Abstract
Auditory processing is presumed to be influenced by cognitive processes – including attentional control – in a top-down manner. In bilinguals, activation of both languages during daily communication hones inhibitory skills, which subsequently bolster attentional control. We hypothesize that the heightened attentional demands of bilingual communication strengthens connections between cognitive (i.e., attentional control) and auditory processing, leading to greater across-trial consistency in the auditory evoked response (i.e., neural consistency) in bilinguals. To assess this, we collected passively-elicited auditory evoked responses to the syllable [da] and separately obtained measures of attentional control and language ability in adolescent Spanish-English bilinguals and English monolinguals. Bilinguals demonstrated enhanced attentional control and more consistent brainstem and cortical responses. In bilinguals, but not monolinguals, brainstem consistency tracked with language proficiency and attentional control. We interpret these enhancements in neural consistency as the outcome of strengthened attentional control that emerged from experience communicating in two languages.
Keywords: bilingualism, brainstem, electrophysiology, auditory
1. Introduction
Every moment, our ears are bombarded with millions of bits of data that inform us about our acoustic environment. To best utilize this flood of information, the brain has developed ways to adaptively respond to sensory input (Engel, Fries, & Singer, 2001). One mechanism by which sensory signaling is improved is by cognitive (i.e., executive) functions biasing the encoding of contextually or behaviorally relevant signals over irrelevant ones. The executive functions that guide this selection are based in the frontal and parietal cortex (Miller & Cohen, 2001; Smith & Jonides, 1999; Weissman, Roberts, Visscher, & Woldorff, 2006) and exert their influence on sensory processing via top-down mechanisms (Bar et al., 2006; Hochstein & Ahissar, 2002) These top-down mechanisms enable the executive system to influence a variety of auditory processing tasks (see McLachlan & Wilson, 2010 for review), including focusing the “searchlight” on a target sound (Fritz, Elhilali, David, & Shamma, 2007; Luo, Wang, Kashani, & Yan, 2008).
The executive system follows a protracted maturational time course that extends through adolescence (Sowell, Thompson, Holmes, Jernigan, & Toga, 1999; Spear, 2000). As evidenced by research on profoundly deaf children, development of the executive system may be shaped by making sound-to-meaning connections through auditory-based language experience (Conway, Pisoni, & Kronenberger, 2009). In further support of a link between language experience and executive function, in bilinguals, the mapping of sound-to-meaning connections across two languages can further tune the ability to selectively attend to important stimuli and ignore irrelevant ones, an executive function called inhibitory control. (Bialystok, 2011; Blumenfeld & Marian, 2011; Carlson & Meltzoff, 2008; Soveri, Laine, Hamalainen, & Hugdahl, 2011). Given that bilingualism can improve attentional control abilities and that the executive system can influence sensory encoding via top-down signaling, we hypothesize that the greater attentional control in bilinguals exerts a stronger influence on auditory processing enabling the bilingual auditory system to more effectively hone-in on the behaviorally-meaningful features of the incoming signal. We predict that this strengthened interaction between cognitive and sensory processing manifests as greater across-trial consistency in the far-field (i.e., scalp-recorded) population evoked response to sound for bilinguals relative to monolinguals (Hornickel & Kraus, 2013). To test this hypothesis, we compared bilingual and monolingual adolescents on their attentional control abilities and the consistency of their auditory cortical and brainstem evoked response potentials to a speech syllable evoked under passive listening conditions.
Auditory evoked cortical and subcortical responses, though obligatory, can be influenced via top-down signaling (Hairston, Letowski, & McDowell, 2013; Woldorff et al., 1993; Wu, Weissman, Roberts, & Woldorff, 2007). For example, the auditory cortex is sensitive to attentional state (Coch, Sanders, & Neville, 2005; Lutz, Slagter, Dunne, & Davidson, 2008; Näätänen, 1990; Winer, 2006; Woldorff et al., 1993; Wu et al., 2007) and is innervated by areas of the brain thought to be involved in directing attention (Gao & Suga, 2000; Huffman & Henson, 1990; Malmierca & Ryugo, 2011). Moreover, recent evidence suggests that the inferior colliculus, the primary generator of the auditory brainstem response to complex sounds (Chandrasekaran & Kraus, 2010; Skoe & Kraus, 2010) is also sensitive to the effects of attentional control (Hairston et al., 2013; Krizman, Marian, Shook, Skoe, & Kraus, 2012; Raizada & Poldrack, 2007; Rinne et al., 2008; Ruggles, Bharadwaj, & Shinn-Cunningham, 2011). This coupling between the executive system and the inferior colliculus is presumed to take place through the extensive network of efferent connections that link cortical to subcortical structures (Gao & Suga, 1998, 2000) and can be measured by the auditory brainstem response to complex sounds (Kraus & Chandrasekaran, 2010).
The auditory brainstem response to complex sounds provides a neurophysiologic snapshot of how lifelong experience has re-wired the auditory system’s automatic brainstem response to sound. For example, lifelong experiences such as native-language learning, bilingualism, and protracted music training leave their mark on auditory brainstem encoding (Krishnan et al. 2008, Krizman et al. 2012, Musacchia et al., 2007). This type of experience-dependent plasticity is thought to arise via top-down mechanisms and can be observed even when the response is evoked under passive listening conditions (Kraus & Chandrasekaran, 2010). Thus, if second language experience hones attentional control, if attentional control strengthens neural consistency through top-down processes, and if subcortical and cortical auditory structures are sensitive to attentional control throughout life, then bilinguals by virtue of having better attentional control abilities, are predicted to have greater consistency in their auditory evoked cortical and brainstem response to speech sounds. This greater neural consistency should in turn relate to attentional control abilities in bilinguals. Moreover, if neural consistency, like attentional control, is shaped by language experience, then we further predict that greater proficiency across a bilingual’s two languages would relate to more consistent neural responses, given that reading abilities (another linguistic skill) have been positively related to neural consistency (Centanni et al., 2013; Hornickel & Kraus, 2013).
2. Results
2.1 Summary of results
Bilingual adolescents had greater consistency in both their brainstem and cortical responses to the speech sound [da] than monolinguals. In both groups, cortical and brainstem consistency were highly related; however, in bilinguals, brainstem consistency, and not cortical consistency, tracked with attentional control and language proficiency.
2.2 Auditory Response Control (Attentional Control)
Bilinguals outperformed monolinguals on behavioral measures of attentional control (F(1,54)=7.363 p=0.009), with bilinguals having a mean standard score (+/− 1 SE) of 84.37 +/− 3.52 and monolinguals having a mean standard score of 69.19 +/− 4.21.
2.3 Response consistency
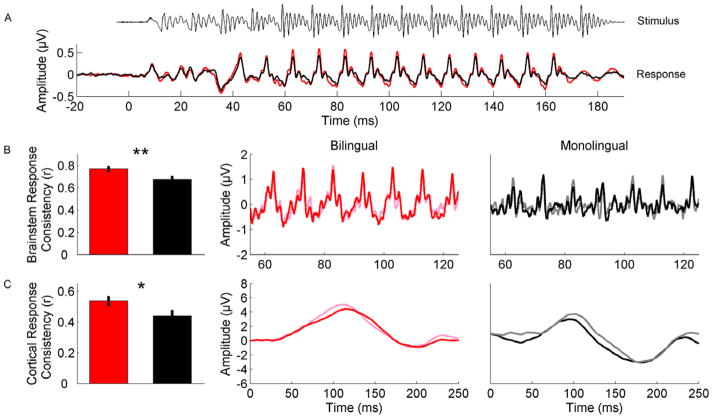
Bilinguals demonstrated more consistent brainstem (F(1,54)=7.874, p=0.007) and cortical (F(1,54)=4.302, p=0.043) responses to the syllable [da] compared to monolinguals (fig 1). For the bilinguals, the mean r-value was 0.769 +/− 0.026 for the brainstem response consistency and 0.538 +/− 0.033 for the cortical response consistency. Monolinguals had mean r-values of 0.675 +/− 0.032 and 0.439 +/− 0.039 for brainstem and cortical responses, respectively.
Figure 1.
Differences in evoked response consistency between bilinguals and monolinguals. (A) shows the grand average monolingual (black) and bilingual (red) brainstem responses to the speech stimulus [da], which has been time shifted to align with the response onset. (B) Brainstem and (C) cortical responses to the syllable [da] show greater consistency over the course of the testing session for bilinguals relative to monolinguals as evidenced by greater overlap between the pink (average of first half of responses) and red (average of last half of responses) compared to gray (average of first half of responses) and black (average of last half of responses). Brainstem responses in B have been zoomed in to illustrate differences in evoked response consistency to the vowel [a] portion of [da]. Waveforms in B and C are from representative subjects.
2.4 Relationships among attentional control, subcortical and cortical response consistency
For all participants, there was a strong correlation between the consistency of the cortical and brainstem responses (r=0.797, p<0.0005; fig 2). This relationship was only seen when correlating cortical consistency to consistency of the brainstem response but not when correlating the electrical activity recorded during the silence preceding each stimulus (r = 0.225, p = 0.109). Only bilinguals demonstrated a relationship between brainstem response consistency and Auditory Response (attentional) Control (r=0.418, p=0.038; fig. 2) and language proficiency (r=0.479, p=0.015; fig. 2), with more consistent responses linked to better attentional control and greater language proficiency. Monolinguals did not show a relationship between brainstem response consistency and Auditory Response Control (r=0.21, p=0.314) or language proficiency (r=−0.092, p=0.662). There was no relationship between cortical response consistency and attentional control abilities for either group (bilinguals: r=0.308, p=0.135; monolinguals: r=−0.252, p=0.225).
Figure 2.
Brainstem response consistency relationships with cortical response consistency, auditory response control, and language proficiency for bilinguals (red dots, A–C) and monolinguals (black dots, D–F). (A and D.) Cortical response consistency and brainstem response consistency are highly correlated in both bilinguals and monolinguals. (B and E.) Only in bilinguals, and not monolinguals, was there a relationship between auditory attentional control abilities and brainstem response consistency, suggesting that experience using two languages sharpens the brain’s response to sound. (C and F.) Bilingual subjects with higher proficiency in both languages had more stable neural responses, while response consistency did not relate to monolingual language proficiency.
3. Discussion
We show that the bilingual auditory system processes sound in ways that are both different from and similar to the monolingual system. Specifically, we demonstrate that although both groups showed consistent cortical and subcortical responses, bilinguals had greater consistency in their neural responses relative to monolinguals. We also observed that consistency of the cortical and subcortical evoked responses was related in both monolinguals and bilinguals. However, specific to bilinguals was a relationship between subcortical response consistency and both attentional control abilities and language proficiency, while neither group showed a relationship between cortical response consistency and these abilities.
All participants demonstrated a relationship between cortical and brainstem consistency that was specific to the evoked responses (and not the preceding neural background activity), suggesting that consistency in processing sound, as indexed by auditory evoked potentials, is maintained throughout the auditory system. This synching of brainstem and cortical responses is argued to result from signaling between afferent and efferent auditory pathways which link the generators of these responses to facilitate encoding of the signal in a behaviorally-relevant manner (Gao & Suga, 2000; Huffman & Henson, 1990). However, given that both the cortical and subcortical responses were recorded simultaneously, it is possible that the relationship may partly reflect a byproduct of the recording procedure.
Although reciprocal connections exist between the frontal cortex -- an area known to be involved in attentional control (Raizada & Poldrack, 2007; Weissman et al., 2006) -- and the auditory cortex (Huffman & Henson, 1990), no relationship was seen between attentional control abilities and cortical response consistency. The lack of a relationship between attentional control and cortical speech processing may be due to the inherently higher variability in cortical relative to subcortical evoked responses, as evidenced by the smaller across-trial correlation values for the cortical response. On the other hand we do find that in bilinguals, but not monolinguals, there is a relationship between consistency of the auditory brainstem response and attentional control abilities. This relationship may suggest that efferent pathways connecting the executive system to the subcortical auditory system are preferentially strengthened through experience communicating in two languages. Whether this connection between the executive system and subcortical auditory processing is independent of or mediated by the auditory cortex cannot be determined from the current data. Given that previous studies establishing a link between attention and auditory processing (both cortically and subcortically) have used active listening paradigms (e.g., Coch et al., 2005; Hairston et al., 2013; Woldorff et al., 1993; Wu et al., 2007), future research should investigate the relationships among language experience, cortical response consistency, subcortical response consistency, and attentional control using active listening conditions that require the overt use of attentional control for encoding of auditory stimuli.
Though the mechanisms of language control in production remain under debate (e.g., see Costa & Santesteban, 2004), it has been repeatedly shown that inhibitory control is engaged during bilingual language perception. When hearing speech, both of a bilingual’s languages are automatically activated (Hermans, Bongaerts, De Bot, & Schreuder, 1998; Kuipers & Thierry, 2010; Viorica Marian & Spivey, 2003; Spivey & Marian, 1999), and so for accurate perception to occur, one language must be inhibited (Kroll, Bobb, Misra, & Guo, 2008; Van Heuven, Schriefers, Dijkstra, & Hagoort, 2008). Inhibition of the non-target language occurs quite rapidly (< 500 ms, Blumenfeld & Marian, 2011; Treccani, Argyri, Sorace, & Della Sala, 2009) and within this time window, bilinguals are acutely tuned into the auditory input, as evidenced by their ability to rapidly detect a language switch (Kuipers & Thierry, 2010). The bilingual’s experience of cross-language activation may re-wire auditory and cognitive (i.e., attentional control) neural circuitry so that over time the neural architecture of the bilingual brain is a reflection of the constant need for attentional control and auditory circuits to interact during speech perception.
We propose that the need to engage executive and auditory systems concurrently during bilingual communication facilitates this distinct relationship in bilinguals between brainstem response consistency and attentional control abilities. We theorize that this relationship arises from the rapid co-activation of both auditory and attentional control processing that occurs during bilingual communication. This sensory-cognitive co-activation we propose, leads to a rewiring of the bilingual brain and how it automatically encodes sound. Indeed, this re-wiring in the bilingual brain is so extensive that it can be seen when recording the evoked subcortical response under passive listening conditions and may suggest a privileged pathway between the executive system and the subcortical auditory system in bilinguals. Through extensive experience engaging attentional control during bilingual communication, and the repeated modulation of subcortical structures by top-down mechanisms during active listening, the obligatory response to sound has been changed to reflect past experiences when they were actively attending to sound. A second possible interpretation that may act in tandem with the first is that through a lifetime of concurrently engaging both attentional control and auditory processing these two systems become synergistically connected, such that top-down mechanisms are activated even under passive listening conditions, resulting in a unique relationship between attentional control and subcortical auditory processing in bilinguals.
The relationship between attentional control and auditory brainstem encoding in bilinguals suggests that in this population the executive system biases neural encoding of the auditory cues that aid in rapid selection of the appropriate language. One of these biasing cues may be the fundamental frequency (F0, Krizman, Marian, et al., 2012), a cue that a bilingual presumably manipulates when switching between languages (Altenberg & Ferrand, 2006). Through lifelong experience mediating two languages, the neural pathway encoding these relevant cues (e.g., F0) is repeatedly selected via feedback from higher order processing centers (Miller & Cohen, 2001). Through Hebbian plasticity mechanisms (Hebb, 2002), neural pathways providing top-down feedback and pathways carrying biased bottom-up sensory processing are strengthened and stabilized. This plasticity which arises from the demands of cross-language activation during bilingual communication would therefore lead to a heightened cognitive-sensory relationship in bilinguals. In support of this, previous work has shown a distinct relationship between auditory perception and inhibitory control during auditory comprehension for bilinguals relative to monolinguals (Blumenfeld & Marian, 2011) and here we show enhanced attentional control abilities in bilinguals relate to their enhanced auditory brainstem response consistency.
Relative to the larger variability typically seen in the cortical response to sensory stimuli (Arieli, Sterkin, Grinvald, & Aertsen, 1996; Shadlen & Newsome, 1998), the auditory brainstem operates with microsecond precision. The auditory brainstem represents the incoming auditory signal with a high degree of fidelity, such that, when the response is averaged over a number of presentations, it closely resembles the evoking stimulus (Galbraith, Arbagey, Branski, Comerci, & Rector, 1995; E. Skoe & Kraus, 2010). This fidelity is requisite for normal auditory function and is purportedly driven by precise, well-orchestrated firing of brainstem neurons (Hall, 2006). In rare cases, this stereotypical precision breaks down substantially, resulting in a condition known as auditory neuropathy. While auditory neuropathy represents an extreme case of neural dysynchrony, milder forms of neural dysynchrony appear to exist in children with reading impairments relative to both average and above average readers (Hornickel & Kraus, 2013). At the opposite end of the spectrum, bilinguals have a heightened form of neural stability that exceeds the normal range seen in the monolingual (i.e., baseline) population suggestive of enhanced neural synchrony within and across trials.
Combined with other findings, our data suggest that greater neural stability is an index of an expert population (e.g., bilinguals, musicians) whereas an unstable response provides an objective biological index of a disordered population (e.g. dyslexia, neuropathy) (Berlin, Morlet, & Hood, 2003; Centanni et al., 2013; Hornickel & Kraus, 2013; Parbery-Clark, Anderson, Hittner, & Kraus, 2012; Erika Skoe & Kraus, 2013). Given the positive relationship between evoked response consistency and language proficiency in bilinguals, we propose that a stable neural response provides a platform on which stronger sound-to-meaning representations can be made. Inconsistency may be at the root of the dyslexic’s difficulty in mapping sound-to-meaning while enhanced neural consistency may bolster language abilities, such as proficiency in a bilingual’s two languages. Stability in the bilingual auditory system may provide the foundation for auditory advantages observed in bilinguals, including enhancements in the neural encoding of the fundamental frequency of speech sounds (Krizman, Marian, et al., 2012), novel language learning (Bartolotti & Marian, 2012; Viorica Marian & Kaushanskaya, 2009), and pattern detection abilities (i.e., statistical learning, Bartolotti, Marian, Schroeder, & Shook, 2011). In populations that have inconsistent neural responses, it is possible to strengthen neural consistency through training (Hornickel, Zecker, Bradlow, & Kraus, 2012; Parbery-Clark et al., 2012; Erika Skoe & Kraus, 2013); and so, in addition to other forms of auditory-based training, such as computer-based training or music lessons, second-language learning may be another type of training that can be implemented to help stabilize an inconsistent neural response.
3.1 Conclusions
Bilingual experience enhances cortical and subcortical neural encoding and cognitive abilities, underscoring the interplay between sensory and cognitive processing in driving experience-dependent neuroplasticity. In bilinguals, we demonstrate that better attentional control is coupled with greater consistency in the subcortical evoked response, which further relates to their proficiency in both languages. This relationship suggests that language experience influences automatic sensory encoding of auditory stimuli, likely through enhancement of top-down processing streams that act to refine bottom-up signal transmission.
4. Methods
4.1 Subjects
Fifty-four freshman from three Chicago-area public high schools participated in this study. Subjects were divided into two groups based on language experience: English monolinguals (n = 27; 48% female, 44% low socioeconomic standing (SES, indexed by maternal education, D’Angiulli, Herdman, Stapells, & Hertzman, 2008; Hollingshead, 1975)) and Spanish-English bilinguals (n=27; 59% female; 59% low SES). Given that sex and SES affect cognitive and neural function (Hackman, Farah, & Meaney, 2010; Krizman, Skoe, & Kraus, 2012), these measures were covaried in all statistical analyses. Inclusionary criteria were normal IQ (Wechsler Abbreviated Scale of Intelligence, WASI; monolinguals: 96.2 +/− 11.6; bilinguals: 98.6 +/− 7.2; F = 0.834, p = 0.365, Wechsler, 1999), air conduction thresholds of < 20 dB normal hearing level (nHL) for octaves from 125–8000 Hz, click-evoked brainstem response latencies within lab-internal normal limits (5.41–5.97 ms, 80 dB sound pressure level, 31/s), and no external diagnosis of an attention disorder (ADHD or ADD). The two language groups were age-matched (monolinguals: 14.56 +/− 0.371 years; bilinguals: 14.63 +/− 0.372 years; F =0.536, p = 0.467). The Northwestern University Institutional Review Board approved all procedures and the children and their parent/guardian gave their informed assent and consent, respectively.
The Language Experience and Proficiency Questionnaire (LEAP-Q, V. Marian, Blumenfeld, & Kaushanskaya, 2007) and a parental report of the child’s language abilities were used to measure language proficiency. All subjects reported high English proficiency (≥7 out of 10 on English speaking and understanding proficiency). Spanish-English bilinguals also reported high Spanish proficiency (≥ 7 out of 10 on Spanish speaking and understanding proficiency) and parents of bilingual children confirmed that the child was highly proficient in both English (≥ 7 out of 10) and Spanish (≥ 7 out of 10). The parent and the bilingual child indicated that the child learned both languages early (English: 3.07 +/− 1.77 years; Spanish: 2.11 +/− 1.69 years). Bilingual subjects reported daily exposure to English (61.3%) and Spanish (38.7%). Fifteen of the 27 bilinguals identified Spanish as their native language. A composite measure of language proficiency was generated for the bilingual subjects by averaging the child self-rating and parent’s rating of the child’s proficiency in English (maximum value = 10) and Spanish (maximum value = 10). The average proficiency ratings for each language were then added (maximum value = 20, range 15.25 – 19.75). Monolingual subjects reported English as their native language (age of acquisition 1.3 +/− 0.95 years), no familiarity with a second language, and their parents also reported that the child only knew English. Similar to the bilingual subjects, a composite proficiency score was determined for the monolingual subjects’ English abilities (maximum value = 10, range 8–10). Given that expert language proficiency was an inclusionary criterion, the range of language proficiencies for the English monolinguals was fairly narrow.
4.2 Auditory Response Control (attentional control)
Attentional control was assessed by the Integrated Visual and Auditory Continuous Performance Test (IVA+Plus, www.braintrain.com, Richmond, VA), a 20 minute, Go/No-go test in which 1’s and 2’s are pseudo-randomly presented auditorily or visually. The subject responds only when a 1, but not a 2, is seen or heard. This test was administered in English at the high school using Sennheiser headphones and a laptop computer placed 60 cm from the subject. Performance for each subject was converted to age-normed standard scores and comparisons were made between bilinguals and monolinguals on the “Auditory Response Control” composite measure, which assesses attentional control in the auditory domain. All of the behavioral testing, including the IVA, occurred separately and was independent from the electrophysiological measurement.
4.3 Electrophysiological Recording
4.3.1. Stimulus and recording
Stimulus and recording parameters have been described previously (E. Skoe & Kraus, 2010). Briefly, the stimulus [da], a dynamic, six-formant, 170 ms sound synthesized at a 20 kHz sampling rate using a Klatt-based formant synthesizer (Klatt, 1980), was presented to the right ear at 80 dB SPL at a rate of 3.98/s through an insert earphone (ER-3, Etymotic Research) using the stimulus presentation software NeuroScan Stim2 (Sound module, Compumedics). Responses were differentially recorded with Ag/Ag-Cl electrodes applied in a vertical montage (Cz-to-right earlobe with forehead as ground) using NeuroScan Acquire4 at a sampling rate of 20 kHz. Brainstem and cortical responses were measured concurrently as part of a passive listening paradigm. During electrophysiological testing, the participant sat in a comfortable reclining chair in an electrically-shielded, sound-attenuated chamber and watched a self-selected movie. The left ear was unblocked allowing the participant to hear the movie soundtrack played in English at < 40 dB SPL, an insufficient intensity to mask the stimulus.
4.4. Data averaging
Subcortical auditory evoked potential responses were off-line bandpass filtered in Neuroscan Edit from 70 to 2000 Hz (12 dB/octave, zero phase-shift), which captures the limits of brainstem phase-locking (Chandrasekaran & Kraus, 2010; Liu, Palmer, & Wallace, 2006). Responses were then averaged over a −40 to 190 ms window, relative to stimulus onset, after first baselining to the pre-stimulus level. Two subaverages were created: one represented the first 3000 sweeps of the recording and the other represented the 3000 sweeps collected during the second half of the recording. An artifact reject criterion of +/−35 μV was applied before averaging..
Cortical evoked potential responses were off-line bandpass filtered from 0.1 to 30 Hz (12 dB/octave, zero phase-shift) using Neuroscan Edit. Next, responses were averaged over a 0 to 250 ms window, relative to stimulus onset to create two sub-averages of 3000 sweeps representing the first half of the recording and the second half of the recording. An artifact reject criterion of +/− 100 μV was applied. Both the cortical and brainstem responses comprised 6000 sweeps.
4.5. Assessing cortical and brainstem consistency
Measurements of brainstem response consistency focused on the response to the vowel [a] (60 – 180 ms). This analysis window was chosen to identify potential mechanisms underlying neural enhancements previously found in bilinguals (Krizman, Marian, et al., 2012).
Cortical response consistency was calculated over the entire 0–250 ms response. Both cortical and brainstem response consistency were obtained by correlating the first 3000 trials to the last 3000 trials (Hornickel & Kraus, 2013), where an r value closer to 1 represents more morphologically consistent subaverages and an r-value of 0 represents no consistency between the two subaverages. The resultant r-values for each subject were Fisher z-transformed for subsequent statistical analyses.
Supplementary Material
Highlights.
Speech-evoked brainstem activity relates to language proficiency in bilinguals.
Bilinguals show more consistent speech-evoked responses than monolinguals.
Attentional control may stabilize the neural response to speech in bilinguals.
Acknowledgments
The authors thank the members of the Auditory Neuroscience Laboratory, especially Trent Nicol, Alexandra Parbery-Clark, Jessica Slater, and the Bilingualism and the members of the Psycholinguistics Research Group for their helpful comments on earlier versions of the manuscript. This research is funded by NSF SMA1015614, NIH DC009399 and HD059858, the Mathers Foundation, and the Knowles Hearing Center, Northwestern University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altenberg EP, Ferrand CT. Fundamental Frequency in Monolingual English, Bilingual English/Russian, and Bilingual English/Cantonese Young Adult Women. Journal of Voice. 2006;20(1):89–96. doi: 10.1016/j.jvoice.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of Ongoing Activity: Explanation of the Large Variability in Evoked Cortical Responses. Science. 1996;273(5283):1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, et al. Top-down facilitation of visual recognition. Proc Natl Acad Sci U S A. 2006;103(2):449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolotti J, Marian V. Language learning and control in monolinguals and bilinguals. Cognitive science. 2012;36(6):1129–1147. doi: 10.1111/j.1551-6709.2012.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolotti J, Marian V, Schroeder SR, Shook A. Bilingualism and Inhibitory Control Influence Statistical Learning of Novel Word Forms. Frontiers in Psychology. 2011;2(324) doi: 10.3389/fpsyg.2011.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin CI, Morlet T, Hood LJ. Auditory neuropathy/dyssynchrony: its diagnosis and management. Pediatric Clinics of North America. 2003;50(2):331–340. doi: 10.1016/s0031-3955(03)00031-2. [DOI] [PubMed] [Google Scholar]
- Bialystok E. How does experience change cognition? Evaluating the evidence. British Journal of Psychology. 2011;102(3):303–305. doi: 10.1111/j.2044-8295.2011.02008.x. [DOI] [PubMed] [Google Scholar]
- Blumenfeld HK, Marian V. Bilingualism influences inhibitory control in auditory comprehension. Cognition. 2011;118(2):245–257. doi: 10.1016/j.cognition.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Meltzoff AN. Bilingual experience and executive functioning in young children. Developmental Science. 2008;11(2):282–298. doi: 10.1111/j.1467-7687.2008.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni T, Booker A, Sloan A, Chen F, Maher B, Carraway R, et al. Knockdown of the Dyslexia-Associated Gene Kiaa0319 Impairs Temporal Responses to Speech Stimuli in Rat Primary Auditory Cortex. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B, Kraus N. The scalp-recorded brainstem response to speech: Neural origins and plasticity. Psychophysiology. 2010;47:236–246. doi: 10.1111/j.1469-8986.2009.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coch D, Sanders LD, Neville HJ. An Event-related Potential Study of Selective Auditory Attention in Children and Adults. Journal of Cognitive Neuroscience. 2005;17(4):605–622. doi: 10.1162/0898929053467631. [DOI] [PubMed] [Google Scholar]
- Conway CM, Pisoni DB, Kronenberger WG. The Importance of Sound for Cognitive Sequencing Abilities. Current Directions in Psychological Science. 2009;18(5):275–279. doi: 10.1111/j.1467-8721.2009.01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Santesteban M. Lexical access in bilingual speech production: Evidence from language switching in highly proficient bilinguals and L2 learners. Journal of Memory and Language. 2004;50(4):491–511. [Google Scholar]
- D’Angiulli A, Herdman A, Stapells D, Hertzman C. Children’s event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology. 2008;22(3):293–300. doi: 10.1037/0894-4105.22.3.293. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nature Reviews Neuroscience. 2001;2(10):704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, David SV, Shamma SA. Auditory attention - focusing the searchlight on sound. Current Opinion in Neurobiology. 2007;17(4):437–455. doi: 10.1016/j.conb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Galbraith GC, Arbagey PW, Branski R, Comerci N, Rector PM. Intelligible speech encoded in the human brain stem frequency-following response. Neuroreport. 1995;6(17):2363–2367. doi: 10.1097/00001756-199511270-00021. [DOI] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proc Natl Acad Sci U S A. 1998;95(21):12663–12670. doi: 10.1073/pnas.95.21.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent plasticity in the auditory cortex and the inferior colliculus of bats: role of the corticofugal system. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(14):8081–8086. doi: 10.1073/pnas.97.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature Reviews Neuroscience. 2010;11(9):651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairston WD, Letowski TR, McDowell K. Task-Related Suppression of the Brainstem Frequency following Response. PLoS ONE. 2013;8(2):e55215. doi: 10.1371/journal.pone.0055215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JW. New handbook of auditory evoked responses. Boston Mass: Pearson; 2006. [Google Scholar]
- Hebb DO. The organization of behavior: A neuropsychological theory. Psychology Press; 2002. [Google Scholar]
- Hermans D, Bongaerts T, De Bot K, Schreuder R. Producing words in a foreign language: Can speakers prevent interference from their first language? Bilingualism: Language and Cognition. 1998;1(03):213–229. [Google Scholar]
- Hochstein S, Ahissar M. View from the top-hierarchies and reverse hierarchies in the visual system. Neuron. 2002;36(5):791–804. doi: 10.1016/s0896-6273(02)01091-7. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four Factor Index of Social Status. New Haven, Conn: Yale University; 1975. [Google Scholar]
- Hornickel J, Kraus N. Unstable Representation of Sound: A Biological Marker of Dyslexia. The Journal of Neuroscience. 2013;33(8):3500–3504. doi: 10.1523/JNEUROSCI.4205-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J, Zecker SG, Bradlow AR, Kraus N. Assistive listening devices drive neuroplasticity in children with dyslexia. Proceedings of the National Academy of Sciences. 2012;109(41):16731–16736. doi: 10.1073/pnas.1206628109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman RF, Henson O. The descending auditory pathway and acousticomotor systems: connections with the inferior colliculus. Brain Research Reviews. 1990;15(3):295–323. doi: 10.1016/0165-0173(90)90005-9. [DOI] [PubMed] [Google Scholar]
- Klatt D. Software for cascade/parallel formant synthesizer. Journal of the Acoustical Society of America. 1980;67:971–975. [Google Scholar]
- Kraus N, Chandrasekaran B. Music training for the development of auditory skills. Nature Reviews Neuroscience. 2010;11(8):599–605. doi: 10.1038/nrn2882. [DOI] [PubMed] [Google Scholar]
- Krizman J, Marian V, Shook A, Skoe E, Kraus N. Subcortical encoding of sound is enhanced in bilinguals and relates to executive function advantages. Proceedings of the National Academy of Sciences. 2012;109(20):7877–7881. doi: 10.1073/pnas.1201575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizman J, Skoe E, Kraus N. Sex differences in auditory subcortical function. Clinical Neurophysiology. 2012;123(3):590–597. doi: 10.1016/j.clinph.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll JF, Bobb SC, Misra M, Guo T. Language selection in bilingual speech: evidence for inhibitory processes. Acta Psychologica. 2008;128:416–430. doi: 10.1016/j.actpsy.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers JR, Thierry G. Event-related brain potentials reveal the time-course of language change detection in early bilinguals. NeuroImage. 2010;50(4):1633–1638. doi: 10.1016/j.neuroimage.2010.01.076. [DOI] [PubMed] [Google Scholar]
- Liu LF, Palmer AR, Wallace MN. Phase-Locked Responses to Pure Tones in the Inferior Colliculus. Journal of Neurophysiology. 2006;95:1926–1935. doi: 10.1152/jn.00497.2005. [DOI] [PubMed] [Google Scholar]
- Luo F, Wang Q, Kashani A, Yan J. Corticofugal modulation of initial sound processing in the brain. The Journal of Neuroscience. 2008;28(45):11615–11621. doi: 10.1523/JNEUROSCI.3972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in cognitive sciences. 2008;12(4):163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Ryugo DK. The auditory cortex. Springer; 2011. Descending connections of auditory cortex to the midbrain and brain stem; pp. 189–208. [Google Scholar]
- Marian V, Blumenfeld HK, Kaushanskaya M. The language experience and proficiency questionnaire (LEAP-Q): Assessing language profiles in bilinguals and multilinguals. Journal of Speech, Language, and Hearing Research. 2007;50(4):940. doi: 10.1044/1092-4388(2007/067). [DOI] [PubMed] [Google Scholar]
- Marian V, Kaushanskaya M. The bilingual advantage in novel word learning. Psychonomic Review Bulletin. 2009;16(4):705–710. doi: 10.3758/PBR.16.4.705. [DOI] [PubMed] [Google Scholar]
- Marian V, Spivey M. Bilingual and monolingual processing of competing lexical items. Applied Psycholinguistics. 2003;24(02):173–193. [Google Scholar]
- McLachlan N, Wilson S. The central role of recognition in auditory perception: A neurobiological model. Psychological Review. 2010;117(1):175. doi: 10.1037/a0018063. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Neuroscience. 2001;24(1):167. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behavioral and Brain Sciences. 1990;13(2):201–233. [Google Scholar]
- Parbery-Clark A, Anderson S, Hittner E, Kraus N. Musical experience offsets age-related delays in neural timing. Neurobiology of Aging. 2012;33(7):1483, e1481. doi: 10.1016/j.neurobiolaging.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Raizada RD, Poldrack RA. Challenge-driven attention: interacting frontal and brainstem systems. Frontiers in human neuroscience. 2007:1. doi: 10.3389/neuro.09.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne T, Balk MH, Koistinen S, Autti T, Alho K, Sams M. Auditory selective attention modulates activation of human inferior colliculus. Journal of Neurophysiology. 2008;100(6):3323–3327. doi: 10.1152/jn.90607.2008. [DOI] [PubMed] [Google Scholar]
- Ruggles D, Bharadwaj H, Shinn-Cunningham BG. Normal hearing is not enough to guarantee robust encoding of suprathreshold features important in everyday communication. Proceedings of the National Academy of Sciences. 2011;108(37):15516–15521. doi: 10.1073/pnas.1108912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The Variable Discharge of Cortical Neurons: Implications for Connectivity, Computation, and Information Coding. The Journal of Neuroscience. 1998;18(10):3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoe E, Kraus N. Auditory brain stem response to complex sounds: A tutorial. Ear and Hearing. 2010;31(3):302–324. doi: 10.1097/AUD.0b013e3181cdb272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoe E, Kraus N. Musical training heightens auditory brainstem function during sensitive periods in development. Frontiers in Psychology. 2013;4:622. doi: 10.3389/fpsyg.2013.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Soveri A, Laine M, Hamalainen H, Hugdahl K. Bilingual advantage in attentional control: Evidence from the forced-attention dichotic listening paradigm. Bilingualism: Language and Cognition. 2011;14:371–378. [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for postadolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–860. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear LP. Neurobehavioral Changes in Adolescence. Current Directions in Psychological Science. 2000;9(4):111–114. [Google Scholar]
- Spivey M, Marian V. Cross talk between native and second languages: Partial activation of an irrelevant lexicon. Psychological Science. 1999;10(3):281–284. [Google Scholar]
- Treccani B, Argyri E, Sorace A, Della Sala S. Spatial negative priming in bilingualism. Psychonomic Bulletin & Review. 2009;16(2):320–327. doi: 10.3758/PBR.16.2.320. [DOI] [PubMed] [Google Scholar]
- Van Heuven WJ, Schriefers H, Dijkstra T, Hagoort P. Language conflict in the bilingual brain. Cerebral Cortex. 2008;18(11):2706–2716. doi: 10.1093/cercor/bhn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Winer JA. Decoding the auditory corticofugal systems. Hearing Research. 2006;212(1):1–8. doi: 10.1016/j.heares.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Gallen CC, Hampson SA, Hillyard SA, Pantev C, Sobel D, et al. Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proceedings of the National Academy of Sciences. 1993;90(18):8722–8726. doi: 10.1073/pnas.90.18.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Weissman D, Roberts K, Woldorff M. The neural circuitry underlying the executive control of auditory spatial attention. Brain Research. 2007;1134(1):187. doi: 10.1016/j.brainres.2006.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.