Abstract
Purpose
Although 3DCRT is the worldwide standard for the treatment of esophageal cancers, IMRT improves dose conformality and reduces radiation exposure to normal tissues. We hypothesized that the dosimetric advantages of IMRT should translate to substantive benefits in clinical outcomes compared to 3DCRT.
Methods and Materials
Analysis was performed on 676 nonrandomized patients (3DCRT=413, IMRT=263) with stage Ib-IVa (AJCC 2002) esophageal cancers treated with chemoradiation at a single institution from 1998–2008. An inverse probability of treatment weighting (IPW) and inclusion of propensity score (treatment probability) as a covariate were used to compare overall survival (OS) time, time to local failure, and time to distant metastasis, while accounting for effects of other clinically relevant covariates. Propensity scores were estimated using logistic regression.
Results
A fitted multivariate inverse probability weighted (IPW)-adjusted Cox model showed that OS time was significantly associated with several well-known prognostic factors, along with radiation modality (IMRT vs 3DCRT, HR=0.72, p<0.001). Compared to IMRT, 3DCRT patients had a significantly greater risk of dying (72.6% vs 52.9%, IPW log rank test: p<0.0001) and for local-regional recurrence (LRR) (p=0.0038). There was no difference in cancer-specific mortality (Gray’s test, p=0.86), or distant metastasis (p=0.99) between the two groups. An increased cumulative incidence of cardiac deaths was seen in the 3DCRT group (p=0.049), but most deaths were undocumented (5 year estimate: 11.7% in 3DCRT vs 5.4% in IMRT, Gray’s test, p=0.0029).
Conclusions
Overall survival, locoregional control, and non-cancer related deaths were significantly better for IMRT compared to 3DCRT. Although these results need confirmation, IMRT should be considered for the treatment of esophageal cancer.
Keywords: IMRT, 3D-conformal radiation therapy, chemoradiation, esophageal cancer, propensity score
Introduction
Esophageal cancer affects over 16,000 people per year in the United States(1). Radiation with concurrent chemotherapy is a standard of care in the United States for stage II-III cancers, both preoperatively and in patients not eligible for surgery. This treatment can cause significant toxicities that are largely determined by the quality and techniques of radiation used.
While two-dimensional (2D) treatment planning was once an accepted standard of care, the advent of CT-based 3-dimensional (3D) treatment planning have allowed better anatomic visualization and improved target delineation for the dose avoidance of normal structures. However, substantial doses still were being delivered to normal tissues due to lack of dose modulation for each of the 3–4 beams used for treatment. Intensity Modulated Radiation Therapy (IMRT) utilizes multiple beams (typically 5–7), with each beam modulated further using computer-controlled multi-leaf collimation to dynamically block the path of the radiation when the beam is on. This effectively allows the dose to be “painted” with various intensities, thus producing the greatest treatment conformality to the tumor and dose avoidance to the normal structures. Despite the distinct dosimetric advantages of IMRT as demonstrated by numerous planning studies(2–6), IMRT is more costly to implement, and is logistically more demanding, from treatment planning to the physics QA process. Consequently, given the paucity of clinical data to support the superiority of IMRT, 3D-conformal radiotherapy (3DCRT) technique is widely viewed as the current standard of care.
At our institution, we have adopted IMRT for use in esophageal cancer treatment since 2002. Up to 2010, over 350 patients have been treated with IMRT and concurrent chemotherapy with or without surgery. In this report, we compared the clinical outcomes of IMRT to 3DCRT in patients treated from 1998 to 2008. To carry out a comparative analysis of this non-randomized patient cohort, we utilized two statistical methods: an inverse probability of treatment weighting (IPW) method and a propensity score based analysis. These methods correct for potential selection bias and covariate imbalances between groups inherent in observational studies, and thus allow meaningful comparisons in the outcomes of patients treated with these two radiation techniques.
Methods and Materials
Study Cohort
We identified 852 consecutive patients from 1998 to 2008 with biopsy confirmed esophageal carcinoma who received radiation therapy. Patients were excluded if they initially presented with metastatic disease (N=101), had post-operative chemoradiation therapy (N=29), received prior palliative radiation (N=17) or proton therapy (N=18), or had radiation alone without chemotherapy (N=11). After exclusions, 676 patients were used as the final analysis cohort (3DCRT=413, IMRT=263). Staging was determined based on the 6th edition (2002) of the American Joint Committee on Cancer TNM staging.
Treatment
Patients with stage II-IVA (and subsets of stage Ib patients) typically were treated with neoadjuvant chemoradiation to 50.4 Gy at 1.8 Gy per fraction. Prior to 2004, most patients were treated using 3DCRT and simulated without 4D CT imaging. Chemotherapy often is administered in combinations of 5-flurouracil and taxane, or with platinum-based compounds. Five to 6 weeks following the completion of neoadjuvant therapy, most patients were restaged using CT, PET/CT, and/or EGD with biopsy of the primary disease site, and evaluated for surgical management. The most common esophagectomy procedure was Ivor Lewis. A minority of patients also received transhiatal, left thoracotomy, radical (en block) resection, or minimally invasive esophagectomy.
Outcome Measures
Typical follow up schedules after completion of all therapies were every 3–4 months in the first two years, followed by every 4–6 months from years 3 to 5. Recurrence of any type (LRR, distant) were documented pathologically (by EGD or CT guided biopsy) or clinically (CT or PET/CT), depending on the decision of the individual physician or consensus of multidisciplinary group. Dates and causes of death were determined by review of clinical follow-up information in medical records and the Social Security Death Index (SSDI). OS was calculated from the date of diagnosis to the date of death or censoring at last look in the SSDI on 5/01/2011. Local-regional recurrence (LRR) is defined as any recurrence at the initial primary site of disease or in regional lymph nodes. Nodal regional recurrence could either be in-field or out-field, but recurrences outside potential areas of coverage by radiation therapy (celiac nodes for proximal disease or supraclavicular nodes for distal disease) were scored as distant metastases. Cancer-specific survival (CSS) was defined by deaths that followed a recurrence of any type, unless there is clear documentation that the death is not due to cancer. Patients who had not experienced progression or death by the last follow-up were administratively censored. Time to recurrence of any type was calculated from the completion of radiotherapy.
Statistical methods
Because patients were not randomized between IMRT and 3DCRT, covariate-adjusted comparisons of outcomes with these two modalities were carried out using two alternative statistical methods to correct for potential bias. The first method is an Inverse Probability Weighted (IPW) Cox model analysis(7, 8), in which each patient is reweighted to create a pseudo population that mimics what would have been obtained by a randomized trial. Goodness-of-fit was assessed using martingale residual plots(9) and the Grambsch-Therneau test(10). In the second method, a Cox model is fit including the propensity score (estimated probability of receiving IMRT) as a covariate(11). Propensity scores were estimated using logistic regression. Descriptive statistics, including frequencies and percentages for categorical variables, and mean and standard deviation for quantitative variables, were computed to summarize patient characteristics for the overall patient cohort and for each treatment group. Between-group comparisons to evaluate imbalances in covariates were conducted using two sample t-tests, Wilcoxon tests, or Fisher’s exact test. To avoid colinearity in the regression models, associations between covariates were assessed using the Wilcoxon rank sum test. Unadjusted OS time, LRR-free survival time, and distant metastatic free survival time were estimated for each treatment group using IPW Kaplan-Meier (KM) plots to adjust for covariate imbalances. Both a conventional log rank test and an IPW adjusted log rank test were carried out to compare event time distributions. A competing risks analysis was carried out to compare different types of deaths between IMRT and 3DCRT. All computations were conducted in R 2.13.0.
Results
Patient and treatment characteristics
The median follow up time for living patients was 82.4 months (range 4.6 to 150.6) for 3DCRT, and 40.3 months (range 3.1 to 77.0) for IMRT. Vital statuses could not be determined for 5 patients. Patient, tumor, and treatment characteristics are summarized in Table 1. IMRT patients were less likely to receive induction chemotherapy (35.7% for IMRT vs. 46.7% for 3DCRT, p<0.01), had worse performance status (PS≤80: 66.5% for IMRT vs. 50.0% for 3DCRT, p<0.01), were less likely to die (52.9% for IMRT vs. 72.6% for 3DCRT, p<0.01), but were more likely to have the site of first recurrence to be distant (46.4% for IMRT vs. 39.7% for 3DCRT, p=0.08). Statistically significant differences in the distribution of degree of tumor differentiation and distribution of FEV1 percentage of predicted levels also were observed (p<0.01).
Table 1.
Patient, Tumor and Treatment Characteristics, Overall and by Radiation Modality
| Factor | Overall (n=676) |
3DCRT group (n=413, 61%) |
IMRT group (n=263, 39%) |
P-Value |
|---|---|---|---|---|
| Age at diagnosis | 63.48 (10.67) | 63.50 (10.33) | 63.45 (11.20) | P=0.95 |
| Gender | ||||
| female | 97 (14.3%) | 56 (13.6%) | 41 (15.6%) | P=0.47 |
| male | 579 (85.7%) | 357 (86.4%) | 222 (84.4%) | |
| Overall Stage | ||||
| 1 | 11 (1.6%) | 5 (1.2%) | 6 (2.3%) | P=0.26 |
| 2 | 234 (34.6%) | 149 (36.1%) | 85 (32.3%) | |
| 3 | 379 (56.1%) | 223 (54.0%) | 156 (59.3%) | |
| 4a | 52 (7.7%) | 36 (8.7%) | 16 (6.1%) | |
| Lesion Location | ||||
| Upper | 56 (8.3%) | 29 (7.0%) | 27 (10.2%) | P=0.15 |
| Middle | 39 (5.8%) | 23 (5.6%) | 16 (6.1%) | |
| Low | 578 (85.5%) | 359 (86.9%) | 219 (83.3%) | |
| Degree of tumor | ||||
| Differentiation | ||||
| Well differentiated | 8 (1.2%) | 5 (1.2%) | 3 (1.1%) | P<0.01 |
| Moderately | 238 (35.2%) | 129 (31.2%) | 109 (41.5%) | |
| Poorly | 383 (56.7%) | 233 (56.4%) | 150 (57.0%) | |
| Unknown | 47 (6.9%) | 46 (11.1%) | 1 (0.4%) | |
| Surgery | ||||
| Yes | 312 (46.2%) | 197 (47.7%) | 115 (43.7%) | P=0.31 |
| No | 364 (53.8%) | 216 (52.3%) | 148 (56.3%) | |
| Induction Chemo | ||||
| Yes | 287 (42.5%) | 193 (46.7%) | 94 (35.7%) | P<0.01 |
| No | 389 (57.5%) | 220 (53.3%) | 169 (64.3%) | |
| Performance Status | ||||
| 90–100 | 293 (43.3%) | 205 (49.6%) | 88 (33.5%) | P<0.01 |
| 80 | 298 (44.1%) | 162 (39.2%) | 136 (51.7%) | |
| ≤70 | 83 (12.3%) | 44 (10.7%) | 39 (14.8%) | |
| Radiation Dose (median, range) | 50.4 Gy (6.6–66) | 50.4 Gy (6.6–66) | 50.4 Gy (16.2–66) | P=0.84 |
| Baseline PET (N, (%)) | 478 (70.7%) | 226 (54.7%) | 252 (95.8%) | P<0.01 |
| FEV1 (L) (mean, (95%CI))* | 3.12 (3.03, 3.21) | 3.22 (3.09, 3.34) | 3.04 (2.92, 3.17) | P=0.09 |
| FEV1 (% pred) (mean, (95% CI)) * | 93.1 (90.9, 95.3) | 89.6 (86.4, 92.7) | 95.9 (92.9, 98.9) | P<0.01 |
| CHF (N, %) | 13 (1.92) | 9 (2.18) | 4 (1.52) | P=0.53 |
Only 161 3DCRT and 204 IMRT pts had PFT values; CHF=Congestive Heart Failure
Overall and Cancer-Specific Survival comparison between groups
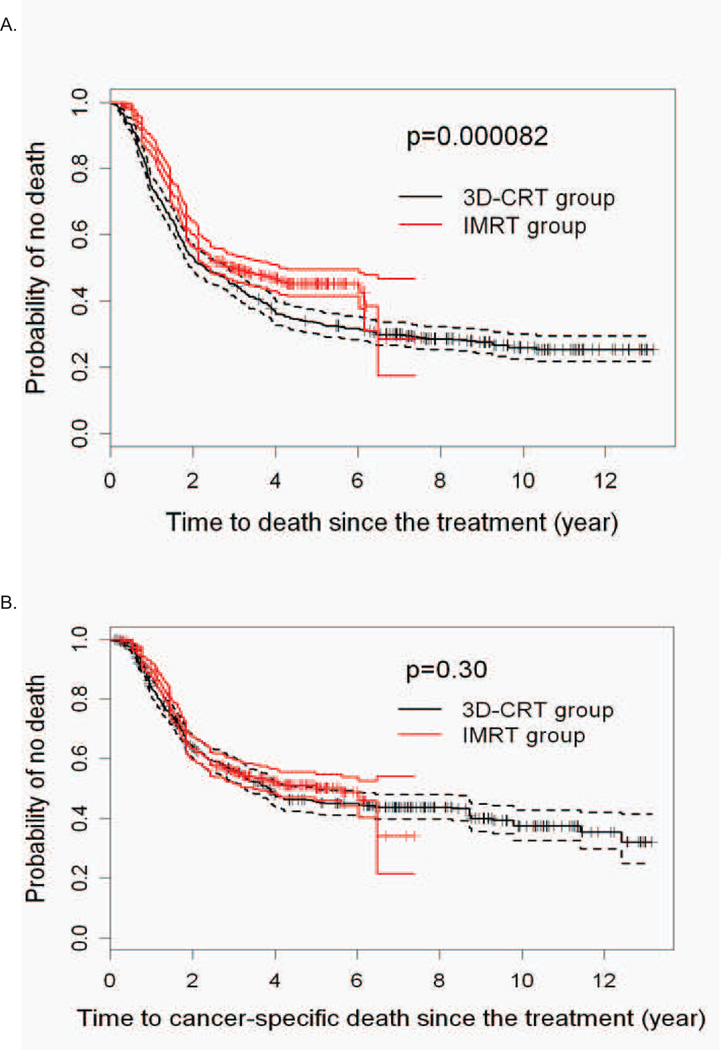
Logistic regression was used to estimate the probability of receiving IMRT (propensity score). We evaluated the association with treatment assignment for each variable listed in Table 1. The only significant predictors of receiving IMRT were baseline PET for staging, performance status, and lesion location (Supplementary Table 1). Receiving IMRT was positively associated with getting PET for staging (OR 22.37, p<0.0001), having worse performance status (KPS 80: OR 1.75, p=0.0036; KPS<=70: OR 1.81, p=0.042), and negatively correlated with a lower esophageal tumor location (OR 0.35, p=0.0042). Conventional KM estimates and IPW adjusted KM estimates of OS time probabilities were computed for each treatment group (Supplementary Figure 1). In the 3DCRT group, the IPW weighted survival curve is very similar to that of the conventional KM curve, while in the IMRT group, the IPW weighted curve is slightly lower than the conventional curve. With either approach, the IMRT group had a significantly better survival time than the 3DCRT group (Figure 1A). The IPW method showed a larger estimated difference in OS time between the two treatment groups, with the IMRT advantage larger than what conventional analyses would indicate (Supplementary Figure 1). The median survival time was 25.2 months for 3DCRT versus 43.2 months for IMRT, and the 3- and 5-year OS were 43% (95%CI, 39% –46%) and 34% (95%CI, 30% –37%) for 3DCRT, and 53% (95%CI, 49% –57%) and 44% (95%CI, 40% –48%) for IMRT, respectively. However, when we analyzed for the IPW-adjusted CSS, we found no statistically significant differences between the two groups (Figure 1B).
Figure 1. Overall and disease-specific survival of 3DCRT and IMRT treated patients.
A. IPW adjusted Kaplan-Meier estimates of the overall survival time curves, with 95% confidence intervals. B. IPW adjusted Kaplan-Meier estimates of cancer-specific survival, with 95% confidence intervals. C. IPW adjusted Kaplan-Meier estimates of the LRR free survival curves, with 95% confidence intervals. D. IPW adjusted Kaplan-Meier estimates of the distant metastatic free survival curves, with 95% confidence intervals.
Table 2 summarizes the fitted multivariate IPW-adjusted Cox model for OS. Besides having 3DCRT as an independent risk factor for lower survival, worse survival also was associated with stage 3 or 4a disease, tumors in the mid- and lower esophagus, not having surgery or induction chemo, older age, poorer performance status, and not having PET. An alternative method in which a Cox model is fit using the propensity score as a covariate gave substantively similar results as the IPW method (not shown).
Table 2.
Fitted Cox model using inverse probability of treatment weights (IPWs)
| Risk Factor | Coefficient Estimate |
Hazard Risk Ratio |
S.E | p-Value |
|---|---|---|---|---|
| Treatment | ||||
| IMRT vs. 3DCRT | −0.33 | 0.72 | 0.07 | 1.1e–05 |
| Overall Stage | ||||
| 2 vs. 1 | 0.54 | 1.72 | 0.34 | 0.106 |
| 3 vs. 1 | 0.93 | 2.54 | 0.33 | 0.005 |
| 4a vs. 1 | 1.10 | 2.99 | 0.36 | 0.002 |
| Lesion Location | ||||
| Middle vs. Upper | 0.54 | 1.72 | 0.20 | 0.006 |
| Low vs. Upper | 0.51 | 1.67 | 0.13 | 7.6e–5 |
| Surgery (Yes vs. No) | −0.65 | 0.52 | 0.09 | 1.9e–14 |
| Induction Chemo (Yes vs. No) | −0.27 | 0.76 | 0.08 | 3.9e–4 |
| Age at Diagnosis | 0.007 | 1.01 | 0.004 | 0.051 |
| Performance Status | ||||
| 80 vs. 90–100 | 0.12 | 1.12 | 0.08 | 0.15 |
| <=70 vs. 90–100 | 0.44 | 1.55 | 0.11 | 4.5e–5 |
| PET (Yes vs. No) | −0.25 | 0.78 | 0.08 | 0.002 |
Disease recurrence pattern comparison
To determine if the differences in overall survival between the two groups could be accounted for by differences in disease recurrence, we evaluated IPW-adjusted LRR and distant metastatic recurrence for the two groups. Figure 1C provides the IPW weighted KM estimates of the LRR free probabilities for each treatment group. Compared to the IMRT group, LRR is significantly worse for the 3DCRT group (IPW log-rank p=0.0038). The fitted IPW adjusted Cox regression model for LRR showed that older age, higher stage, lower lesion location, and not having PET all were associated with increased hazard of LRR. For the IPW weighted KM estimates of distant metastatic free probabilities, there were no differences between the two groups (Figure 1D). A fitted IPW-adjusted Cox model for distant metastatic free survival only showed age at diagnosis and having induction chemotherapy to be significant (HR=0.99, p=0.037 and HR=0.76, p=0.003, respectively). Similar findings were seen using the alternative method of including propensity score as a covariate (not shown).
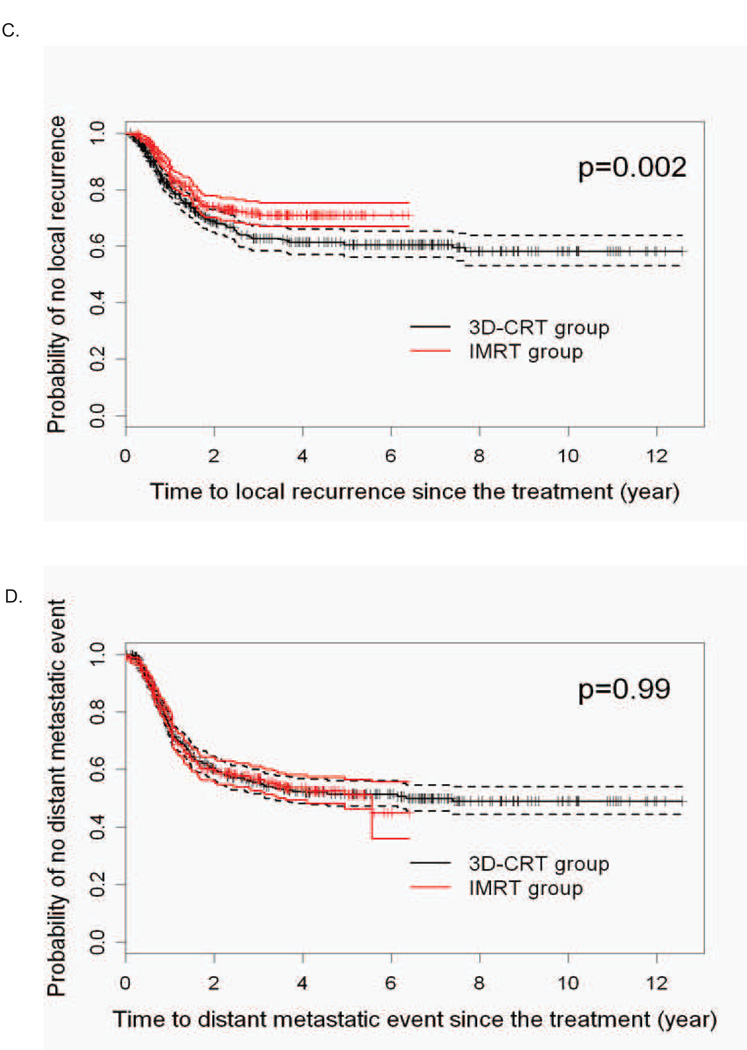
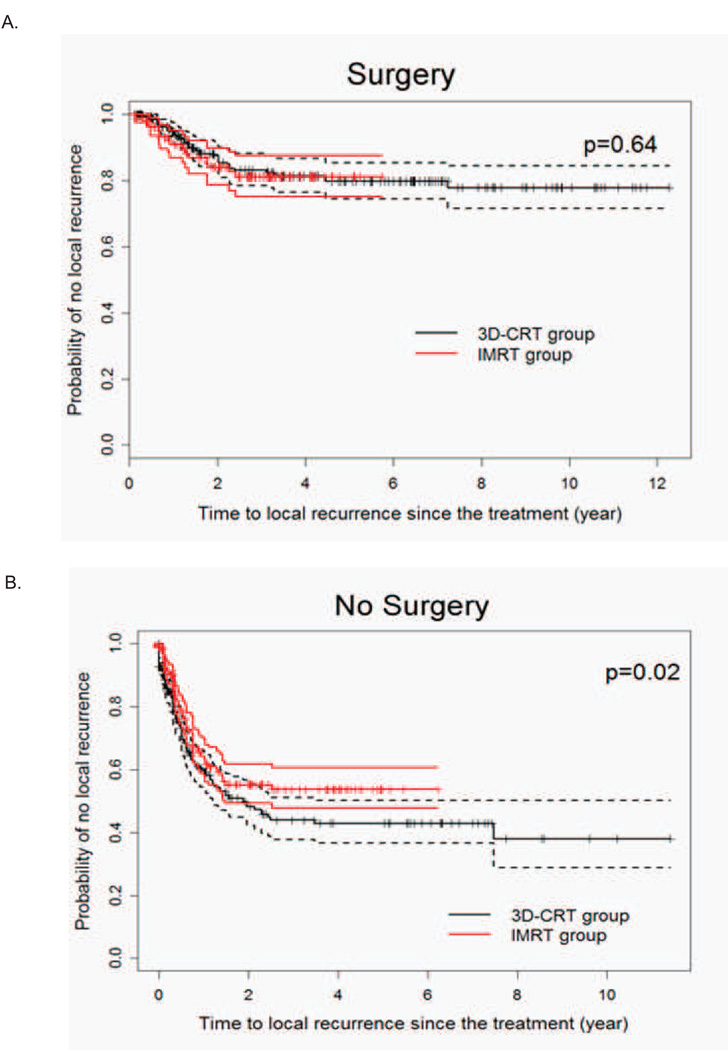
We next evaluated the IPW-adjusted LRR in the two radiation groups based on whether patients had surgery after chemoradiation. We found that the LRR advantage of IMRT was restricted to the non-surgical patients (Figure 2). We next determined specifically where the increased failures were seen in the 3DCRT group. While there were no statistical differences in terms of time to in-field or time to out-field regional nodal recurrences, the probability of recurrence in the primary site being the first site of local failure over time was statistically significantly higher for 3DCRT patients (Figure 3).
Figure 2. Local-regional recurrence in 3DCRT and IMRT treated patients stratified according to surgery.
A. IPW adjusted Kaplan-Meier estimates of the LRR time in the patients who had surgery after chemoradiotherapy. B. IPW adjusted Kaplan-Meier estimates of the LRR time in definitively treated patients with chemoradiotherapy.
Figure 3. Comparison of radiation technique in the patterns of local-regional failure in the definitively treated esophageal cancer patients.
IPW adjusted Kaplan-Meier estimates of the time-to-recurrence in the A) primary, B) in-field nodal, or C) outfield nodal disease sites.
Competing risks analysis for cause-specific mortality
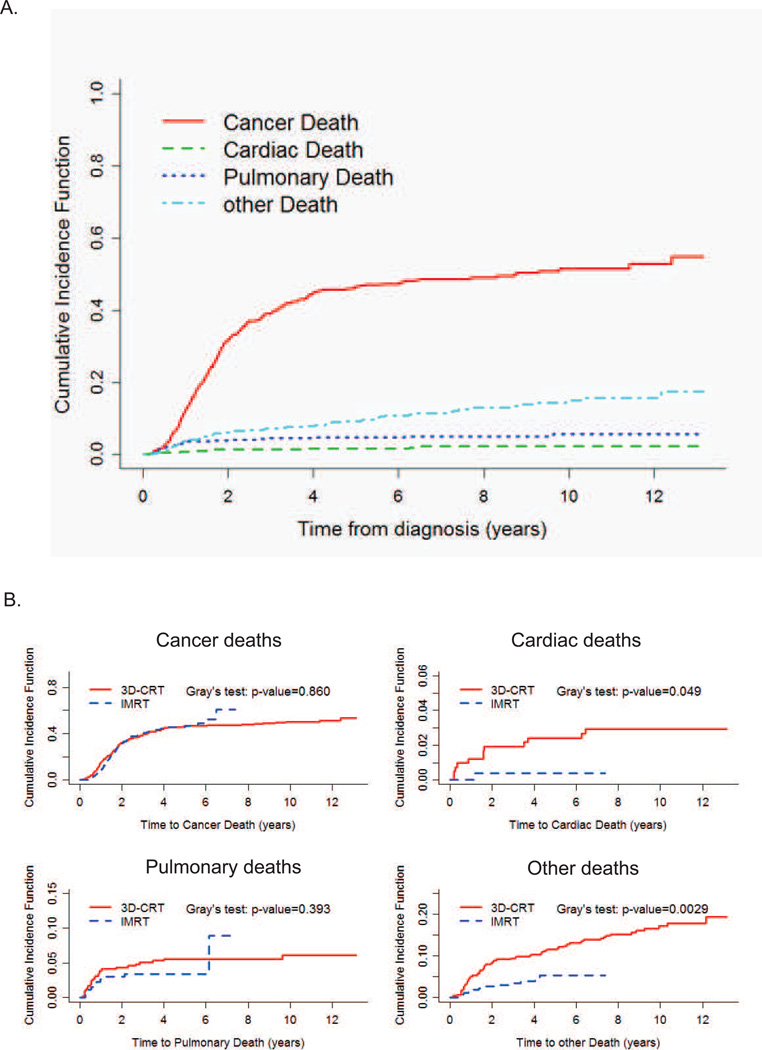
We analyzed the cumulative risk of cause-specific mortality. Cancer-related deaths were responsible for the great majority of documented deaths for this group of patients, accounting for nearly 50% of all deaths within 5 years (Figure 4A). While there were no differences in cumulative incidence of cancer specific deaths (p=0.86) or pulmonary deaths (p=0.39), there was a significantly higher cumulative incidence in documented cardiac-related deaths in the 3DCRT group (p=0.049) (Figure 4B). Excess deaths due to indeterminate causes were also significantly higher for 3DCRT compared to IMRT (5 year estimate: 11.7% 3DCRT vs. 5.4% IMRT, p=0.0029, Gray’s test).
Figure 4. Cumulative Incidence Function for all cause mortalities of 3DCRT and IMRT treated patients.
A. Overall cumulative incidence function for the various causes of deaths. B. Cumulative incidence function of each death type for the two treatment groups.
We next assessed postoperative deaths (defined as deaths within 30 or 60 days after surgery) in the 312 patients who underwent surgical resection after neoadjuvant chemoradiation. Of the 171 deaths in the surgery group, 9 were postoperative deaths. We saw no differences between the two groups at either postoperative period (30 days: 3 (2.44%) in 3DCRT versus 0 (0%) in IMRT, p=0.56; 60 days: 6 (4.88%) in 3DCRT vs. 3 (6.25%) in IMRT, p=0.71).
Discussion
IMRT improves target conformality and reduces radiation dose to adjacent organs(2–6), but how this translates to clinical benefit for esophageal cancer patients is largely unknown. Using two statistical methods to adjust for potential imbalances inherent in observational studies, we compared survival and disease specific outcomes of patients treated with 3DCRT versus IMRT in a large group of esophageal cancer patients with long term follow up. While we found no differences between 3DCRT and IMRT in the times to distant recurrence, or in cancer specific survival, we found that there was a significant improvement in OS and LRR in the IMRT treated patients compared to 3DCRT. LRR was worse for 3DCRT only in the non-surgical patients, with the primary site of failures being responsible for the difference seen. We also observed a higher incidence of documented cardiac-related deaths with 3DCRT, but more patients were lost to follow up in the 3DCRT patients without clear documentation of the cause of death. While the total number of deaths is expected to be higher in the 3DCRT group because of the longer follow up time, most of the deaths in the 3DCRT group occurred during an earlier time period, generally within the first two years after completion of radiotherapy (figure 4B). The excess deaths seen in the 3DCRT group could be due, in part, to the toxic sequelae of treatment. Patients that die from sudden cardiac arrests from myocardial infarctions (MI), pneumonias, pneumonitis, etc, will go undocumented unless we are notified of their deaths, which is rarely the case. Patients who die as a consequence of their cancer but were lost to follow up could certainly contribute to this group of undocumented deaths.
Thoracic radiation is associated with substantial late, treatment-related cardiopulmonary morbidity and mortality. In patients treated for Hodgkin’s lymphoma, cardiac morbidities (MI, pericarditis) have commonly been described, despite the lower relative radiation doses used for treatment(12–14). A number of studies also have reported complications of radiation treatment for esophageal cancer, many of which used the older radiation technique of 2DCRT. In the Intergroup 0123 dose comparison trial(15), there were 37% (50.4 Gy arm) to 46% (64 Gy arm) grade 3 or greater late toxicities. Interestingly, deaths (n=11) were higher in the 64 Gy arm compared to the standard 50.4 Gy dose group (n=3), but mysteriously 7 of the 11 deaths on the high dose arm occurred before reaching 50.4 Gy. In a series of 139 patients(16), grade 3 or higher cardiopulmonary complications occurred in 26 of 78 patients (33%), with two deaths due to MI. In another study(17), 69 patients treated with definitive chemoradiation had toxicities that appear related to the age of the patient, as the 2-year cumulative incidences of grade 3 or higher cardiopulmonary toxic events were 29% for patients 75 years or greater versus 3% for younger patients (p=0.005).
The existing literature on the use of IMRT for esophageal cancer has been primarily early clinical experiences from small institutional series(18, 19). Recently, Lin et al. from China reported clinical outcomes in 60 patients treated with IMRT or 3DCRT(20). Although the clinical response rate and the 1-, 2-, and 3-year survival rates appear to be numerically higher in the IMRT group, none of these endpoints were significantly better than 3DCRT. Unfortunately, definitive conclusions cannot be drawn from these results given the short follow up and the small patient numbers.
Our study is limited by its retrospective nature and by our inability to determine the specific cause of the deaths in many of the 3DCRT patients due to unexplained loss to follow up. The main strength of our analyses is that we have used well established statistical methods to adjust for the clinical variables with the most likely imbalances that are inherent in observational studies, in order to provide the best comparison possible between 3DCRT and IMRT based on the available data. We also have used as a comparison group the largest cohort of patients treated with IMRT ever reported in the literature. While we recognize that the 3DCRT and IMRT patients were mostly treated in two different time periods, with imbalances that were due to differences in how certain technologies were adopted for use in the clinic at the various times, such as PET scans for staging, treatment approaches and clinical personnel have been similar for the two groups of patients. For this reason, we believe that heterogeneities in the outcomes due to time differences were accounted for by the IPW methodology.
In conclusion, we report significantly improved overall survival and local-regional control in IMRT treated compared to 3DCRT treated esophageal cancer patients. There was an excess of non-cancer related deaths in the 3DCRT group. These results suggest that the dosimetric advantages of IMRT may translate to clinical benefit. Ideally, these results should be confirmed in a large randomized study comparing these two modalities. Before that occurs, we believe our results demonstrate that IMRT has the potential to improve outcomes over traditional approaches, and should be strongly considered for the treatment of esophageal cancers.
Supplementary Material
Acknowledgments
Research Support: Funding was provided in part by The University of Texas MD Anderson Cancer Center and by the National Cancer Institute Cancer Center Support Grant CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: The authors have no conflicts of interest to report.
Meeting Presentation: A portion of this work has been presented in abstract form at the 2012 American Society of Clinical Oncology, Gastrointestinal Cancers Symposium, San Francisco, California, January 19–21, 2012.
References
- 1.ACS. Esophageal Cancer. Cancer Statistics; 2010. www.cancer.org/acs/groups/content/.../esophagealcancerpdf.pdf. [Google Scholar]
- 2.Fenkell L, Kaminsky I, Breen S, et al. Dosimetric comparison of IMRT vs. 3D conformal radiotherapy in the treatment of cancer of the cervical esophagus. Radiotherapy and Oncology. 2008;89:287–291. doi: 10.1016/j.radonc.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Chandra A, Guerrero TM, Liu HH, et al. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiotherapy and Oncology. 2005;77:247–253. doi: 10.1016/j.radonc.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Nutting CM, Bedford JL, Cosgrove VP, et al. A comparison of conformal and intensity-modulated techniques for oesophageal radiotherapy. Radiotherapy and Oncology. 2001;61:157–163. doi: 10.1016/s0167-8140(01)00438-8. [DOI] [PubMed] [Google Scholar]
- 5.Wu VWC, Kwong DLW, Sham JST. Target dose conformity in 3-dimensional conformal radiotherapy and intensity modulated radiotherapy. Radiotherapy and Oncology. 2004;71:201–206. doi: 10.1016/j.radonc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Wu VWC, Sham JST, Kwong DLW. Inverse planning in three-dimensional conformal and intensity-modulated radiotherapy of mid-thoracic oesophageal cancer. British Journal of Radiology. 2004;77:568–572. doi: 10.1259/bjr/19972578. [DOI] [PubMed] [Google Scholar]
- 7.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Sugihara M. Survival analysis using inverse probability of treatment weighted methods based on the generalized propensity score. Pharmaceutical Statistics. 2009;9:21–34. doi: 10.1002/pst.365. [DOI] [PubMed] [Google Scholar]
- 9.Therneau TM, Grambsch PM, Fleming TR. Martingale-based residuals for survival models. Biometrika. 1990;77:147–160. [Google Scholar]
- 10.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 11.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in Medicine. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Boivin JF, Hutchison GB, Lubin JH, et al. Coronary artery disease mortality in patients treated for Hodgkin's disease. Cancer. 1992;69:1241–1247. doi: 10.1002/cncr.2820690528. [DOI] [PubMed] [Google Scholar]
- 13.Cosset JM, Henry-Amar M, Pellae-Cosset B, et al. Pericarditis and myocardial infarctions after Hodgkin's disease therapy. International Journal of Radiation Oncology Biology Physics. 1991;21:447–449. doi: 10.1016/0360-3016(91)90794-5. [DOI] [PubMed] [Google Scholar]
- 14.Joensuu H. Acute myocardial infarction after heart irradiation in young patients with Hodgkin's disease. Chest. 1989;95:388–390. doi: 10.1378/chest.95.2.388. [DOI] [PubMed] [Google Scholar]
- 15.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation therapy oncology group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. Journal of Clinical Oncology. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 16.Ishikura S, Nihei K, Ohtsu A, et al. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. Journal of Clinical Oncology. 2003;21:2697–2702. doi: 10.1200/JCO.2003.03.055. [DOI] [PubMed] [Google Scholar]
- 17.Morota M, Gomi K, Kozuka T, et al. Late Toxicity After Definitive Concurrent Chemoradiotherapy for Thoracic Esophageal Carcinoma. International Journal of Radiation Oncology Biology Physics. 2009;75:122–128. doi: 10.1016/j.ijrobp.2008.10.075. [DOI] [PubMed] [Google Scholar]
- 18.La TH, Minn AY, Su Z, et al. Multimodality treatment with intensity modulated radiation therapy for esophageal cancer. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus /I.S.D.E. 23:300–308. doi: 10.1111/j.1442-2050.2009.01004.x. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Ishikawa H, Ebara T, et al. The role of intensity modulated radiotherapy (IMRT) for locally advanced esophageal cancer. Japanese Journal of Clinical Radiology. 2010;55:1114–1120. [Google Scholar]
- 20.Lin XD, Shi XY, Zhou TC, et al. Intensity-modulated or 3-D conformal radiotherapy combined with chemotherapy with docetaxel and cisplatin for locally advanced esophageal carcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31:1264–1267. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.