Abstract
OBJECTIVES
The objective of this study was to examine the association between tobacco and alcohol dose and type and the age of onset of pancreatic adenocarcinoma (PancCa).
METHODS
Prospective data from the Pancreatic Cancer Collaborative Registry were used to examine the association between age of onset and variables of interest including: gender, race, birth country, educational status, family history of PancCa, diabetes status, and tobacco and alcohol use. Statistical analysis included logistic and linear regression, Cox proportional hazard regression, and time-to-event analysis.
RESULTS
The median age to diagnosis for PancCa was 66.3 years (95% confidence intervals (CIs), 64.5–68.0). Males were more likely than females to be smokers (77% vs. 69%, P = 0.0002) and heavy alcohol and beer consumers (19% vs. 6%, 34% vs. 19%, P < 0.0001). In univariate analysis for effects on PancCa presentation age, the following were significant: gender, alcohol and tobacco use (amount, status and type), family history of PancCa, and body mass index. Both alcohol and tobacco had dose-dependent effects. In multivariate analysis, alcohol status and dose were independently associated with increased risk for earlier PancCa onset with greatest risk occurring in heavy drinkers (HR 1.62, 95% CI 1.04–2.54). Smoking status had the highest risk for earlier onset pancreatic cancer with a HR of 2.69 (95% CI, 1.97–3.68) for active smokers and independent effects for dose (P = 0.019). The deleterious effects for alcohol and tobacco appear to resolve after 10 years of abstinence.
CONCLUSIONS
Alcohol and tobacco use are associated with a dose-related increased risk for earlier age of onset of PancCa. Although beer drinkers develop pancreatic cancer at an earlier age than nondrinkers, alcohol type did not have a significant effect after controlling for alcohol dose.
INTRODUCTION
The effectiveness of screening programs relies on the implementation of screening at the appropriate age for a given patient (1). For example, experts recommend colonoscopy to screen for polyps or colon cancer beginning at age 50 years for average-risk patients (2). In contrast, individuals with a family history of one or more first-degree relatives with sporadic colon cancer should undergo colonoscopy beginning at age 40 or 10 years younger than the affected relative, whichever is earlier. Currently, there are no accepted screening tests for pancreatic cancer and screening is not recommended for the general population. Screening for patients with a strong family history of pancreatic cancer using endoscopic ultrasound has had limited success but what age at which to initiate screening remains unclear (3–5).
In those patients with a genetic predisposition to pancreatic cancer, use of tobacco and alcohol has been associated with an earlier age of diagnosis for pancreatic adenocarcinoma (PancCa) (6,7). More recent studies have suggested that there is an association between both alcohol and tobacco use and the age of diagnosis for sporadic pancreatic cancer (8,9); however, dose dependence for these effects has been largely unexplored. Moreover, no reported studies have examined the differential effects of the type of alcohol consumed on the age of diagnosis for pancreatic cancer.
In this study, we have used a multicenter, international database, the Pancreatic Cancer Collaborative Registry (PCCR), to examine the association between alcohol and tobacco use and the age of presentation of pancreatic cancer. We hypothesized that alcohol and tobacco would lower the age of presentation of pancreatic cancer in a dose-dependent manner. Owing to the detailed nature of the survey instrument used by centers that participate in the PCCR, we were able to examine dose effects for both alcohol and tobacco on the age of presentation of pancreatic cancer while adjusting for other important covariates, such as family history of pancreatic cancer, race and body mass index (BMI). For the first time, we were able to study the effects of particular alcohol types on age of diagnosis of pancreatic cancer.
METHODS
The PCCR (http://pccrproject.com) is a multicenter, international effort to prospectively collect data and biospecimens on patients diagnosed with pancreatic cancer (10). The PCCR data set contains patient data from the following institutions: University of Nebraska Medical Center (Omaha, NE), University of Genoa (Genoa, Italy), Creighton University School of Medicine (Omaha, NE), University of Pittsburgh Medical Center (Pittsburgh, PA), NorthShore University Health Systems formerly known as Evanston Northwestern Healthcare (Evanston, IL), University of Michigan Health System (Ann Arbor, MI), and University of Alabama at Birmingham (Birmingham, AL). No subjects included in our previous work on age of diagnosis in pancreatic cancer (enrolled from January 2004 to October 2007) were included in the present study, which adopted a much more detailed survey instrument later in 2007 (8). The PCCR gathers information on patients with pancreatic cancer and individuals at high risk for developing pancreatic cancer. Participation is voluntary and subjects receive no compensation for their participation. Patients are eligible for entry into the database if they: (i) had a tissue-based diagnosis of pancreatic cancer (including PancCa, neuroendocrine tumors of the pancreas, acinar tumors of the pancreas, and mucinous tumors of the pancreas) or a pancreatic mass visible on standard imaging (e.g., computed tomography) and a clinical presentation and course consistent with pancreatic cancer or (ii) have a family history of pancreatic cancer with two or more relatives with pancreatic cancer. All subjects provide informed consent using institution-specific institutional review board consent forms, which explicitly allow for the transfer of de-identified information into the central database of the PCCR and sharing of data between centers. A standardized survey instrument is administered to the subject to gather core data from every subject as well as additional optional information (10). Patient-derived data are transferred to the database via a web-based, secure site by trained study coordinators. The data warehouse is designed as a separate, read-only database, which is a Cancer Biomedical Informatics Grid (caBIG) bronze level-certified system. Users access the database via a front-end application. For the purpose of this study, only data from patients with adenocarcinoma of the pancreas were included (Figure 1). Patients who did not respond to questions regarding tobacco and alcohol use were excluded. The database was queried for demographic factors, including: date of birth and age at diagnosis, gender, race, country of birth, maximally achieved educational status (including college graduate, college experience, postgraduate training, technical/vocational training, high school graduate, high school experience, and grade school experience), diabetes status and years since diagnosis, and family history of pancreatic cancer. Self-reported BMI (“before onset of illness”) was used to classify subjects as normal weight (BMI <25), overweight (BMI >25 but <30), or obese (BMI >30). Tobacco and alcohol use: tobacco (type (cigarette, cigar, tobacco chewers, pipe, and snuff), packs per day (PPD; for cigarette users; <1 PPD or > 1 PPD with specified quantity), age started smoking, age ceased smoking) and alcohol use (type (white wine, red wine, hard liquor, and beer), drinks per day for each alcohol type (<1 drink per day, > 1 drink per day with specified daily quantity), age started drinking alcohol, and age ceased drinking alcohol). The database lacks information on second-hand smoke exposure and so that variable could not be examined in this model.
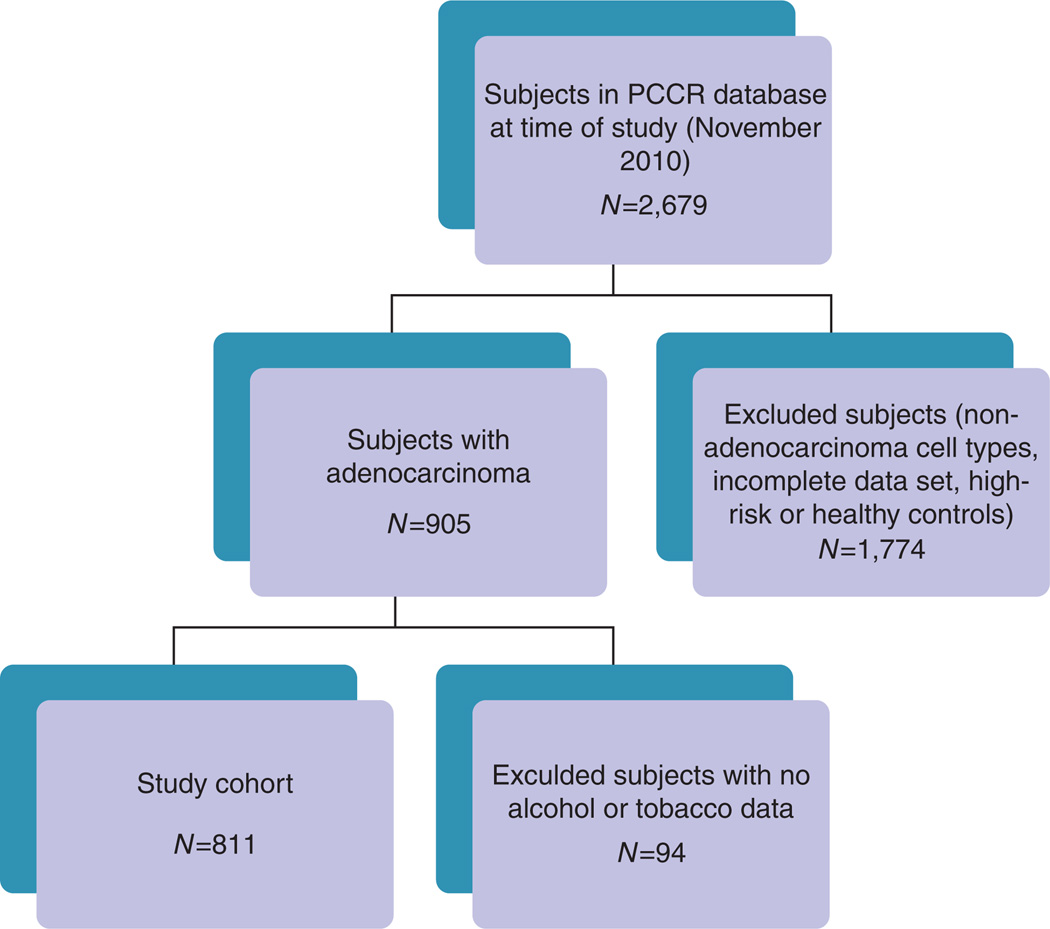
Figure 1.
Consort diagram. Flow diagram showing patients included or excluded to produce final study group. PCCR, Pancreatic Cancer Collaborative Registry.
Specific calculations for alcohol and tobacco use data were performed. Duration of alcohol and tobacco use was calculated as age ceased– age started (for each alcohol type and each tobacco type). Time interval of alcohol and smoking cessation before diagnosis was calculated as (age at diagnosis – age ceased (for alcohol and for tobacco)). Tobacco quantity was classified as Active (ongoing use of tobacco or cessation of tobacco use < 1 year before diagnosis with pancreatic cancer), 1–10YPTD (quit 1–10 years before diagnosis), or > 10YPTD (quit greater than 10 years before diagnosis). These intervals were selected based on previous data showing that a minimum of 2 years is required before pancreatic cancer risk begins to return toward levels of nonsmokers and is nearly equal to that of nonsmokers after 10 years (11). Moreover, our own work using an independent data set has shown that while patients who had quit < 10 years before diagnosis had an age of presentation similar to actively smoking patients, patients who had quit more than 10 years before diagnosis were no different than patients who had never smoked (8). For uniformity in data interpretation, alcohol cessation categories were selected to mirror those of tobacco. Alcohol consumption was grouped in ranges: mild (<13gm/ day)—all who reported drinking one or less drinks per day for all alcohol types combined; moderate (> 13–39gmi’day)—all who reported drinking more than one drink per day and less than or equal to three drinks per day; and heavy (> 39gm/day)—all who reported greater than three drinks per day. The Center for Disease Control has estimated that a standard drink contains 13.7g of alcohol (http://www.cdc.gov/alcohol/faqs.htm). This is the amount of alcohol found in 12-ounces of beer, 5-ounces of wine, or 1.5– ounces of 80-proof distilled spirits or liquor (e.g., gin, rum, vodka, or whiskey). Pack year history of tobacco use was calculated by PPD×duration of cigarette use (≤1PPD category was assumed to equal 1PPD).
Because of the difficulty in quantifying non-cigarette tobacco use, all analyses that examined quantitative (rather than categorical) effects of tobacco on age of presentation in pancreatic cancer used only those subjects whose primary form of tobacco use was cigarettes. To accurately classify subjects for type of alcohol used, a set of indicator variables was created for each drink type. For example, if a subject drank beer and wine but not hard liquor, that subject’s indicator variables would be beer = 1, wine= 1, and hard liquor = 0. In this way, a parameter estimate for a particular alcohol type is the effect for that alcohol type after adjustment for use of the other two alcohol types.
Statistical analysis
The study end point is the age of presentation of pancreatic cancer. Data were analyzed using time-to-event analysis. The pancreatic cancer-free probability and median time to present with pancreatic cancer were estimated from date of birth using Kaplan–Meier methods and illustrated using Kaplan–Meier curves. In multivariate analysis, a Cox proportional hazard model was fitted to assess the effects of alcohol and cigarettes on the hazard of developing pancreatic cancer at any given age while adjusting for other covariates. For multivariate models, selection was determined based on univariate results. All covariates significant at P < 0.10 at the univariate level were included in the multivariate model. The only exception to this was gender, which was not significant at the univariate level, however, this covariate was included in the multivariate model, as it was associated with cigarette and alcohol use. Statistical analysis was performed using SAS, version 9.1 (SAS Institute, Cary, NC) and a P-value <0.05 was considered statistically significant.
Association between age of presentation of pancreatic cancer and other continuous covariates was evaluated using Pearson correlation coefficient matrices. χ2 tests and logistic regression analysis were used to assess the association between discrete variables.
RESULTS
At the time of study, the PCCR database contained records on 2,679 subjects, 905 of these having PancCa. Among these, there were 811 subjects whose records contained detailed information on tobacco and alcohol use and who formed the final cohort for this analysis (Figure 1). The demographic composition of the cohort is summarized in Table 1. Alcohol and tobacco use is shown in Table 2. Most of the subjects were born within the United States. The study population was predominantly white (95%) with half of the subjects (51%) having some form of education or training beyond high school. Three percent of subjects had a family history of pancreatic cancer. Although 46% of the subjects were known diabetics, nearly half of these (112/240) had been diagnosed <2 years before their diagnosis of pancreatic cancer. More than one-quarter of the patients were nondrinkers (244/772 (32%)) or never smokers (193/730(26%)). There were 537 subjects (74%) who reported tobacco use (ever smokers) and 63 subjects (9%) who gave no designation or an unclear designation of smoking status. Among tobacco users, cigarette use was the most common form of tobacco used with a small minority of patients using other forms of tobacco, such as cigars, pipe tobacco, and snuff. There were 528 subjects (68%) who reported alcohol use history (ever drinkers). Among alcohol users, beer was the most frequently consumed alcoholic beverage and 60% of subjects reported consumption of only one type of alcohol. Thirty-nine subjects (5%) did not provide data regarding alcohol use. More than half (72%) of the subjects were overweight or obese, although specific BMI was only available in 715/811 (88%) patients.
Table 1.
Distribution of selected characteristics in sample
| Variable | Numbera | Percentb(%) |
|---|---|---|
| Gender and race | ||
| Male | 440 | 54 |
| White | 758 | 95 |
| Educational status | ||
| Postgraduate training | 316 | 45 |
| Technical/vocational | 43 | 6 |
| High school | 232 | 33 |
| Grade school | 119 | 17 |
| Country of birth | ||
| United states | 635 | 84 |
| Italy | 93 | 12 |
| Other | 31 | 4 |
| Family history of pancreatic Cancer | 28 | 3 |
| Diabetes status | ||
| No known history of DM | 307 | 53 |
| Yes | 266 | 46 |
| Established Dx (≥2 years) | 128 | 53 |
| Recent Dx (<2 years) | 112 | 47 |
| BMI | ||
| Normal (BMI<25) | 198 | 28 |
| Overweight (25≤BMI≤30) | 285 | 40 |
| Obese (BMI>30) | 232 | 32 |
| Early onset (<=55 years at age of diagnosis) | 113 | 14 |
BMI, body mass index; DM, diabetes mellitus; Dx, diagnosis.
Observations may not total 811 due to missing responses.
Percentages may not equal 100% due to rounding.
Table 2.
Tobacco and alcohol use in the cohort
| Variable | Numbera | Percentb (%) |
|---|---|---|
| Smoking and alcohol history | ||
| Never tobacco users or drinkers | 87 | 13 |
| Tobacco use | ||
| Never-tobacco users | 193 | 26 |
| Non-cigarette tobacco users | 25 | 3 |
| Multiple types of tobacco users | 56 | 8 |
| Tobacco user: unknown type or quantity | 63 | 9 |
| Cigarette only users | 393 | 54 |
| Status of cigarette use at Dx | ||
| Active | 133 | 35 |
| Quit 1–10 years before Dx | 56 | 15 |
| Quit > 10 years before Dx | 187 | 50 |
| Pack per day history | ||
| ≤1 Pack per day | 262 | 71 |
| > 1 Pack per day | 109 | 29 |
| Alcohol use | ||
| Nondrinkers | 244 | 32 |
| Alcohol users | 528 | 68 |
| Drink type | ||
| Beer | 134 | 29 |
| Red wine | 58 | 12 |
| White wine | 28 | 6 |
| Hard liquor | 61 | 13 |
| Multiple types | 188 | 40 |
| Status of alcohol use at Dx | ||
| Active | 272 | 77 |
| Quit 1–10 years before Dx | 30 | 9 |
| Quit > 10 years before Dx | 50 | 14 |
| Amount of alcohol use | ||
| <13g/day (mild) | 269 | 65 |
| 13–39g/day (moderate) | 84 | 20 |
| >39g/day (heavy) | 60 | 5 |
Dx, diagnosis.
Observations may not total 811 due to missing responses.
Percentages may not equal 100% due to rounding.
When we examined the associations between gender, alcohol use (both amount and type), and tobacco use, we found that women were more likely than men to be nondrinkers (47% vs. 18%, P < 0.0001). Males rather than females were more likely to be heavy (> 1 PPD) smokers (40% vs. 16% (males vs. females for all comparisons),P < 0.0001), heavy (>39g/day) drinkers (19% vs. 6%, P < 0.0001), and to consume beer or multiple types of alcohol, rather than wine or hard liquor. Among subjects who consumed alcohol, heavy alcohol use was associated with type of alcohol with three times as many beer drinkers reporting heavy alcohol (19%) use compared with wine drinkers (6%) or hard liquor drinkers (6%) (P= 0.012). BMI was associated with alcohol use with fewer wine drinkers being obese (11%) than nondrinkers (39%) or drinkers of other types of alcohol or multiple type users (24–31%) (P = 0.001). There was no association between heavy alcohol use and heavy tobacco use.
Univariate effects of gender, BMI, alcohol, and tobacco on age of presentation in pancreatic cancer
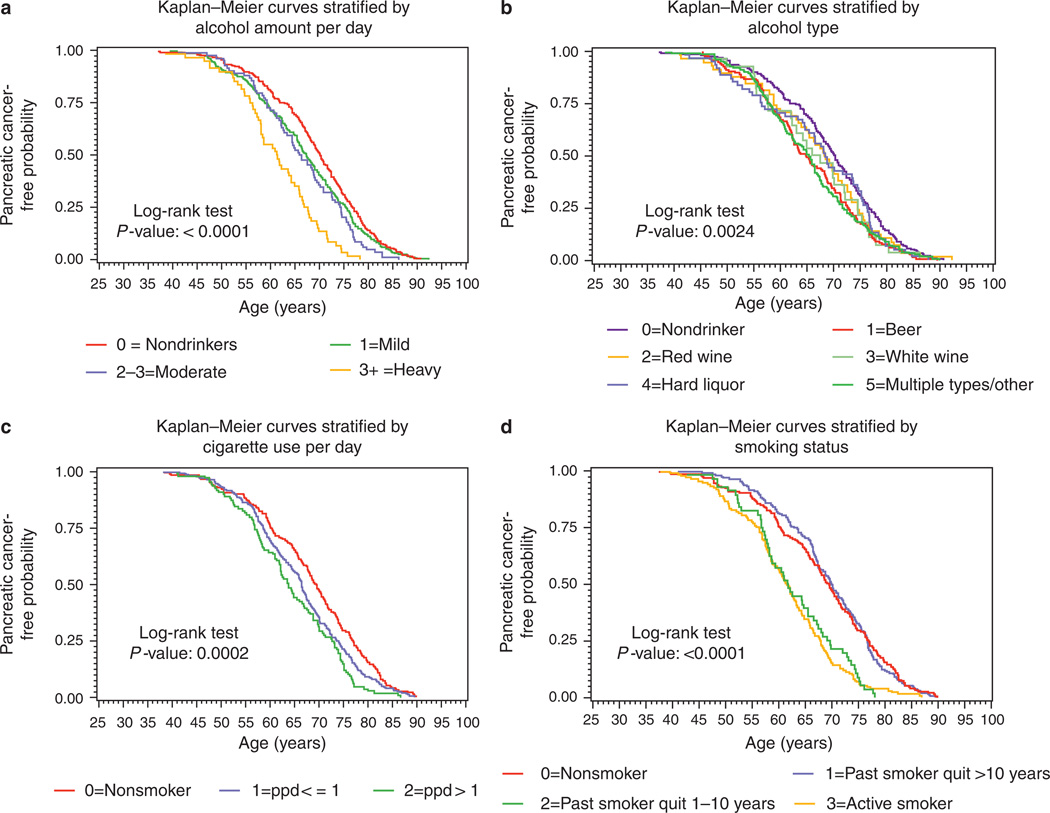
The effects of examined variables on median age (95% CI) in years at time of presentation of pancreatic cancer are summarized in Table 3. Categorical effects on time to event (pancreatic cancer) are shown using individual Kaplan–Meier curves (Figure 2a – d). The median age of diagnosis of pancreatic cancer for the cohort as a whole was 66.3 (64.5–68.0) years. Male patients presented earlier than female patients (P = 0.0193). There was a dose-dependent effect for alcohol on age of presentation for pancreatic cancer with heavy (> 39 g/day) alcohol being associated with a significantly earlier (8.8 years) median age of presentation compared with nondrinkers (Figure 2a). When we examined the effects of alcohol type on age of presentation of pancreatic cancer, we found that drinking beer had a significant effect on age of presentation with beer drinkers presenting 5.3 years earlier than nondrinkers and 1.8–4.7 years earlier than patients who drank other types of alcohol (Figure 2b). Among cigarette smokers, there was a dose-related decrease in the age of presentation for pancreatic cancer such that one pack or less per day smokers presented 3 years younger than nonsmokers. Those smoking more than a pack per day presented almost 6 years earlier than nonsmokers (Figure 2c). The deleterious effects of tobacco use resolved after 10 years of nonuse such that the age of presentation for patients who had quit smoking more than 10 years before their pancreatic cancer diagnosis was at or later than their never-smoked counterparts (Figure 2d). Conversely, patients who had quit smoking <10 years before their pancreatic cancer diagnosis had a median age of presentation similar to those patients who were active smokers (61.8 vs. 61.6 years). Patients who were obese presented at a younger age than normal weight subjects (66.3 vs. 69.6 years; Table 3). Patients who had a family history of pancreatic cancer were more likely to present at an earlier age than those without a family history. Finally, patients enrolled by our center in Genoa, Italy, tended to present at an earlier age than those enrolled by US centers.
Table 3.
Univariate effects of selected variables on age of presentation of pancreatic cancer
| Variable | Median age |
95% CI | Log-rank P-value |
|---|---|---|---|
| Center | 0.0611 | ||
| Italy | 65.3 | 62.9–68.3 | |
| US centers | 67.8 | 66.7–68.9 | |
| Gender | 0.0193 | ||
| Male | 66.6 | 65.7–68.4 | |
| Female | 68.2 | 67.1–69.8 | |
| BMI | 0.0463 | ||
| Normal weight (BMI<25) | 69.6 | 68.0–71.5 | |
| Overweight (25≤BMI≤30) | 67.3 | 65.5–68.9 | |
| Obese (BMI>30) | 66.3 | 64.5–68.0 | |
| Race | 0.2648 | ||
| White | 67.7 | 66.6–68.5 | |
| Other | 65.9 | 57.6–68.2 | |
| Diabetes status | 0.5173 | ||
| Established diabetes (>2 years) | 69.5 | 66.6–71.4 | |
| Recent Dx (≤2 years) Unknown Dx date | 69.3 | 66.3–70.8 | |
| Unknown Dx date | 72.5 | 63.8–76.6 | |
| No known Hx diabetes | 66.6 | 65.6–67.8 | |
| Family Hx pancreas Ca | 0.0216 | ||
| No | 67.7 | 66.6–68.6 | |
| Yes | 65.6 | 58.8–69.8 | |
| Smoking status | <0.0001 | ||
| Nonsmokers | 69.5 | 67.7–71.2 | |
| Past smokers (quit > 10 years ago) | 70.0 | 67.6–72.2 | |
| Past smokers (quit ≤10 years go) | 61.8 | 58.3–65.5 | |
| Active smokers | 61.6 | 59.0–63.4 | |
| Cigarette (PPD) | 0.0002 | ||
| Nonsmokers | 69.5 | 67.7–71.2 | |
| 0 <PPD≤1 | 66.5 | 64.7–67.5 | |
| PPD >1 | 63.8 | 61.8–66.9 | |
| Alcohol status | 0.0015 | ||
| Nondrinker | 70.2 | 68.4–71.8 | |
| Quit > 10 years before Dx | 66.4 | 62.7–69.1 | |
| Quit 1–10 years before Dx | 66.5 | 55.9–72.3 | |
| Active drinker | 65.9 | 64.3–67.6 | |
| Alcohol amount/day | <0.0001 | ||
| Nondrinker | 70.2 | 68.4–71.8 | |
| <13g | 67.4 | 65.7–69.1 | |
| 13–39g | 66.1 | 62.9–69.0 | |
| >39g | 61.4 | 58.0–64.3 | |
| Types of alcohol used | 0.0024 | ||
| Nondrinker | 70.2 | 68.4–71.8 | |
| Beer | 64.9 | 62.4–68.4 | |
| Red wine White wine | 68.4 | 64.9–71.7 | |
| White wine | 66.8 | 62.1–72.2 | |
| Hard liquor | 68.4 | 64.7–73.7 | |
| Multiple types | 65.5 | 62.7–66.6 | |
| Entire cohort | 66.3 | 64.5–68.0 |
BMI, body mass index; CI, confidence interval; Dx, diagnosis; Hx, history of; PPD, packs per day.
Figure 2.
(a) Kaplan-Meier curves showing age at presentation for pancreatic cancer as a function of the amount of alcohol subjects consumed. Subjects who were heavy drinkers (>39g of alcohol per day) developed pancreatic cancer at a significantly earlier age. (b) Kaplan-Meier curves showing age at presentation for pancreatic cancer as a function of the type of alcohol subjects consumed. Beer drinkers developed pancreatic cancer at a significantly earlier age than nondrinkers or those who drank wine or liquor alone. (c) Kaplan-Meier curves showing age at presentation for pancreatic cancer as a function of tobacco dose. Subjects who smoked experienced pancreatic cancer at an earlier age and in a dose-dependent manner. (d) Kaplan–Meier curves showing age at presentation for pancreatic cancer as a function of smoking status. Subjects who were active smoking or who had quit < 10 years before diagnosis presented at an earlier age than those who had never smoked or quit smoking more than 10 years before diagnosis.
Multivariate analysis of the effects of alcohol and tobacco on age of presentation in pancreatic cancer
In multivariate analysis using a Cox proportional hazards model, we found that heavy alcohol consumption (>39g/day) was associated with the highest risk for diagnosis of pancreatic cancer at an earlier age (HR 1.62, 95% confidence interval (CI), 1.04–2.54) relative to those who drank mild-to-moderate amounts of alcohol (Table 4). Independent of the dose effects of alcohol, we found that subjects who had stopped drinking more than 10 years before their diagnosis of pancreatic cancer had a risk for earlier age of onset, which was nearly identical (HR 0.95) to that of subjects who had never drank alcohol, and significantly lower than subjects who has quit <10 years before diagnosis (HR 1.27) or who were actively drinking at the time of diagnosis (HR 1.65). In this multivariate model, there was a strong association between tobacco and age of presentation for pancreatic cancer. Subjects who were actively smoking had a risk more than threefold greater than that for subjects who had stopped smoking 10 or more years before diagnosis (HR 2.69 (95% CI 1.97–3.68) vs. HR 0.88 (95% CI 0.66–1.18) for active vs. quit >10YPTD). Moreover, there was an independent effect for tobacco dose (P = 0.019). Similar to the effects seen with alcohol cessation, subjects who had stopped smoking 10 or more years before diagnosis had a risk similar to subjects who had never been smokers. Although there appeared to be a trend for BMI and risk, this did not reach statistical significance. After adjustment for amount of alcohol used, the type of alcohol had no significant effect on age of presentation for pancreatic cancer (P = 0.815). Similarly, there was no statistically significant effect seen for gender, center of enrollment, or family history of pancreatic cancer although there was a trend toward significance for family history. After adjusting for other covariates, an interaction term between cigarette use (status or dose) and heavy drinking was not significantly associated with age of presentation (P-values 0.1689 and 0.3173, respectively).
Table 4.
Cox proportional hazards regression model for age of presentation of pancreatic cancer
| Variable | Hazard ratio | 95% CI | χ2 P value |
|---|---|---|---|
| Center | |||
| United States vs. Italy | 1.03 | 0.56–1.88 | 0.930 |
| Gender | |||
| Male vs. female | 1.06 | 0.81–1.37 | 0.684 |
| BMI | 0.057 | ||
| Normal (BMI<25) | Ref | ||
| Overweight (25≤BMI≤30) | 1.16 | 0.89–1.51 | |
| Obese (BMI>30) | 1.42 | 1.06–1.89 | |
| Family Hx pancreas Ca | |||
| Yes vs. no | 1.98 | 0.79–4.96 | 0.144 |
| Smoking status | <.0001 | ||
| Nonsmokers | Ref | ||
| Past smokers (quit ≥10yearsago) | 0.88 | 0.66–1.18 | |
| Past smokers (quit 1–10years ago) | 1.64 | 1.07–2.53* | |
| Active smokers | 2.69 | 1.97–3.68** | |
| Cigarette (PPD) | |||
| PPD >1 vs. 0 <PPD≤1 | 1.47 | 1.06–2.02* | 0.019 |
| Status of alcohol use at Dx | 0.030 | ||
| Never drinker | Ref | ||
| Quit ≥10 years before Dx | 0.95 | 0.54–1.68 | |
| Quit 1–10 years before Dx | 1.27 | 0.66–2.43 | |
| Active | 1.65 | 0.96–2.81 | |
| Alcohol amount per day | |||
| Heavy (>39g) vs. mild/mod. (≤39g) | 1.62 | 1.04–2.54* | 0.035 |
| Type of alcohol used (includes multiple type users) | 0.815 | ||
| Beer indicator | 1.06 | 0.66–1.68 | |
| Wine indicator | 0.88 | 0.47–1.64 | |
| Hard liquor indicator | 0.90 | 0.59–1.39 |
BMI, body mass index; CI, confidence interval; Dx, diagnosis; Hx, history of; mod., moderate; PPD, packs per day.
Significant at 0.05 level.
Significant at 0.01 level.
Includes multiple type users.
Association between beer drinking and BMI
To investigate a potential interaction between beer drinking and obesity, a post-hoc analysis was conducted. Excluding beer drinkers who were also multiple-type users, 24% of beer drinkers were obese, whereas 21% of non-beer drinkers were obese (P = 0.851). In addition, an interaction term for BMI and beer use was not significant in the multivariate model for age of presentation, P-value = 0.1984.
DISCUSSION
Pancreatic cancer mortality is nearly 100% and screening programs for the general population are neither feasible nor effective. This is directly related to the relatively low incidence of the disease. Even when highly sensitive modalities such as endoscopic ultrasound are used, repeated administration of screening leads to an unacceptable number of false-positive tests and can be expected to decrease rather than improve survival (12). Two measures that would likely improve the effectiveness of screening include defining subpopulations at increased risk (thereby increasing the pretest probability and related specificity) and optimizing the timing of screening interventions in a given patient (thereby limiting exposure to the screening test and its related risks). Doing one without the other is unlikely to change outcomes in this disease. This was illustrated in a recent study by Ludwig et al. (13), who found that screening among patients with a familial pancreatic cancer history had a higher yield in those who were older than 65 years of age. As screening programs are developed, an understanding of how personal features influence the age of presentation will be important to optimize the timing of such screening. PancPRO is a risk prediction model for pancreatic cancer, which incorporates detailed, multi-generational family history to predict the probability that an individual carries a gene mutation, which might make them susceptible to pancreatic cancer as well as that individual’s absolute risk of developing pancreatic cancer at a specific age and over a specified time frame (14). This model does not currently incorporate other risk factors or personal behaviors, which might affect overall risk or age of presentation for pancreatic cancer such as a history of chronic pancreatitis or tobacco or alcohol exposure. Previous work by others has shown that patients from familial pancreatic cancer kindreds and those with hereditary pancreatitis develop pancreatic cancer at a significantly earlier age than those with sporadic pancreatic cancer (15–17). In one nested case-control study, patients from familial pancreatic cancer kindreds, defined as individuals with two or more relatives with pancreatic cancer, the median age of diagnosis was 59.6 years (7). In another study from France, researchers found that patients who suffered from hereditary pancreatitis presented with pancreatic cancer at a median age of 55 years (18). By comparison, in our study, patients who reported heavy alcohol use (> 39 g of alcohol per day) had a median age of presentation of 61.4 years, well within the range of these populations who are considered high risk and in whom annual screening has been advocated by some.
Pancreatic carcinogenesis is a complex process in which specific genetic mutations, some sporadic, and others inherited, lead to progressively disordered alterations in pancreatic ductal epithelium and ultimately adenocarcinoma. Although a small proportion of cases arise in the setting of an as-yet-unknown dominant susceptibility gene (19), the vast majority of cases result from a combination of genetic mutations and environmental insults. Multiple environmental and lifestyle factors have been implicated in pancreatic cancer ranging from a diet high in animal fat to excessive intake of carbonated soft drinks (20,21). Smoking is a well-established risk factor for pancreatic cancer and has been shown in this study and others to be associated with early-onset disease in sporadic as well as familial adenocarcinoma of the pancreas (6–9,16). Modifications in the age of presentation by smoking status have been described for other cancers. For example, at least four independent studies have reported a decrease in the age of presentation for colon cancer among smokers (22–25). Data such as these can influence professional societies to tailor recommendations for cancer screening (26).
Alcohol has been shown to cause oxidative damage to the pancreas promoting inflammatory pathways and may be the mechanism through which alcohol could promote pancreatic carcinogenesis (mitogenic and inflammatory/carcinogenesis (27–29)). Indeed, it is this inflammation-carcinogenesis paradigm that has been proposed as an explanation for the increased risk of pancreatic cancer in patients with chronic pancreatitis, obesity, and diabetes (30–33). The influence of alcohol on pancreatic cancer risk from epidemiologic studies is conflicting with some studies showing increased risk associated with consumption and others showing no increased risk (34–39). The majority of the studies, most of which were conducted in the USA and Japan among populations with low-to-moderate alcoholic beverage intake, have not found a significant association between alcoholic beverage intake and pancreatic cancer. A recently published International Agency for Research on Cancer (IARC) monograph on the evaluation of carcinogenic risks to humans concluded that there was insufficient evidence to support a role of alcohol in pancreatic cancer development (40). However, recent publications support the role of alcohol and occurrence of pancreatic cancer in the subset of patients with heavy alcohol consumption independent of cigarette smoking (34–36).
In a recent study, we demonstrated that cigarette smoking and alcohol consumption were associated with a younger age of presentation for pancreatic cancer with a synergistic effect for users of both alcohol and tobacco (8). Although we were able to show dose effects for tobacco among nondrinkers and for alcohol among nonsmokers, the study lacked sufficient power to examine these variables in a multivariate model or to control for other potential disease mediators such as BMI, family history of pancreatic cancer, or personal history of diabetes. In the current study, using an independent, international, multicenter cohort, we have confirmed that alcohol and tobacco use are associated with earlier onset of pancreatic cancer and shown for the first time that alcohol dose and tobacco dose have significant effects on age of presentation independent of other important factors. In univariate analysis, we found that alcohol type appeared to have a significant effect on age of presentation for pancreatic cancer with beer drinkers presenting a median of 5.3 years earlier than nondrinkers and an average of 4.7 to 1.8 years earlier than subjects who consumed other forms of alcohol such as wine or hard liquor. However, when the effect for alcohol type was adjusted for alcohol dose and tobacco use, we discovered that the type of alcohol used did not have a significant effect on the age of diagnosis. Similarly, when we controlled for alcohol and tobacco use, we found no statistically significant effect for a family history of pancreatic cancer on the age of presentation. Given previously published work showing an effect for family history on age of presentation in patients with hereditary pancreatitis and among patients from familial pancreatic cancer kindreds, our finding of a lack of effect was somewhat surprising (6,15,16). One possible explanation of these results is that the number of patients with a family history of pancreatic cancer in our study was small (only 28 patients) and that one might see a true effect in a larger study. This is essentially a power limitation and is born out by the wide range for the effect in the model (0.79–4.96). Another possible explanation is that the age of presentation of pancreatic cancer may be modified more significantly by tobacco use than by family history, essentially “drowning out” that variable in the multivariate model. Another potential confounder we considered in our study was the country of enrollment. Although there were not notable differences in tobacco use by center, the story was different for alcohol. Here we found that not only were Italians more likely to drink alcohol (99% vs. 71%), but they were also more likely to drinkheavily (21% vs. 13%). Therefore, it may not be surprising that once alcohol dose was adjusted for in the multivariate model, the center of enrollment was no longer statistically significant. Such findings illustrate the importance of a multivariate analysis that can control for important covariables in this complex disease and support the efforts of large, multicenter consortiums in defining the epidemiology of PancCa. Another important finding of the current study is that for both alcohol and tobacco, the risk associated with use appears to return to that of never-users after a period of abstinence lasting 10 or more years. This finding supports the belief that early intervention and counseling could positively impact natural history.
The current study is not without limitations. Perhaps the greatest limitation stems from the lack of normal, healthy controls in the database. Because all of the subjects had pancreatic cancer, no conclusions or inferences regarding causation or risk for pancreatic cancer can be made. Put simply, this study looks specifically at the association between the age of onset in pancreatic cancer and the variables included in the model. Although the detailed nature of the PCCR questionnaire allowed us to examine several important variables for pancreatic cancer, it is in essence a cross-sectional, cohort study. As such, it would be inappropriate to draw conclusions about causation. Another limitation is our inability to control for a multitude of potential confounders known or suspected to affect pancreatic cancer risk including coffee exposure, exposure to second-hand smoke, whether the cancer arose in the setting of a mucinous tumor or if there was a history of sporadic or hereditary chronic pancreatitis. Similarly, the infrequency of non-white patients (~5% total for all nonwhite categories combined) in this cohort prohibited valid statistical analysis in the multivariate model and therefore limits the generalization of the findings to nonwhite populations.
It is important to note that current prospective pancreatic cancer screening programs in high-risk groups often lead to surgery for lesions which have no or undefined risk for the eventual development of PancCa (3,4). For this reason, the US Preventative Service Task Force presently recommends against screening (by any manner) for pancreatic cancer in asymptomatic adults (41). In the current study, we have demonstrated that the age of presentation for sporadic pancreatic cancer is modified by tobacco dose and alcohol dose but not by the type of alcohol. In patients who consume alcohol, particularly those who use heavy amounts of alcohol defined as greater than 39 g/day, the age of presentation for pancreatic cancer is significantly earlier than in those who consumed no or low amounts of alcohol. Although prospective studies are needed to validate these results, these provocative findings support the hypothesis that exogenous factors may accelerate pancreatic cancer progression and may provide guidance in the development of pancreatic cancer surveillance programs in the future.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
√
Alcohol and tobacco decrease age of presentation of pancreatic cancer among patients with associated genetic syndromes.
-
√
It is not known how alcohol and tobacco modify the age of presentation of sporadic pancreatic cancer.
WHAT IS NEW HERE
-
√
Alcohol and tobacco have a dose-dependent effect in lowering the age of presentation of sporadic pancreatic cancer.
-
√
These deleterious effects appear to resolve after 10 years of abstinence.
ACKNOWLEDGMENTS
We acknowledge the assistance of the Genoa Pancreatic Cancer Study Group Contributors: PI Giovanna Bianchi Scarra, PhD, University and San Martino Hospital; Giacomo Borgonovo, MD, Renato Dulbecco, MD, Luca Mastracci, MD, Francesco Papadia, MD, Vincenzo Savarino, MD, University and San Martino Hospital; Giuseppe Fornarini, MD, Gianni Sacchi, MD, Stefania Sciallero, MD, San Martino Hospital; Fuigina Bonelli, MD, National Cancer Institute; Forenza Belli, MD, Andrea Decensi, MD, Marco Filauro, MD, Alberto Gozza, MD, Paola Romagnoli, MD, Ospedale Galliera. We are also grateful to following individuals for data entry and processing and for manuscript review and editing: Nalina Singh, BS, and Sobia Sadiq, BS. Finally, we recognize the invaluable assistance of Dr James Scheiman for his extensive review, critique, and editing of the final manuscript.
Financial Support: This work was supported by the following grants: NIH K23 DK082097 (MAA), NCI T32 CA 083654 (SL), NIH R01 CA140940 (SS), and Italian Ministry of Health DGRST.4/4235-P1.9.A.B. (PG).
Footnotes
CONFLICT OF INTEREST
Gurantor of the aritcle: Michelle A. Anderson, MD, MSc.
Specific author contributions: Study conception and design, enrollment of subjects, analysis and interpretation of the data, drafting of the article, and final approval of the article: Michelle A. Anderson and Randall E. Brand; drafting of the article, analysis and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the article: Eugene Zolotarevsky; statistical design, analysis and interpretation of the data, critical revision of the article for important intellectual content and final approval of the article: Kristine L. Cooper, Shih-Yuan Lee, and Daniel Normolle; design and maintenance of database, preparation of the data for analysis, critical revision of the article for important intellectual content, and final approval of the article: Simon Sherman; maintenance of database, preparation of the data for analysis, critical revision of the article for important intellectual content, and final approval of the article: Oleg Shat; enrollment of subjects, critical revision of the article for important intellectual content, and final approval of the article: David C. Whitcomb, Henr) T. Lynch, Paola Ghiorzo, Wendy S. Rubenstein, Kristen J. Vogel, Aaron R. Sasson, William E. Grizzle, Caitlyn Plonka, Amy Mertens, and Renee C. Tripon; registry maintenance, enrollment of subjects, critical revision of the article for important intellectual content, and final approval of the article: Marsha A. Ketcham.
Potential competing interests: None.
REFERENCES
- 1.Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727–737. doi: 10.1059/0003-4819-151-10-200911170-00009. W237-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davila RE, Rajan E, Baron TH, et al. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc. 2006;63:546–557. doi: 10.1016/j.gie.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Langer P, Kann PH, Fendrich V, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58:1410–1418. doi: 10.1136/gut.2008.171611. [DOI] [PubMed] [Google Scholar]
- 4.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–781. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 5.Poley JW, Kluijt I, Gouma DJ, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175–2181. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 6.Lowenfels AB, Maisonneuve P, Whitcomb DC, et al. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. IAMA. 2001;286:169–170. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 7.Rulyak SJ, Lowenfels AB, Maisonneuve P, et al. Risk factors for the development of pancreatic cancer in familial pancreatic cancer kindreds. Gastroenterology. 2003;124:1292–1299. doi: 10.1016/s0016-5085(03)00272-5. [DOI] [PubMed] [Google Scholar]
- 8.Brand RE, Greer JB, Zolotarevsky E, et al. Pancreatic cancer patients who smoke and drink are diagnosed at younger ages. Clin Gastroenterol Hepatol. 2009;7:1007–1012. doi: 10.1016/j.cgh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raimondi S, Maisonneuve P, Lohr JM, et al. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol Biomarkers Prev. 2007;16:1894–1897. doi: 10.1158/1055-9965.EPI-07-0341. [DOI] [PubMed] [Google Scholar]
- 10.Sherman S, Shats O, Ketcham MA, et al. PCCR: Pancreatic Cancer Collaborative Registry. Cancer Inform. 2011;10:83–91. doi: 10.4137/CIN.S6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs CS, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med. 1996;156:2255–2260. [PubMed] [Google Scholar]
- 12.Rubenstein JH, Scheiman JM, Anderson MA. A clinical and economic evaluation of endoscopic ultrasound for patients at risk for familial pancreatic adenocarcinoma. Pancreatology. 2007;7:514–525. doi: 10.1159/000108969. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig E, Olson SH, Bayuga S, et al. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol. 2011;106:946–954. doi: 10.1038/ajg.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Chen S, Brune KA, et al. PancPRO: risk assessment for individuals with a family history of pancreatic cancer. J Clin Oncol. 2007;25:1417–1422. doi: 10.1200/JCO.2006.09.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442–446. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 16.James TA, Sheldon DG, Rajput A, et al. Risk factors associated with earlier age of onset in familial pancreatic carcinoma. Cancer. 2004;101:2722–2726. doi: 10.1002/cncr.20700. [DOI] [PubMed] [Google Scholar]
- 17.Verna EC, Hwang C, Stevens PD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res. 2010;16:5028–5037. doi: 10.1158/1078-0432.CCR-09-3209. [DOI] [PubMed] [Google Scholar]
- 18.Rebours V, Boutron-Ruault MC, Schnee M, et al. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am J Gastroenterol. 2008;103:111–119. doi: 10.1111/j.1572-0241.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 19.Klein AP, Beaty TH, Bailey-Wilson JE, et al. Evidence for a major gene influencing risk of pancreatic cancer. Genet Epidemiol. 2002;23:133–149. doi: 10.1002/gepi.1102. [DOI] [PubMed] [Google Scholar]
- 20.Thiebaut AC, Jiao L, Silverman DT, et al. Dietary fatty acids and pancreatic cancer in the NIH-AARP diet and health study. J Natl Cancer Inst. 2009;101:1001–1011. doi: 10.1093/jnci/djp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller NT, Odegaard A, Anderson K, et al. Soft drink and juice consumption and risk of pancreatic cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19:447–455. doi: 10.1158/1055-9965.EPI-09-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zisman AL, Nickolov A, Brand RE, et al. Associations between the age at diagnosis and location of colorectal cancer and the use of alcohol and tobacco: implications for screening. Arch Intern Med. 2006;166:629–634. doi: 10.1001/archinte.166.6.629. [DOI] [PubMed] [Google Scholar]
- 23.Peppone LJ, Mahoney MC, Cummings KM, et al. Colorectal cancer occurs earlier in those exposed to tobacco smoke: implications for screening. J Cancer Res Clin Oncol. 2008;134:743–751. doi: 10.1007/s00432-007-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buc E, Kwiatkowski F, Alves A, et al. Tobacco smoking: a factor of early onset of colorectal cancer. Dis Colon Rectum. 2006;49:1893–1896. doi: 10.1007/s10350-006-0704-1. [DOI] [PubMed] [Google Scholar]
- 25.Acott AA, Theus SA, Marchant-Miros KE, et al. Association of tobacco and alcohol use with earlier development of colorectal cancer: should we modify screening guidelines? Am J Surg. 2008;196:915–918. doi: 10.1016/j.amjsurg.2008.07.033. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 26.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Sun T, Wang L, et al. Functional variants in cell death pathway genes and risk of pancreatic cancer. Clin Cancer Res. 2008;14:3230–3236. doi: 10.1158/1078-0432.CCR-08-0177. [DOI] [PubMed] [Google Scholar]
- 28.Wheatley-Price P, Asomaning K, Reid A, et al. Myeloperoxidase and superoxide dismutase polymorphisms are associated with an increased risk of developing pancreatic adenocarcinoma. Cancer. 2008;112:1037–1042. doi: 10.1002/cncr.23267. [DOI] [PubMed] [Google Scholar]
- 29.Go VL, Gukovskaya A, Pandol SJ. Alcohol and pancreatic cancer. Alcohol. 2005;35:205–211. doi: 10.1016/j.alcohol.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 32.Stolzenberg-Solomon RZ, Adams K, Leitzmann M, et al. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am J Epidemiol. 2008;167:586–597. doi: 10.1093/aje/kwm361. [DOI] [PubMed] [Google Scholar]
- 33.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta S, Wang F, Holly EA, et al. Risk of pancreatic cancer by alcohol dose, duration, and pattern of consumption, including binge drinking: a population-based study. Cancer Causes Control. 2010;21:1047–1059. doi: 10.1007/s10552-010-9533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaud DS, Vrieling A, Jiao L, et al. Alcohol intake and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium (PanScan) Cancer Causes Control. 2010;21:1213–1225. doi: 10.1007/s10552-010-9548-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucenteforte E, La Vecchia C, Silverman D, et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Ann Oncol. 2012;23:374–382. doi: 10.1093/annonc/mdr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michaud DS, Giovannucci E, Willett WC, et al. Coffee and alcohol consumption and the risk of pancreatic cancer in two prospective United States cohorts. Cancer Epidemiol Biomarkers Prev. 2001;10:429–437. [PubMed] [Google Scholar]
- 38.Rohrmann S, Linseisen J, Vrieling A, et al. Ethanol intake and the risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Causes Control. 2009;20:785–794. doi: 10.1007/s10552-008-9293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gapstur SM, Jacobs EJ, Deka A, et al. Association of alcohol intake with pancreatic cancer mortality in never smokers. Arch Intern Med. 2011;171:444–451. doi: 10.1001/archinternmed.2010.536. [DOI] [PubMed] [Google Scholar]
- 40.Secretan B, Straif K, Baan R, et al. A review of human carcinogens—Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 41.Calonge N Screening for Pancreatic Cancer. AHRQ Pub No 05-0554-A [serial on the Internet] 2004 [Google Scholar]