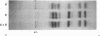Abstract
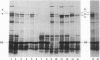
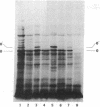
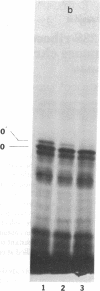
In a DNA-dependent protein-synthesizing system that contains streptomycin-sensitive ribosomes, lambda DNA directs the synthesis of two proteins that are products of the O gene. The larger is produced as a result of read-through of a UGA termination codon. In the system containing streptomycin-resistant ribosomes this read-through protein is not synthesized, indicating that the mutational alteration in the ribosomal protein S12 restricts the read-through. The mutant ribosomes also fail to synthesize the read-through coat protein of RNA phage Qbeta. In addition, the mutant ribosomes restrict suppression of amber mutations in vitro, similar to their effect in vivo.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfort M., Noff D., Oppenheim A. B. Isolation, characterization and deletion mapping of amber mutations in the cll gene of phage lambda. Virology. 1975 Jan;63(1):147–159. doi: 10.1016/0042-6822(75)90380-3. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- CAMPBELL A. Sensitive mutants of bacteriophage lambda. Virology. 1961 May;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- Folkmanis A., Takeda Y., Simuth J., Gussin G., Echols H. Purification and properties of a DNA-binding protein with characteristics expected for the Cro protein of bacteriophage lambda, a repressor essential for lytic growth. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2249–2253. doi: 10.1073/pnas.73.7.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GESTELAND R. F., BOEDTKER H. SOME PHYSICAL PROPERTIES OF BACTERIOPHAGE R17 AND ITS RIBONUCLEIC ACID. J Mol Biol. 1964 Apr;8:496–507. doi: 10.1016/s0022-2836(64)80007-3. [DOI] [PubMed] [Google Scholar]
- Gorini L. Ribosomal discrimination of tRNAs. Nat New Biol. 1971 Dec 29;234(52):261–264. doi: 10.1038/newbio234261a0. [DOI] [PubMed] [Google Scholar]
- Hofstetter H., Monstein H. J., Weissmann C. The readthrough protein A1 is essential for the formation of viable Q beta particles. Biochim Biophys Acta. 1974 Dec 6;374(2):238–251. doi: 10.1016/0005-2787(74)90366-9. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Kahan L., Nomura M. In vitro synthesis of ribosomal proteins directed by Escherichia coli DNA. Proc Natl Acad Sci U S A. 1974 Feb;71(2):446–450. doi: 10.1073/pnas.71.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L., Post L., Nomura M. DNA-dependent in vitro synthesis of fibosomal proteins, protein elongation factors, and RNA polymerase subunit alpha: inhibition by ppGpp. Cell. 1976 Nov;9(3):439–448. doi: 10.1016/0092-8674(76)90089-1. [DOI] [PubMed] [Google Scholar]
- Ninio J. A semi-quantitative treatment of missense and nonsense suppression in the strA and ram ribosomal mutants of Escherichia coli. Evaluation of some molecular parameters of translation in vivo. J Mol Biol. 1974 Apr 5;84(2):297–313. doi: 10.1016/0022-2836(74)90586-5. [DOI] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J. F., Fan D. P., Brenner S. A strong suppressor specific for UGA. Nature. 1967 Apr 29;214(5087):452–453. doi: 10.1038/214452a0. [DOI] [PubMed] [Google Scholar]
- Strigini P., Gorini L. Ribosomal mutations affecting efficiency of amber suppression. J Mol Biol. 1970 Feb 14;47(3):517–530. doi: 10.1016/0022-2836(70)90319-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiner A. M., Weber K. A single UGA codon functions as a natural termination signal in the coliphage q beta coat protein cistron. J Mol Biol. 1973 Nov 15;80(4):837–855. doi: 10.1016/0022-2836(73)90213-1. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Weber K. Natural read-through at the UGA termination signal of Q-beta coat protein cistron. Nat New Biol. 1971 Sep 15;234(50):206–209. doi: 10.1038/newbio234206a0. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]