Abstract
Brönsted acid-catalyzed one-pot synthesis of indoles from o-aminobenzyl alcohols and furans has been developed. This method operates via the in situ formation of aminobenzylfuran, followed by its recyclization into the indole core. The method proved to be efficient for substrates possessing different functional groups, including -OMe, -CO2Cy, and -Br. The resulting indoles can easily be transformed into diverse scaffolds, including 2,3- and 1,2-fused indoles, and indole possessing an α,β-unsaturated ketone moiety at the C-2 position.
1. Introduction
Low resonance energy of furan ring1 allows for its facile recyclization reactions into different carbo-2 and heterocycles.3 Of particular interest, is recyclization reaction of furan into indole, since valuable indole4 fragments can be obtained from readily available starting materials.5 Hence, a few reports document an intramolecular Pd-catalyzed arylative recyclization of furan-containing aryl- or hetaryl halides A into indoles B (Scheme 1-1).3f,3g Recently, one of us developed a Brönsted acid-mediated process for recyclization of furan derivatives 4 into indoles 3 (Scheme 1–2a).6 This method needs preparation of furylmethyl aniline 4, which is accomplished by condensation of benzyl alcohol 1 with furan 2. The recyclization step requires the use of overstoichiometric amounts of HCl/AcOH, which substantially limits the scope of this method. We thought that the development of more environmentally benign and general catalytic method for recyclization of furan, which would exhibit wider substrate scope and potentially would allow for a one-pot synthesis of indoles 3 directly from 1 and 2 is justified. Herein, we wish to report general and efficient Brönsted acid-catalyzed synthesis of indoles from o-aminobenzyl alcohols and furans, which features a one-pot protocol, wider substrate scope, and higher functional group tolerance than the previously published two-step procedure (Scheme 1–2b).
Scheme 1.
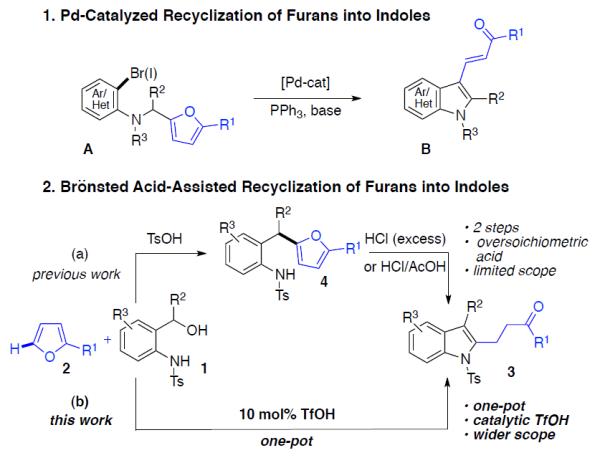
Methods for Recyclization of Furans into Indoles
2. Results and Discussion
Optimization of the One-Pot Synthesis of Indole 3 from o-Aminobenzyl Alcohol 1a
Recognizing that both steps of the sequence depicted in Scheme 1–2a proceed under electrophilic conditions, we hypothesized that finding conditions for formation of indole 3 directly from aminobenzyl alcohol 1 and 2-methylfuran 2 is feasible. To this end, different Lewis acid catalysts were first screened for the one-pot recyclization of 1a and 2a into 3aa (Table 1). Thus, employment of FeCl3 resulted in efficient formation of intermediate 4aa with only traces of target indole 3aa produced (entry 1). Employment of copper, indium, and silver triflates was more efficient for the second step leading to better ratios of 3aa/4aa (entries 2–4). Use of Sc(OTf)3 resulted in nearly complete conversion of starting materials into indole 3aa (entry 5). In order to verify whether Sc(OTf)3 is the true catalyst in this recyclization reaction, or if it is catalyzed by the Brönsted acid7 (TfOH) produced by a hydrolysis of Sc(OTf)3 during the first dehydrocondensation step, the following test experiments were performed. First, addition of activated molecular sieves, which scavenged the forming water and thus suppressed the hydrolysis of Sc(OTf)3, resulted in much more sluggish recyclization reaction, producing intermediate 4aa as a major product (entry 6). Moreover, addition of 30 mol% of a proton scavenger 2,4,6-tritertbutylpyrimidine (TTBP)8 had a similar inhibition effect on the second step of the reaction (entry 7). Finally, addition of both water- and a proton scavengers completely shut down the recyclization step, thus producing furylmethyl aniline 4aa as a single reaction product (entry 8). These results unambiguously demonstrate that TfOH is the true catalyst of the recyclization step (4aa→3aa). Consequently, we examined several other Brönsted acids in the recyclization reaction of 1a and 2a into 3aa. It was found that employment of HNTf2, HCl, and CF3CO2H produced only intermediate 4aa in low to moderate yields (entries 9–12). Use of H2SO4 and TsOH gave mixtures of indole 3aa and furyl aniline derivative 4aa (entries 13, 14). Remarkably, in the presence of MsOH and TfOH indole 3aa was produced in 87% and 95% yields, respectively (entries 15, 16). The following optimization with TfOH as the catalyst (entries 17–19) revealed optimal conditions for this Brönsted acid-catalyzed transformation (entry 18), which allowed for a facile and complete conversion of 1a and 2a into indole 3aa.
Table 1.
Optimization of the One-Pot Synthesis of Indole 3 from o-Aminobenzyl Alcohol 1aa
| entry | catalyst | time, h | yield 3 (4), %b |
|---|---|---|---|
| 1 | 10% FeCl3 | 12 | <5 (91) |
| 2 | 10% Cu(OTf)2 | 16 | 53 (38) |
| 3 | 10% In(OTf)3 | 2 | 87 (10) |
| 4 | 10% AgOTf | 1 | 80 (13) |
| 5 | 10% Sc(OTf)3 | 12 | 92 (<1) |
| 6c | 10% Sc(OTf)3 | 24 | 29 (61) |
| 7d | 10% Sc(OTf)3 | 24 | 22 (70) |
| 8c,d | 10% Sc(OTf)3 | 24 | -- (94) |
| 9 | 10% HNTf2 | 12 | -- (68) |
| 10e | 10% HCl | 12 | -- (38) |
| 11e | 100% HCl | 12 | -- (21) |
| 12 | 10% CF3COOH | 12 | -- (58) |
| 13 | 10% H2SO4 | 12 | 39 (50) |
| 14 | 10% TsOH | 12 | 41 (48) |
| 15 | 10% MsOH | 4 | 87 (0) |
| 16 | 10% TfOH | 1 | 95 (0) |
| 17 | 5% TfOH | 4 | 91 (0) |
| 18f | 10% TfOH | 1 | 94 (0) |
| 19g | 10% TfOH | 1 | 73 (0) |
Reaction conditions: o-Aminobenzyl alcohol 1a (0.2 mmol), 2-methylfuran 2a (2 equiv, 0.4 mmol, 35.8 μL), and catalyst were stirred in DCE (0.7 mL, 0.3 M) at 80°C. The progress of the reaction was monitored by TLC.
NMR Yield.
In the presense of 50 mg molecular sieves 4Å.
In the presence of 30 mol% 2,4,6-tritertbutylpyrimidine.
4M HCl in dioxane was used.
1.5 equiv of 2-methylfuran was used.
1.2 equiv of 2-methylfuran was used.
Scope of Furans in the One-Pot Synthesis of Indoles
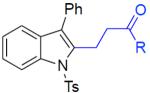
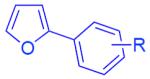
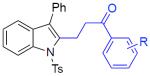
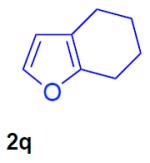
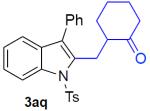
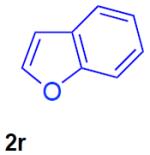
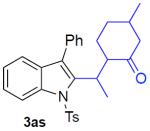
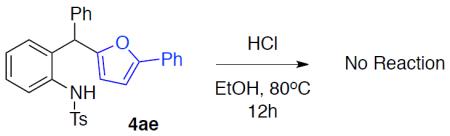
With the optimized conditions in hand, we explored the generality of this one-pot recyclization reaction. First, the scope of recyclization reaction of aminoalcohol 1a with different furans 2 was examined (Table 2). Thus, 2-alkyl- and 2-cycloalkylfurans 2a-d were smoothly converted into the corresponding indoles 3ab–3ad in good to excellent yields (entries 1–5). We also showed that recyclization reaction could be easily scaled up to a gram-scale synthesis of indole 3aa (entry 2). Different 2-arylfurans 2e-m, possessing MeO-, Br-, F-, and CF3- groups were also efficiently transformed into indoles 3ae–3am (entries 6–14). Remarkably, it was found that sterically bulky 2-arylfurans 2g–2i are also competent reactants in this reaction providing the corresponding indoles 3ag–3ai in good yields (entries 8–10). Furans 2n and 2o, possessing an ester group in a side chain, recyclized uneventfully producing indoles 3an and 3ao in 61% and 68% yield, respectively (entry 15–16). Likewise, 2,3-disubstituted furans, such as 2,3-dimethylfuran 2p and 4,5,6,7-tetrahydrobenzofuran 2q, produced the corresponding indoles 3ap and 3aq in high yields (entries 17–18). Benzofuran 2r also underwent recyclization to produce benzylphenoloindole derivative 3ar in moderate yield (entry 19). However, when C-4 substituted menthofuran 2s was used, no recyclization product 3as was formed (entry 20). Noteworthy, not only these catalytic one-pot conditions are more convenient experimentally, but they also exhibit wider substrate scope. Thus, attempts on recyclization of intermediate 4ae under previously developed conditions (HCl-EtOH)6 in 12 hours produced no detectable amounts of indole 3ae. Moreover, recyclization of furylmethyl aniline 4ao using HCl-EtOH produced transesterified indole 3an.9
Table 2.
Scope of Furans in the One-Pot Synthesis of Indoles from o-Aminobenzyl Alcohola
| entry | furan | product | 3, yield |
|---|---|---|---|

|
|
||
| 1 | 2a: R = Me | 3aa: R = Me | 90% |
| 2b | 2a: R = Me | 3aa: R = Me | 83% |
| 3 | 2b: R = n-Bu | 3ab: R = n-Bu | 95% |
| 4 | 2c: R = Cpent | 3ac: R = Cpent | 93% |
| 5 | 2d: R = Cy | 3ad: R = Cy | 75% |
|
|
||
| 6c | 2e: R = H | 3ae: R = H | 87% |
| 7 | 2f: R = 2-OMe | 3af: R = 2-OMe | 85% |
| 8 | 2g: R = 2-CF3 | 3ag: R = 2-CF3 | 56% |
| 9 | 2h: R = 2,3-benzo | 3ah: R = 2,3-benzo | 53% |
| 10 | 2i: R = 2,4,6-tri Pr | 3ai: R = 2,4,6-tri Pr | 48% |
| 11 | 2j: R = 4-CF3 | 3aj: R = 4-CF3 | 60% |
| 25 | 2k: R = 4-F | 3ak: R = 4-F | 83% |
| 13 | 2l: R = 4-Br | 3al: R = 4-Br | 70% |
| 14 | 2m: R = 4-OMe | 3am: R = 4-OMe | 56% |
|
|
||
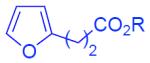
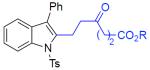
| 15 | 2n: R = Et | 3an: R = Et | 61% |
| 16 | 2n: R = Et | 3ao: R = Cy | 68% |
| 17 |
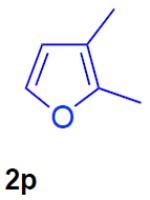
|
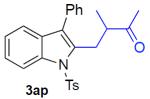
|
87% |
| 18 |
|
|
70% |
| 19 |
|

|
45% |
| 20 |

|
|
0%d |
Reaction conditions: o-Aminobenzyl alcohol 1a (0.5 mmol), furan 2 (1.5 equiv, 0.75 mmol), and TfOH (10 mol%, 4.5 μL) were stirred in DCE (1.6 mL, 0.3 M) at 80°C. The progress of the reaction was monitored by TLC.
Reaction was performed on 4.0 mmol scale in a pressure tube.
Reaction mixture (entries 6–14) was heated at 110°C for 2h.
Furylmethyl aniline intermediate 4ar was isolated in 95% yield.
Scope of o-Aminobenzyl Alcohols in the One-Pot Synthesis of Indoles
Next, the scope of the reaction with regard to the R1 substituent at the α-position of aminobenzyl alcohol 1 was examined (Table 3, entries 1–8). It was found that alkyl-substituted benzyl alcohols, such as methyl-, iso-propyl-, tert-butyl-, and cyclohexyl-, can be easily transformed into indoles 3ba–3ea in good to excellent yields (entries 1–4). Likewise, aryl-containing aminoalcohols, possessing F-, Me-, and MeO- groups efficiently cyclized into indoles 3fa–3ha (entries 5–7). Recyclization of 2-thienyl-substituted benzyl alcohol 1i produced the corresponding indole 3ia in 41% yield (entry 8).
Table 3.
Variation of Substituents at α-Position and at Aniline Phenyl Ring of o-Aminobenzyl Alcoholsa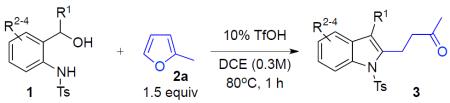
| entry | aminoalcohol | product | 3, yieldb |
|---|---|---|---|
|
|
||
| 1 | 1b: R1 = Me | 3ba: R1 = Me | 88% |
| 2 | 1c: R1 = iPr | 3ca: R1 = iPr | 76% |
| 3 | 1d: R1 = tBu | 3da: R1 = tBu | 63% |
| 4 | 1e: R1 = Cy | 3ea: R1 = Cy | 83% |
| 5 | 1f: R1 = 4-FC6H4- | 3fa: R1 = 4-FC6H4- | 72% |
| 6 | 1g: R1 = 4-MeC6H4- | 3ga: R1 = 4-MeC6H4- | 60% |
| 7 | 1h: R1 = 4-MeOC6H4- | 3ha: R1 = 4-MeOC6H4- | 71% |
| 8 | 1i: R1 = 2-thienyl | 3ia: R1 = 2-thienyl | 41% |
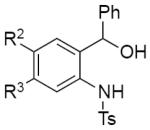
|
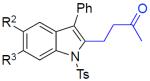
|
||
| 9 | 1j: R2 = R3 = F | 3ja: R2 = R3 = F | 79% |
| 10 | 1k: R2 = R3 = OMe | 3ka: R2 = R3 = OMe | 60% |
| 11 | 1l: R2 = H, R3 = CF3 | 3la: R2 = H, R3 = CF3 | 63% |
| 12 | 1m: R2 = Br, R3 = H | 3ma: R2 = Br, R3 = H | 61% |
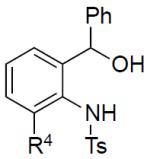
|

|
||
| 13 | 1n: R4 = Me | 3na: R4 = Me, G = Ts | traces |
| 14 | 1o: R4 = Cl | 3oa: R4 = Cl, G = H | 58% |
Reaction conditions: o-Aminobenzyl alcohol 1 (0.5 mmol), 2-methylfuran 2a (1.5 equiv, 0.75 mmol, 67.1 μL), and TfOH (10 mol%, 4.5 μL) were stirred in DCE (1.6 mL, 0.3 M) at 80°C. The progress of the reaction was monitored by TLC.
Next, aminoalcohols possessing different substituents at the aniline ring were tested in this recyclization reaction (Table 3, entries 9–14). Thus, reaction of 4,5-difluoro- and 4,5-dimethoxybenzyl alcohols 1j and 1k led to the formation of indoles 3ja and 3ka in good yields (entries 9–10). 5-Trifluoromethylbenzyl alcohol 1l and 4-bromobenzyl alcohol 1m provided indoles 3la and 3ma in good yields, as well (entries 11–12). It is worth mentioning that 6-methyl aminoalcohol 1n was not efficient in this reaction (entry 13), whereas recyclization of 6-chloro aminoalcohol 1o provided N-detosylated indole 3oa in reasonable yield (entry 14).
Further Modifications of the Obtained Indoles
After establishing the scope of this one-pot recyclization reaction, we then performed several transformations that highlight the utility of the synthesized indoles. Thus, the formed indole 3ae, upon reduction of its carbonyl group into alcohol group and subsequent Friedel-Crafts-type cyclization, was smoothly converted into tetracyclic indole core 5 (Scheme 2).10 Removal of the tosyl group with sodium naphthalenide,11 produced N–H indole 6, which upon oxidation with DDQ, furnished α,β-unsaturated ketone 7. Alternatively, upon reaction with excess Mg in methanol,12 the tosyl group in indole 3aa was efficiently removed with simultaneous reduction of the carbonyl group to produce alcohol 8. The latter, upon mesylation and subsequent intramolecular N-alkylation,13 was easily converted into dihydropyrroloindole 9, an important motif found in a number of drug candidates and natural products.14
Scheme 2.
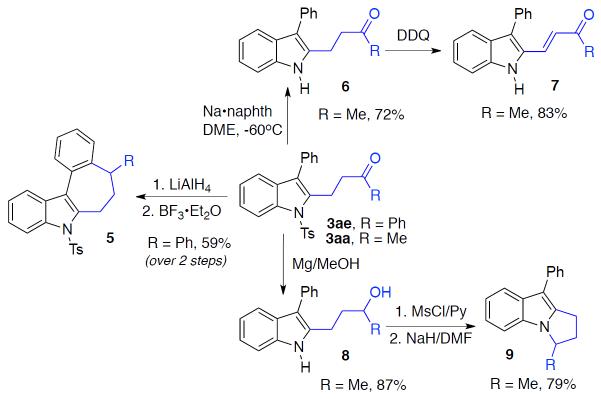
Further Modifications of the Obtained Indoles
3. Conclusion
In summary, we developed an efficient one-pot Brönsted acid-catalyzed method for synthesis of indoles from o-aminobenzyl alcohols and furans. This method features initial formation of aminobenzylfuran, followed by its recyclization into indole possessing ethyl ketone moiety. This method proved to have wider substrate scope and higher functional group tolerance than the previously reported two-step procedure. The formed indoles can be further transformed into valuable 2,3- and 1,2-fused indoles, and into indoles possessing an α,β-unsaturated ketone moiety.
4. Experimental Section
General Information
Column chromatography was carried out employing Silicycle Silica-P flash silica gel (40–63 μm). Precoated silica gel plates F-254 were used for thin-layer analytical chromatography (TLC). Chemical shifts for 1H and 13C nuclear magnetic resonance (NMR) spectra were reported in parts per million (ppm, δ). HRMS analyses were performed on either Micromass 70-VSE mass spectrometer by using electron ionization (EI) technique with Spector analyzer or by using electrospray ionization (ESI) with time-of-flight (TOF) analyzer. Anhydrous solvents purchased from suppliers were additionally purified on solvent purification system and/or stored over calcium hydride/molecular sieves. 2-Aryl/alkylfurans, if not commercially available, were synthesized from 2-bromofuran.15
Synthesis of Starting Materials
2-(N-tosylamino)benzaldehyde ab1 was prepared as described.16
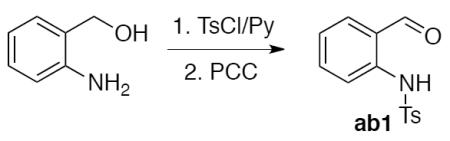 To a solution of 2-aminobenzyl alcohol (6.2 g, 50.0 mmol) and pyridine (1.2 equiv, 4.8 mL, 60.0 mmol) in CHCl3 (165 mL), TsCl (0.98 equiv, 9.3 g, 49.0 mmol) in CHCl3 (50 mL) was added dropwise at room temperature. The reaction mixture was stirred for 3 h and then quenched with water (50 mL). The organic phase was separated, washed with water, dried over Na2SO4, and concentrated to give the 2-(N-tosylamino)benzyl alcohol (13.5 g, 48,6 mmol, 97%), which was used directly in the next step.
To a solution of 2-aminobenzyl alcohol (6.2 g, 50.0 mmol) and pyridine (1.2 equiv, 4.8 mL, 60.0 mmol) in CHCl3 (165 mL), TsCl (0.98 equiv, 9.3 g, 49.0 mmol) in CHCl3 (50 mL) was added dropwise at room temperature. The reaction mixture was stirred for 3 h and then quenched with water (50 mL). The organic phase was separated, washed with water, dried over Na2SO4, and concentrated to give the 2-(N-tosylamino)benzyl alcohol (13.5 g, 48,6 mmol, 97%), which was used directly in the next step.
To a stirred suspension of PCC (1.5 equiv, 15.7 g, 72.9 mmol) in CH2Cl2 (240 mL), a solution of the 2-(N-tosylamino)benzyl alcohol in CH2Cl2 (360 mL) was added dropwise at room temperature. The mixture was stirred for 3 h. The liquid was decanted from the solid, and the remaining solid was washed several times with Et2O (3×100 mL). The combined organic fractions were passed through a short pad of silica gel. The silica gel was additionally washed with CH2Cl2 (3×100 mL) and the combined organic solutions were evaporated to give the crude product. The product was recrystallized from CHCl3/EtOH (1/5, 100 mL) to yield aldehyde ab1 as a white-yellow solid (11.2 g, 40.8 mmol, 84%), which was used directly in the synthesis of alcohols 1.
General procedure for the synthesis of 2-(N-tosylamino)-4,5-difluorobenzaldehyde ab2, 2-(N-tosylamino)-4-trifluoromethylbenzaldehyde ab3, 2-(N-tosylamino)-5-bromobenzaldehyde ab4, and 2-(N-tosylamino)-3-chlorobenzaldehyde ab5.17

To a solution of a proper 2-aminobenzoic acid (5 mmol) and Na2CO3 (2.5 equiv) in distilled water (0.4 M) heated to 60 °C, TsCl (1.25 equiv) was added during the course of 15 min. The mixture was then heated to 85 °C and stirred at this temperature for 3 h. The hot mixture was then cooled down and mixed with ice. HCl (6 N) was added dropwise to the resulted mixture until slightly acidic pH (caution: CO2 evolution!). The resulted white suspension was extracted with EtOAc (3×10 mL), and combined organic solutions were additionally washed with HCl (1 N), dried over Na2SO4, and evaporated to give the 2-(N-tosylamino)benzoic acid. The crude product was used directly in the next step.
Under a nitrogen atmosphere, to an ice-cooled stirred suspension of LiAlH4 (2.0 equiv) in THF (0.2M), a solution of 2-(N-tosylamino)benzoic acid in minimum amount of THF was added dropwise. The reaction mixture was allowed to warm up to room temperature and stirred for 3 h. After completion of the reaction (determined by TLC, eluent hexanes:EtOAc = 2:1), the reaction mixture was cooled down (ice bath) and saturated aqueous NH4Cl (2.0 mL) was added dropwise (caution: H2 evolution!). The resulting sluggish mixture was filtered through Celite® and concentrated. The crude product was used directly in the next step.
The resulted alcohol was oxidized to aldehyde using PCC as described earlier. The obtained aldehydes ab2–ab5 were used directly in the synthesis of alcohols 1.
General procedure for the synthesis of 2-(N-tosylamino)-4,5-dimethoxybenzaldehyde ab6 and 2-(N-tosylamino)-3-methylbenzaldehyde ab7.18
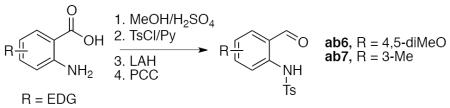
A solution of a proper 2-aminobenzoic acid (5 mmol), methanol (10 mL), and concentrated H2SO4 (2 mL) was heated under reflux for 24 h. The reaction mixture was cooled down, concentrated, poured into ice, neutralized with solid Na2CO3 (caution: CO2 evolution!), and extracted with CH2Cl2 (3×10 mL). The combined organic layers were washed with water, saturated aqueous NaHCO3, dried over Na2SO4, and concentrated to provide a crude methyl 2-aminobenzoate, which was used directly in the next step.
To a solution of crude methyl 2-aminobenzoate and pyridine (3 equiv) in CH2Cl2 (10 mL), a solution of TsCl (0.98 equiv) in CH2Cl2 (5 mL) was added dropwise at room temperature. The reaction mixture was stirred for 3 h and then quenched with water (10 mL). The organic layer was separated, washed with water, dried over Na2SO4, and concentrated. The crude product was used directly in the next step.
Reduction of methyl ester with LiAlH4 and subsequent oxidation of the resulted alcohol to aldehyde with PCC was done as described earlier. The obtained aldehydes ab6–ab7 were used directly in the synthesis of alcohols 1.
General procedure for the synthesis of 2-(N-tosylamino)benzyl alcohols 1 from 2-(N-tosylamino)benzaldehydes ab.
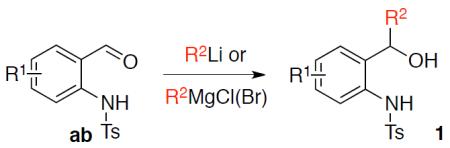
An oven dried, argon flushed 25 mL flask was loaded with 2-(N-tosylamino)benzaldehyde ab (1.0 mmol) and THF (8.0 mL, 0.125 M). The flask was cooled to −78 °C and a solution of R2Li/R2MgCl(Br) (2.2 mmol, 2.2 equiv) was added dropwise. The resulting mixture was allowed to warm up to 0 °C, and saturated NH4Cl (5 mL) was added. The organic layer was separated and the water layer was washed with ethyl acetate (2×5 mL). The combined organic solutions were dried over Na2SO4 and the solvents were evaporated. The resulted crude product was purified by recrystallization from iPrOH to give pure alcohol 1 as a white solid.
N-(2-(hydroxy(phenyl)methyl)phenyl)-4-methylbenzenesulfonamide (1a) was obtained from aldehyde ab1 and PhLi, 332 mg (94%), Rf = 0,09 (hexanes/EtOAc = 4/1). The spectral data matched that of the previously synthesized material.61H NMR (500 MHz, CDCl3) δ 8.00 (s, 1H), 7.48–7.46 (m, 3H), 7.32–7.30 (m, 3H), 7.23 (t, J = 7.3 Hz, 1H), 7.17–7.14 (m, 4H), 7.02 (t, J = 7.7 Hz, 1H), 6.92 (d, J = 7.3 Hz, 1H), 5.67 (s, 1H), 2.69 (broad s, 1H), 2.36 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 143.6, 141.0, 136.6, 135.8, 133.1, 129.5, 129.0 (2C), 128.6, 127.9, 127.2, 126.3, 124.6, 122.1, 74.7, 21.5. HRMS (EI+) calcd. for C20H19NO3S [M]+: 353.1086, measured: 353.1087.
N-(2-(1-hydroxyethyl)phenyl)-4-methylbenzenesulfonamide (1b) was obtained from aldehyde ab1 and MeLi, 255 mg (88%), Rf = 0,18 (hexanes/EtOAc = 4/1). The spectral data matched that of the previously synthesized material.191H NMR (500 MHz, CDCl3) δ 8.51 (broad s, 1H), 7.68–7.67 (m, 2H), 7.41 (d, J = 8.1 Hz, 1H), 7.22–7.20 (m, 2H), 7.17 (dt, J = 7.3, 1.7 Hz, 1H), 7.09–7.02 (m, 2H), 4.84 (q, J = 6.6 Hz, 1H), 2.68 (broad s, 1H), 2.36 (s, 3H), 1.34 (d, J = 6.6 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 143.7, 136.9, 135.7, 134.0, 129.6, 128.5, 127.1, 127.0, 124.6, 121.8, 69.8, 22.8, 21.5. HRMS (EI+) calcd. for C15H17NO3S [M]+: 291.0929, measured: 291.0935.
N-(2-(1-hydroxy-2-methylpropyl)phenyl)-4-methylbenzenesulfonamide (1c) was obtained from aldehyde ab1 and iPrMgCl, 213 mg (67%), Rf = 0,21 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.48 (broad s, 1H), 7.67–7.66 (m, 2H), 7.48 (d, J = 7.5 Hz, 1H), 7.21–7.15 (m, 3H), 7.00–6.95 (m, 2H), 4.23 (d, J = 9.0 Hz, 1H), 2.70 (broad s, 1H), 2.36 (s, 3H), 1.66 (m, 1H), 1.00 (d, J = 6.6 Hz, 3H), 0.43 (d, J = 6.8 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 143.7, 136.8, 135.8, 132.0, 129.5, 129.2, 128.3, 127.1, 123.9, 121.3, 81.3, 33.4, 21.5, 19.4, 19.2. HRMS (EI+) calcd. for C17H21NO3S [M]+: 319.1242, measured: 319.1245.
N-(2-(1-hydroxy-2,2-dimethylpropyl)phenyl)-4-methylbenzenesulfonamide (1d) was obtained from aldehyde ab1 and tBuMgCl, 209 mg (63%), Rf = 0,23 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.86 (broad s, 1H), 7.76–7.74 (m, 2H), 7.38 (d, J = 8.3 Hz, 1H), 7.24–7.23 (m, 2H), 7.14 (m, 1H), 6.99–6.93 (m, 2H), 4.51 (s, 1H), 2.53 (broad s, 1H), 2.38 (s, 3H), 0.98 (s, 9H). 13C NMR (125 MHz, CDCl3) δ 143.6, 137.1, 136.6, 130.5, 129.6, 128.4, 128.1, 127.3, 122.6, 119.4, 84.0, 37.3, 26.3, 21.5. HRMS (EI+) calcd. for C18H23NO3S [M]+: 333.1399, measured: 333.1404.
N-(2-(cyclohexyl(hydroxy)methyl)phenyl)-4-methylbenzenesulfonamide (1e) was obtained from aldehyde ab1 and CyMgBr, 305 mg (85%), Rf = 0,20 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.62 (broad s, 1H), 7.70–7.69 (m, 2H), 7.58 (d, J = 8.1 Hz, 1H), 7.23–7.17 (m, 3H), 7.00–6.97 (dt, J = 7.5, 1.1 Hz, 1H), 6.92 (dd, J = 7.7, 1.5 Hz, 1H), 4.29 (d, J = 9.0 Hz, 1H), 2.65 (broad s, 1H), 2.37 (s, 3H), 2.05 (m, 1H), 1.73 (m, 1H), 1.58 (m, 1H), 1.48 (m, 1H), 1.30 (m, 1H), 1.14–1.01 (m, 2H), 0.95–0.89 (m, 2H), 0.79 (m, 1H), 0.67 (m, 1H). 13C NMR (125 MHz, CDCl3) δ 143.7, 137.0, 135.9, 131.2, 129.6, 129.3, 128.3, 127.1, 123.6, 120.9, 80.7, 42.5, 29.6, 29.5, 26.1, 25.6, 25.4, 21.5. HRMS (EI+) calcd. for C20H25NO3S [M]+: 359.1555, measured: 333.1558.
N-(2-((4-fluorophenyl)(hydroxy)methyl)phenyl)-4-methylbenzenesulfonamide (1f) was obtained from aldehyde ab1 and 4-FC6H4MgBr, 248 mg (67%), Rf = 0,13 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.06 (broad s, 1H), 7.47 (d, J = 7.9 Hz, 1H), 7.44–7.43 (m, 2H), 7.21 (dt, J = 8.1, 1.5 Hz, 1H), 7.13–7.12 (m, 2H), 7.10–7.08 (m, 2H), 7.02 (dt, J = 7.5, 0.7 Hz, 1H), 6.94–6.90 (m, 3H), 5.72 (s, 1H), 3.10 (broad s, 1H), 2.38 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 163.2 (d, JC-F = 246.0 Hz), 143.7, 136.9 (d, JC-F = 2.8 Hz), 136.4, 135.7, 133.0, 129.5, 129.1 (d, JC-F = 3.7 Hz), 127.9 (d, JC-F = 8.3 Hz), 127.0, 124.7, 122.1, 115.3 (d, JC-F = 22.2 Hz), 74.1, 21.5. HRMS (EI+) calcd. for C20H18NO3SF [M]+: 371.0991, measured: 371.0986.
N-(2-(hydroxy(p-tolyl)methyl)phenyl)-4-methylbenzenesulfonamide (1g) was obtained from aldehyde ab1 and 4-MeC6H4MgBr, 256 mg (70%), Rf = 0,12 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.08 (broad s, 1H), 7.49–7.47 (m, 3H), 7.22 (m, 1H), 7.15–7.10 (m, 4H), 7.04–7.00 (m, 3H), 6.93 (d, J = 7.7 Hz, 1H), 5.62 (d, J = 3.1 Hz, 1H), 2.81 (d, J = 3.5 Hz, 1H), 2.39 (s, 3H), 2.37 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 143.5, 138.0, 137.5, 136.5, 135.8, 133.1, 129.4, 129.3, 128.9, 128.8, 127.1, 126.2, 124.5, 121.9, 74.5, 21.5, 21.1. HRMS (EI+) calcd. for C21H21NO3S [M]+: 367.1242, measured: 367.1240.
N-(2-(hydroxy(4-methoxyphenyl)methyl)phenyl)-4-methylbenzenesulfonamide (1h) was obtained from aldehyde ab1 and 4-MeOC6H4MgBr, 321 mg (84%), Rf = 0,06 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.19 (broad s, 1H), 7.47–7.45 (m, 3H), 7.19 (m, 1H), 7.13–7.11 (m, 2H), 7.05–6.99 (m, 3H), 6.91 (d, J = 7.7 Hz, 1H), 6.80–6.78 (m, 2H), 5.59 (broad s, 1H), 3.79 (s, 3H), 3.11 (m, 1H), 2.37 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 159.1, 143.5, 136.5, 135.7, 133.2, 129.4, 128.9, 128.7, 127.6, 127.1, 124.4, 121.8, 113.9, 74.3, 55.2, 21.4. HRMS (EI+) calcd. for C21H21NO4S [M]+: 383.1191, measured: 383.1194.
N-(2-(hydroxy(thiophen-2-yl)methyl)phenyl)-4-methylbenzenesulfonamide (1i) was obtained from aldehyde ab1 and thiophen-2-yl lithium (prepared by mixing 2-bromothiophene with 1.1 equiv of n-BuLi in THF at −78 °C), 341 mg (95%), Rf = 0,15 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.10 (broad s, 1H), 7.49–7.48 (m, 2H), 7.45 (d, J = 8.1 Hz, 1H), 7.26–7.21 (m, 2H), 7.15–7.13 (m, 2H), 7.10–7.03 (m, 2H), 6.87 (dd, J = 5.1, 3.5 Hz, 1H), 6.59 (m, 1H), 5.93 (m, 1H), 3.41 (m, 1H), 2.43 (s, 3H). 13C NMR (125 MHz, acetone-d6) δ 148.7, 144.9, 138.3, 137.4, 134.7, 130.8, 129.9 (2C), 128.4, 126.4, 125.6, 125.3, 121.9, 72.6, 21.8. HRMS (EI+) calcd. for C18H17NO3S2 [M]+: 359.0650, measured: 359.0648.
N-(4,5-difluoro-2-(hydroxy(phenyl)methyl)phenyl)-4-methylbenzenesulfonamide (1j) was obtained from aldehyde ab2 and PhLi, 303 mg (78%), Rf = 0,19 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 7.46–7.45 (m, 2H), 7.30–7.26 (m, 4H), 7.18–7.16 (m, 2H), 7.09–7.08 (m, 2H), 6.66 (dd, J = 10.8, 8.6 Hz, 1H), 5.53 (s, 1H), 2.39 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 149.5 (dd, JC-F = 249.7, 13.9 Hz), 147.1 (dd, JC-F = 247.8, 12.9 Hz), 144.3, 140.4, 135.8, 131.8 (JC-F = 6.5 Hz), 131.0, 129.8, 128.8, 128.2, 127.1, 126.3, 117.6 (JC-F = 19.4 Hz), 112.2 (JC-F = 21.3 Hz), 73.2, 21.5. HRMS (EI+) calcd. for C20H17NO3SF2 [M]+: 389.0897, measured: 389.0895.
N-(4,5-dimethoxy-2-(hydroxy(phenyl)methyl)phenyl)-4-methylbenzenesulfonamide (1k) was obtained from aldehyde ab6 and PhLi, 313 mg (76%), Rf = 0,10 (hexanes/EtOAc = 2/1). 1H NMR (500 MHz, CDCl3) δ 7.53–7.51 (m, 2H), 7.31–7.27 (m, 4H), 7.21–7.19 (m, 2H), 7.15–7.13 (m, 2H), 6.83 (s, 1H), 6.45 (s, 1H), 5.62 (d, J = 3.3 Hz, 1H), 3.75 (s, 3H), 3.68 (s, 3H), 2.73 (broad s, 1H), 2.41 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 148.7, 146.8, 143.8, 141.6, 136.3, 129.6, 128.7, 128.5, 127.7, 127.4, 126.3, 111.5, 108.6, 73.0, 55.9, 21.5. HRMS (EI+) calcd. for C22H23NO5S [M]+: 413.1297, measured: 413.1294.
N-(2-(hydroxy(phenyl)methyl)-5-(trifluoromethyl)phenyl)-4-methylbenzenesulfonamide (1l) was obtained from aldehyde ab3 and PhLi, 341 mg (81%), Rf = 0,16 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.34 (broad s, 1H), 7.72 (s, 1H), 7.45–7.44 (m, 2H), 7.33–7.31 (m, 3H), 7.26–7.24 (d, J = 8.1 Hz, 1H), 7.18–7.14 (m, 4H), 7.07 (d, J = 8.1 Hz, 1H), 5.77 (s, 1H), 3.18 (s, 1H), 2.38 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 144.2, 140.3, 136.4, 136.0, 135.7, 131.1 (q, JC-F = 32.4 Hz), 129.7, 129.5, 128.8, 128.2, 127.2, 126.2, 123.5 (q, JC-F = 272.8 Hz), 120.8, 118.0, 74.5, 21.5. HRMS (EI+) calcd. for C21H18NO3SF3 [M]+: 421.0960, measured: 421.0940.
N-(4-bromo-2-(hydroxy(phenyl)methyl)phenyl)-4-methylbenzenesulfonamide (1m) was obtained from aldehyde ab4 and PhLi, 401 mg (93%), Rf = 0,12 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.02 (broad s, 1H), 7.45–7.43 (m, 2H), 7.33–7.32 (m, 5H), 7.16–7.14 (m, 4H), 7.07 (d, J = 1.8 Hz, 1H), 5.61 (s, 1H), 2.96 (broad s, 1H), 2.39 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 143.9, 140.2, 136.1, 135.1, 134.6, 131.8, 131.7, 129.7, 128.8, 128.2, 127.1, 126.3, 123.5, 117.7, 74.2, 21.5. HRMS (ES+) calcd. for C20H18NO3SBrNa [M+Na]+: 454.0088, measured: 454.0090.
N-(2-(hydroxy(phenyl)methyl)-6-methylphenyl)-4-methylbenzenesulfonamide (1n) was obtained from aldehyde ab7 and PhLi, 235 mg (64%), Rf = 0,08 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 7.63–7.61 (m, 2H), 7.33–7.27 (m, 5H), 7.21–7.19 (m, 2H), 7.11–7.07 (m, 2H), 6.94 (d, J = 9.0 Hz, 1H), 6.73 (s, 1H), 5.96 (s, 1H), 3.11 (broad s, 1H), 2.45 (s, 3H), 1.94 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 144.1, 142.8, 142.5, 137.1, 137.0, 132.0, 130.7, 129.8, 128.3, 128.0, 127.3, 127.2, 127.1, 126.5, 71.3, 21.6, 18.4. HRMS (EI+) calcd. for C21H21NO3S [M]+: 367.1242, measured: 367.1241.
N-(2-chloro-6-(hydroxy(phenyl)methyl)phenyl)-4-methylbenzenesulfonamide (1o) was obtained from aldehyde ab5 and PhLi, 232 mg (60%), Rf = 0,10 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 7.55–7.54 (m, 2H), 7.37–7.35 (m, 2H), 7.32–7.21 (m, 6H), 7.14–7.13 (m, 2H), 6.67 (broad s, 1H), 6.61 (s, 1H), 3.78 (broad s, 1H), 2.42 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 146.4, 144.5, 142.9, 135.6, 132.0, 130.2, 129.6, 129.2, 129.0, 128.6, 128.1, 127.6, 127.0, 126.1, 70.2, 21.6. HRMS (EI+) calcd. for C20H18NO3SCl [M]+: 387.0696, measured: 387.0697.
One-Pot Synthesis of Indoles from o-Aminobenzyl Alcohols
Optimization of the One-Pot Synthesis of Indoles from o-Aminobenzyl Alcohols (Table 1)
In a 1 mL Wheaton microreactor vial, equipped with a Teflon pressure cap, a mixture of o-aminobenzyl alcohol 1a (0.1 mmol, 35.3 mg), 2-methylfuran 2a (0.2 mmol, 2 equiv, 18 μL), catalyst, and DCE (0.4 mL, 0.25M) was stirred at 80°C. The progress of the reaction was monitored by TLC (eluent hexanes:EtOAc = 4:1). After completion, the reaction mixture was filtered through Celite®, the solvent was evaporated and crude product was analyzed by 1H NMR.
General procedure for one-pot synthesis of indoles from aminobenzylalcohols and furans
In a 3 mL Wheaton microreactor vial, to a stirred suspension of aminobenzylalcohol 1 (0.4 mmol) and furan 2 (0.6 mmol, 1.5 equiv) in DCE (1.3 mL, 0.3M), triflic acid (0.04 mmol, 10 mol%, 3.6 μL) was added. The microreactor was capped with a Teflon pressure cap and placed into pre-heated (80μC) aluminum block. The resulted solution was stirred for 1–2 h at this temperature. After completion of the reaction (determined by TLC, eluent hexanes:EtOAc = 4:1), microreactor, containing product 3, was cooled down and the reaction mixture was diluted with 1.5 mL of hexanes. The product was purified using flash silica gel column chromatography.
N-tosyl-2-(3-oxobutyl)-3-phenylindole (3aa)
150 mg (90%), yellow oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,42 (hexanes/EtOAc = 4/1). The spectral data matched that of the previously synthesized material.61H NMR (500 MHz, CDCl3) δ 8.26 (d, J = 8.4 Hz, 1H), 7.65 (d, J = 8.1 Hz, 2H), 7.46–7.43 (m, 2H), 7.39–7.28 (m, 5H), 7.23–7.18 (m, 3H), 3.35 (t, J = 7.7 Hz, 2H), 2.94 (t, J = 7.7 Hz, 2H), 2.34 (s, 3H), 2.14 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 207.3, 144.9, 136.7, 136.3, 135.5, 132.6, 130.6, 129.9, 129.8, 128.7, 127.7, 126.4, 124.7, 124.4, 123.9, 119.5, 115.2, 44.7, 29.8, 21.6, 21.3. HRMS (EI+) calcd. for C25H23NO3S [M]+: 417.1399, measured: 417.1388.
N-tosyl-2-(3-oxoheptyl)-3-phenylindole (3ab)
174 mg (95%), yellow oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,42 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.25 (d, J = 8.0 Hz, 1H), 7.65 (d, J = 8.4 Hz, 2H), 7.45–7.42 (m, 2H), 7.39–7.29 (m, 4H), 7.26–7.22 (m, 2H), 7.19 (d, J = 8.4 Hz, 2H), 3.22 (t, J = 7.7 Hz, 2H), 2.89 (t, J = 7.7 Hz, 2H), 2.37 (t, J = 7.3 Hz, 2H), 2.32 (s, 3H), 1.58–1.52 (m, 2H), 1.34–1.26 (m, 2H), 0.90 (t, J = 7.3 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 209.7, 144.9, 136.6, 136.5, 135.5, 132.7, 130.5, 129.8 (2C), 128.7, 127.6, 126.4, 124.7, 124.3, 123.9, 119.5, 115.2, 43.7, 42.4, 26.0, 22.3, 21.6, 21.3, 13.9. HRMS (EI+) calcd. for C28H29NO3S [M]+ : 459.1868, measured: 459.1867.
N-tosyl-2-(3-oxo-3-cyclopentylpropyl)-3-phenylindole (3ac)
175 mg (93%), yellow oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,36 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.27 (d, J = 9.1 Hz, 1H), 7.65 (d, J = 8.1 Hz, 2H), 7.45–7.42 (m, 2H), 7.38–7.28 (m, 5H), 7.24–7.22 (m, 1H), 7.19 (d, J = 8.1 Hz, 2H), 3.24 (t, J = 7.7 Hz, 2H), 2.96 (t, J = 7.7 Hz, 2H), 2.85 (pent, J = 8.4 Hz, 1H), 2.34 (s, 3H), 1.84–1.70 (m, 4H), 1.68–1.54 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 211.6, 144.8, 136.7, 136.7, 135.7, 132.8, 130.6, 129.8, 129.8, 128.7, 127.6, 126.4, 124.6, 124.2, 123.8, 119.5, 115.2, 51.3, 42.8, 28.8, 26.0, 21.5, 21.4. HRMS (EI+) calcd. for C29H29NO3S [M]+: 471.1868, measured: 471.1877.
N-tosyl-2-(3-oxo-3-cyclohexylpropyl)-3-phenylindole (3ad)
146 mg (75%), yellow oil, eluent: hexanes/EtOAc = 9/1; Rf = 0,41. 1H NMR (500 MHz, CDCl3) δ 8.27 (d, J = 8.4 Hz, 1H), 7.64 (d, J = 8.0 Hz, 2H), 7.45–7.42 (m, 2H), 7.38–7.27 (m, 5H), 7.26–7.21 (m, 1H), 7.19 (d, J = 8.0 Hz, 2H), 3.21 (t, J = 7.7 Hz, 2H), 2.94 (t, J = 7.7 Hz, 2H), 2.34 (s, 3H), 1.85–1.75 (m, 4H), 1.66–1.64 (m, 1H), 1.34–1.17 (m, 6H); 13C NMR (125 MHz, CDCl3) δ 212.5, 144.8, 136.8, 136.7, 135.6, 132.7, 130.6, 129.8, 129.7, 128.6, 127.6, 126.4, 124.6, 124.2, 123.8, 119.4, 115.2, 50.7, 41.7, 28.5, 25.9, 25.7, 21.5, 21.3. HRMS (ES+) calcd. for C30H32NO3S [M+H]+: 486.2103, measured 486.2081.
N-tosyl-2-(3-phenyl-3-oxopropyl)-3-phenylindole (3ae)
166 mg (87%), yellow oil, eluent: hexanes/EtOAc = 9/1; Rf = 0,38. 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 9.7 Hz, 1H), 7.95–7.93 (m, 2H), 7.68 (d, J = 8.4 Hz, 2H), 7.56–7.53 (m, 1H), 7.45–7.42 (m, 4H), 7.38–7.31 (m, 5H), 7.25–7.19 (m, 3H), 3.49–3.42 (m, 4H), 2.34 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 198.6, 144.9, 136.8, 136.6, 136.5, 135.6, 133.0, 132.7, 130.6, 129.9, 129.8, 128.7, 128.5, 128.1, 127.7, 126.4, 124.7, 124.5, 123.9, 119.5, 115.2, 40.1, 21.8, 21.5. HRMS (ES+) calcd. for C30H25NO3S [M+H]+: 480.1633, measured: 480.1638.
N-tosyl-2-[3-(2-methoxyphenyl)-3-oxopropyl]-3-phenylindole (3af)
173 mg (85%), colorless oil, eluent: hexanes/EtOAc = 5/1; Rf = 0,24. 1H NMR (500 MHz, CDCl3) δ 8.30 (d, J = 8.4 Hz, 1H), 7.74–7.72 (m, 1H), 7.68 (d, J = 8.0 Hz, 2H), 7.44–7.42 (m, 3H), 7.36–7.32 (m, 5H), 7.24–7.22 (m, 1H), 7.19 (d, J = 8.0 Hz, 2H), 7.01–6.92 (m, 2H), 3.84 (s, 3H), 3.49 (t, J = 7.1 Hz, 2H), 3.41 (t, J = 7.1 Hz, 2H), 2.34 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 200.4, 158.8, 144.7, 137.2, 136.7, 135.8, 133.4, 132.9, 130.7, 130.4, 129.9 (2C), 129.7 (2C), 128.7 (2C), 127.8, 127.5, 126.4 (2C), 124.5, 124.1, 123.8, 120.5, 119.4, 115.2, 111.5, 55.5, 45.0, 21.8, 21.5. HRMS (EI+) calcd. for C31H27NO4S [M]+ : 509.1661, measured: 509.1663.
N-tosyl-2-[3-(2-trifluoromethylphenyl)-3-oxopropyl]-3-phenylindole (3ag)
122 mg (56%), colorless oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,26. 1H NMR (500 MHz, CDCl3) δ 8.27 (d, J = 8.4 Hz, 1H), 7.71–7.69 (m, 1H), 7.67 (d, J = 8.4 Hz, 2H), 7.61–7.58 (m, 1H), 7.56–7.50 (m, 2H), 7.47–7.44 (m, 2H), 7.41–7.29 (m, 5H), 7.24–7.20 (m, 3H), 3.43–3.36 (m, 4H), 2.35 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 202.7, 144.9, 139.9, 136.7, 135.8, 135.6, 132.5, 131.8, 130.5, 130.0, 129.9, 129.7, 128.7, 127.8, 127.1, 126.9 (q, JC–F = 32.4 Hz), 126.6 (q, JC–F = 4.4 Hz), 126.4, 124.8, 124.6, 123.9, 123.6 (q, JC–F = 272.5 Hz), 119.6, 115.1, 44.4, 21.6, 21.4. HRMS (EI+) calcd. for C31H24NO3SF3 [M]+ : 547.1429, measured: 547.1435.
N-tosyl-2-[3-(naphthyl-1)-3-oxopropyl]-3-phenylindole (3ah)
112 mg (53%), yellow oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,28. 1H NMR (500 MHz, CDCl3) δ 8.60 (d, J = 8.4 Hz, 1H), 8.29 (d, J = 8.8 Hz, 1H), 7.97 (d, J = 8.4 Hz, 1H), 7.90–7.86 (m, 2H), 7.69 (d, J = 8.0 Hz, 2H), 7.59–7.51 (m, 2H), 7.48–7.43 (m, 3H), 7.40–7.32 (m, 5H), 7.25–7.22 (m, 1H), 7.20 (d, J = 8.0 Hz, 2H), 3.53–3.49 (m, 4H), 2.34 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 202.8, 144.9, 136.8, 136.5, 135.7, 135.5, 133.9, 133.2, 132.9, 132.7, 132.6, 130.5, 130.1, 129.8, 128.8, 128.4, 127.8, 127.7, 126.4, 126.3, 125.8, 124.7, 124.5, 124.4, 123.9, 119.5, 115.2, 43.3, 22.1, 21.5. HRMS (ES+) calcd. for C34H27NO3S [M+H]+ : 530.1790, measured: 530.1791.
N-tosyl-2-[3-(2,4,6-tri-iso-propylphenyl)-3-oxopropyl]-3-phenylindole (3ai)
116 mg (48%), colorless oil, eluent: hexanes/EtOAc = 9/1; Rf = 0,33. 1H NMR (500 MHz, CDCl3) δ 8.25 (d, J = 8.4 Hz, 1H), 7.71 (d, J = 8.4 Hz, 2H), 7.48–7.39 (m, 3H), 7.34–7.31 (m, 4H), 7.23–7.20 (m, 3H), 6.98 (s, 2H), 3.45 (t, J = 7.5 Hz, 2H), 3.25 (t, J = 7.5 Hz, 2H), 2.88 (sept, J = 6.6 Hz, 1H), 2.60 (sept, J = 6.6 Hz, 2H), 2.36 (s, 3H), 1.25 (d, J = 6.6 Hz, 6H), 1.20 (d, J = 6.6 Hz, 12H); 13C NMR (125 MHz, CDCl3) δ 209.4, 149.4, 144.8, 143.6, 137.5, 136.6, 136.3, 135.8, 132.9, 130.5, 129.9, 129.8, 128.7, 127.7, 126.4, 124.6, 124.4, 123.8, 121.0, 119.4, 115.1, 47.7, 34.3, 30.9, 24.5, 24.0, 21.6, 21.1. HRMS (EI+) calcd. for C39H43NO3S [M]+ : 605.2964, measured: 605.2958.
N-tosyl-2-[3-(4-trifluoromethylphenyl)-3-oxopropyl]-3-phenylindole (3aj)
131 mg (60%), colorless oil; eluent: hexanes/EtOAc = 9/1; Rf = 0,23. 1H NMR (500 MHz, CDCl3) δ 8.29 (d, J = 8.4 Hz, 1H), 8.04 (d, J = 8.4 Hz, 2H), 7.70 (d, J = 8.0 Hz, 2H), 7.66 (d, J = 8.4 Hz, 2H) 7.45–7.42 (m, 2H), 7.39–7.30 (m, 5H), 7.25–7.22 (m, 1H), 7.20 (d, J = 8.4 Hz, 2H), 3.51–3.43 (m, 4H), 2.34 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.7, 145.0, 139.2, 136.8, 136.0, 135.5, 134.3 (q, JC–F = 32.4 Hz), 132.6, 130.5, 129.9, 129.8, 128.8, 128.4, 127.8, 126.4, 125.6 (q, JC–F = 3.0 Hz), 124.9, 124.8, 124.0, 123.7 (q, JC–F = 272.7 Hz), 119.6, 115.2, 40.4, 21.8, 21.5. HRMS (EI+) calcd. for C31H24NO3SF3 [M]+: 547.1429, measured: 547.1437.
N-tosyl-2-[3-(4-fluorophenyl)-3-oxopropyl]-3-phenylindole (3ak)
165 mg (83%), light blue oil; eluent: hexanes/EtOAc = 7/1; Rf = 0,31. 1H NMR (500 MHz, CDCl3) δ 8.30 (d, J = 8.8 Hz, 1H), 7.98–7.95 (m, 2H), 7.67 (d, J = 7.7 Hz, 2H), 7.45–7.42 (m, 2H), 7.38–7.31 (m, 5H), 7.25–7.19 (m, 3H), 7.11–7.08 (m, 2H), 3.44 (s, 4H), 2.34 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.0, 165.7 (d, JC–F = 255 Hz), 144.9, 136.8, 136.4, 135.6, 133.1, 132.6, 130.7 (d, JC–F = 9.25 Hz), 130.5, 129.9, 129.8, 128.8, 127.7, 126.4, 124.6, 124.4 (d, JC–F = 105 Hz), 119.6, 115.7, 115.5, 115.2, 40.0, 21.8, 21.5. HRMS (EI+) calcd. for C30H24NO3SF [M]+ : 497.1461, measured: 497.1455.
N-tosyl-2-[3-(4-bromophenyl)-3-oxopropyl]-3-phenylindole (3al)
156 mg (70%), colorless oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,28. 1H NMR (400 MHz, CDCl3) δ 8.29 (d, J = 8.0 Hz, 1H), 7.80 (d, J = 8.4 Hz, 2H), 7.67 (d, J = 8.0 Hz, 2H), 7.57 (d, J = 8.8 Hz, 2H), 7.45–7.45 (m, 2H), 7.39–7.30 (m, 5H), 7.25–7.19 (m, 3H), 3.43 (s, 4H), 2.34 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 197.6, 144.9, 136.8, 136.2, 135.5, 135.3, 132.6, 131.8, 130.5, 129.9, 129.8, 129.6, 128.8, 128.2, 127.7, 126.4, 124.8, 124.7, 123.9, 119.6, 115.2, 40.0, 21.8, 21.5. HRMS (ES+) calcd. for C30H24NO3SBr [M+H]+ : 558.0739, measured: 558.0742.
N-tosyl-2-[3-(4-methoxyphenyl)-3-oxopropyl]-3-phenylindole (3am)
114 mg (56%), colorless oil, eluent: hexanes/EtOAc = 4/1; Rf = 0,33. 1H NMR (500 MHz, CDCl3) δ 8.29 (d, J = 9.1 Hz, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.68–7.66 (m, 2H), 7.44–7.41 (m, 2H), 7.37–7.29 (m, 5H), 7.24–7.21 (m, 1H), 7.19 (d, J = 8.4 Hz, 2H), 6.91–6.89 (m, 2H), 3.86 (s, 3H), 3.41 (s, 4H), 2.34 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.2, 163.4, 144.8, 136.8, 132.7, 130.6, 130.3, 129.9, 129.5, 128.9, 128.7, 128.0, 127.6, 126.4, 125.9, 124.7, 124.3, 123.9, 119.5, 115.2, 113.6, 55.4, 39.8, 21.9, 21.5. HRMS (ES+) calcd. for C31H28NO4S [M+H]+ : 510.1739, measured: 510.1741.
N-tosyl-2-[3-oxo-5-(etoxycarbonyl)pentyl]-3-phenylindole (3an)
123 mg (61%), yellow oil, eluent: hexanes/EtOAc = 5/1; Rf = 0,20. 1H NMR (500 MHz, CDCl3) δ 8.26 (d, J = 9.7 Hz, 1H), 7.64 (d, J = 8.4 Hz, 2H), 7.45–7.42 (m, 2H), 7.38–7.27 (m, 5H), 7.23–7.18 (m, 3H), 4.12 (q, J = 7.0 Hz, 2H), 3.26 (t, J = 7.7 Hz, 2H), 2.96 (t, J = 7.7 Hz, 2H), 2.73 (t, J = 6.6 Hz, 2H), 2.58 (t, J = 6.6 Hz, 2H), 2.34 (s, 3H), 1.24 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 207.3, 172.6, 144.8, 136.7, 136.3, 135.6, 132.6, 130.5, 129.8, 129.7, 128.7, 127.7, 126.3, 124.7, 124.4, 123.9, 119.5, 115.2, 60.6, 43.7, 36.9, 28.0, 21.5, 21.5, 14.2. HRMS (EI+) calcd. for C29H29NO5S [M]+: 503.1766, measured: 503.1759.
N-tosyl-2-[3-oxo-5-(cyclohexyloxycarbonyl)pentyl]-3-phenylindole (3ao)
189 mg (68%), colorless oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,21. 1H NMR (400 MHz, CDCl3) δ 8.26 (d, J = 8.0 Hz, 1H), 7.65–7.63 (m, 2H), 7.45–7.42 (m, 2H), 7.39–7.36 (m, 1H), 7.34–7.26 (m, 4H), 7.23–7.17 (m, 3H), 4.74 (sept, J = 4.2 Hz, 1H), 3.28–3.24 (m, 2H), 2.98–2.94 (m, 2H), 2.73 (t, J = 6.7 Hz, 2H), 2.56 (t, J = 6.6 Hz, 2H), 2.33 (s, 3H), 1.83–1.80 (m, 2H), 1.73–1.68 (m, 2H), 1.55–1.50 (m, 1H), 1.44–1.22 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 207.3, 172.1, 144.9, 136.7, 136.3, 135.6, 132.6, 130.5, 129.8, 129.8, 128.7, 127.7, 126.4, 124.7, 124.4, 123.9, 119.5, 115.2, 72.9, 43.7, 37.1, 31.5, 28.4, 25.3, 23.7, 21.6, 21.3. HRMS (EI+) calcd. for C30H35NO5S [M]+: 557.2236, measured: 557.2243.
N-tosyl-2-(2-methyl-3-oxobutyl)-3-phenylindole (3ap)
150 mg (87%), yellow oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,31. 1H NMR (500 MHz, CDCl3) δ 8.25 (d, J = 8.0 Hz, 1H), 7.61 (d, J = 8.0 Hz, 2H), 7.45–7.42 (m, 2H), 7.39–7.27 (m, 5H),7.23–7.20 (m, 1H), 7.18 (d, J = 8.0 Hz, 2H), 3.47 (dd, J = 14.4, 4.4 Hz, 1H), 3.32–3.25 (m, 1H), 2.97 (dd, J = 14.4, 9.5 Hz, 1H), 2.33 (s, 3H), 2.13 (s, 3H), 0.82 (d, J = 7.3 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 211.8, 144.8, 137.0, 135.3, 135.1, 132.7, 130.8, 130.2, 129.7, 128.7, 127.7, 126.8, 126.3, 124.8, 124.1, 119.6, 115.5, 47.5, 29.4, 28.5, 21.5, 15.4. HRMS (EI+) calcd. for C26H25NO3S [M]+: 431.1555, measured: 431.1559.
N-tosyl-2-[(2-oxocyclohexyl)methyl]-3-phenylindole (3aq)
128 mg (70%), yellow oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,16. 1H NMR (500 MHz, CDCl3) δ 8.26 (d, J = 8.7 Hz, 1H), 7.62 (d, J = 8.2 Hz, 2H), 7.44–7.40 (m, 2H), 7.35–7.26 (m, 3H), 7.25–7.16 (m, 5H), 3.65 (dd, J = 14.6, 3.5 Hz, 1H), 3.08–3.00 (m, 1H), 2.89 (dd, J = 14.6, 9.9 Hz, 1H), 2.33 (s, 3H), 2.34–2.31 (m, 2H), 2.05–1.99 (m, 1H), 1.81–1.76 (m, 1H), 1.68–1.65 (m, 1H), 1.59–1.48 (m, 2H), 0.90–0.83 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 212.0, 144.8, 137.0, 135.6, 135.2, 133.0, 130.8, 130.2, 129.7, 128.6, 127.6, 126.6, 126.4, 124.6, 124.0, 119.4, 115.5, 51.2, 42.0, 32.7, 27.8, 26.7, 25.1, 21.5. HRMS (EI+) calcd. for C28H27NO3S [M]+: 457.1712, measured: 457.1716.
N-tosyl-2-(2-hydroxyphenylmethyl)-3-phenylindole (3ar)
82 mg (45%), colorless oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,39. 1H NMR (500 MHz, CDCl3) δ 8.19 (d, J = 8.4 Hz, 1H), 7.46–7.33 (m, 9H), 7.27–7.25 (m, 1H), 7.08–7.03 (m, 3H), 6.80–6.65 (m, 3H), 5.53 (s, 1H), 4.45 (s, 2H), 2.31 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 153.0, 144.6, 136.7, 135.6, 134.7, 132.7, 130.2, 129.8 129.6, 129.6, 126.4, 128.8, 127.7, 127.4, 126.5, 126.1, 124.8, 123.7, 120.9, 119.7, 116.2, 115.1, 26.0, 21.5. HRMS (EI+) calcd. for C28H23NO3S [M]+: 453.1399, measured: 453.1407.
2-N-Tosylaminophenyl(phenyl)(menthofuryl-2)methane (4as)
184 mg (95%), colorless oil, eluent: hexanes/EtOAc = 9/1; Rf = 0,39. 1H NMR (500 MHz, CDCl3) δ 7.58 (d, J = 8.1 Hz, 2H), 7.48 (d, J = 7.6 Hz, 1H), 7.27–7.25 (m, 1H), 7.24–7.20 (m, 3H), 7.06 (dd, J = 8.1, 7.0 Hz, 1H), 6.81–6.78 (m, 3H), 6.43 (d, J = 7.0 Hz, 1H), 4.88 (d, J = 4.0 Hz, 1H), 2.60–2.52 (m, 1H), 2.43 (s, 3H), 2.37–2.27 (m, 2H), 2.13–2.05 (m, 1H), 1.91–1.79 (m, 2H), 2.66 (d, J = 4.0 Hz, 3H), 1.38–1.26 (m, 2H), 1.05 (d, J = 7.0 Hz, 3H), 1.01–0.84 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 150.0, 145.4, 143.8, 140.1, 137.0, 135.4, 135.3, 134.4, 129.7, 128.7, 128.6, 127.7, 127.1, 126.9, 126.0, 124.9, 124.8, 118.2, 116.5, 44.7, 31.4, 31.2, 29.6, 21.5, 20.1, 7.9. HRMS (EI+) calcd. for C30H31NO3S [M]+: 485.2025, measured: 485.2015.
N-tosyl-2-(3-oxobutyl)-3-methylindole (3ba)
125 mg (88%), colorless oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,31. 1H NMR (500 MHz, CDCl3) δ 8.19 (d, J = 8.4 Hz, 1H), 7.57–7.56 (m, 2H), 7.37 (d, J = 7.7 Hz, 1H), 7.31–7.23 (m, 2H), 7.15–7.14 (m, 2H), 3.23–3.20 (m, 2H), 2.94–2.91 (m, 2H), 2.31 (s, 3H), 2.17 (s, 3H), 2.15 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 207.7, 144.5, 136.6, 135.6, 135.4, 131.4, 129.7, 126.2, 124.3, 123.5, 118.5, 117.6, 115.0, 43.9, 29.9, 21.5, 20.7, 8.9. HRMS (ES+) calcd for C20H22NO3S [M+H]+: 356.1320, measured: 356.1323.
N-tosyl-2-(3-oxobutyl)-3-isopropylindole (3ca)
117 mg (76%), colorless oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,35. 1H NMR (500 MHz, CDCl3) δ 8.21 (d, J = 8.3 Hz, 1H), 7.59 (d, J = 7.7 Hz, 1H), 7.55–7.53 (m, 2H), 7.26 (m, 1H), 7.21 (m, 1H), 7.16–7.14 (m, 2H), 3.24–3.20 (m, 2H), 3.14 (sept, J = 7.0 Hz, 1H), 2.90–2.87 (m, 2H), 2.32 (s, 3H), 2.18 (s, 3H), 1.32 (d, J = 7.2 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ 207.7, 144.5, 137.2, 135.7, 134.2, 129.7, 129.2, 127.3, 126.2, 123.8, 123.0, 120.2, 115.4, 44.6, 30.0, 25.9, 22.1, 21.5, 20.8. HRMS (ES+) calcd for C22H26NO3S [M+H]+: 384.1633, measured: 384.1636.
N-tosyl-2-(3-oxobutyl)-3-tertbutylindole (3da)
100 mg (63%), colorless oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,33 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.28 (d, J = 8.4 Hz, 1H), 7.77 (d, J = 8.1 Hz, 1H), 7.49–7.48 (m, 2H), 7.24 (m, 1H), 7.19 (m, 1H), 7.15–7.14 (m, 2H), 3.45–3.42 (m, 2H), 2.91 (broad s, 2H), 2.32 (s, 3H), 2.19 (s, 3H), 1.46 (s, 9H). 13C NMR (125 MHz, CDCl3) δ 207.6, 144.5, 137.7, 135.7, 135.0, 130.3, 129.6, 129.0, 126.1, 123.7, 122.9, 122.3, 115.8, 45.4, 34.0, 31.6, 29.9, 21.6, 21.5. HRMS (ES+) calcd for C23H28NO3S [M+H]+: 398.1790, measured: 398.1797.
N-tosyl-2-(3-oxobutyl)-3-cyclohexylindole (3ea)
141 mg (83%), colorless oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,36 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.21 (d, J = 8.3 Hz, 1H), 7.64 (d, J = 7.7 Hz, 1H), 7.54–7.52 (m, 2H), 7.24 (t, J = 8.3 Hz, 1H), 7.19 (t, J = 7.9 Hz, 1H), 7.15–7.13 (m, 2H), 3.25–3.22 (m, 2H), 2.90–2.87 (m, 2H), 2.72 (m, 1H), 2.31 (s, 3H), 2.18 (s, 3H), 1.90–1.77 (m, 5H), 1.61–1.59 (m, 2H), 1.42–1.26 (m, 3H). 13C NMR (125 MHz, CDCl3) δ 207.8, 144.5, 137.2, 135.7, 124.6, 129.7, 126.6, 126.2, 123.8, 123.0, 120.5, 115.3, 44.7, 36.7, 32.0, 30.0, 26.9, 26.1, 21.5, 20.8. HRMS (ES+) calcd for C25H30NO3S [M+H]+: 424.1946, measured: 424.1944.
N-tosyl-2-(3-oxobutyl)-3-(4-fluorophenyl)indole (3fa)
126 mg (72%), yellow oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,23 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.26 (d, J = 8.4 Hz, 1H), 7.65–7.64 (m, 2H), 7.33 (t, J = 8.3 Hz, 1H), 7.28–7.22 (m, 4H), 7.20–7.19 (m, 2H), 7.14 (d, J = 8.6 Hz, 2H), 3.24–3.20 (m, 2H), 2.95–2.91 (m, 2H), 2.34 (s, 3H), 2.14 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 207.1, 162.3 (d, JC–F = 247.8 Hz), 145.0, 136.6, 136.5, 135.5, 131.5 (d, JC–F = 7.4 Hz), 130.5, 129.9, 128.6 (d, JC–F = 2.8 Hz), 126.4, 124.4 (d, JC–F = 105.4 Hz), 123.4, 119.3, 115.9, 115.7, 115.2, 44.6, 29.7, 21.6, 21.2. HRMS (ES+) calcd for C25H23FNO3S [M+H]+: 436.1383, measured: 436.1376.
N-tosyl-2-(3-oxobutyl)-3-(4-methylphenyl)indole (3ga)
103 mg (60%), yellow oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,25 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.26 (d, J = 9.0 Hz, 1H), 7.65–7.64 (m, 2H), 7.33–7.31 (m, 2H), 7.28–7.26 (m, 2H), 7.23 (m, 1H), 7.20–7.18 (m, 4H), 3.24–3.20 (m, 2H), 2.95–2.91 (m, 2H), 2.42 (s, 3H), 2.34 (s, 3H), 2.14 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 207.3, 144.8, 137.4, 136.6, 136.1, 135.5, 130.6, 129.8, 129.6, 129.5, 129.4, 126.3, 124.6, 124.4, 123.8, 119.5, 115.1, 44.7, 29.7, 21.5, 21.3, 21.2. HRMS (EI+) calcd for C26H25NO3S [M]+: 431.1555, measured: 431.1561.
N-tosyl-2-(3-oxobutyl)-3-(4-methoxyphenyl)indole (3ha)
127 mg (71%), yellow oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,19 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.25 (d, J = 8.8 Hz, 1H), 7.65–7.63 (m, 2H), 7.33–7.30 (m, 2H), 7.21–7.17 (m, 5H), 6.99–6.97 (m, 2H), 3.85 (s, 3H), 3.26–3.23 (m, 2H), 2.95–2.92 (m, 2H), 2.33 (s, 3H), 2.13 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 207.3, 159.1, 144.8, 136.6, 136.0, 135.5, 130.9, 130.8, 129.8, 126.3, 124.7, 124.6, 124.1, 123.8, 119.4, 115.1, 114.2, 55.2, 44.6, 29.7, 21.5, 21.3. HRMS (EI+) calcd for C26H25NO4+ 25 4S [M] : 447.1504, measured: 447.1509.
N-tosyl-2-(3-oxobutyl)-3-(thiophen-2-yl)indole (3ia)
69 mg (41%), yellow oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,22 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.25 (d, J = 8.4 Hz, 1H), 7.67–7.65 (m, 2H), 7.54 (d, J = 7.7 Hz, 1H), 7.39 (dd, J = 5.3, 0.7 Hz, 1H), 7.34 (m, 1H), 7.26 (m, 1H), 7.21–7.20 (m, 2H), 7.14 (m, 1H), 7.05 (dd, J = 3.5, 0.7 Hz, 1H), 3.36–3.33 (m, 2H), 3.00–2.97 (m, 2H), 2.34 (s, 3H), 2.18 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 207.1, 145.1, 137.6, 136.3, 135.6, 133.1, 130.1, 129.9, 127.5, 127.4, 126.4, 126.0, 124.9, 124.0, 119.6, 116.8, 115.0, 44.7, 29.8, 21.6, 21.4. HRMS (ES+) calcd for C23H22NO3S2 [M+H]+: 424.1041, measured: 424.1042.
N-tosyl-2-(3-oxobutyl)-3-phenyl-5,6-difluoroindole (3ja)
143 mg (79%), colorless solid, eluent: hexanes/EtOAc = 7/1; Rf = 0,27 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.12 (dd, J = 11.4, 7.0 Hz, 1H), 7.64–7.62 (m, 2H), 7.46–7.43 (m, 2H), 7.38 (m, 1H), 7.25–7.22 (m, 4H), 7.12 (dd, J = 9.9, 7.9 Hz, 1H), 3.21–3.18 (m, 2H), 2.93–2.90 (m, 2H), 2.37 (s, 3H), 2.13 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 206.9, 148.8 (ddd, JC–F = 244.1, 19.4, 14.8 Hz), 145.4, 137.6 (d, JC–F = 4.6 Hz), 135.1, 131.9, 131.6, 131.6, 130.0, 129.5, 128.9, 128.0, 126.3, 123.8, 107.6 (d, JC–F = 19.4 Hz), 104.5 (d, JC–F = 24.0 Hz), 44.5, 29.7, 21.6, 21.3. HRMS (ES+) calcd for C25H22+NO3SF2 [M+H] : 454.1288, measured: 454.1291.
N-tosyl-2-(3-oxobutyl)-3-phenyl-5,6-dimethoxyindole (3ka)
114 mg (60%), yellow solid, eluent: hexanes/EtOAc = 3/1; Rf = 0,19. The spectral data matched that of the previously synthesized material.61H NMR (500 MHz, CDCl3) δ 8.85 (s, 1H), 7.59–7.57 (m, 2H), 7.46–7.43 (m, 2H), 7.37 (m, 1H), 7.26–7.25 (m, 2H), 7.19–7.17 (m, 2H), 6.72 (s, 1H), 4.00 (s, 3H), 3.79 (s, 3H), 3.19–3.16 (m, 2H), 2.93–2.90 (m, 2H), 2.33 (s, 3H), 2.12 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 207.3, 147.8, 147.4, 144.7, 135.3, 134.8, 132.8, 130.7, 129.7, 129.6, 128.7, 127.6, 126.1, 124.7, 123.5, 100.8, 99.3, 56.4, 56.0, 44.7, 29.7, 21.5, 21.4. HRMS (ES+) calcd for C27H28NO5S [M+H]+: 478.1688, measured: 478.1679.
N-tosyl-2-(3-oxobutyl)-3-phenyl-6-trifluoromethylindole (3la)
122 mg (63%), colorless solid, eluent: hexanes/EtOAc = 7/1; Rf = 0,24 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.57 (d, J = 0.7 Hz, 1H), 7.67–7.65 (m, 2H), 7.48–7.45 (m, 3H), 7.42–7.38 (m, 2H), 7.28–7.23 (m, 4H), 3.28–3.25 (m, 2H), 2.95–2.92 (m, 2H), 2.36 (s, 3H), 2.14 (d, J = 1.3 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 206.8, 145.4, 139.1, 135.8, 135.2, 132.9, 131.9, 130.0, 129.7, 128.9, 128.0, 126.7 (q, JC-F = 31.4 Hz), 126.4, 124.6 (q, JC-F = 271.9 Hz), 123.9, 120.6 (d, JC-F = 2.8 Hz), 119.8, 112.5 (d, JC-F = 3.7 Hz), 44.4, 29.7, 21.5, 21.2. HRMS (ES+) calcd for C26H23NO3SF3 [M+H]+: 486.1351, measured: 486.1350.
N-tosyl-2-(3-oxobutyl)-3-phenyl-5-bromoindole (3ma)
121 mg (61%), yellowish solid, eluent: hexanes/EtOAc = 7/1; Rf = 0,21 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 8.13 (d, J = 8.6 Hz, 1H), 7.63–7.62 (m, 2H), 7.46–7.38 (m, 5H), 7.26–7.21 (m, 4H), 3.24–3.20 (m, 2H), 2.94–2.91 (m, 2H), 2.35 (s, 3H), 2.13 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 206.9, 145.2, 137.7, 135.3, 135.2, 132.3, 131.9, 129.9, 129.7, 128.9, 128.0, 127.5, 126.3, 123.6, 122.2, 117.5, 116.6, 44.5, 29.7, 21.6, 21.2. HRMS (ES+) calcd for C25H23BrNO3S [M+H]+: 496.0582, measured: 496.0583.
2-(3-oxobutyl)-3-phenyl-7-chloroindole (3oa)
68 mg (58%), colorless solid, eluent: hexanes/EtOAc = 7/1; Rf = 0,23 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3) δ 9.05 (broad s, 1H), 7.52–7.44 (m, 5H), 7.37 (m, 1H), 7.19 (d, J = 7.5 Hz, 1H), 7.04 (t, J = 7.7 Hz, 1H), 3.14–3.12 (m, 2H), 2.92–2.89 (m, 2H), 2.22 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 209.5, 135.8, 134.8, 132.4, 129.6, 129.0, 128.6, 126.3, 121.1, 120.5, 117.5, 116.2, 115.5, 43.8, 30.0, 19.6. HRMS (EI+) calcd for C18H16ClNO [M]+: 297.0920, measured: 297.0923.
Procedure for a gram-scale synthesis of indole 3aa from aminobenzylalcohol 1a and furan 2a
In a 25 mL pressure tube, to a stirred suspension of aminobenzylalcohol 1a (4.0 mmol, 1.41 g) and furan 2a (6.0 mmol, 1.5 equiv, 537.0 μL) in DCE (13.3 mL, 0.3M), triflic acid (0.4 mmol, 10 mol%, 36.0 μL) was added. The tube was capped with a Teflon pressure cap and placed into pre-heated (80°C) oil bath. The resulted solution was stirred for 2 h at this temperature. After completion of the reaction (determined by TLC, eluent hexanes:EtOAc = 4:1), the pressure tube, containing product 3aa, was cooled down and the solvent was evaporated. The product was purified using silica gel column chromatography (eluent hexanes:EtOAc = 7:1) to yield indole 3aa (1.38 g, 83%) as a yellow oil, crystallizing upon staying.
General Procedure for the Synthesis of Furylmethyl Aniline Derivatives 4.6
A mixture of alcohol 1a (0.5 mmol), p-TsOH (4.3 mg, 5 mol%), and furan (0.6 mmol, 1.2 equiv) in benzene (5 mL) was refluxed with azeotropic removal of water for 30 min (TLC monitoring, eluent: hexanes:EtOAc = 4:1). After completion, the reaction flask was cooled down and the product was purified using flash silica gel column chromatography.
N-Tosyl-(2-(phenyl(5-phenylfuran-2-yl)methyl))aniline (4ae)
179 mg (75%), yellowish solid, eluent: hexanes/EtOAc = 7/1; Rf = 0,16. 1H NMR (400 MHz, CDCl3) δ 7.61–7.58 (m, 2H), 7.57–7.54 (m, 2H), 7.47 (dd, J = 8.0, 1.2 Hz, 1H), 7.38–7.34 (m, 2H), 7.30–7.23 (m, 7H), 7.13 (dt, J = 7.7, 1.3 Hz, 1H), 6.94–6.92 (m, 2H), 7.86 (dd, J = 7.8, 1.2 Hz, 1H), 6.54 (d, J = 3.3 Hz, 1H), 6.28 (s, 1H), 5.80 (dd, J = 3.4, 0.8 Hz, 1H), 5.05 (s, 1H), 2.39 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 153.4, 143.9, 139.5, 136.8, 125.6, 134.1, 130.6, 129.8, 129.6, 129.0, 128.7, 128.6, 128.0, 127.5, 127.4, 127.1, 126.6, 125.9, 123.6, 111.3, 105.6, 46.1, 21.5. HRMS (EI+) calcd. for C30H25NO3S [M]+: 479.1555, measured: 479.1546.
Cyclohexyl 3-(5-((2-N-tosylaminophenyl)(phenyl)methyl)furan-2-yl)propanoate (4ao)
189 mg (68%), colorless oil, eluent: hexanes/EtOAc = 7/1; Rf = 0,19. 1H NMR (400 MHz, CDCl3) δ 7.58–7.56 (m, 2H), 7.45 (dd, J = 8.0, 1.0 Hz, 1H), 7.27–7.21 (m, 6H), 7.10 (dt, J = 7.7, 1.2 Hz, 1H), 6.80–6.78 (m, 2H), 7.73 (dd, J = 7.8, 1.3 Hz, 1H), 6.28 (s, 1H), 5.90 (d, J = 3.1 Hz, 1H), 5.59 (d, J = 3.1 Hz, 1H), 4.91 (s, 1H), 4.75 (sept, J = 4.2 Hz, 1H), 2.91–2.87 (m, 2H), 2.59–2.56 (m, 2H), 2.43 (s, 3H), 1.82–1.79 (m, 2H), 1.73–1.69 (m, 2H), 1.56–1.51 (m, 1H), 1.42–1.22 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 171.8, 154.4, 152.8, 143.8, 139.6, 136.9, 135.6, 134.1, 129.8, 129.5, 128.8, 128.7, 127.9, 127.3, 127.1, 126.5, 125.7, 109.8, 105.9, 72.8, 45.9, 33.0, 31.6, 25.3, 23.7, 23.7, 21.5. HRMS (EI+) calcd. for C33H35NO5S [M]+: 557.2236, measured: 557.2231.
General Procedure for Recyclization Reaction of 4 into Indole 3.6
A solution of compound 4 (0.2 mmol) in 2 mL of HCl-EtOH (1.25M) was stirred under reflux for 12h, after what the solvent was evaporated, and the product was purified using flash silica gel column chromatography.
-
(1)
4ae→3ae: 94% of 4ae was recovered.
-
(2)
4ao→3ao: Conversion of 4ao (>95%). Compound 3an was isolated instead of 3ao as a result of transesterification reaction.

Formation of Tetrahydrobenzo[3,4]cyclohepta[1,2-b]indole 5 from Indole 3ae
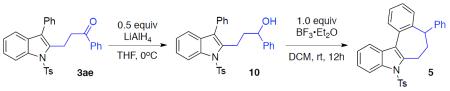
Reduction of ketone (3ae→10)
To an ice-cooled stirred suspension of LiAlH4 (0.5 mmol, 0.5 equiv, 18.0 mg) in THF (5.0 mL), a solution of indole 3ae (1.0 mmol, 417.0 mg) in THF (2.0 mL) was added. The reaction mixture was allowed to warm up to room temperature and stirred for 30 min. After completion of the reaction (determined by TLC, eluent hexanes:EtOAc = 4:1), reaction mixture was cooled down (ice bath) and aqueous sat. NH4Cl (1.0 mL) was added dropwise (caution: H2 evolution!). The resulting sluggish mixture was filtered through Celite® and concentrated. The alcohol 10 was purified using flash silica gel column chromatography. 406 mg (97%), yellow-brown solid, eluent: hexanes/EtOAc = 3/1; Rf = 0,13. 1H NMR (500 MHz, CDCl3): δ ppm 8.27 (d, J = 8.4 Hz, 1H), 7.62–7.61 (m, 2H), 7.46–7.43 (m, 2H), 7.39 (s, 1H), 7.36–7.29 (m, 8H), 7.26–7.21 (m, 2H), 7.19–7.17 (m, 2H), 4.69 (m, 1H), 3.23–3.17 (m, 1H), 3.11–3.05 (m, 1H), 2.34 (s, 3H), 2.28–2.19 (m, 2H), 2.07 (broad s, 1H). 13C NMR (125 MHz, CDCl3): δ ppm 144.7, 144.0, 137.3, 136.7, 135.7, 132.9, 130.5, 129.7, 128.7, 128.3, 127.5, 127.3, 126.2, 125.8, 124.5, 124.0, 124.0, 123.8, 119.4, 115.2, 73.6, 40.1, 23.2, 21.5. HRMS (EI+) calcd. for C30H27NO3S [M]+: 481.1712, measured: 481.1712.
Cyclization (10→5)
In a 3 mL Wheaton vial, to a stirred solution of alcohol 10 (0.2 mmol, 96.2 mg) in DCM (1.0 mL), a solution of BF3•Et2O (0.2 mmol, 1.0 equiv, 24.7 μL) was added. The reaction mixture was stirred at room temperature for 12 h. After completion of the reaction (determined by TLC, eluent hexanes:EtOAc = 4:1) the resulting mixture was diluted with hexanes (1.0 mL). The cyclized product 5 was purified using flash silica gel column chromatography. 56 mg (61%), colorless solid, eluent: hexanes/EtOAc = 25/1; Rf = 0,45 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3): δ ppm 8.35 (d, J = 8.5 Hz, 1H), 7.75 (d, J = 7.9 Hz, 1H), 7.69–7.67 (m, 2H), 7.64 (d, J = 7.5 Hz, 1H), 7.38 (t, J = 6.1 Hz, 1H), 7.35–7.30 (m, 4H), 7.27–7.26 (m, 1H), 7.18–7.14 (m, 5H), 6.88 (d, J = 7.9 Hz, 1H), 3.92–3.88 (m, 1H), 3.74–3.69 (m, 1H), 2.77–2.67 (m, 2H), 2.53–2.46 (m, 1H), 2.30 (s, 3H). 13C NMR (125 MHz, CDCl3): δ ppm 144.8, 143.5, 132.1, 137.9, 137.1, 136.1, 133.1, 129.8, 129.1, 128.6, 128.3, 128.2, 128.1, 126.9, 126.5, 126.3, 126.0, 124.2, 123.8, 121.3, 119.1, 115.2, 46.5, 39.1, 23.7, 21.5. HRMS (EI+) calcd. for C30H25NO2S [M]+: 463.1606, measured: 463.1599.
Formation of α,β-Unsaturated Ketone 7 from Indole 3aa

Tosyl- group deprotection (3aa→6)
In a 25 mL round-bottom argon flushed flask, equipped with a stirring bar and septum, to a dry-ice cooled solution of indole 3aa (1 mmol, 417 mg) in DME (10 mL), a solution of sodium naphthalenide in DME (1.0–1.1 equiv) was added dropwise (addition of sodium naphthalenide continues until the dark green color of its solution stops disappearing. It is important not to add excess of sodium naphthalenide!). The resulting yellow-green solution was allowed to warm up to room temperature (turned yellow) and the solvent was evaporated. The mixture, containing product 6 and naphthalene was purified using flash silica gel column chromatography. 189 mg (72%), colorless solid, eluent: hexanes/EtOAc = 7/1; Rf = 0,18 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3): δ ppm 8.81 (broad s, 1H), 7.64 (d, J = 7.9 Hz, 1H), 7.49–7.47 (m, 4H), 7.38–7.33 (m, 2H), 7.20 (t, J = 7.2 Hz, 1H), 7.12 (t, J = 7.3 Hz, 1H), 3.13–3.10 (m, 2H), 2.91–2.88 (m, 2H), 2.21 (s, 3H). 13C NMR (125 MHz, CDCl3): δ ppm 210.0, 135.3, 135.0 (2C), 129.7, 128.5, 127.5, 126.0, 121.8, 119.8, 118.9, 114.4, 110.7, 44.1, 30.1, 19.5. HRMS (EI+) calcd. for C18H17ON [M]+: 263.1310, measured: 263.1313.
Oxidation (6→7)
In a 3 mL Wheaton vial, to a stirred solution of ketone 6 (0.2 mmol, 52.6 mg) in DCE (2.0 mL), DDQ (0.24 mmol, 1.2 equiv, 54.5 mg) was added. The reaction mixture was stirred at room temperature for 2 h. After completion of the reaction (determined by TLC, eluent hexanes:EtOAc = 4:1) the resulting mixture was diluted with hexanes (2.0 mL). The product 7 was purified using flash silica gel column chromatography. 43 mg (83%), deep yellow solid, eluent: hexanes/EtOAc = 7/1; Rf = 0,15 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3): δ ppm 8.81 (broad s, 1H), 7.71 (d, J = 8.1 Hz, 1H), 7.63 (d, J = 16.3 Hz, 1H), 7.55–7.53 (m, 4H), 7.47–7.43 (m, 2H), 7.35 (t, J = 7.3 Hz, 1H), 7.17 (t, J = 7.2 Hz, 1H), 7.61 (d, J = 16.3 Hz, 1H), 2.36 (s, 3H). 13C NMR (125 MHz, CDCl3): δ ppm 198.6, 137.6, 133.5, 132.8, 130.2, 129.8, 128.8, 127.7, 127.3, 125.6, 125.1, 124.8, 120.9, 120.7, 111.5, 26.8. HRMS (EI+) calcd. for C18H15ON [M]+: 261.1154, measured: 261.1157.
Formation of Dihydropyrroloindole 9 from Indole 3aa
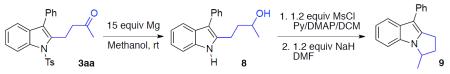
Tosyl- group deprotection/reduction of carbonyl group (3aa→8)
To a 25 mL round-bottom argon flushed flask, indole 3aa (1 mmol, 417 mg), Mg (15 mmol, 15 equiv, 360 mg), and methanol (15 mL) were added. The flask was sonicated in the ultrasound bath until the start of the dihydrogen evolution from magnesium surface, and then reaction mixture was stirred at room temperature until all magnesium reacted (1–2 h). The resulting mixture was filtered through Celite® and concentrated. The alcohol 8 was purified using flash silica gel column chromatography. 230 mg (87%), yellow oil, eluent: hexanes/EtOAc = 4/1; Rf = 0,08. 1H NMR (500 MHz, CDCl3): δ ppm 8.67 (broad s, 1H), 7.70 (d, J = 7.9 Hz, 1H), 7.56–7.49 (m, 4H), 7.37–7.34 (m, 2H), 7.22 (t, J = 7.0 Hz, 1H), 7.16 (d, J = 7.9 Hz, 1H), 3.86 (m, 1H), 3.05–2.96 (m, 2H), 1.88–1.78 (m, 3H), 1.22 (d, J = 5.7 Hz, 3H). 13C NMR (125 MHz, CDCl3): δ ppm 135.6, 135.5, 135.3, 129.6, 128.6, 127.8, 126.0, 121.6, 119.8, 118.9, 114.4, 110.6, 67.6, 38.6, 23.8, 22.6. HRMS (EI+) calcd. for C18H19ON [M]+: 265.1467, measured: 265.1468.
Cyclization (8→9)
In a 10 mL round-bottom flask, to a solution of alcohol 8 (0.2 mmol, 53.0 mg), triethylamine (0.3 mmol, 1.5 equiv, 42.5 μL), and DMAP (0.02 mmol, 0.1 equiv, 2.4 mg) in DCM (1.0 mL), MsCl (0.24 mmol, 1.2 equiv, 19.0 μL) was added. The reaction mixture was stirred at room temperature for 2 h and then filtered through Celite® and concentrated. The crude product was dissolved in 0.2 mL of DMF and added to an ice-cooled suspension of NaH (0.24 mmol, 1.2 equiv, 5.8 mg) in DMF (0.8 mL). The resulting mixture was allowed to warm up to room temperature and stirred for 2 h. After, the reaction mixture was poured into water (10.0 mL) and the product 9 was extracted with diethyl ether (3×5 mL). The combined organic fractions were washed with brine, dried over Na2SO4, and concentrated. The crude dihydropyrroloindole 9 was purified using flash silica gel column chromatography. 39 mg (79%), colorless oil, eluent: hexanes/EtOAc = 25/1; Rf = 0,50 (hexanes/EtOAc = 4/1). 1H NMR (500 MHz, CDCl3): δ ppm 7.94 (d, J = 7.2 Hz, 1H), 7.68–7.67 (m, 2H), 7.50–7.47 (t, J = 7.5 Hz, 2H), 7.40 (d, J = 6.1 Hz, 1H), 7.26 (m, 1H), 7.23–7.17 (m, 2H), 4.66 (m, 1H), 3.27 (m, 1H), 3.16 (m, 1H), 2.83 (m, 1H), 2.29 (m, 1H), 1.61 (d, J = 6.4 Hz, 3H). 13C NMR (125 MHz, CDCl3): δ ppm 141.7, 136.2, 132.3, 130.8, 128.6, 127.4, 124.9, 120.6, 119.5, 109.6, 107.5, 52.6, 36.0, 23.7, 20.3. HRMS (EI+) calcd. for C18H17N [M]+: 247.1361, measured: 247.1365.
Supplementary Material
Acknowledgments
Financial support of this work by the National Institutes of Health (VG, GM-64444) and the Ministry of Education of the Perm Krai (AVB, Russian Federation) is gratefully acknowledged.
Footnotes
Supporting Information availability statement 1H and 13C NMR spectra for compounds 1a-1o, 3aa-3ar, 4as, 3ba-3oa, 4ae, 4ao, 10, and 5–9. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).For a reviw, see: Balaban AT, Oniciu DC, Katritzky AR. Chem. Rev. 2004;104:2777. doi: 10.1021/cr0306790.
- (2).For recent examples on synthesis of aromatic compounds via furan recyclization reactions, see: Hashmi ASK, Frost TM, Bats JW. J. Am. Chem. Soc. 2000;122:11553.. Hashmi ASK, Weyrauch JP, Rudolph M, Kurpejovic E. Angew. Chem., Int. Ed. 2004;43:6545. doi: 10.1002/anie.200460232.. Liu L, Gao Y, Che C, Wu N, Wang DZ, Li C-C, Yang Z. Chem. Commun. 2009:662. doi: 10.1039/b817376a.. Chen Y, Lu Y, Li G, Liu Y. Org. Lett. 2009;11:3838. doi: 10.1021/ol901408u.. Chen Y, Li G, Liu Y. Adv. Synth. Catal. 2011;353:392.. Chen Y, Liu Y. J. Org. Chem. 2011;76:5274. doi: 10.1021/jo2004023.. Petronijevic FR, Wipf P. J. Am. Chem. Soc. 2011;133:7704. doi: 10.1021/ja2026882.. Zubkov FI, Airiyan IK, Ershova JD, Galeev TR, Zaytsev VP, Nikitina EV, Varlamov AV. RSC Advances. 2012;2:4103.. Huguet N, Lebœuf D, Echavarren AM. Chem. Eur. J. 2013;19:6581. doi: 10.1002/chem.201300646.
- (3).For recent examples on heterocycles synthesis via furan recyclization reactions, see: Harris JM, Padwa A. J. Org. Chem. 2003;68:4371. doi: 10.1021/jo034324s.. Kelly AR, Kerrigan MH, Walsh PJ. J. Am. Chem. Soc. 2008;130:4097. doi: 10.1021/ja710988q.. Bi J, Aggarwal VK. Chem. Commun. 2008:120. doi: 10.1039/b713447a.. Zhou X, Wu W, Liu X, Lee C-S. Org. Lett. 2008;10:5525. doi: 10.1021/ol8022787.. Fructos MR, Alvarez E, Mar Diaz-Requejo M, Perez PJ. J. Am. Chem. Soc. 2010;132:4600. doi: 10.1021/ja1006614.. El Kaïm L, Grimaud L, Wagschal S. Chem. Commun. 2011:1887. doi: 10.1039/c0cc04164e.. Yin B, Cai C, Zeng G, Zhang R, Li X, Jiang H. Org. Lett. 2012;14:1098. doi: 10.1021/ol300008d.. Uchuskin MG, Pilipenko AS, Serdyuk OV, Trushkov IV, Butin AV. Org. Biomol. Chem. 2012;10:7262. doi: 10.1039/c2ob25836f.. Trushkov IV, Nevolina TA, Shcherbinin VA, Sorotskaya LN, Butin AV. Tetrahedron Lett. 2013;54:3974.. Parr BT, Green SA, Davies HML. J. Am. Chem. Soc. 2013;135:4716. doi: 10.1021/ja401386z.. Zhang S-Y, Tu Y-Q, Cao X-P. Chem. Eur. J. 2013;19:5246. doi: 10.1002/chem.201300205.
- (4).For reviews on synthesis and application of indoles, see: Sundberg RJ, editor. Indoles. Academic Press; London: 1996. . Joule JA. Indole and its Derivatives. In: Thomas EJ, editor. Science of Synthesis, Houben–Weyl Methods of Molecular Transformations. Chapter 10 13. Vol. 10. George Thieme Verlag; Stuttgart, Germany: 2000. . Maes BUW. Top. Heterocycl. Chem. In: Gribble GW, editor. Heterocyclic Scaffolds II: Reactions and Applications of Indoles. Vol. 26. Springer-Verlag; Berlin, Heidelberg: 2010. . Shiri M. Chem. Rev. 2012;112:3508. doi: 10.1021/cr2003954.. Shiri M, Zolfigol MA, Kruger HG, Tanbakouchian Z. Chem. Rev. 2010;110:2250. doi: 10.1021/cr900195a.
- (5).For reviews on furan synthesis, see: Gallezot P. Chem. Soc. Rev. 2012;41:1538. doi: 10.1039/c1cs15147a.. van Putten R-J, van der Waal JC, de Jong E, Rasrendra CB, Heeres HJ, de Vries JG. Chem. Rev. 2013;113:1499. doi: 10.1021/cr300182k.. Gulevich AV, Dudnik AS, Chernyak N, Gevorgyan V. Chem. Rev. 2013;113:3084. doi: 10.1021/cr300333u.
- (6).Butin AV, Smirnov SK, Stroganova TA, Bender W, Krapivin GD. Tetrahedron. 2007;63:474. [Google Scholar]
- (7).For discussion on Bronsted acid catalysis in metal-catalyzed reactions, see for example: Rhee JU, Krische MJ. Org. Lett. 2005;7:2493. doi: 10.1021/ol050838x.. Li Z, Zhang J, Brouwer C, Yang C-G, Reich NW, He C. Org. Lett. 2006;8:4175. doi: 10.1021/ol0610035.. Rosenfeld DC, Shekhar S, Takemiya A, Utsunomiya M, Hartwig JF. Org. Lett. 2006;8:4179. doi: 10.1021/ol061174+.. Hashmi ASK. Catal. Today. 2007;122:211.
- (8).For employment of TTBP as proton scavenger, see for example: Crich D, Vinogradova O. J. Org. Chem. 2006;71:8473. doi: 10.1021/jo061417b.. Dudnik AS, Sromek AW, Rubina M, Kim JT, Kel'in AV, Gevorgyan V. J. Am. Chem. Soc. 2008;130:1440. doi: 10.1021/ja0773507.
- (9).See, Experimental Section for details
- (10).For transition metal-catalyzed synthesis of of 2,3-fused indoles, see: Sun K, Liu S, Bec PM, Driver TG. Angew. Chem., Int. Ed. 2011;50:1702. doi: 10.1002/anie.201006917.. Maity S, Zheng N. Angew. Chem., Int. Ed. 2012;51:9562. doi: 10.1002/anie.201205137.
- (11).McIntosh JM, Matassa LC. J. Org. Chem. 1988;53:4452. [Google Scholar]
- (12).Fleming I, Frackenpohl J, Ila H. J. Chem. Soc., Perkin Trans. 1998;1:1229. [Google Scholar]
- (13).Schrader TO, Johnson BR, Lopez L, Kasem M, Gharbaoui T, Sengupta D, Buzard D, Basmadjian C, Jones RM. Org. Lett. 2012;14:6306. doi: 10.1021/ol303070k. [DOI] [PubMed] [Google Scholar]
- (14).For importance of dihydropyrroloindoles, see: Ding Z, Yoshikai N. Angew. Chem., Int. Ed. 2013;52:8574. doi: 10.1002/anie.201305151. and references cited therein.
- (15).Haner J, Jack K, Nagireddy JR, Raheem M-A, Durham R, Tam W. Synthesis. 2011:731. [Google Scholar]
- (16).Hechavarría Fonseca M, Eibler E, Zabel M, König B. Tetrahedron: Asymmetry. 2003;14:1989. [Google Scholar]
- (17).Fan C, Vederas JC. Org. Biomol. Chem. 2012;10:5815. doi: 10.1039/c2ob00040g. [DOI] [PubMed] [Google Scholar]
- (18).Poloukhtine A, Rassadin V, Kuzmin A, Popik VV. J. Org. Chem. 2010;75:5953. doi: 10.1021/jo101238x. [DOI] [PubMed] [Google Scholar]
- (19).Gao W-C, Jiang S, Wang R-L, Zhang C. Chem. Commun. 2013:4890. doi: 10.1039/c3cc40797g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


