798. [DOI] [PubMed] [Google Scholar]
- 6.McAuley E, Konopack JF, Motl RW, Morris KS, Doerksen SE, Rosengren KR. Physical activity and quality of life in older adults: influence of health status and self-efficacy. Ann Behav Med. 2006;31:99–103. doi: 10.1207/s15324796abm3101_14. [DOI] [PubMed] [Google Scholar]
- 7.McAuley E, Doerksen SE, Morris KS, et al. Pathways from physical activity to quality of life in older women. Ann Behav Med. 2008;36:13–20. doi: 10.1007/s12160-008-9036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg M. Society and the adolescent self-image. Prinston, NJ: Prinstin University Press; 1965. [Google Scholar]
- 9.Sonstroem RJ, Morgan WP. Exercise and self-esteem: rationale and model. Med Sci Sports Exerc. 1989;21:329–337. [PubMed] [Google Scholar]
- 10.McAuley E, Blissmer B, Katula J, Duncan TE, Mihalko SL. Physical activity, self-esteem, and self-efficacy relationships in older adults: a randomized controlled trial. Ann Behav Med. 2000;22:131–139. doi: 10.1007/BF02895777. [DOI] [PubMed] [Google Scholar]
- 11.Bowen DJ, Fesinmeyer MD, Yasui Y, et al. Randomized trial of exercise in sedentary middle aged women: effects on quality of life. Int J Behav Nutr Phys Act. 2006;3:34. doi: 10.1186/1479-5868-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 13.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care. 2003;12:347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 14.Courneya KS, Sellar CM, Stevinson C, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27:4605–4612. doi: 10.1200/JCO.2008.20.0634. [DOI] [PubMed] [Google Scholar]
- 15.Evangelista LS, Hamilton MA, Fonarow GC, Dracup K. Is exercise adherence associated with clinical outcomes in patients with advanced heart failure? Phys Sportsmed. 2010;38:28–36. doi: 10.3810/psm.2010.04.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aurilio LAM. PhD thesis. Pittsburgh, PA: University of Pittsburgh; 2000. Promotion of adoption and adherence to regular leisure-time walking behavior in healthy mid-life women: A randomized controlled study. [Google Scholar]
- 17.McTiernan A, Yasui Y, Sorensen B, et al. Effect of a 12-month exercise intervention on patterns of cellular proliferation in colonic crypts: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1588–1597. doi: 10.1158/1055-9965.EPI-06-0223. [DOI] [PubMed] [Google Scholar]
- 18.Pate RR, Blair SN, Durstine JL, et al. Guidelines for Exercise Testing and Prescription. 4. Philadelphia, PA: Lea & Febiger; 1991. [Google Scholar]
- 19.McTiernan A, Sorensen B, Irwin ML, et al. Exercise effect on weight and body fat in men and women. Obesity. 2007;15:1496–1512. doi: 10.1038/oby.2007.178. [DOI] [PubMed] [Google Scholar]
- 20.Schauer JE, Hanson P. Usefulness of a branching treadmill protocol for evaluation of cardiac functional capacity. Am J Cardiol. 1987;60:1373–1377. doi: 10.1016/0002-9149(87)90622-9. [DOI] [PubMed] [Google Scholar]
- 21.Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1992;63:60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- 22.Yates T, Davies M, Gorely T, Bull F, Khunti K. Effectiveness of a pragmatic education program designed to promote walking activity in individuals with impaired glucose tolerance: a randomized controlled trial. Diabetes Care. 2009;32:1404–1410. doi: 10.2337/dc09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folta SC, Lichtenstein AH, Seguin RA, Goldberg JP, Kuder JF, Nelson ME. The Strong Women-Healthy Hearts program: reducing cardiovascular disease risk factors in rural sedentary, overweight, and obese midlife and older women. Am J Public Health. 2009;99:1271–1277. doi: 10.2105/AJPH.2008.145581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ware JE. SF 36 health survey: manual and interpretation guide. Boston, MA: The Health Institute New England Medical Center; 1993. [Google Scholar]
- 25.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Imayama I, Alfano CM, Cadmus Bertram LA, et al. Effects of 12-month exercise on health-related quality of life: a randomized controlled trial. Prev Med. 2011;52:344–351. doi: 10.1016/j.ypmed.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher KI, Jakicic JM, Napolitano MA, Marcus BH. Psychosocial factors related to physical activity and weight loss in overweight women. Med Sci Sports Exerc. 2006;38:971–980. doi: 10.1249/01.mss.0000218137.25970.c6. [DOI] [PubMed] [Google Scholar]
- 28.Castro CM, Sallis JF, Hickmann SA, Lee RE, Chen AH. A prospective study of psychosocial correlates of physical activity for ethnic minority women. Psychol Health. 1999;14:277–293. [Google Scholar]
- 29.Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med. 2009;169:163–171. doi: 10.1001/archinternmed.2008.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontaine KR, Barofsky I, Andersen RE, et al. Impact of weight loss on health-related quality of life. Qual Life Res. 1999;8:275–277. doi: 10.1023/a:1008835602894. [DOI] [PubMed] [Google Scholar]
- 31.Palmeira AL, Markland DA, Silva MN, et al. Reciprocal effects among changes in weight, body image, and other psychological factors during behavioral obesity treatment: a mediation analysis. Int J Behav Nutr Phys Act. 2009;6:9. doi: 10.1186/1479-5868-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross KM, Milsom VA, Rickel KA, et al. The contributions of weight loss and increased physical fitness to improvements in health-related quality of life. Eat Behav. 2009;10:84–88. doi: 10.1016/j.eatbeh.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darga LL, Magnan M, Mood D, Hryniuk WM, DiLaura NM, Djuric Z. Quality of life as a predictor of weight loss in obese, early-stage breast cancer survivors. Oncol Nurs Forum. 2007;34:86–92. doi: 10.1188/07.ONF.86-92. [DOI] [PubMed] [Google Scholar]
- 34.Martin CK, Church TS, Thompson AM, Earnest CP, Blair SN. Exercise dose and quality of life: a randomized controlled trial. Arch Intern Med. 2009;169:269–278. doi: 10.1001/archinternmed.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21:1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 36.Skender ML, Goodrick GK, Del Junco DJ, et al. Comparison of 2-year weight loss trends in behavioral treatments of obesity: diet, exercise, and combination interventions. J Am Diet Assoc. 1996;96:342–346. doi: 10.1016/S0002-8223(96)00096-X. [DOI] [PubMed] [Google Scholar]
- 37.Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of Diet and Exercise, Alone or Combined, on Weight and Body Composition in Overweight-to-Obese Postmenopausal Women. Obesity. 2011 doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burns CM, Tijhuis MA, Seidell JC. The relationship between quality of life and perceived body weight and dieting history in Dutch men and women. Int J Obes Relat Metab Disord. 2001;25:1386–1392. doi: 10.1038/sj.ijo.0801714. [DOI] [PubMed] [Google Scholar]
- 39.Atlantis E, Chow CM, Kirby A, Singh MF. An effective exercise-based intervention for improving mental health and quality of life measures: a randomized controlled trial. Prev Med. 2004;39:424–434. doi: 10.1016/j.ypmed.2004.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.