Abstract
Background
Prognosis and treatment options differ for each molecular subtype of breast cancer, but risk of regional lymph node (LN) metastasis for each subtype has not been well-studied. Since LN status is the most important predictor for prognosis, the aim of this study is to investigate the propensity for LN metastasis in each of the five breast cancer molecular subtypes.
Methods
Under an IRB-approved protocol, we retrospectively reviewed the charts of all pathologically confirmed breast cancer cases from 1/2004 to 6/2012. Five subtypes were defined as luminal A (hormone receptor +, Ki67 low), luminal B (hormone receptor+, Ki67 high), luminal-human epidermal growth factor receptor 2 (HER-2), HER-2-enriched (hormone receptor negative), and triple negative (TN).
Results
A total of 375 patients with complete data were classified by subtype: 95 (25.3%) luminal A, 120 (32%) luminal B, 69 (18.4%) luminal-HER-2, 26 (6.9%) HER-2-enriched, and 65 (17.3%) TN. On univariate analysis, age (<50), higher tumor grade, HER-2 + status, tumor size, and molecular subtype were significant for LN positivity. Molecular subtype correlated strongly with tumors size (X2; p=0.0004); therefore, multivariable logistic regression did not identify molecular subtype as an independent variable to predict LN positivity.
Conclusions
Luminal A tumors have the lowest risk of LN metastasis, while luminal HER-2 subtype has the highest risk of LN metastasis. Immunohistochemical-based molecular classification can be readily performed and knowledge of the factors that affect LN status may help with treatment decisions.
Keywords: molecular subtypes of breast cancer, immunohistochemistry
INTRODUCTION
Gene expression profiling has shown that breast cancer is a heterogenous disease that can be classified into molecularly distinct subtypes [1]. Perou and colleagues [1] identified breast cancer cells that shared gene expression patterns resembling luminal epithelial cells (luminal), myoepithelial (or basal) cells, and/or cells with over-expression of the human epidermal growth factor receptor 2 (c-Erb-2 or HER-2) gene. High cost and special processing of tumor samples have limited the use of genomic profiling in routine clinical practice. Immunohistochemical (IHC) evaluation for the presence of several proteins: estrogen receptor (ER), progesterone receptor (PR), HER-2, and Ki-67 [2, 3] correlate closely, although not exactly, with tumor subtypes identified by DNA microarray expression profiles. The IHC-based subtype classification is a convenient surrogate, and multiple studies have shown reproducible associations between specific IHC-defined subtypes with their characteristic clinical and biologic behaviors/prognostic outcomes [4], especially for the triple negative phenotype [5-8]. Risk factors [9, 10], prognosis [11, 12], and treatment response rates [13, 14] are well-known to differ between the subtypes. Thus, these IHC-derived molecular characteristics can inform us of the potential biological behavior of each subtype. However, the risk of regional nodal metastasis for each subtype has not been well-studied. Because lymph node (LN) status is the most important predictor for prognosis and treatment, the aim of this study is to investigate the relationship between the breast cancer molecular subtype and LN status. We hypothesized that there will be a significant difference in propensity for LN metastasis among the different breast cancer subtypes.
In this study, the five subtypes are defined as luminal A (Estrogen Receptor [ER] and/or Progesterone Receptor [PR]) positive, proliferation marker Ki-67 low, HER-2 negative), luminal B (ER/PR positive, Ki-67 high, HER-2 negative), luminal HER-2 (ER/PR positive, HER-2 over-expressed or amplified), HER-2-enriched (ER/PR negative, HER-2 over-expressed or amplified), and triple negative (TN, ER/PR negative, HER-2 negative). The TN can be further subdivided into basal (cytokeratin [CK] 5/6-positive, and/or epidermal growth factor receptor 1 [EGFR]) and non-basal.
MATERIALS AND METHODS
Under an IRB-approved protocol, we retrospectively reviewed the charts of all pathologically confirmed breast cancer cases at the University of Texas Medical Branch in Galveston, TX from 1/2004 to 6/2012. A total of 749 breast cancer cases were identified. Patients were excluded if their diagnosis was in-situ cancer only, if definitive LN staging was not performed prior to neoadjuvant therapy, or if there was insufficient clinicopathologic data available. After applying exclusion criteria, only 375 patients with invasive breast cancer and complete clinicopathological data were analyzed. These cases were classified according to molecular subtype and LN status. Data gathered included demographic factors (age at diagnosis, race/ethnicity, type of medical insurance), tumor characteristics (breast cancer histological type, tumor grade, tumor size) and LN status (positive LN is >0.2 mm tumor deposit detected by routine histology), and immunohistochemical (IHC) biomarkers for subtyping (ER, PR, Ki-67, HER-2 neu, EGFR, and CK 5/6). IHC staining was performed at the time of breast cancer diagnosis, except for 25 TN cases, where some information was missing but tissue blocks were available. These cases underwent retrospective biomarker staining for CK5/6 and EGFR. A total of 5 cases underwent retrospective fluorescence in-situ hybridization (FISH) analysis to evaluate for HER-2 amplification.
Tumor grade was determined by the Bloom-Richardson scoring system (tubules, pleomorphism, and mitotic rate). ER or PR was considered a positive result with ≥1% tumor cells with strong nuclear staining [15]. ER or PR was considered negative if 0% of the tumor cells demonstrated nuclear staining. Ki-67 antigen, a marker of mitoses, was considered “low” if the number of positively stained nuclei represented <14% of the total nuclei evaluated, and “high” if ≥14% [3]. Her-2-neu was determined using a two-step process. The initial IHC-based evaluation was performed using a polyclonal rabbit anti-human c-erbB-2/neu antibody and reported semiquantitatively as either negative (0, no staining or 1+ staining defined as <10% of tumor cells with weak, incomplete, membranous staining) or positive (3+ staining, defined as at least 30% of the tumor cells with uniform, complete, intense membranous staining). Equivocal results (2+ staining, membrane staining that was non-uniform or weak but with obvious circumferential distribution in at least 10% of cells or intense complete membrane staining in 30% or less of tumor cells) were reflexively studied with a FISH assay using the Pat Vysion HER-2 neu DNA Probe Kit on formalin-fixed, paraffin-embedded tissue. Positive controls (high-level protein expression, low-level protein expression and internal control) and negative control were routinely used in the evaluation of the assay. Fixation of the examined tissue as well as the scoring technique [16] were performed in accordance with the College of American Pathologists and the American Society of Clinical Oncology joint guideline on testing for HER-2 status in invasive breast cancer. Specimens were scored and categorized as HER-2 negative if the result is “HER-2 not amplified” and positive if “amplified”.
IHC Antibodies
Slides were stained on an automated immunostainer (Dako autostainer, Carpintaria, CA, USA). Primary antibodies were all purchased from Dako and included the ER/PR PharmDx kit (# SK310 ER clone1D5, and PgR1294), c-erB-2/neu antibody (# A0485, 1:400), Ki67 (clone MIB-1, # IR626), EGFR (# M3563, clone H11, 1:200), CK5/6 (# M723, clone D5/16B4, 1:100), and negative controls mouse IgG antibodies (# IR750). Antigen retrieval, secondary antibodies, and detection were performed using the Envision FLEX kit (Dako, # K8002).
Molecular classification by IHC
Tumors were classified into five major molecular subtypes using IHC and/or FISH for HER-2, where appropriate: luminal A (ER+ and/or PR+, HER-2−, Ki67 ≤14%), luminal B (ER+ and/or PR+, HER-2−, Ki67 >14%), luminal HER-2 (ER+ and/or PR+, HER-2+), HER-2-enriched (ER−, PR−, HER-2+), and TN (ER−, PR−, HER-2−). The TN subtype can be further subdivided into basal-like (TN, EGFR+ and/or CK5/6+) and non-basal-like (TN, EGFR−, CK5/6−) [6, 17].
Statistical Methods
Frequencies and percentages were reported for categorical variables (such as histology, grade, and LN status). Chi-squared test and Fisher's exact test were used to evaluate the association between LN status and patient's demographic and tumor characteristics. Uni-covariate and multi-covariate logistic regression models estimated the odds ratios for LN metastasis. Since HER-2 expression was strongly correlated with molecular subtype, we excluded HER-2 from the multivariate logistic regression in order to assess the impact of molecular subtype. Otherwise, all other variables in the uni-covariate model were included. P-values were two-sided and were considered statistically significant when less than 0.05. All analyses examining the association between LN status, tumor characteristics, and demographic data were conducted using SAS (version 9.1.3, Cary, NC) statistical software.
RESULTS
A total of 749 breast cancer cases were identified during the study period. Patients with ductal carcinoma in situ only, without pathologically determined LN metastasis prior to neoadjuvant chemotherapy, or incomplete clinical data were excluded from the study. The demographic and clinicopathological characteristics of the 375 eligible patients are shown in Table 1. Approximately one-third of the patients were diagnosed with breast cancer before age 50. The most common breast cancer subtype was luminal B (32%), followed by luminal A (25.3%), luminal HER-2 (18.4%), TN (17.3%), and HER-2 enriched (6.9%). For patients <50 years, luminal B (28.9%) and luminal HER-2 (24.8%) were the most prevalent subtypes, whereas luminal B (33.5%) and luminal A (27.6%) characterized the most common tumors for patients ≥50 years. There were differences in subtypes by ethnicity (Table 1): among the White and Hispanic populations, the most prevalent subtypes were luminal A and B, representing equivalent distributions (28 and 29%, respectively for White patients; 30.8 and 37%, respectively for Hispanic patients). In contrast, among African-American patients, luminal B was most prevalent at 37%, with luminal A representing the least prevalent subtype (11.9%); HER-2-enriched was 13%, representing the greatest number of patients compared to White (5.6%) or Hispanic (3.8%).
Table 1.
Demographic and tumor characteristics
| Variable | N | % | |
|---|---|---|---|
| Age | <50 | 120 | 32.0 |
| ≥50 | 255 | 68.0 | |
| Financial Class | Insured, other | 144 | 38.4 |
| Uninsured | 14 | 3.7 | |
| Medicare | 120 | 32.0 | |
| Medicaid | 97 | 25.9 | |
| Ethnicity | White | 231 | 61.6 |
| African-American | 84 | 22.4 | |
| Hispanic | 52 | 13.9 | |
| Other | 8 | 2.1 | |
| Histology | Ductal | 346 | 92.3 |
| Lobular | 24 | 6.4 | |
| Other | 5 | 1.3 | |
| Grade | Well-differentiated | 65 | 17.3 |
| Moderately-differentiated | 149 | 39.7 | |
| Poorly differentiated | 150 | 40.0 | |
| N/A | 11 | 2.9 | |
| ER Status | Positive (≥1%) | 279 | 74.4 |
| Negative (<1%) | 96 | 25.6 | |
| PR Status | Positive (≥1%) | 253 | 67.5 |
| Negative (<1%) | 122 | 32.5 | |
| Ki-67 | N.A. | 1 | |
| < 14% | 112 | 29.9 | |
| ≥ 14% | 262 | 70.1 | |
| HER-2 Status | Positive* | 96 | 25.6 |
| Negative | 279 | 74.4 | |
| LN Status | Positive# | 188 | 50.1 |
| Negative† | 187 | 49.9 | |
| Molecular Subtype | Her-2 Enriched | 26 | 6.9 |
| Luminal/Her-2 | 69 | 18.4 | |
| Luminal A | 95 | 25.3 | |
| Luminal B | 120 | 32.0 | |
| Triple Negative | 65 | 17.3 | |
| Tumor Size | N.A. | 2 | |
| T1 | 142 | 38.1 | |
| T2 | 147 | 39.4 | |
| T3 | 45 | 12.1 | |
| T4 | 39 | 10.5 |
N.A. data not available.
HER-2 is positive if IHC shows 3+ staining or FISH shows amplification.
Eight patients (9/188 or 4.7%) had pN1mi (micrometastases defined as >0.2 mm but <2 mm tumor deposit).
Four patients (4/187 or 2.1%) had pN0i+ (tumor deposit <0.2 mm detected by H&E).
The incidence of LN-positive tumors for all patients was 50.1%, and was highest in the younger age group (<50 yrs), and among the Hispanic patients (62.8% and 71.2%, respectively, Table 2). Additionally, LN-positivity correlated with increasing tumor size (Table 2, p<0.001). Among the IHC-based individual biomarkers (ER, PR, Ki67, HER-2), only HER-2 status was significantly predictive of LN status (p=0.005, Table 2). Both HER-2-enriched and luminal HER-2 subtypes had the highest incidence of LN-positive tumors (58% and 65.2%, respectively; p=0.0104, Chi-Sq, Table 2, Figure 2).
Table 2.
Demographic and tumor characteristics by lymph node status
| Variable | LN positive | LN negative | P value* | |
|---|---|---|---|---|
| Age | <50 | 76 (62.8%) | 45 (37.2%) | .0007 |
| ≥50 | 112 (44.1%) | 142 (55.9%) | ||
| Financial Class | Insured | 74 (51.4%) | 70 (48.6%) | .0019 |
| Uninsured | 8 (57.1%) | 6 (42.9%) | ||
| Medicare | 44 (36.7%) | 76 (63.3%) | ||
| Medicaid | 62 (63.9%) | 35 (36.1%) | ||
| Ethnicity | White | 99 (42.9%) | 132 (57.1%) | .0002 |
| African-American | 50 (59.5%) | 34 (40.5%) | ||
| Hispanic | 37 (71.2%) | 15 (28.8%) | ||
| Other | 2 (25%) | 6 (75%) | ||
| Histology | Ductal | 177 (51.2%) | 169 (48.8%) | .2557 |
| Lobular | 10 (41.7%) | 14 (58.3%) | ||
| Other | 1 (20.0%) | 4 (80.0%) | ||
| Grade | Well-differentiated | 20 (30.8%) | 45 (69.2%) | .0028 |
| Moderately-differentiated | 85 (57%) | 64 (43%) | ||
| Poorly-differentiated | 79 (52.7%) | 71 (47.3%) | ||
| N.A. | 4 (36.4%) | 7 (63.6%) | ||
| ER Status | Positive | 143 (51.3%) | 136 (48.7%) | .4592 |
| Negative | 45 (46.9%) | 51 (53.1%) | ||
| PR Status | Positive | 128 (50.6%) | 125 (49.4%) | .7977 |
| Negative | 60 (49.2%) | 62 (50.8%) | ||
| Ki-67 | <14% | 49 (43.8%) | 63 (56.3%) | .1140 |
| ≥14% | 138 (52.7%) | 124 (47.3%) | ||
| HER-2 | Positive | 60 (62.5%) | 36 (37.5%) | .0050 |
| Negative | 128 (45.9%) | 151 (54.1%) | ||
| Molecular Subtype | Her-2 Enriched | 15 (57.7%) | 11 (42.3%) | .0104 |
| Luminal-HER-2 | 45 (65.2%) | 24 (34.8%) | ||
| Luminal A | 37 (38.9%) | 58 (61.1%) | ||
| Luminal B | 63 (52.5%) | 57 (47.5%) | ||
| Triple Negative | 28 (43.1%) | 37 (56.9%) | ||
| Tumor Size | T1 | 44 (31%) | 98 (69%) | <.0001 |
| T2 | 73 (49.7%) | 74 (50.3%) | ||
| T3 | 37 (82.2%) | 24 (17.8%) | ||
| T4 | 34 (87.2%) | 5 (12.8%) |
N.A. = data not available
P value was based on Chi-squared test or Fisher's exact test.
Figure 2.
Correlation between lymph node statue and molecular subtype (Chi-square analysis. p=0.0104, see Table 2).
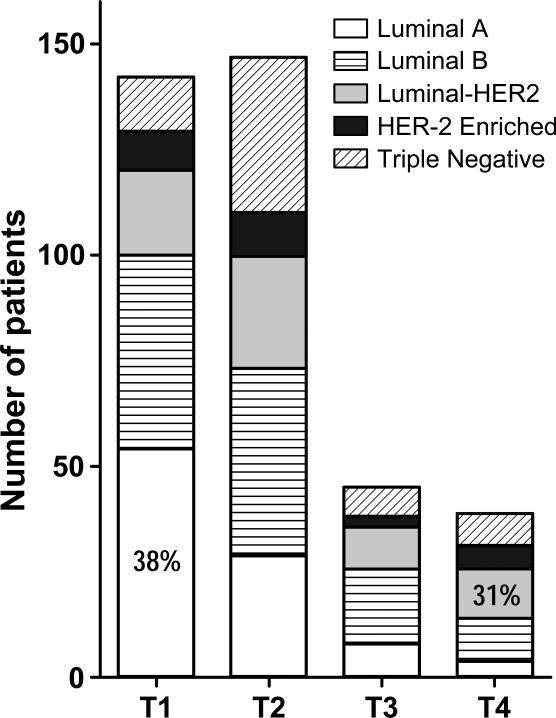
On univariate logistic regression analysis, the association of LN positivity correlated with age (<50), higher tumor grade, HER-2-positive status and molecular subtype, and tumor size (Table 3). African American and Hispanic patients were 1.9 and 3.2 times more likely than White patients to have positive lymph nodes. Patients with Medicaid versus privately insured patients were 1.5 times more likely to have positive nodes at presentation (Table 3). A multivariate logistic regression analysis determined that specific independent predictors of LN positivity were age <50, ethnicity (African American and Hispanic vs. White), tumor grade, and tumor size (Table 3). Since tumor size was strongly associated with molecular subtype (p=0.0004, Figure 1), subtype was not significant on multi-covariate analysis. The most prevalent molecular subtype of T1 tumors was luminal A (56.8%, Figure 1), which was also the subtype that had the lowest propensity for LN metastasis (38.9%, Table 2), whereas luminal HER-2 was the most common subtype for T4 tumors (30.8%, Figure 1), and also the subtype with the highest percentage of LN metastasis (65.2%, Table 3). After adjusting for age, race, grade and T stage, Financial Class was no longer significant.
Table 3.
Uni- and multi-covariate logistic regression models for lymph node positive breast cancer
| Covariate | Uni-covariate | Multi-covariate | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | |
| Age (≥50 vs. <50) | 0.467 (0.300-0.728) | 0.001 | 0.562 (0.337-0.935) | 0.0265 |
| Financial Class (Uninsured vs. insured) | 1.178 (0.384-3.614) | 0.775 | ||
| Financial Class (Medicare vs. insured) | 0.511 (0.303-0.863) | 0.012 | ||
| Financial Class (Medicaid vs. insured) | 1.565 (0.898-2.726) | 0.114 | ||
| Ethnicity (African-American vs. White) | 1.961 (1.180-3.258) | 0.009 | ||
| Ethnicity (Hispanic vs. White) | 3.289 (1.710-6.326) | <0.001 | 3.229 (1.533-6.803) | 0.002 |
| Ethnicity (Other vs. White) | 0.444 (0.088-2.549) | 0.327 | ||
| Histology(Lobular vs. Ductal) | 0.682 (0.295-1.577) | 0.371 | ||
| Histology (Other vs. Ductal) | 0.239 (0.026-2.157) | 0.202 | ||
| Grade (moderate vs. well-differentiated) | 2.987 (1.609-5.545) | 0.001 | 2.692 (1.287-5.630) | 0.0085 |
| Grade (poor vs. well-differentiated) | 2.503 (1.351-4.636) | 0.004 | 1.879 (0.822-4.297) | 0.135 |
| ER Status(negative vs. positive) | 0.839 (0.527-1.336) | 0.460 | ||
| PR Status (negative vs. positive ) | 0.945 (0.613-1.456) | 0.798 | ||
| Ki-67 (≥14% vs. <14%) | 1.431 (0.917-2.233) | 0.115 | ||
| HER-2 Status (negative vs. positive) | 0.509 (0.316-0.818) | 0.005 | NA | |
| Subtype (HER-2 -Enriched vs. Luminal A) | 2.138 (0.886-5.156) | 0.091 | ||
| Subtype (Luminal-HER-2 vs. Luminal A) | 2.939 (1.543-5.599) | 0.001 | ||
| Subtype (Luminal B vs. Luminal A) | 1.733 (1.003-2.992) | 0.049 | ||
| Subtype (Triple Negative vs. Luminal A) | 1.186 (0.625-2.254) | 0.602 | ||
| Tumor Size (T2 vs. T1) | 2.197 (1.359-3.553) | 0.001 | 2188 (1.291-3.710) | 0.0036 |
| Tumor Size (T3 vs. T1) | 10.301 (4.434-23.931) | <0.001 | 9.669 (3.940-23.730) | <0.0001 |
| Tumor Size (T4 vs. T1) | 15.145 (5.550-41.330) | <0.001 | 12.522 (4.402-35.620) | <0.0001 |
CI: confidence interval.
NA: not applicable, HER-2 not included in the model
Figure 1.
Correlation between tumor size and molecular subtype (Chi-square analysis, p=0.0004, see Table 2).
Finally, to examine whether there were differences in LN positivity between the TN basal versus the non-basal tumors, only 40/65 (61%) patients were evaluable with available IHC staining data (Table 4). There were no differences in the incidence of LN positivity between basal and non-basal TN breast cancers (Table 4, p=0.2).
Table 4.
Triple negative breast cancers
| Type | N | LN negative | LN positive | p-value Fisher Exact Test |
|---|---|---|---|---|
| Basal | 27 | 13 | 14 | |
| Non-Basal | 13 | 8 | 5 | |
| Total | 40 | 21 | 19 | p=0.2 |
DISCUSSISON
Perou et al [1] initially identified four “intrinsic” breast cancer subtypes: basal-like, HER-2-enriched, luminal and normal breast-like, based on unsupervised hierarchical clustering in a genome-wide analysis. Subsequent studies have further stratified the luminal into less proliferative A tumors and more proliferative B tumors, and the HER-2 phenotype into triple positive versus hormone receptor-negative, HER-2-positive breast cancer. The normal breast-like phenotype was not reproducible in other studies; this subtype is now considered an artifact and is not used [8]. The 2011 St. Gallen International Expert Consensus Group has endorsed the use of IHC-based molecular subtypes as a surrogate for the intrinsic subtypes of breast cancer [19].
Although IHC-based molecular classification of breast cancer can be performed easily in clinical practice, currently, no consensus or standard definition stratifying breast cancer into IHC-based molecular subtypes exists between published studies. For example, the threshold for ER/PR-positive in some studies is at ≥10% of the cells stained positive [14, 20]. Also, some studies do not stain for the Ki67 antigen [21], while other studies employ tumor grade [14] as a surrogate for cellular proliferation. The number of subclassifications is also varied [11, 14, 22]. We subdivided the HER-2 positive tumors because HER-2-enriched breast cancers had an odds ratio of 2.1-fold risk for positive LN compared to luminal A, whereas luminal HER-2 cancers showed a higher (2.9-fold) risk (Table 3). Our data is in agreement with Van Calster et al [23], who have shown that triple-positive tumors are most likely to be LN positive. Luminal A and TN breast cancers are the least likely to present with positive LN. Among the TN breast cancers, we did not find any differences in the incidence of positive LN between the basal versus the non-basal breast cancers (Table 4). However, it is interesting to note that the basal-like breast cancers have markedly higher recurrence rates, compared to non-basal TN cancers [12], with a higher incidence of visceral metastasis [12, 24, 25], suggesting that the poor prognosis of basal-like breast cancers may be due to a higher propensity for distant (rather than regional) spread, as well as a paucity of adequate systemic therapies [17].
We found that IHC-based molecular subtypes correlated with LN status only by univariate analysis (Table 2, p=.0104; Table 3). Because tumor size was strongly associated with molecular subtype (p=0.0004, Figure 1), molecular subtype was not significant after adjusting for tumor size. Our data suggests that the molecular subtype reflects the biological behavior of the breast cancer, including the primary tumor's ability to achieve a particular size prior to clinical detection (either by imaging or physical examination), and its relative ability to metastasize to regional LN at the time of diagnosis.
There are several limitations to our study. The majority of patients were not included in this study because they had Stage 0 (in-situ) disease and no need for LN evaluation. We also excluded patients with incomplete clinicopathologic data, which introduces inherent selection bias in this study. This is a retrospective, descriptive study spanning eight-and-a-half-year period of time. Our most recent institutional standard for IHC evaluation of TN tumors (the addition of CK5/6 and EGFR staining) was not applied on the majority of TN breast cancers (diagnosed prior to application of the current practice). We subjected 25 cases to CK5/6 and EGFR IHC staining, and 5 cases for FISH analysis of HER-2 amplification for this study since this was not performed at the time of original patient diagnosis. Another limitation is that our study is from a single institution with relatively small sample sizes within each molecular subtype. However, similar to our findings, a study by Voduc et al [12] (N=2,985), independently verified that the highest rates of LN positivity occurred in the luminal HER-2 and HER-2-enriched tumors (54% and 55% of patients), whereas the lowest rates of positive LN were found in luminal A and TN tumors (41%, 36%, respectively).
Contemporary surgical management of axillary LN in breast cancer emphasizes a minimally invasive approach, decreasing the morbidity of axillary interventions while maximizing diagnostic information gained through increased pathologic scrutiny of only the sentinel LN, without compromising patient survival [26]. However, an area of continued controversy exists in the accuracy of sentinel LN biopsy after neoadjuvant chemotherapy. Many surgeons advocate forgoing sentinel LN staging prior to neoadjuvant chemotherapy for select patients who are clinically node-negative (by palpation, ultrasound evaluation and/or negative needle biopsy) [27]. The benefit is that the patient would undergo a single operation upon completion of preoperative chemotherapy. Concurrent with the surgical treatment of their primary breast cancer, the patient would undergo sentinel LN biopsy after chemotherapy [27]. Limitations of this practice include: 1) needle biopsy of a radiographically suspicious axillary LN can have a false-negative result of over 30% [28], and 2) the false-negative rate of sentinel LN biopsies after neoadjuvant chemotherapy is higher at 12% [29] compared to the desirable rate of <5% [30]. False-negative LN results may alter adjuvant systemic treatment recommendations and/or additional recommendations for locoregional control (subsequent axillary lymph node dissection/radiation therapy), potentially impacting care for some patients. Knowledge of molecular subtype and risk of LN metastasis may be useful for surgeons and patients deciding to forgo sentinel LN biopsy prior to neoadjuvant chemotherapy.
Conclusions
The prevalence of LN metastasis among the five breast cancer molecular subtypes is significantly different on univariate analysis. In particular, luminal A tumors have the lowest risk of LN metastasis and the greatest number of T1 tumors, while the luminal HER-2 subtype has the highest risk of LN metastasis and the greatest number of T4 tumors. After adjusting for tumor size, molecular subtype was not significantly correlated with LN status on multivariable logistic regression. IHC-based molecular classification can be readily performed and knowledge of the factors that affect LN status may help with treatment decisions.
ACKNOWLEDGEMENTS
The authors thank Eileen Figueroa and Steve Schuenke for manuscript and figure preparation.
This work is supported by1K08CA125209-01A2 to CC and the Ruth Levy Kempner Endowment to SH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper was presented at the Academic Surgical Congress in New Orleans, LA on Feb. 5, 2013.
REFERENCES
- 1.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 3.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wirapati P, Sotiriou C, Kunkel S, et al. Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008;10:R65. doi: 10.1186/bcr2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 7.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weigelt B, Mackay A, A'Hern R, et al. Breast cancer molecular profiling with single sample predictors: a retrospective analysis. Lancet Oncol. 2010;11:339. doi: 10.1016/S1470-2045(10)70008-5. [DOI] [PubMed] [Google Scholar]
- 9.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phipps AI, Chlebowski RT, Prentice R, et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:454. doi: 10.1158/1055-9965.EPI-10-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhargava R, Beriwal S, Dabbs DJ, et al. Immunohistochemical surrogate markers of breast cancer molecular classes predicts response to neoadjuvant chemotherapy: a single institutional experience with 359 cases. Cancer. 2010;116:1431. doi: 10.1002/cncr.24876. [DOI] [PubMed] [Google Scholar]
- 12.Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 13.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 14.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 15.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 17.Metzger-Filho O, Tutt A, de Azambuja E, et al. Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol. 2012;30:1879. doi: 10.1200/JCO.2011.38.2010. [DOI] [PubMed] [Google Scholar]
- 18.Carter CL, Allen C. Henson DE Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Ronde JJ, Hannemann J, Halfwerk H, et al. Concordance of clinical and molecular breast cancer subtyping in the context of preoperative chemotherapy response. Breast Cancer Res Treat. 2010;119:119. doi: 10.1007/s10549-009-0499-6. [DOI] [PubMed] [Google Scholar]
- 21.Reyal F, Rouzier R, Depont-Hazelzet B, et al. The molecular subtype classification is a determinant of sentinel node positivity in early breast carcinoma. PLoS One. 2011;6:e20297. doi: 10.1371/journal.pone.0020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Calster B, Vanden Bempt I, Drijkoningen M, et al. Axillary lymph node status of operable breast cancers by combined steroid receptor and HER-2 status: triple positive tumours are more likely lymph node positive. Breast Cancer Res Treat. 2009;113:181. doi: 10.1007/s10549-008-9914-7. [DOI] [PubMed] [Google Scholar]
- 24.Crabb SJ, Cheang MC, Leung S, et al. Basal breast cancer molecular subtype predicts for lower incidence of axillary lymph node metastases in primary breast cancer. Clin Breast Cancer. 2008;8:249. doi: 10.3816/CBC.2008.n.028. [DOI] [PubMed] [Google Scholar]
- 25.Dent R, Hanna WM, Trudeau M, et al. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115:423. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 26.Giuliano AE, Han SH. Local and regional control in breast cancer: role of sentinel node biopsy. Adv Surg. 2011;45:101. doi: 10.1016/j.yasu.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Hunt KK, Yi M, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg. 2009;250:558. doi: 10.1097/SLA.0b013e3181b8fd5e. [DOI] [PubMed] [Google Scholar]
- 28.Altomare V, Guerriero G, Carino R, et al. Axillary lymph node echo-guided fine-needle aspiration cytology enables breast cancer patients to avoid a sentinel lymph node biopsy. Preliminary experience and a review of the literature. Surg Today. 2007;37:735. doi: 10.1007/s00595-006-3366-7. [DOI] [PubMed] [Google Scholar]
- 29.Xing Y, Foy M, Cox DD, et al. Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006;93:539. doi: 10.1002/bjs.5209. [DOI] [PubMed] [Google Scholar]
- 30.McMasters KM, Wong SL, Chao C, et al. Defining the optimal surgeon experience for breast cancer sentinel lymph node biopsy: a model for implementation of new surgical techniques. Ann Surg. 2001;234:292. doi: 10.1097/00000658-200109000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]