Abstract
The structural gene for plasmid-mediated ampicillin resistance resides upon a 3.2 X 10(6) dalton transposable sequence (TnA) flanked by short inverted repeated sequences that accompany its insertion. TnA was transposed to pMB8, a 1.8 X 10(6) dalton derivative of the colicingenic plasmid ColE1. Random deletions were introduced in the resultant 5 X 10(6) dalton recombinant plasmid by a combination of nuclease treatments in vitro. From this set of deletions a subset was isolated that contained deletions affecting the transposition of TnA. The deletions were mapped by digestion with restriction nucleases and electron microscopic analysis of DNA hetero-duplexes and were found to include one of the inverted repeated sequences or lie in the central portion of TnA. Complementation experiments were attempted between these plasmids and another compatible plasmid carrying a deletion in TnA that abolished its ampicillin resistance. The results of the deletion data indicate that approximately 2 X 10(6) daltons of TnA is required for transposition; the complementation experiments suggest that the terminal inverted repetition and the central region of TnA play different essential roles in TnA transposition.
Full text
PDF

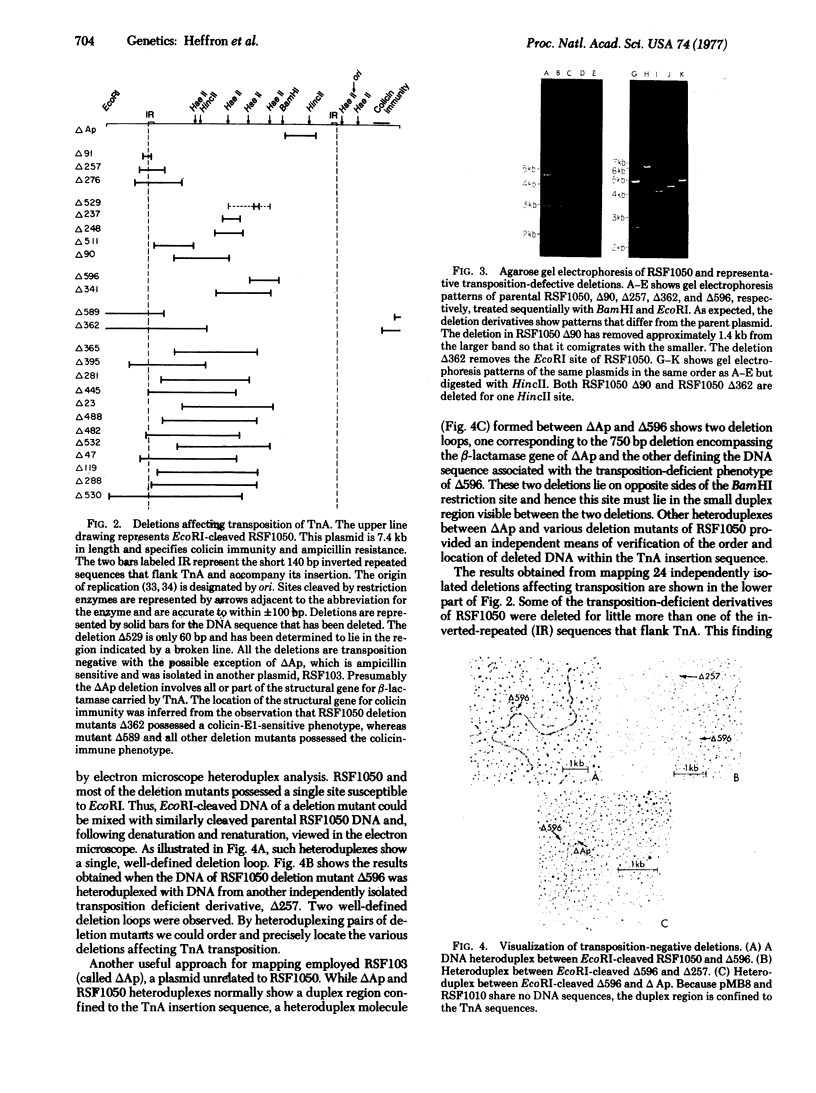


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth P. T., Datta N., Hedges R. W., Grinter N. J. Transposition of a deoxyribonucleic acid sequence encoding trimethoprim and streptomycin resistances from R483 to other replicons. J Bacteriol. 1976 Mar;125(3):800–810. doi: 10.1128/jb.125.3.800-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. M., Richmond M. H. Translocation of a discrete piece of deoxyribonucleic acid carrying an amp gene between replicons in Eschericha coli. J Bacteriol. 1976 Apr;126(1):1–6. doi: 10.1128/jb.126.1.1-6.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon J., Shenk T. E., Berg P. Biochemical procedure for production of small deletions in simian virus 40 DNA. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1392–1396. doi: 10.1073/pnas.72.4.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey C., Richardson D., Carbon J. A method for the deletion of restriction sites in bacterial plasmid deoxyribonucleic acid. Mol Gen Genet. 1976 May 7;145(2):155–158. doi: 10.1007/BF00269587. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Rosner J. L. Acquisition of a determinant for chloramphenicol resistance by coliphage lambda. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5041–5045. doi: 10.1073/pnas.72.12.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J. Replication of colicin E1 plasmid DNA in minicells from a unique replication initiation site. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2256–2259. doi: 10.1073/pnas.71.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan E., Saedler H., Starlinger P. O0 and strong-polar mutations in the gal operon are insertions. Mol Gen Genet. 1968;102(4):353–363. doi: 10.1007/BF00433726. [DOI] [PubMed] [Google Scholar]
- Jordan E., Saedler H., Starlinger P. Strong-polar mutations in the transferase gene of the galactose operon in E.coli. Mol Gen Genet. 1967;100(3):296–306. doi: 10.1007/BF00381825. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor E1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1538–1544. doi: 10.1016/0006-291x(70)90562-0. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- Kopecko D. J., Cohen S. N. Site specific recA--independent recombination between bacterial plasmids: involvement of palindromes at the recombinational loci. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1373–1377. doi: 10.1073/pnas.72.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Deletion mutants of simian virus 40 generated by enzymatic excision of DNA segments from the viral genome. J Mol Biol. 1974 Oct 15;89(1):179–193. doi: 10.1016/0022-2836(74)90169-7. [DOI] [PubMed] [Google Scholar]
- Landy A., Ruedisueli E., Robinson L., Foeller C., Ross W. Digestion of deoxyribonucleic acids from bacteriophage T7, lambda, and phi 80h with site-specific nucleases from Hemophilus influenzae strain Rc and strain Rd. Biochemistry. 1974 May 7;13(10):2134–2142. doi: 10.1021/bi00707a022. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Katz L., Helinski D. R. Unidirectional replication of plasmid ColE1 DNA. Nature. 1974 Sep 27;251(5473):337–340. doi: 10.1038/251337a0. [DOI] [PubMed] [Google Scholar]
- Malamy M. H. Frameshift mutations in the lactose operon of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:189–201. doi: 10.1101/sqb.1966.031.01.027. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Ptashne K., Cohen S. N. Occurrence of insertion sequence (IS) regions on plasmid deoxyribonucleic acid as direct and inverted nucleotide sequence duplications. J Bacteriol. 1975 May;122(2):776–781. doi: 10.1128/jb.122.2.776-781.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedler H., Reif H. J., Hu S., Davidson N. IS2, a genetic element for turn-off and turn-on of gene activity in E. coli. Mol Gen Genet. 1974;132(4):265–289. doi: 10.1007/BF00268569. [DOI] [PubMed] [Google Scholar]
- Saedler H., Starlinger P. 0 degree mutations in the galactose operon in E. coli. I. Genetic characterization. Mol Gen Genet. 1967;100(2):178–189. doi: 10.1007/BF00333604. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Mutations caused by the insertion of genetic material into the galactose operon of Escherichia coli. J Mol Biol. 1969 Feb 28;40(1):93–105. doi: 10.1016/0022-2836(69)90298-8. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Isolation of a sequence-specific endonuclease (BamI) from Bacillus amyloliquefaciens H. J Mol Biol. 1975 Sep 5;97(1):123–125. doi: 10.1016/s0022-2836(75)80028-3. [DOI] [PubMed] [Google Scholar]