Abstract
Tissue and organ replacement is required when there are no alternative therapies available. Although vascular tissue engineering was originally developed to meet the clinical demands of small-diameter vascular conduits as bypass grafts, it has evolved into a highly advanced field where perfusable vasculatures are generated for implantation. Herein, we review several cutting-edge techniques that have led to implantable human blood vessels in clinical trials, the novel approaches that build complex perfusable microvascular networks in functional tissues, the use of stem cells to generate endothelial cells for vascularization, as well as the challenges in bringing vascular tissue engineering technologies into the clinics.
1. Introduction
Tissue engineering is a continuously growing field that provides novel therapeutic strategies to repair and replace damaged and diseased organs and tissues. Among the various aspects of tissue engineering, vascular engineering is of great importance to generate implantable blood vessels, and to provide vascularization in other functional tissues for implantation.
As cardiovascular diseases remain the number one killer in the U.S. and worldwide [1], there is a constantly high demand of vascular grafts [especially small-diameter (< 6 mm) vascular grafts] for replacement therapy. Although autologous vessels (e.g. saphenous veins) and synthetic polymer grafts (e.g. GORE® Hybrid Vascular Graft, Gore Medical) have been commonly used, the shortage of autologous blood vessels and the limitation of synthetic grafts have prompted the search for alternative vascular grafts [2•,3]. In the last two decades, various approaches have been developed to generate biological vascular grafts (diameter > 1 mm) for implantation, and some of these techniques have led to engineered blood vessels already or currently under human clinical trials (Table 1).
Table 1.
Tissue engineered blood vessels in human clinical trials
| Groups | Approaches | Methods | Clinical applications | References |
|---|---|---|---|---|
| Niklason/Humacyte | Biodegradable scaffold-based | Human SMCs cultured on PGA scaffold under pulsatile stretching, decellularized | AV shunts for dialysis | Poland (ClinicalTrials.gov Identifier: NCT01744418) |
| U. S. (ClinicalTrials.gov Identifier: NCT01840956 | ||||
| L'Heureux/Cytograft | Cell self-assembly | ECM sheets containing human fibroblasts rolled into tubular structure, cultured to allow fusion between sheets | AV shunts for dialysis | Argentina and Poland [14,15] |
| Shinoka and Breuer | In vivo bioreactor | Autologous BM-MNC briefly cultured on polycaprolactone–polylactic acid copolymer with PGA, recruiting cells in vivo for remodeling | Extracardiac cavopulmonary conduits | Japan [18,19] |
| U.S. [20,21] |
In contrast, one of the most pressing challenges in the field of tissue engineering is to achieve vascularization in engineered tissues and organs [4]. As neovascularization (growth of new capillaries) upon implantation into host is very slow, prevascularization of thicker tissue constructs by formation of well-connected networks of capillaries prior to implantation followed by anastomosis with host's microvasculature appears to be essential for the success of implantation [5••,6••]. However, building perfusable vascular networks that mimic the complex and highly organized natural vascular architecture in tissue and organ subtends even greater challenges than engineering blood vessels and limits clinical applications.
In this review, we will discuss current state-of-the-art regarding perfusable vasculature, including blood vessels and microvascular networks, with a focus on those already in clinical trials or in large animals with the potential to be translated into humans. As the endothelial cell (EC) is the key player in microvascular engineering, we will also discuss recent development in obtaining autologous ECs for building implantable, perfusable vasculature.
2. Building engineered blood vessels
Utilization of tissue engineering approaches to generate biological vascular grafts started with Bell and colleagues in 1986 when they reported the construction of an artery in vitro for the first time [7]. In this pioneering study, bovine smooth muscle cells (SMCs) were cultured in collagen gels to produce the media layer of an artery, onto which adventitial fibroblasts were seeded to produce the adventitial layer. ECs were then seeded into the lumen. Although these grafts structurally mimicked a native artery, they lacked the functions of a native artery as their burst pressure was very low. Nevertheless, from then on, many approaches have been developed to engineer a vascular graft that mimics native vessel vis-a-vis mechanical properties and extracellular matrix (ECM) composition (mainly collagen and elastin). Overall, these different methods can be grouped into scaffold-based ones and scaffold-free ones, and tissue engineered blood vessels are generated either in a bioreactor or in the body [2•,8]. Notably three vascular engineering technologies have led to products with clinical trials in the U.S. and elsewhere (Table 1), thereby demonstrating the potential of using tissue engineering strategies to generate implantable blood vessels for clinical applications.
2.1 Biodegradable scaffold approach
In 1999, Niklason and colleagues reported the use of polyglycolic acid (PGA) scaffold and cultured SMCs to generate a functional artery. Engineering vessels had a burst pressure of 2150 mmHg, higher than that of human saphenous veins (1680 mmHg [9]), the gold standard small-diameter bypass conduits. When implanted into the right saphenous artery in pigs, the grafts remained patent for at least 24 days. This study demonstrated the potential of using PGA scaffolds to generate robust blood vessels by supporting SMC growth and collagen matrix deposition, and showed the importance of pulsatile stretching in improving mechanical strength. However, polymer remnants in the engineered vessels can substantially degrade tissue mechanics, and the lack of mature elastin impairs vessel compliance [10]. To overcome some of these issues, we have developed various approaches such as designing faster-degrading bio-polymers [11].
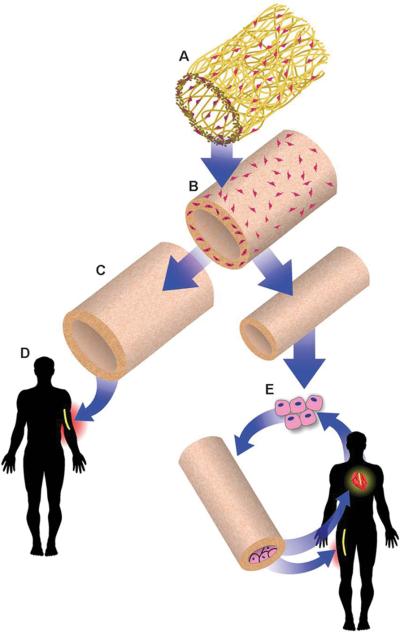
In 2011, Dahl and coworkers from Humacyte Inc. reported the construction of human vascular grafts using cadaver SMCs and the implantation of these vessels after decellularization into baboons as arteriovenous (AV) conduits [12••]. The engineered constructs maintained blood flow for up to 6 months. After this preclinical success, these decellularized allogenic grafts were implanted as AV conduits in a small number of patients with kidney failure in Poland starting in December 2012. This European Phase I trial was followed by the first U.S. clinical implant in June 2013 (Figure 1). These trials are currently ongoing, and the advantage of this approach is that the decellularized grafts can be stored for at least 12 months [12••] and used “off-the-shelf” (Figure 2), reducing patient wait time by weeks to months [13]. Furthermore, the acellular human-based grafts do not appear to cause significant immune responses in (non-human) primate hosts. When seeded with autologous ECs, they can be implanted as small-diameter vascular grafts with low rates of thrombosis [2••]. A remaining challenge, however, is the donor age-dependency and donor-to-donor variation in the abilities of human SMCs to make ECM in vitro. In response, we have used alternative cell sources such as human inducible pluripotent stem cell (iPSC)-derived SMCs [14], which may help overcome these limitations in the long term.
Figure 1.
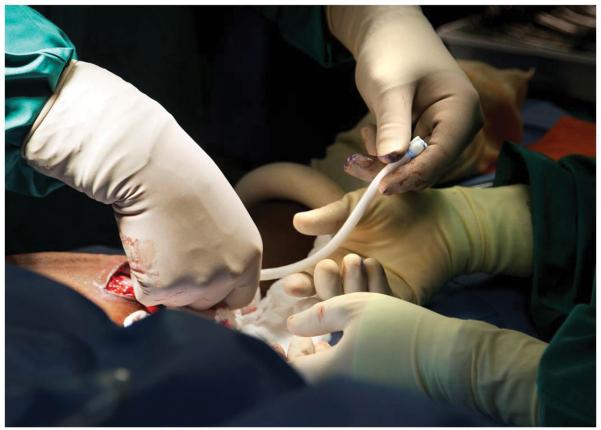
Implantation of an engineered human vascular graft into the arm of a patient as an AV shunt. Reproduced with the permission of Shawn Rocco, Duke Medicine.
Figure 2.
Human SMCs are cultured on the PGA scaffolds (A) to produce the vascular grafts (B), which are then decellularized (C) and stored until the time of patient need. The acellular human-based vascular grafts may be directly implanted without ECs if the diameters are greater than 6 mm (D), or may be seeded with ECs from the patient for small-diameter (3 to 4 mm) applications. From [12]. Reprinted with permission from AAAS.
2.2 Cell self-assembly approach
In 1998, L'Heureux and colleagues reported the generation of a functional blood vessel in the absence of scaffold [15]. Tissue sheets were generated by growing human SMCs and fibroblasts to over-confluency and then assembled to a tubular structure that mimicked a native artery. Engineered grafts had a burst pressure of 2594 mmHg and were implanted into dogs for up to 7 days, however, blood infiltration between cell sheet layers was observed. To improve this unsatisfactory result and simplify the process, later studies used only fibroblast sheets but increased maturation time to allow better fusion between cell sheets [9]. These new grafts had a burst pressure of 3468 mmHg and remained patent without blood filtration into the vessel wall for up to 8 weeks when implanted as interposition arterial grafts in primates [9].
Clinical trials to test the durability of these cell sheet-based human vascular grafts (Lifeline™ grafts, Cytograft) as AV shunts began in 2004 in Argentina and Poland [16,17]. Out of 10 grafts implanted, 5 remained patent for 6-20 months while 3 failed within the first 3 months. Unrolling of tissue sheets under high blood flow was one of the main causes for the failure [16], and has driven researchers to further optimize the manufacturing process. Nevertheless, the cell self-assembly approach generates completely biological and mechanically robust vascular grafts without the potential detrimental effects of biodegradable scaffolds. In addition, grafts can be constructed using either autologous or allogenic cells, devitalized and stored “on-the-shelf” [18]. The amount of time required for engineering the vessels, the lack of mature elastin, and the potential of unrolling, however, may remain limiting factors in bringing this technology directly to patients.
2.3 “Body as a bioreactor” approach
In 2001, Shinoka and coworkers reported the first application of a tissue engineered blood vessel in a human [19]. Cells were harvested from patient's peripheral vein and cultured for 10 days on a tubular scaffold made from polycaprolactone–polylactic acid copolymer that was reinforced with PGA. The engineered blood vessel was subsequently implanted as a pulmonary artery graft into the patient and remained patent for at least 7 months. Since then, tissue engineered constructs were implanted in over 40 children in Japan, either as extracardiac total cavopulmonary connections (25 patients) or as tissue engineered patches (19 patients), and met with unquestionable success [20,21]. There was no graft-related mortality, and grafts remained functional after a mean follow up of 5.8 years and only developed limited stenosis. Over the course of the clinical studies of this graft, two major changes occurred in generating these grafts as compared to the first one used in human. Namely (1) bone marrow-derived mononuclear cells (BM-MNCs) were used instead of cells from the peripheral vein; and (2) cells were only cultured on the polymer scaffold for 2 hours instead of a few days prior to implantation. These innovative changes make the process of cell harvesting less invasive and have allowed the grafts to be constructed directly in the operating room.
Following the satisfactory outcomes in Japan, the first U.S. clinical implant was performed in August 2011. As reported by Shinoka, Breuer and colleagues, the result was positive after 6 months and 3 years [3,22•]. Animal studies suggest that by recruiting SMCs and ECs from the neighboring host vessel, BM-MNC-seeded grafts convert into mature blood vessels after implantation into the body [23]. Their ability to grow with their pediatric recipients makes these grafts particularly attractive for use in young patients. However, compared with other engineered blood vessels, BM-MNC-seeded grafts can only be used in a low-pressure circulatory system, due to the lack of mature ECM and mechanical strength prior to implantation.
These aforementioned tissue engineering approaches vary in many ways (including cells, scaffolds and bioreactors), but they all depend upon the ability of cells to make and deposit ECM to form the wall of a vessel. Based upon recent progress in early trials, it is likely that functional vascular grafts will become standard therapy in the clinic in the not-too-distant future.
3. Engineering perfusable microvascular networks
It has been widely accepted that oxygen is the limiting factor for cell survival in large cultured tissues [24], and the diffusion limit for oxygen is approximately 150 - 200 μm [25]. Therefore, perfusable microvascular networks are needed to transport oxygen and nutrition and to remove waste for cell survival in thicker tissues and organs such as heart, lung, liver and kidney.
Microvessels and capillaries can be generated by two mechanisms, including angiogenesis and vasculogeneis [26,27]. Numerous technologies have been developed to vascularize tissues and organs [4,28,29], and they include cell-based strategies that rely on the ability of ECs to from 3-D microcapillary-like structures on their own in vitro [30], and scaffold-based strategies wherein synthetic or bio-scaffolds support EC survival and promote microvessel formation and maturation. Microfluidic platforms are one the most well-studied techniques for creating microvasculature in tissues to be implanted and for “body-on-a-chip” applications [31••], and excellent reviews can be found in this issue and elsewhere (eg [32-36]). Other approaches to create perfusable 3D microvascular networks in engineered constructs include bio-printing [37•] or 3D sacrificial moulding [38••,39]. Although microfabrication approaches are ideal for the construction of capillary-scale micro-channels for immediate perfusion and prevascularization, they have not been successful in recapitulating the complexity of natural vascular architecture.
3.1 Decellularized scaffold approach
The use of a decellularized whole-organ as a scaffold for vascularization has become an alternative approach having great potential. When done carefully, decellularization removes most cellular components and antigens, creates an ECM scaffold of decreased immunogenicity, while retaining an intact vascular template for EC attachment to form a perfusable vasculature, and at the same time preserving biocompatible ECM proteins for repopulation by tissue-specific cells [40]. Generally, a perfusable bioreactor is used to culture these whole organs by providing oxygen and nutrients, and by providing shear stress that is essential for EC functional differentiation [41].
In 2008, Ott and colleagues first reported the regeneration of a rat heart using a decellularized scaffold [42]. An intact rat heart was decellularized by coronary perfusion and subsequently repopulated with rat ECs and cardiocytes. ECs formed a single layer aligning the lumen of the coronary vessels after 7 days’ perfusion culture, while neonatal rat cardiomyocytes formed thicker myofibers (0.25-1.1 mm in thickness) after up to 28 days’ perfusion culture. Heart's pump function (2% of adult heart function) was observed in the engineered rat heart. Although preliminary, this work opened up new possibilities for culturing whole organs for implantation.
From then on, several groups have published the regeneration of whole organs such as lung, liver, kidney and pancreas, some of which were also implanted into animals and performed organ-specific functions (Supplement Table 1). In 2010, we and others reported the implantation of an engineered lung into rats [43,44]. In our study, an intact rat lung was decellularized, subsequently seeded with endothelial and epithelial cells, and cultured under vascular perfusion and airway ventilation. Neonatal rat lung epithelial cells repopulated the decellularized scaffold and displayed different specific lung epithelial markers, while rat microvascular ECs were found throughout the scaffold vasculature. When the engineered lungs were implanted into rats, they were perfusable for up to 2 hours in vivo and exhibited gas exchange. However, bleeding into the airways was observed in the engineered lungs, due to the damage of capillaries during decellularization and during mechanical ventilation after implantation. In Ott and colleague's study, although no visible airway bleeding was found in the implanted engineered lungs, pulmonary edema developed after 6 hours, indicating damage of the lung matrix. Preserving native ECM architecture thus is critical for whole organ regeneration, and maintaining intact microvascular architecture in decellularized scaffolds for subsequent vascularization remains one of the biggest challenges. Nevertheless, promising results in these pioneering efforts have encouraged us and others to improve decellularization processes to preserve ECM structures, and to develop approaches to regenerate whole organs from large animals (Supplement Table 1).
3.2 Arteriovenous (AV) loop approach
Building perfusable vascular networks can also be achieved by using the body as a bioreactor. One of these approaches utilizes the ability of host macroscopic blood vessels to develop a microvasculature in the engineered constructs within an AV loop. Recent reports by Dalaere and colleagues described the first well-documented implantation of a pre-vascularized trachea into a patient [45]. In this study, the donor's trachea graft was heterotopically implanted into the recipient's forearm by wrapping with fascia and connective tissue. Capillary networks sprouted from the recipient's fascial blood vessels to vascularize the tracheal graft, which was removed from the forearm and transplanted to the defect area in trachea, and remained functional for at least 1 year. The AV loop approach provides a promising alternative method for generating vascularized constructs for clinical applications; however, the need for multiple surgical interventions remains its major disadvantage.
Although it will be a long time before engineered whole organs can be used in the clinic, these developments have shown the promise of generating prevascularized tissues/organs for future implantation.
4. Endothelial cells for vascular engineering
ECs form the inner layer of blood vessel and play an important role in maintaining vessel wall permeability barrier and regulating coagulation, and as mediators of inflammation and many other physiological processes [46]. ECs are the critical element in vascular engineering, as they prevent thrombosis in small-diameter vascular grafts upon implantation and are the essential player for building perfusable microvasculature in tissue engineered constructs. Ideally, in order to use prevascularized engineered constructs in patients, ECs should be autologous and easy to acquire (in large numbers if we are to vascularize an entire organ in vitro). As 30 million ECs were used in the revascularization of a decellularized rat lung, for an animal weighing on 250 g [43], billions or trillions of cells may be required for revascularizing human-sized organs.
Although autologous microvascular ECs and endothelial progenitor cells (EPCs) [47] are capable of forming blood vessels, their clinical applications in prevascularizing engineered tissues/organs are hindered by the limitation of obtaining sufficient numbers from these sources. In comparison, pluripotent stem cells have provided revolutionarily new possibilities for obtaining unlimited amount of ECs for tissue engineering applications. Levenberg and coworkers first reported the differentiation of human embryonic stem cells (ESCs) to ECs through the formation of embryoid bodies (EBs) [48]. These differentiated cells expressed CD31 and other EC-related markers, showed acetylated LDL uptake, and formed tubular structures in vitro, mimicking native ECs. The EB strategy remains one of the most commonly used approach for generating ECs from pluripotent stem cells [49••]. However, issues associated with ethics concerns, tumorigenicity, and immunogenicity have rendered human ESCs inappropriate for clinical applications heretofore.
Human iPSCs, on the other hand, can be derived from autologous cells [50,51], thus eliminating the rejection issues associated with ESCs. In addition, transgene-free and vector-free human iPSCs can be generated using the non-integrating episomal system [52], sendai virus [53], or by directly delivering reprogramming proteins to somatic cells [54], thus eliminating their tumorigenic potential. ECs differentiated from episomal vector-generated human iPSCs exhibit characteristic markers and functions of native ECs and are currently commercially available as iCell® Endothelial Cells (Cellular Dynamics International). As such, human iPSCs offer the advantages of providing a possibly unlimited number of autologous ECs to vascularize tissue engineered constructs for implantation. However, it's worth noting that the phenotypes of ECs vary greatly depending on their origins in body, and ECs change their phenotypes when cultured in vitro due to changes in their microenvironments [55,56]. Rufaihah and coworkers recently discovered that iPSC-derived ECs contained both venous and arterial subtypes [57•], suggesting that special consideration has to be taken when using iPSC-derived ECs in vascular engineering.
5. Conclusion
Tissue engineering approaches may one day provide patients suffering from organ and tissue failure with unprecedented therapeutic options. In particular, development in vascular engineering has made it possible to generate functional tissues/organs for implantation. Thanks to the advancement in human iPSC technology, obtaining unlimited amount of autologous cells including ECs (and tissue specific cells) has become conceivable, although it's still a big challenge to obtain clinical grade human cells sufficient for whole organ regeneration. In addition, improving the cost-effectiveness of current approaches is also to be considered for its translation in to the clinic in the future.
Supplementary Material
Highlights.
Tissue engineering approaches have generated blood vessels in clinical trials
Challenges remain to build perfusable microvasculature for implantation
Decellularization provides a novel strategy for pre-vascularization
Generation of human ECs for vascularization can be achieved using iPSCs
Acknowledgements
This work was supported by National Institutes of Health grants R01HL118245-01A1 (to Dai, Gui Co-I), HL083895-06A1 and P01-HL107205-01A1 (both to Niklason).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
L.E.N. has a financial interest in Humacyte, Inc, a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart Disease and Stroke Statistics--2013 Update: A Report From the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Cleary MA, Geiger E, Grady C, Best C, Naito Y, Breuer C. Vascular tissue engineering: the next generation. Trends Mol Med. 2012;18:394–404. doi: 10.1016/j.molmed.2012.04.013. [An overview of the current development in the generation of blood vessels, with focus on those used in the pulmonary system.] [DOI] [PubMed] [Google Scholar]
- 3.Kurobe H, Maxfield MW, Breuer CK, Shinoka T. Concise review: tissue-engineered vascular grafts for cardiac surgery: past, present, and future. Stem Cells Transl Med. 2012;1:566–571. doi: 10.5966/sctm.2012-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63:300–311. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 5••.Auger FA, Gibot L, Lacroix D. The pivotal role of vascularization in tissue engineering. Annu Rev Biomed Eng. 2013;15:177–200. doi: 10.1146/annurev-bioeng-071812-152428. [A recent comprehensive review on the roles and approaches of vascularization for functional tissues/organs.] [DOI] [PubMed] [Google Scholar]
- 6•.Baiguera S, Ribatti D. Endothelialization approaches for viable engineered tissues. Angiogenesis. 2013;16:1–14. doi: 10.1007/s10456-012-9307-8. [A recent review on different approaches of endothelialization.] [DOI] [PubMed] [Google Scholar]
- 7.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 8.Peck M, Gebhart D, Dusserre N, McAllister TN, L'Heureux N. The Evolution of Vascular Tissue Engineering and Current State of the Art. Cells Tissues Organs. 2011;195:144–158. doi: 10.1159/000331406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.L'Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NA, Kyles AE, Gregory CR, Hoyt G, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361–365. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahl SL, Rhim C, Song YC, Niklason LE. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng. 2007;35:348–355. doi: 10.1007/s10439-006-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gui L, Zhao L, Spencer RW, Burghouwt A, Taylor MS, Shalaby SW, Niklason LE. Development of novel biodegradable polymer scaffolds for vascular tissue engineering. Tissue Eng Part A. 2011;17:1191–1200. doi: 10.1089/ten.tea.2010.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Dahl SLM, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, et al. Readily Available Tissue-Engineered Vascular Grafts. Science Translational Medicine. 2011;3:68ra69–68ra69. doi: 10.1126/scitranslmed.3001426. [Authors describe the construction of human vascular grafts that can be used “off-the-shelf” and the pre-clinical outcome as AV shunts in non-human primates. This study leads to the first U.S. implant of an engineered human artery.] [DOI] [PubMed] [Google Scholar]
- 13.Dahl SL, Blum JL, Niklason LE. Bioengineered vascular grafts: can we make them off-the-shelf? Trends Cardiovasc Med. 2011;21:83–89. doi: 10.1016/j.tcm.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Sundaram S, Niklason LE. Smooth Muscle and Other Cell Sources for Human Blood Vessel Engineering. Cells Tissues Organs. 2011 doi: 10.1159/000331409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.L'Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. Faseb J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 16.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440–1446. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 17.L'Heureux N, McAllister TN, de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med. 2007;357:1451–1453. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]
- 18.Wystrychowski W, Cierpka L, Zagalski K, Garrido S, Dusserre N, Radochonski S, McAllister TN, L'Heureux N. Case study: first implantation of a frozen, devitalized tissue-engineered vascular graft for urgent hemodialysis access. J Vasc Access. 2011;12:67–70. doi: 10.5301/jva.2011.6360. [DOI] [PubMed] [Google Scholar]
- 19.Shin'oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344:532–533. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 20.Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, Shinoka T. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139:431–436. 436, e431–432. doi: 10.1016/j.jtcvs.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 21.Shinoka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–1338. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 22•.Breuer CK, Shinoka T, Snyder E. Seeding Tissue-Engineered Vascular Grafts in a Closed, Disposable Filter–Vacuum System. BioProcess International. 2013;11:52–56. [Authors describe a vacuum seeding system that can simplify the construction of vascular grafts for future clinical applications.] [Google Scholar]
- 23.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, Yi T, Mirensky TL, Nalbandian A, Udelsman B, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107:4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 25.Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J Exp Med. 1973;138:745–753. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 27.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 28.Tian L, George SC. Biomaterials to prevascularize engineered tissues. J Cardiovasc Transl Res. 2011;4:685–698. doi: 10.1007/s12265-011-9301-3. [DOI] [PubMed] [Google Scholar]
- 29.Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 31••.Sung JH, Esch MB, Prot JM, Long CJ, Smith A, Hickman JJ, Shuler ML. Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab Chip. 2013;13:1201–1212. doi: 10.1039/c3lc41017j. [A recent review on the construction of body-on-a-chip system using microfabrication technology.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong KH, Chan JM, Kamm RD, Tien J. Microfluidic models of vascular functions. Annu Rev Biomed Eng. 2012;14:205–230. doi: 10.1146/annurev-bioeng-071811-150052. [DOI] [PubMed] [Google Scholar]
- 33.Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borenstein JT, Vunjak-Novakovic G. Engineering tissue with BioMEMS. IEEE Pulse. 2011;2:28–34. doi: 10.1109/MPUL.2011.942764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borenstein JT, Weinberg EJ, Orrick BK, Sundback C, Kaazempur-Mofrad MR, Vacanti JP. Microfabrication of three-dimensional engineered scaffolds. Tissue Eng. 2007;13:1837–1844. doi: 10.1089/ten.2006.0156. [DOI] [PubMed] [Google Scholar]
- 36.Barber RW, Emerson DR. Biomimetic design of artificial micro-vasculatures for tissue engineering. Altern Lab Anim. 2010;38(Suppl 1):67–79. doi: 10.1177/026119291003801S02. [DOI] [PubMed] [Google Scholar]
- 37•.Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. 2013;60:691–699. doi: 10.1109/TBME.2013.2243912. [An overview on the organ regeneration using rapid prototyping technology.] [DOI] [PubMed] [Google Scholar]
- 38••.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [Authors describe the generation of living tissues with perfusable 3D microvascular networks using a biocompatible carbohydrate glass as the sacrificial template.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellan LM, Singh SP, Henderson PW, Porri TJ, Craighead HG, Spector JA. Fabrication of an artificial 3-dimensional vascular network using sacrificial sugar structures. Soft Matter. 2009;5:1354–1357. [Google Scholar]
- 40.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Bijonowski BM, Miller WM, Wertheim JA. Bioreactor design for perfusion-based, highly-vascularized organ regeneration. Curr Opin Chem Eng. 2013;2:32–40. doi: 10.1016/j.coche.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 43.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010 doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 45.Delaere P, Vranckx J, Verleden G, De Leyn P, Van Raemdonck D, Leuven Tracheal Transplant G. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med. 2010;362:138–145. doi: 10.1056/NEJMoa0810653. [DOI] [PubMed] [Google Scholar]
- 46.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 47.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 48.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Wong WT, Huang NF, Botham CM, Sayed N, Cooke JP. Endothelial cells derived from nuclear reprogramming. Circ Res. 2012;111:1363–1375. doi: 10.1161/CIRCRESAHA.111.247213. [A comprehensive review on the generation of ECs from pluripotent stem cells and their function in tissue vascularization.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 51.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 52.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 56.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 57•.Rufaihah AJ, Huang NF, Kim J, Herold J, Volz KS, Park TS, Lee JC, Zambidis ET, Reijo-Pera R, Cooke JP. Human induced pluripotent stem cell-derived endothelial cells exhibit functional heterogeneity. Am J Transl Res. 2013;5:21–35. [Authors describe the heterogeneity of human iPSC-differentiated ECs and discuss the approach for generating subtype-specific cells for vascular engineering.] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.