Abstract
Background
For breast-conserving surgery (BCS), the method of margin assessment that most frequently achieves negative margins without increasing volume of tissue excised is uncertain. We examined our institutional experience with 3 different margin assessment methods used by 6 experienced breast surgeons.
Methods
Patients undergoing BCS for invasive carcinoma during July-December of a representative year during which each method was performed (Perpendicular, 2003; Tangential, 2004; Cavity-Shave, 2011) were included. Effect of margin method on positive margin rate at first excision, and total volume excised to achieve negative margins, were evaluated by multivariable analysis, by surgeon, and by tumor size and presence of extensive intraductal component (EIC).
Results
555 patients were identified: Perpendicular, 140; Tangential, 124; Cavity-Shave, 291. Tangential method had a higher rate of positive margins at first excision than Perpendicular and Cavity-Shave methods (49%, 15%, 11%, respectively; p<0.0001). Median volumes to achieve negative margins were similar (55ml, Perpendicular; 64ml Tangential; 62ml Cavity-Shave, p=0.24). Four of 6 surgeons had the lowest rate of positive margins with Cavity-Shave method—significant when compared to Tangential (p<0.0001), but not Perpendicular (p=0.37). Comparison of volume excised using the 3 methods was variable by surgeon (p<0.0001). Perpendicular method was optimal for T1 tumors without EIC; Cavity-Shave tended to be superior for T2/3 tumors and/or EIC.
Conclusions
While the Cavity-Shave method may decrease rates of positive margins, its effect on volume is variable among surgeons and may result in an increase in total volume excised for some surgeons, and for small tumors without EIC.
Keywords: Margins, breast conservation, lumpectomy, breast cancer
INTRODUCTION
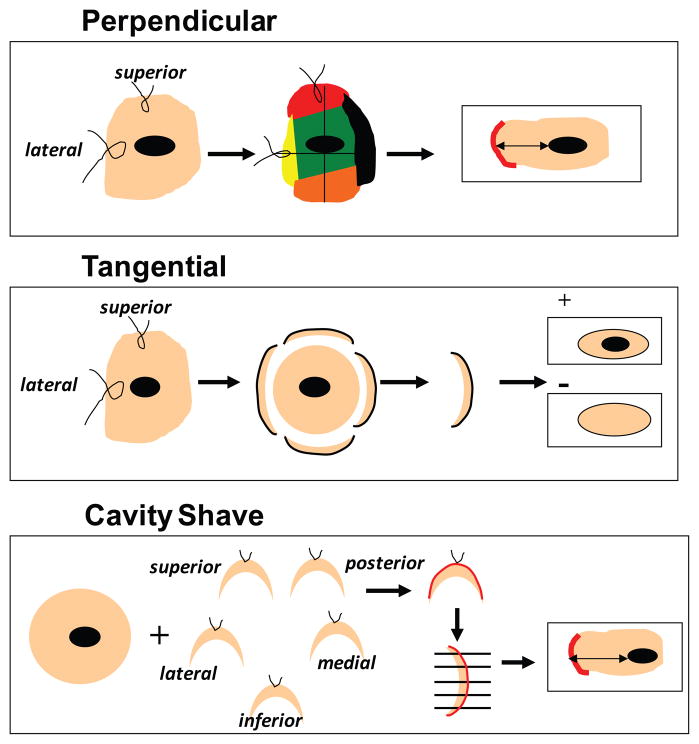
Currently, in the United States, most women treated for early-stage breast cancer will undergo breast-conserving surgery (BCS).1 The goal of BCS is achieving oncologic outcomes equivalent to mastectomy while preserving cosmesis. A critical component of BCS is achieving negative margins, as positive margins have been shown to increase rates of local recurrence.2–6 The determination of negative margins on a lumpectomy specimen depends not only on adequacy of excision, but also on the method of margin assessment used to evaluate the specimen. There exist multiple methods of margin assessment, and there is no consensus as to the ideal method. An ideal margin assessment method would enable the surgeon to excise enough tissue beyond the cancer to achieve negative margins while maintaining low excision volume. Three methods of margin assessment are the Perpendicular, Tangential, and Cavity-Shave methods (FIG 1).
FIG 1.
Techniques of margin assessment. Perpendicular method—specimen is oriented intraoperatively with sutures on two surfaces: short superior and long lateral. The pathologist then inks the specimen with 6 different colors, and it is sectioned perpendicular to the long axis into 2–3 mm slices. The closest distance between tumor and ink is measured microscopically. Tangential method—specimen is oriented with 2 sutures intraoperatively. The entire specimen is inked in a single color, and 2–3 mm margins are tangentially shaved from each side by the pathologist. The shaved margin is examined microscopically; if tumor cells are present, the margin is reported as positive. Cavity-Shave method—primary specimen is removed, then an additional rim of tissue is shaved from the surgical cavity in each direction. Each additional margin is oriented with a suture. The surgeon-designated margin is inked by the pathologist and sectioned perpendicular to the long axis. The closest distance between tumor and ink is measured microscopically.
Another variable that may influence positive margin rate and excision volume is surgical technique. Therefore, it is possible that the effect of margin assessment method on positive margin rate or total volume of excision may vary by surgeon. To our knowledge, this has not been previously studied. We compared 3 different methods of margin assessment, used at different times by the same 6 surgeons at our institution. Lastly, we hypothesized that the effect of margin assessment method on the positive margin rate and volume of excision necessary to achieve negative margins would vary by tumor size and presence of an extensive intraductal component (EIC).
Therefore, the purpose of this analysis was to evaluate the overall and surgeon-specific effect of margin assessment method on positive margin rate and total excision volume, and to examine how the margin method affects these outcomes in women with tumors expected to occupy a small volume of breast tissue (T1 without EIC) or large volume (T2/T3 and/or EIC).
MATERIALS AND METHODS
This retrospective study was approved by the institutional review board at Memorial Sloan-Kettering Cancer Center. A prospectively maintained database was used to identify all patients with invasive breast cancer who underwent BCS and sentinel lymph node biopsy after percutaneous needle biopsy diagnosis of breast cancer from July through December of 2003, 2004, and 2011. Patients were excluded from analysis if they had neoadjuvant therapy.
Six surgeons were practicing during all 3 time periods; only their cases were included. During these time periods, 3 different methods of margin assessment were used: Perpendicular (2003); Tangential (2004); and Cavity-Shave (2011).
In the Perpendicular method, the specimen is oriented by the surgeon in the operating room by placing sutures on 2 surfaces (lateral and superior) of the specimen. The pathologist then processes the specimen to identify each of 4 margins with different colors [(superior (blue), inferior (green), anterior (yellow), posterior (black)] with medial and lateral margins submitted in separate cassettes. The specimen is then sequentially sectioned into 2–3mm slices perpendicular to its longest axis. The closest distance between the tumor and the inked edge of the tissue sections is assessed microscopically and reported. A positive margin is defined as tumor on ink.
In the Tangential method, the specimen is also oriented by the surgeon in the operating room by placing sutures on 2 surfaces (lateral and superior) of the specimen. The pathologist inks the entire outer surface of the specimen with a single color, then tangentially shaves 2–3mm margin samples from each side of the specimen. The tissue shaved off from each margin surface is submitted together, keeping record of the margin designation (anterior, superior, inferior, medial, lateral, and posterior). The shaved tissue is submitted entirely “en face” and evaluated microscopically for the presence of tumor cells. If tumor cells are present in a section, the corresponding margin is reported as “positive.” According to this method, a “positive” margin designates presence of the tumor anywhere between 2–3mm from the margin or at the margin. With this method, “close” and “positive” margins are indistinguishable; no estimate of margin width is reported to the clinician as all cases with tumor within 2–3mm of the margin are reported as “positive.”
The Cavity-Shave method involves the surgeon excising and labeling each wall of the lumpectomy cavity as a separate margin intraoperatively. The surgeon designates with a suture the surface of the specimen representing each “final” margin. The pathologist inks each “final” margin surface, and the margin specimen is sectioned into 2–3mm slices perpendicular to the longest axis of the specimen. All tissue is submitted for microscopic evaluation in up to 6 cassettes, including any gross or palpable abnormality. If the specimen cannot be entirely submitted in 6 cassettes, every other section is submitted up to a maximum of 6 cassettes. Whenever atypia or carcinoma is identified microscopically in the initial 6 sections, any remaining tissue is also entirely submitted for microscopic examination. If invasive carcinoma or DCIS is identified microscopically, its distance from the closest inked tissue edge is reported. A positive margin is defined as tumor on ink.
For the calculation of volume of excision, specimen dimensions were obtained from the pathology reports. Volume of the primary specimen was calculated using the formula for an ellipsoid: 4/3π × ½ length × ½ width × ½ height, and volume of a cavity-shaved or re-excised margin was calculated using the formula for an elliptical cylinder: π × ½ length × ½ width × height. Total volume of excision was calculated by summing the volume of the initial excision, any margins, and any re-excisions necessary to achieve negative margins.
Statistical Analysis
Kruskal-Wallis, chi-squared, and Fisher’s exact tests were used to compare variables across the 3 margin assessment methods. Outcome variables were positive margin rates at first excision and total volume excised (including all re-excision volumes) to achieve a negative margin (defined as tumor not on ink). Other variables examined included tumor size, presence of EIC (defined as >25% DCIS), presence of lymphovascular invasion (LVI), presence of multifocality, and surgeon. To examine predictors of positive margins at first excision, univariable and multivariable logistic regression models were utilized. Because total volume excised to achieve a negative margin was not normally distributed, this outcome was analyzed with univariable and multivariable regression models after natural logarithm transformation. All statistical analyses were done in SAS (Version 9.2, Cary, NC).
RESULTS
Cases
A total of 555 patients met inclusion criteria; 140 with Perpendicular, 124 with Tangential, and 291 with Cavity-Shave margin assessment methods. Clinicopathologic characteristics for the entire study population by margin assessment method are shown in Table 1. Age, tumor size, T stage, and EIC were not significantly different between groups. There were significantly fewer patients with LVI in the Tangential group (9%)(p=0.02), and more patients with multifocal disease in the Perpendicular group (19%)(p=0.007). Of patients who had a re-excision, residual carcinoma was found at the new margin (i.e., positive margin on re-excision) in 6% of those with Perpendicular, 14% of those with Tangential, and 6% of those with Cavity-Shave margin assessment methods.
Table 1.
Comparison of patient and tumor characteristics for 3 margin assessment methods.
| Perpendicular (n=140) | Tangential (n=124) | Cavity-Shave (n=291) | ||
|---|---|---|---|---|
|
| ||||
| Median (range) | Median (range) | Median (range) | p-value | |
| Age, years | 63 (25–84) | 60 (31–86) | 61 (30–92) | 0.16 |
| Tumor size (invasive component), cm | 1.3 (0.1–6.0) | 1.2(0.2–4.2) | 1.2(0.1–5.5) | 0.61 |
|
| ||||
| N (%) | N (%) | N (%) | ||
|
| ||||
| AJCC T stage | 0.55 | |||
| T1 | 113 (81%) | 97 (78%) | 254 (87%) | |
| T2 | 21 (15%) | 20 (16%) | 36 (12%) | |
| T3 | 1 (0.7%) | 0 | 1 (0.3%) | |
| Unknown | 5 (4%) | 7 (6%) | 0 | |
| Extensive intraductal component (> 25% DCIS) | 0.40 | |||
| No | 116 (83%) | 88 (71%) | 256 (88%) | |
| Yes | 21 (15%) | 17 (14%) | 34 (12%) | |
| Unknown | 3 (2%) | 19 (15%) | 1 (0.3%) | |
| Lymphovascular invasion | 0.02 | |||
| No | 112 (80%) | 113 (91%) | 231 (79%) | |
| Yes | 28 (20%) | 11 (9%) | 57 (20%) | |
| Unknown | 0 | 0 | 3 (1%) | |
| Multifocality | 0.007 | |||
| No | 114 (81%) | 111 (90%) | 266 (91%) | |
| Yes | 26 (19%) | 13 (10%) | 24 (8%) | |
| Unknown | 0 | 0 | 1 (0.3%) | |
AJCC, American Joint Committee on Cancer Staging System, 7th edition, 2010; DCIS, ductal carcinoma in situ
Kruskal-Wallis tests used for continuous variable comparisons. Chi-squared and Fisher’s exact tests used for categorical variable comparisons where appropriate. All comparisons exclude unknown values.
Positive Margins at First Excision
Overall, positive margin rates at first excision were higher for Tangential (49%) than for Perpendicular (15%) or Cavity-Shave (11%)(p<0.0001). After controlling for size, EIC, LVI, multifocality, and surgeon, the Tangential method remained associated with positive margins on first excision (when compared to Cavity Shave, odds ratio (OR), 9.0; 95% confidence interval (CI), 5.1–15.8; p<0.0001)(Table 2). On multivariable analysis, surgeon was of borderline significance (p=0.06), and there was a trend toward higher rate of positive margins with larger tumor size (OR, 1.3; 95% CI, 0.9–1.7; p=0.10) and multifocality (OR, 1.7; 95% CI, 0.9–3.5; p=0.11).
Table 2.
Univariable and multivariable logistic regression models for factors associated with positive margins on first excision, and univariable and multivariable regression models for factors associated with total volume excised to achieve negative margins (after natural logarithm transformation)
| Logistic regression models for factors associated with positive margins on first excision | Regression models for factors associated with total volume excised to achieve negative margins | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable
|
Multivariable
|
Univariable
|
Multivariable
|
|||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | Estimate | 95% CI | p-value | Estimate | 95% CI | p-value | |
| Method of margin assessment | < 0.0001 | < 0.0001 | 0.24 | 0.007 | ||||||||
| Perpendicular | 1.3 | 0.7–2.3 | 0.39 | 1.3 | 0.7–2.5 | 0.37 | −0.13 | −0.20, 0.03 | 0.10 | −0.23 | −0.38, −0.09 | 0.002 |
| Tangential | 7.1 | 4.3–11.7 | < 0.0001 | 9.0 | 5.1–15.8 | < 0.0001 | −0.008 | −0.17, 0.16 | 0.92 | −0.11 | −0.27, 0.05 | 0.16 |
| Cavity-Shave | 1.0 | -- | -- | 1.0 | -- | -- | Reference | -- | -- | Reference | -- | -- |
| Histopathologic characteristics | ||||||||||||
| Tumor size (each cm increase) | 1.3 | 1.0–1.7 | 0.03 | 1.3 | 0.9–1.7 | 0.10 | 0.22 | 0.14, 0.31 | < 0.0001 | 0.23 | 0.15, 0.31 | < 0.0001 |
| Extensive intraductal component | 1.4 | 0.8–2.5 | 0.29 | 1.5 | 0.8–3.0 | 0.22 | 0.15 | −0.04, 0.35 | 0.12 | 0.18 | −0.004, 0.36 | 0.056 |
| Lymphovascular invasion | 1.2 | 0.7–2.0 | 0.47 | 1.5 | 0.8–2.8 | 0.22 | 0.11 | −0.6, 0.29 | 0.20 | −0.09 | −0.26, 0.07 | 0.28 |
| Multifocality | 1.7 | 1.0–3.1 | 0.06 | 1.7 | 0.9–3.5 | 0.11 | 0.26 | 0.05, 0.46 | 0.01 | 0.28 | 0.09, 0.46 | 0.004 |
| Surgeon (n) | 0.10 | 0.06 | < 0.0001 | < 0.0001 | ||||||||
| A (n=139) | 1.0 | -- | -- | 1.0 | -- | -- | Reference | -- | -- | Reference | -- | -- |
| B (n=53) | 0.6 | 0.3–1.5 | 0.27 | 0.9 | 0.4–2.4 | 0.89 | −0.69 | −0.92, −0.47 | < 0.0001 | −0.68 | −0.91, −0.46 | < 0.0001 |
| C (n=164) | 1.4 | 0.8–2.3 | 0.25 | 1.7 | 0.9–3.1 | 0.09 | −0.68 | −0.84, −0.52 | < 0.0001 | −0.67 | −0.83, 0.51 | < 0.0001 |
| D (n=50) | 0.8 | 0.3–1.7 | 0.52 | 0.9 | 0.3–2.3 | 0.80 | 0.25 | 0.02, 0.48 | 0.04 | 0.17 | −0.06, 0.40 | 0.15 |
| E (n=78) | 0.6 | 0.3–1.2 | 0.15 | 0.5 | 0.2–1.2 | 0.13 | −0.13 | −0.33, 0.07 | 0.20 | −0.12 | −0.31, 0.08 | 0.23 |
| F (n=71) | 0.7 | 0.3–1.5 | 0.36 | 0.7 | 0.3–1.7 | 0.44 | −0.18 | −0.38, 0.03 | 0.09 | −0.10 | −0.30, 0.11 | 0.35 |
OR, odds ratio; CI, confidence interval.
Multivariable models included all variables listed in table.
Overall, the Perpendicular and Cavity-Shave methods were not significantly different in achieving negative margins (p=0.37). However, when examined by surgeon, there was variability in the effect of the 3 methods. All but 1 surgeon obtained the highest rate of positive margins with the Tangential method (FIG 2A). Comparison of Perpendicular and Cavity-Shave methods revealed that 4 of 6 surgeons had lower rates of positive margins with the Cavity-Shave method; 1 (Surgeon D) had statistically significant (p=0.04) lower rates.
FIG 2.
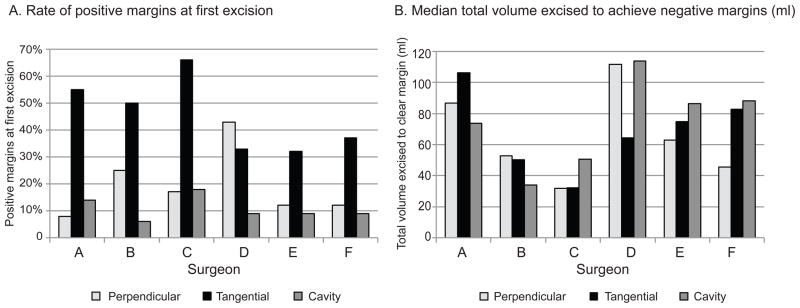
A comparison of methods of margin assessment, by surgeon. (A) Rate of positive margins on first excision; and (B) median total volume excised to achieve negative margins (ml).
Total Volume Excised to Achieve Negative Margin
Overall, the median total volume excised to achieve a negative margin was 55ml for the Perpendicular method, 64ml for Tangential, and 62ml for Cavity-Shave (p=0.24). While this difference among margin assessment methods was not significant in univariate analysis, when adjusted for tumor size, EIC, LVI, multifocality, and surgeon, the Perpendicular method was a significant predictor of lower volume excised to achieve a negative margin (p=0.002). Increasing tumor size (p<0.0001) and the presence of multifocality (p=0.004) were associated with an increased volume necessary to achieve a negative margin (Table 2). EIC was associated with a larger total volume excised to achieve negative margins, and this approached statistical significance (p=0.056). There were statistically significant differences in total volume excised among surgeons (p<0.0001). These differences generally persisted after adjustment for method of margin assessment, tumor size, EIC, LVI, and multifocality. Of the 6 surgeons examined, 1 had the lowest volume with the Tangential method, 2 with Cavity-Shave, and 3 with Perpendicular (FIG 2B).
Comparison of Cavity-Shave and Perpendicular Methods, by Tumor Characteristics
In an attempt to identify clinical situations in which one method may be superior, patients were dichotomized into 2 groups according to factors that could be estimated preoperatively: those with small tumors (T1, ≤2cm) without EIC, and those with large tumors (T2/T3, >2cm) and/or EIC (Table 3). In the group with small tumors without EIC, the Perpendicular method resulted in smaller volume excised (45ml versus 63 ml, p=0.01) without increasing positive margin rates significantly (13% versus 11%, p=0.56). In the group with larger tumors and/or EIC, the Cavity-Shave method resulted in a non-statistically significant reduction in positive margins (15% versus 23%, p=0.34), and lower total volume excised to achieve negative margins (61ml versus 86ml, p=0.14).
Table 3.
Rate of positive margins on first excision and total volume excised to achieve negative margins: a comparison of Perpendicular and Cavity Shave methods, by T stage and presence of EIC.
| Smaller extent of disease
|
Larger extent of disease
|
||||||
|---|---|---|---|---|---|---|---|
| Perpendicular | Cavity-Shave | p-value | Perpendicular | Cavity-Shave | p-value | ||
| Rate of positive margins on first excision | |||||||
| T1 (≤2cm) | 13% | 12% | 0.69 | T2/3 (>2cm) | 27% | 14% | 0.20 |
| No EIC | 14% | 11% | 0.50 | EIC | 19% | 15% | 0.67 |
| T1 and no EIC | 13% | 11% | 0.56 | T2/T3 or EIC | 23% | 15% | 0.34 |
|
| |||||||
| Median total volume excised to achieve negative margins (ml) | |||||||
| T1 (≤2cm) | 47ml | 61ml | 0.03 | T2/3 (>2cm) | 95ml | 69ml | 0.47 |
| No EIC | 48ml | 63ml | 0.02 | EIC | 86ml | 55ml | 0.34 |
| T1 and no EIC | 45ml | 63ml | 0.01 | T2/T3 or EIC | 86ml | 61ml | 0.14 |
T stage, American Joint Commission on Cancer tumor stage; EIC, extensive intraductal component
Note: p-values derived from linear regression models using natural logarithm of total volume to negative margins.
DISCUSSION
The method of margin assessment used affects positive margin rate and the total tissue volume excised in breast conservation. Our institution has had experience with three methods; Perpendicular, Tangential, and Cavity-Shave. The Perpendicular method was the earliest method used, and in this study, it was associated with low positive margin rates on initial excision as well as low excision volumes required for negative margins. This method has been the traditional one used at most centers over the past 20 years. The advantage of this method is the ability to precisely measure the margin width between tumor and inked edge of the specimen, and allow differentiation between a positive (tumor on ink) versus close margin. A disadvantage of this method is the potential for lack of concordance of orientation between pathologist and surgeon. Molina et al found that with the surgeon placing 2 standard orientation sutures on a breast specimen, there was disagreement between the surgeon and pathologist as to the location of a third suture in 31% of specimens examined.7 Another disadvantage of Perpendicular is that the microscopic assessment can be very time consuming for the pathology staff. The entire specimen is sectioned into 2–3mm slices, and margins of invasive carcinoma and DCIS are evaluated microscopically based on proximity of the lesion to the inked edge of the specimen. Sections with the index lesion within 0.5cm of the margin are completely examined, while more distant margins are representatively sampled.8 As each tissue section may have 2 or more edges inked with different colors, this results in a high workload for the pathology staff, especially in a high-volume center as ours.
Adoption of the Tangential method at our institution was made in 2004 in an attempt to avoid some of the drawbacks of the Perpendicular method. Tangential allows a more complete analysis of margins as the entire surface area of the specimen is shaved and examined. It also reduces the workload for the pathologist, since the margins are classified as “positive” or “negative” and no exact distance is measured. However, this method does not distinguish between truly positive margins (tumor on ink) and tumor within 2–3mm of the inked margin, resulting in a higher rate of “positive” margins than Perpendicular and Cavity-Shave, and results in no decrease in total volume of tissue excised. Implementation of the Tangential method of margin assessment resulted in a marked increase in positive margin rate, from the previous rate of 15% with Perpendicular, to 49% with Tangential. This clearly led to a higher rate of re-excision and a lower yield of additional disease detected on re-excision.9 Guidi et al correlated Tangential versus Perpendicular margin assessment in 22 consecutively obtained specimens and found that when margins judged to be positive by the Tangential method were re-analyzed with cross sectioning, nearly half were reclassified as negative.10 Similarly, Mendez et al had a 78% re-excision rate with the Tangential method because of “positive” margins, although analysis of the tissue re-excised revealed that only half had residual tumor.11
The final method examined in this study, the Cavity-Shave method, was implemented in 2008 because of the unacceptably high positive-margin rate with the Tangential method and the advantage of having the surgeon designate margin orientation intraoperatively. Several studies have shown that the Cavity-Shave method decreases rates of positive margin and prevents re-excision in 11–59% of patients12–15; although in some series, it was associated with higher volumes of excision.16–18
We undertook this study to assess not only the positive margin rate, but also the total volume excised, for each of these methods as applied in our institution. We controlled for patient and tumor factors as well as surgeon. In our series, the overall rate of positive margins with Cavity-Shave was 11%, compared to 15% with Perpendicular and 49% with Tangential. When examined by surgeon, most but not all surgeons obtained the lowest rate of positive margins with Cavity-Shave (FIG 2A). The overall volume excised to achieve a negative margin with the Cavity-Shave method was comparable to Tangential (62ml versus 64ml), but greater than Perpendicular (55ml). This outcome was variable by surgeon, with each method resulting in the lowest volume of excision for at least one surgeon.
Examination of the data by surgeon shows that there are some surgeons for whom a particular method both lowered their positive margin rate and decreased the total volume excised (probably due to fewer re-excisions). For one surgeon, the Perpendicular method was superior for both outcomes, and for one, the Cavity-Shave method was superior. However, for the remaining 4 surgeons, there was a “cost” to the method that achieved the lowest positive margin rate: a higher total volume excised.
When cases were categorized into those of limited extent (T1, no EIC), the Perpendicular method was optimal, with smaller total volume and an insignificant increase in positive margin rate. For those with larger tumors or EIC, Cavity-Shave was superior for both outcomes, although not statistically significant.
In summary, the Tangential method results in the highest positive margin rate and is associated with high volume of excision. The Perpendicular and Cavity-Shave methods result in similar positive margin rates, with Cavity-Shave trending toward a lower rate of positive margins—an effect that was more pronounced in patients with EIC and/or T2-T3 tumors. Volume excised for a negative margin varied by surgeon, with no single method achieving low volumes among all surgeons. Use of the Cavity-Shave method may decrease rates of positive margins, particularly in the presence of EIC or T2-T3 tumors. However, the reduction in positive margin rate associated with Cavity-Shave may be associated with a larger volume excised for some surgeons, and for T1 invasive cancers without EIC.
Synopsis.
Cavity-Shave margin assessment method may decrease rates of positive margins; its effect on volume is variable among surgeons and may result in an increase in the total volume excised for some surgeons and for T1 tumors without extensive intraductal component.
Acknowledgments
This study was presented in part at the 2013 Society of Surgical Oncology Annual Cancer Symposium, National Harbor, MD. March 7–9, 2013. This study was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
CONFLICT OF INTEREST None.
References
- 1.Showalter SL, Grover S, Sharma S, et al. Factors Influencing Surgical and Adjuvant Therapy in Stage I Breast Cancer: A SEER 18 Database Analysis. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2693-8. [DOI] [PubMed] [Google Scholar]
- 2.Gage I, Schnitt SJ, Nixon AJ, et al. Pathologic margin involvement and the risk of recurrence in patients treated with breast-conserving therapy. Cancer. 1996;78(9):1921–8. doi: 10.1002/(sici)1097-0142(19961101)78:9<1921::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Holland PA, Gandhi A, Knox WF, et al. The importance of complete excision in the prevention of local recurrence of ductal carcinoma in situ. Br J Cancer. 1998;77(1):110–4. doi: 10.1038/bjc.1998.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Punglia RS, Morrow M, Winer EP, et al. Local therapy and survival in breast cancer. N Engl J Med. 2007;356(23):2399–405. doi: 10.1056/NEJMra065241. [DOI] [PubMed] [Google Scholar]
- 5.Kreike B, Hart AA, van de Velde T, et al. Continuing risk of ipsilateral breast relapse after breast-conserving therapy at long-term follow-up. Int J Radiat Oncol Biol Phys. 2008;71(4):1014–21. doi: 10.1016/j.ijrobp.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010;46(18):3219–32. doi: 10.1016/j.ejca.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 7.Molina MA, Snell S, Franceschi D, et al. Breast specimen orientation. Ann Surg Oncol. 2009;16(2):285–8. doi: 10.1245/s10434-008-0245-z. [DOI] [PubMed] [Google Scholar]
- 8.Kuerer HM. Kuerer’s breast surgical oncology. New York: McGraw-Hill Medical; 2010. [Google Scholar]
- 9.Wright MJ, Park J, Fey JV, et al. Perpendicular inked versus tangential shaved margins in breast-conserving surgery: does the method matter? J Am Coll Surg. 2007;204(4):541–9. doi: 10.1016/j.jamcollsurg.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Guidi AJ, Connolly JL, Harris JR, et al. The relationship between shaved margin and inked margin status in breast excision specimens. Cancer. 1997;79(8):1568–73. [PubMed] [Google Scholar]
- 11.Mendez JE, Lamorte WW, de Las Morenas A, et al. Influence of breast cancer margin assessment method on the rates of positive margins and residual carcinoma. Am J Surg. 2006;192(4):538–40. doi: 10.1016/j.amjsurg.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Cao D, Lin C, Woo SH, et al. Separate cavity margin sampling at the time of initial breast lumpectomy significantly reduces the need for reexcisions. Am J Surg Pathol. 2005;29(12):1625–32. doi: 10.1097/01.pas.0000180448.08203.70. [DOI] [PubMed] [Google Scholar]
- 13.Hewes JC, Imkampe A, Haji A, et al. Importance of routine cavity sampling in breast conservation surgery. Br J Surg. 2009;96(1):47–53. doi: 10.1002/bjs.6435. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson AF, Asad J, Boolbol SK, et al. Do additional shaved margins at the time of lumpectomy eliminate the need for re-excision? Am J Surg. 2008;196(4):556–8. doi: 10.1016/j.amjsurg.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Povoski SP, Jimenez RE, Wang WP, et al. Standardized and reproducible methodology for the comprehensive and systematic assessment of surgical resection margins during breast-conserving surgery for invasive breast cancer. BMC Cancer. 2009;9:254. doi: 10.1186/1471-2407-9-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marudanayagam R, Singhal R, Tanchel B, et al. Effect of cavity shaving on reoperation rate following breast-conserving surgery. Breast J. 2008;14(6):570–3. doi: 10.1111/j.1524-4741.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 17.Rizzo M, Iyengar R, Gabram SG, et al. The effects of additional tumor cavity sampling at the time of breast-conserving surgery on final margin status, volume of resection, and pathologist workload. Ann Surg Oncol. 2010;17(1):228–34. doi: 10.1245/s10434-009-0643-x. [DOI] [PubMed] [Google Scholar]
- 18.Millar EK, Leong AS. Significance and assessment of margin status in ductal carcinoma in situ of the breast. Adv Anat Pathol. 2001;8(6):338–44. doi: 10.1097/00125480-200111000-00004. [DOI] [PubMed] [Google Scholar]