Abstract
Purpose/Objective
Proton beam therapy (PBT) is a promising modality for the management of thoracic malignancies. We report our preliminary experience of treating esophageal cancer patients with concurrent chemotherapy (CChT) and PBT at MD Anderson Cancer Center.
Materials/Methods
This is an analysis of 62 esophageal cancer patients enrolled on a prospective study evaluating normal tissue toxicity from CChT/PBT from 2006 to 2010. Patients were treated with Passive Scattering PBT with 2 or 3 field beam arrangement using 180–250 MV protons. We used the method of Kaplan and Meier to assess time to event outcomes and compared the distributions between groups using the log-rank test.
Results
The median follow-up time was 20.1 months for survivors. The median age was 68 years (range 38–86). Most were males (82%), had adenocarcinomas (76%) and had stage II-III disease (84%). The median radiation dose was 50.4 Gray-Equivalence (Gy(RBE)) (range 36–57.6). The most common grade 2–3 acute toxicities from CChT/PBT were esophagitis (46.8%), fatigue (43.6%), nausea (33.9%), anorexia (30.1%), and radiation dermatitis (16.1%). There were two cases of grade 2 and 3 radiation pneumonitis and two grade 5 toxicities. A total of 29 patients (46.8%) received preoperative CChT/PBT with one postoperative death. The pathologic complete response (pCR) rate for the surgical cohort was 28%, and the pCR and near CR rate (0–1% residual cells) was 50%. While there were significantly fewer local-regional recurrences in the preoperative group (3/29) as compared to the definitive CChT/PBT group (16/33) (log-rank test p=0.005), there were no differences in DM free interval or OS between the two groups.
Conclusions
This is the first report of patients treated with PBT/CChT for esophageal cancer. Our data suggest that this modality is associated with a few severe toxicities but the pathologic response and clinical outcomes are encouraging. Prospective comparison with more traditional approach is warranted.
Keywords: Protons, Esophageal cancer, Chemotherapy, Chemoradiation
INTRODUCTION
Esophageal cancer is a deadly disease that afflicts over 16,000 people in the United States, causing 14,500 deaths(1). While surgery is standard for early stage disease, chemotherapy and radiation therapy is a standard treatment approach for non-metastatic locally advanced disease, either preoperatively or definitively. Given the location of the esophagus, radiation delivery must take into careful consideration the need to minimize dose to surrounding normal organs in order to limit the morbidity of treatment, improve the chance that the patient can be fit to go to surgery, and minimize the potential for postoperative complications.
While three-dimensional conformal RT (3DCRT) is considered the standard in the United States and worldwide, it imposes relatively high doses to regions of the heart, lung, spinal cord, bowel, and liver. Charged particles such as protons have little exit dose beyond the target volume, thereby greatly sparing adjacent normal tissues. This physical characteristic allows PBT to improve the therapeutic ratio by limiting toxicities while at the same time delivering higher radiation doses. While the dosimetric advantages of protons in the treatment of esophageal cancers have been demonstrated in numerous planning studies compared to 3DCRT and IMRT(2–4), the clinical experience has been somewhat limited. The use of protons for the treatment of esophageal cancer has been reported, mostly delivered without chemotherapy as definitive treatment for the management of squamous cell carcinoma of the esophagus(5, 6). To our knowledge, there are no reported experiences in the literature of the use of PBT with chemotherapy in preoperative or definitive management of esophageal cancers. We report here the toxicities and outcomes of the first 62 patients treated using CChT/PBT at MD Anderson Cancer Center.
MATERIALS AND METHODS
Patients
This is an Institutional Review Board approved retrospective analysis of a prospective study assessing normal tissue toxicities for patients treated with CChT/PBT. This study (PCR05-0207, “Data Collection of Normal Tissue Toxicity for Proton Therapy” was intended to prospectively collected acute and late toxicity data of all patients treated with proton beam therapy, regardless of site, stage, or the types of concurrent chemotherapy administered, with the goal to correlate normal tissue response with radiation dose distribution and imaging data. Between May 15, 2006 to April 12, 2010, 62 patients with stage Ib to IV (based on AJCC 6th edition) esophageal cancers received CChT/PBT as either definitive treatment, consolidation treatment (for stage IV disease after induction chemotherapy with good response or as required based on institutional protocol) or as preoperative therapy. Follow-up was last updated on April 24, 2011. All patients were staged with PET/CT scans, CT scans with contrast, and esophagoscopy (EGD) with endoscopic ultrasound. The types of chemotherapy administered were according to the discretion of the treating gastrointestinal medical oncologist (supplementary table 1). Hematologic and radiation related toxicites were assessed on a weekly basis. Four weeks after completion of treatment, restaging with PET/CT and EGD with biopsies were performed and resectability determined based on the patient’s performance status and results from the restaging studies.
Radiation planning and treatment
Based on our department policy, patients were instructed to not eat anything except for sips of water for at least 3 hours prior to simulation. Four-Dimensional (4D) CT simulation was performed for all patients to assess tumor and normal tissue excursion relative to respiratory motion. Contouring and treatment planning were performed on the Eclipse treatment planning system (Varian Medical Systems) based on passive scattering technique. Gross tumor volume (GTV) is defined as all disease seen on PET and EGD, and clinical tumor volume (CTV) included all areas of potential disease spread. PBT range uncertainties of stopping power was based on the Moyer approximation(7), and for inter- and intra-fractional motion and possible alignment variability of the compensator, the smear margin radius was determined using the Urie approximation(8). This is typically 1-to-1.5cm from the CTV. Patients were treated with either a two field Anterior-Posterior (AP) and Posterior-Anterior (PA) beam arrangement, PA and left lateral oblique, or a three beam approach using a RPO or PA, a left lateral (with slight left posterior oblique (LPO) angle tilt), and a LPO arrangement. The optimal beam arrangements were determined on a case-by-case basis. Customized brass blocks and plexiglass tissue compensators were created for each plan to deliver the spread out Bragg Peak (SOBP) necessary to encompass the treatment volume. Depending on the depth of treatment, typically 150–250 MeV protons were utilized. Patients were aligned on the treatment table using skin marks and daily orthogonal KV imaging. The prescribed dose is 50.4 Gy(RBE) in 28 fractions.
Dose constraints used were total lung V20 <35% (ideally <20% for preoperative patients unless difficult due to treatment volume), mean lung dose < 20 Gy, heart V40 < 40%, liver V30 < 30%, spinal cord < 45 Gy.
Disease related outcomes reporting
Local-regional recurrence (LRR) was defined as disease recurrence in the primary site or regional nodes. Regional nodes for cervical and upper thoracic disease are the supraclavicular/mediastinal nodes; mid-thoracic esophagus are the mediastinal nodes; and distal esophagus are the celiac/left gastric/mediastinal nodes. Local-regional control interval was calculated from the date of diagnosis to the date of first time LRR or last follow-up. The date of LRR for patients who never achieved disease free was set to the end of radiation therapy. Distant metastasis control interval was calculated from the date of diagnosis to the date of first time distant metastasis or last follow-up. Disease recurrence control interval was calculated from the date of diagnosis to the date of first time LRR or distant metastasis or last follow-up which ever occur first. Deaths without relapse were censored at the time of death. The date of relapse for patients who never achieved disease free was set to the end of radiation therapy. Overall survival interval was calculated from the date of diagnosis to the dates of deaths or last follow-up. Relapse free survival interval was calculated from the date of diagnosis to the earliest date of relapse or deaths or last follow-up.
Statistical considerations
Due to small sample size, all time to event analysis are univariable analysis using the method of Kaplan and Meier (KM) and the comparison of distributions between groups were made using the log-rank test. Analysis was performed using R (http://www.r-project.org/).
RESULTS
Dosimetry and DVH analysis of PBT plans
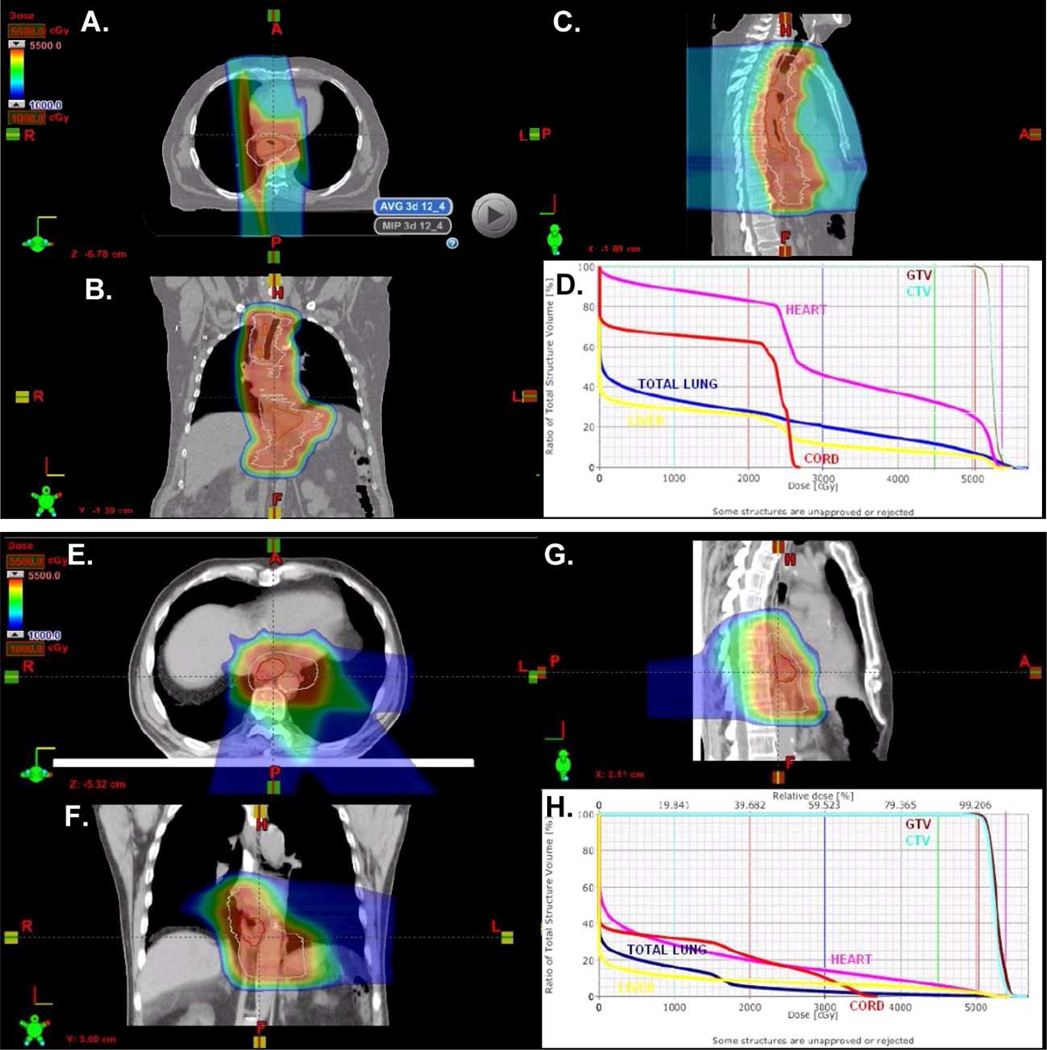
Figure 1 illustrates the treatment plans and dose-volume histogram of two cases reflecting two commonly used beam arrangements for this cohort of patients. The first case (Figure 1A-D), was an 8 cm tumor that extended from the mid esophagus to the distal esophagus and involved nodes in the paratracheal and celiac axis. Patient was treated using an AP/PA beam arrangement and 250 MeV protons to 50.4 Gy(RBE) in 28 fractions. The second case is a T3N0 adenocarcinoma of the GE junction treated with a 3 field plan (RPO, LPO, Left lateral posterior oblique (LLPO)) (Figure 1E-H). Although the 3 field arrangement was done initially, we now commonly treat using a two field plan (PA and LLPO) for GEJ tumors.
Figure 1. PBT treatment plans for two patients.
A-C) Two field AP/PA plan for this patient with a mid-to-distal esophageal cancer. D) DVH analysis of the plan for this patient shows a MLD=12.5 Gy, Heart V40=37%, cord maximum 27 Gy, Liver V30=12%. E-G) Three field RPO, LPO, left lateral posterior oblique PBT plan for this patient with GEJ tumor adenocarcinoma. H) DVH analysis of the plan. MLD=3.8 Gy, Heart V40=8%, cord maximum 37.1 Gy, Liver V30=7%.
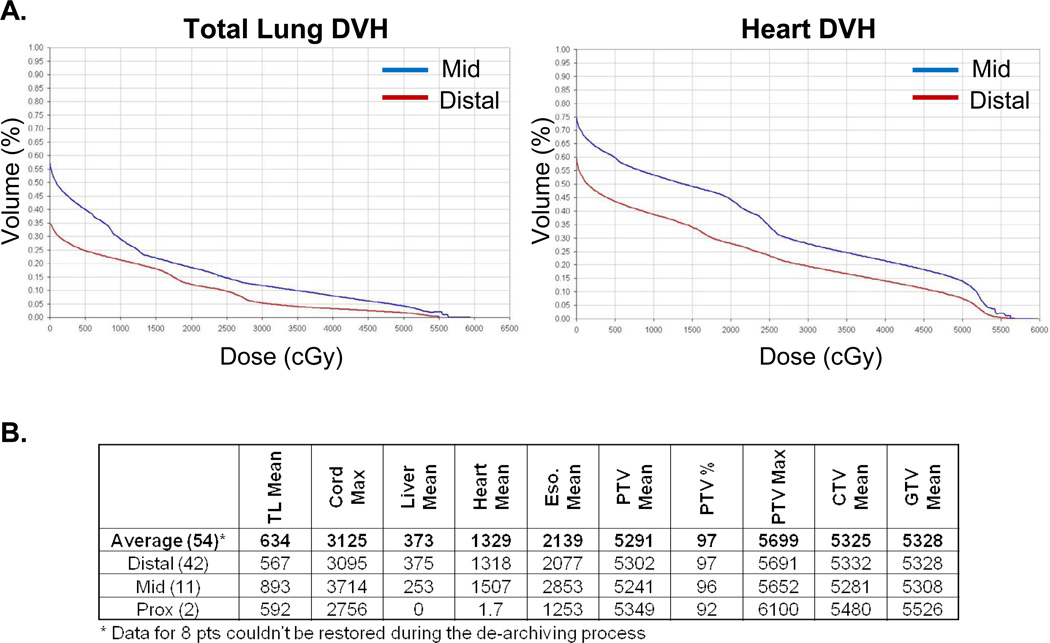
Figure 2A are the summary DVH analysis for total lung and heart doses for the data accessible patients (N=54), based on whether the primary site was in the distal or mid thoracic esophagus (two cases of proximal tumors were not included in this analysis). The average dose to the total lung and heart in the distal esophagus was lower than the mid esophageal regions. Figure 2B tabulates the average mean and maximum normal tissue dose and target volume coverage stratified based on the site of disease. Target volumes were well covered within the high dose regions, and the average dose to normal tissues were well below our dose constraints.
Figure 2. Dose Volume Histogram (DVH) analysis of the accessible patients treated with CChT/PBT.
A-B) Summary DVH curves of the total lung and heart depending on whether the tumors were in the distal or mid-esophageal regions. C) Tabular summary of the mean and maximal doses to normal and target structures for the entire cohort (average) or for the primary sites of disease (distal, middle (mid), proximal (prox) esophagus).
Patient demographics and tumor characteristics
Table 1 summarizes the demographics and tumor characteristics of this patient cohort. The median age is 68 years old. The majority of the patients were Caucasian (95.2%), male (82%) with adenocarcinomas (75.8%). While the majority of patients had stage 2 to 3 disease (83.9%), some patients with more advanced disease (celiac lymph node involvement or distant metastasis) were treated with consolidative CChT/PBT after induction chemotherapy. Twenty-nine patients (46.8%) were treated with CChT/PBT followed by surgical resection. Induction chemotherapy was given to 26 patients (41.9%).
Table 1.
Patient demographic and tumor characteristics
| No. of Patients | 62 |
|---|---|
| Gender, n (%) | |
| Male | 51 (82.3%) |
| Female | 11 (17.7%) |
| Median Age (range) | 68 (38 – 86) |
| Race, n (%) | |
| Caucasians | 59 (95.2%) |
| African Americans | 1 (1.6%) |
| Hispanics | 1 (1.6%) |
| Asians | 1 (1.6%) |
| Histology, n (%) | |
| Adenocarcinoma | 47 (75.8%) |
| Squamous Cell Carcinoma | 14 (22.6%) |
| Mixed Adeno, neuroendocrine | 1 (1.6%) |
| Stage, n (%) | |
| I | 2 (3.2%) |
| II | 20 (32.3%) |
| III | 32 (51.6%) |
| IVa | 2 (3.2%) |
| IVb | 6 (9.7%) |
| Tumor Location, n (%) | |
| Upper | 3 (4.8%) |
| Mid | 11 (17.7%) |
| Distal/GEJ | 48 (77.5%) |
| Treatment, n (%) | |
| Definitive | 33 (53.2%) |
| Neoadjuvant + Surgery | 29 (46.8%) |
| Induction Chemotherapy, n (%) | |
| Yes | 26 (41.9%) |
| No | 36 (58.1%) |
| Median Proton Dose in Gy(RBE) (range) | 50.4 (36 – 57.6) |
Abbreviations: RBE: Relative Biologic Equivalence
Treatment related toxicities and deaths attributed to CChT/PBT
The most common grade 2–3 adverse events were dysphagia (43.6%), esophagitis (46.8%), fatigue (43.6%), nausea (33.9%), anorexia (30.1%), and radiation dermatitis (16.1%) (Table 2). There was one case each for grade 2, 3, and 5 radiation pneumonitis (see below). Treatment was discontinued for one patient after 36 Gy(RBE) because of intolerance to esophagitis. Two patients were scored as having grade 5 toxicities. One patient died after 45 Gy(RBE) due to ventricular tachycardia leading to ICU admission and subsequent death due to cardiac arrest. A second patient died 374 days after CChT/PBT and 250 days after surgery. This patient was admitted for increasing pleural effusion, respiratory failure and became ventilator dependent for presumed radiation pneumonitis. Patient was discharged in stable condition to skilled nursing facility but died 2.5 months later.
Table 2.
Treatment related toxicities
| Toxicity | Grade | Frequency, N (%) |
|---|---|---|
| Esophagitis | 0–1 | 33(53.2%) |
| 2 | 23(37.1%) | |
| 3 | 6(9.7%) | |
| Dysphagia | 0–1 | 35(58.5%) |
| 2 | 21(33.9%) | |
| 3 | 6(9.7%) | |
| Nausea/Vomiting | 0–1 | 41(66.1%) |
| 2 | 16(25.8% | |
| 3 | 5(8.1%) | |
| Radiation Dermatitis | 0–1 | 52(83.9%) |
| 2 | 8(12.9%) | |
| 3 | 2(3.2%) | |
| Fatigue | 0–1 | 35(56.5%) |
| 2 | 22(35.5%) | |
| 3 | 5(8.1%) | |
| Anorexia | 0–1 | 44(71.0%) |
| 2 | 15(24.2%) | |
| 3 | 3(4.8%) | |
| Pneumonitis | 0–1 | 60(96.7%) |
| 2 | 1(1.6%) | |
| 3 | 1(1.6%) |
Disease associated outcomes
The median follow-up time for survivors is 20.1 months. At last follow-up, there were 21 deaths, 16 distant metastatic events, and 19 LRR. The patterns of LRR are summarized in supplementary table 2. The LRR in the definitively treated patients were either in the primary site (N=10 or 16) or nodal regions (3 in field and 3 out of field). All LRR (N=3) for the surgical patients were in nodes outside of the radiation fields. Nine patients died without LRR, 8 patients died without evidence of distant metastasis, and 3 died not due to disease recurrence, 1 died due to unknown cause 274 days after definitive chemoradiation. The estimated 3 year overall survival is 51.7% (95%CI 0.31, 0.69). The 3 year relapse free survival, distant metastastic free survival, and local-regional control are 40.5% (95%CI 22.4, 0.58), 66.7% (95%CI 48.3, 79.8), and 56.5% (95%CI 0.34, 0.74), respectively. The clinical factors that predicted for overall survival, distant metastasis, and local-regional control based on Kaplan Meier statistics and log-rank test are summarized in Table 3.
Table 3.
Factors associated poorer outcomes in various clinical endpoints comparing distribution of time to event based on Log-rank test. P-values are shown*
| Variables | Overall Survival |
Relapse Free Survival |
DM Control |
Locoregional Control |
|---|---|---|---|---|
| Induction Chemotherapy | 0.50 | 0.59 | 0.042 | 0.82 |
| Grade 3 (vs Grade 1–2) | 0.22 | 0.22 | 0.0020 | 0.79 |
| Stages 3–4 (vs 1–2) | 0.12 | 0.14 | 0.028 | 0.13 |
| Clinical N1 (vs N0) | 0.072 | 0.12 | 0.051 | 0.21 |
| Clinical M1 (vs M0) | 0.012 | 0.080 | 1.6E-05 | 0.22 |
| Gender (F vs M) | 0.72 | 0.58 | 0.52 | 0.059 |
| Adenocarcinoma (vs SCCA) | 0.63 | 0.54 | 0.51 | 0.058 |
| PET CR (vs <CR) | 0.68 | 0.38 | 0.31 | 0.95 |
| Surgery (vs no surgery) | 0.34 | 0.047 | 0.24 | 0.0051 |
| Positive post-tx biopsy | 0.012 | 0.0094 | 0.0045 | 0.0043 |
| Tumor length ≥ median | 0.21 | 0.14 | 0.018 | 0.43 |
p<0.05 are bolded
Pathologic response and perioperative morbidities of preoperative CChT/PBT
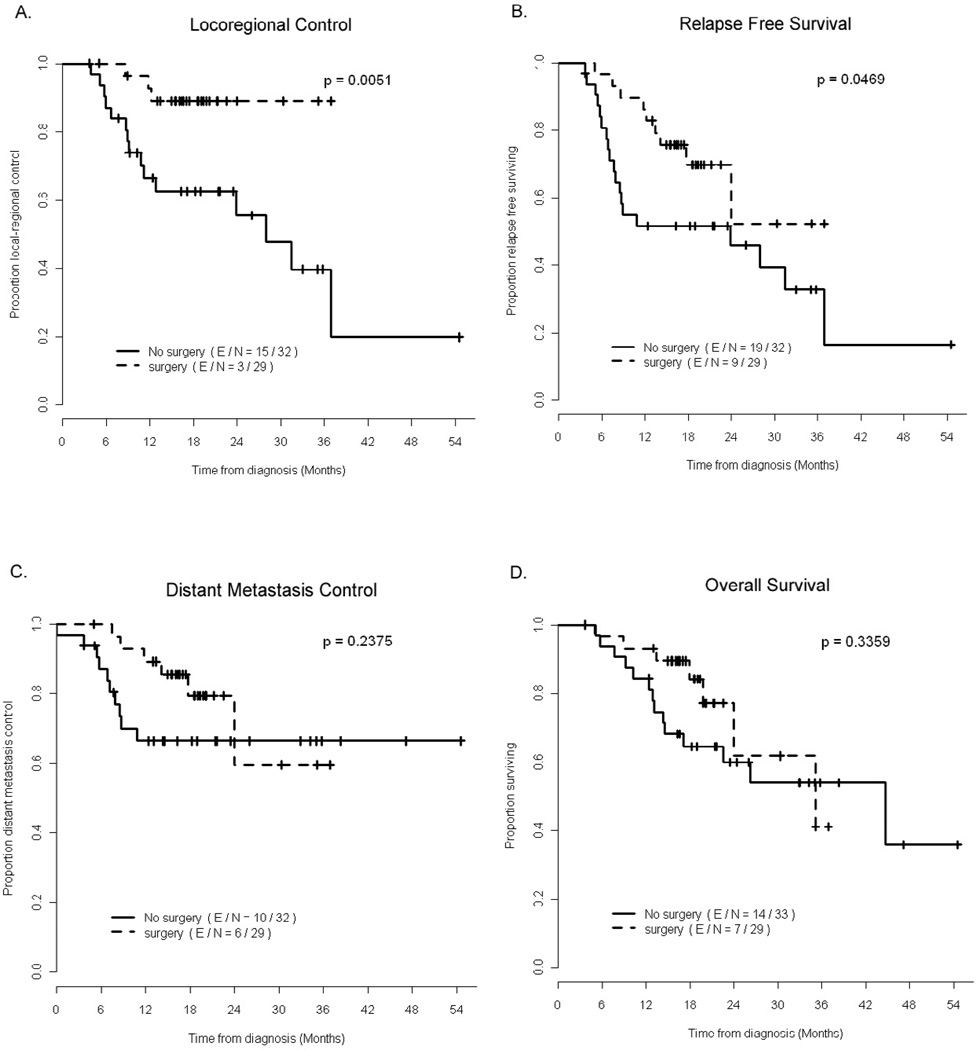
Twenty-nine patients (46.8%) had surgical resection after chemoradiation. Patients who undergo surgery vs. not were typically younger (mean(SD) 60(9.3) vs 71(10.3) years old, p<0.001), have adenocarcinoma (100% vs 54.5%, p<0.001), have less weight loss after chemoradiation (mean(SD) 3.4 (3.4) vs 5.7 (3.6) kg, p=0.0116), and have fewer grade 2–3 treatment related fatigue (54.6% vs 31%, p=0.029) or anorexia (45.5% vs 10.3%, p=0.015). Mean hospital stay is 8.3 ± 3.5 days. Four patients (6.5%) had postoperative pulmonary complications of pulmonary embolism, pneumonia, TE fistula, and/or empyema, 4 (6.5%) patients had anastomatic leaks, 5 (8.1%) had atrial fibrillation and 2 (3.2%) had wound infection. Seven patients had readmission within 6 days of discharge due to anastomotic leakage, and one patient died 29 days after surgey from Klebsiella pneumonia sepsis. The complete pathologic response (pCR) rate is 28%, and pCR and near CR (0–1%) is 50%. Local-regional control and relapse free survival were better in patients who had surgical resection, but there was no statistically significant difference in distant metastatic free survival or overall survival with surgical resection (figure 3). The 6, 12, 24, and 36 month-disease outcomes between surgical and non-surgical patients are summarized in supplementary table 3.
Figure 3.
Survival and disease specific outcomes in the preoperative (n=29) and definitively treated (n=33) patients.
DISCUSSION
To our knowledge, this is the first reported clinical experience of the treatment of esophageal cancers using CChT/PBT. The safety and efficacy of CChT/PBT appear promising. Majority of the patients experienced grade 0–2 toxicities, and there were low postoperative complications. The pathologic complete response rate (28%) is in keeping with what has been reported in the literature, and the near complete response (0–1%) was 50%.
Theoretical advantages of protons for esophageal cancer
The current standard beam arrangement for the treatment of esophageal cancer is 3D conformal radiation therapy (3DCRT), where 3 or 4 beams are arranged and weighted more heavily in the AP/PA direction in order to spare more lung but increases the dose to the heart and spinal cord. Intensity modulated radiation therapy (IMRT) can better spare the surrounding normal structures in several planning studies(9–12). IMRT improves in mean lung dose (MLD) without an advantage seen for the heart and liver compared to 3DCRT. Charged particles, such as protons, have the property of the Bragg peak which can limit the dose to the target tissue(13). In a study comparing protons with X-ray in 5 patients(3), proton plans were able to spare better all structures (spinal cord, lung, heart and kidneys) and enhances the tumor control probability value by an average of 20%-units (range 2–23% units) using 5% NTCP in any risk organ. This advantage is not limited to 3DCRT radiation, but also extends to IMRT treatment planning. In a recent study, 4D CT-based planning was compared between IMRT and 2F (AP/PA) or 3F (AP/two posterior obliques) proton beam for 15 patients(4). Both the 2F and 3F proton plans were able to improve lung sparing by reducing the V5, 10, and 20 Gy(RBE) and the MLD and cord sparing, but there was no improvement in heart sparing using the beam arrangements as prescribed. A more recent planning study demonstrated that Intensity Modulated Proton Therapy (IMPT) was able further reduce the dose to the lung, heart and liver compared to IMRT(14). These studies demonstrate the promise of protons to improve the therapeutic ratio in the treatment of esophageal cancer.
Clinical experiences with the use of protons for esophageal cancers
While the dosimetric advantage of protons is clear, the reported clinical experience using proton beam in the treatment of esophageal cancers is limited. All of the reported studies come out of the University of Tsukuba, with the first report in 1989 in one patient treated with proton beam without chemotherapy as a preoperative measure(15). Several updates has since been published from the same group(5, 6, 16), with the most recent reported for 51 patients treated between 1985 to 2005(6). All but one patient had squamous cell carcinoma. Thirty-three patients were treated with a combination of x-rays (median dose 46 Gy) and protons (median 36 Gy(RBE)) as a boost, with a combined total dose of 80 Gy(RBE) (range 70–90 Gy(RBE)), and 18 were treated with proton beam alone (median dose 79 Gy(RBE), range 62–98 Gy(RBE)). There were no treatment interruptions due to radiation esophagitis or hematologic toxicities for any patients. The 5 year actuarial survival for all 51 patients was 21.1% and a median survival of 20.5 months. While 22 patients (43%) remained disease free, LRRwas the most common first site of failure for the 29 patients, occurring in the primary site for 17 patients, in field nodal disease in 6 patients, and 1 out-of-field nodal failure. This updated experience demonstrates the promise of proton beam dose escalation for the treatment of esophageal cancers. However, the tolerability of combining protons with chemotherapy and the efficacy of extending the treatment to adenocarcinomas, which are far more common in the United States and Western Europe, are currently unknown.
For our current study using CChT/PBT for the treatment of esophageal cancer, we also found the treatment to be well tolerated, but unlike the Japanese studies, nearly 80% of our patients were adenocarcinomas and nearly half of our patients were treated with preoperative therapy. We found complete pathologic response in 28% of patients, and a near complete response (0–1% viable cells) for 50%. The postoperative complications for pulmonary, cardiac, gastrointestinal, or wound infections were each less than 10%. Although we found that surgical resection is a strong prognostic factor for better local-regional control, there were no statistically significant differences in distant metastatic free survival or overall survival between the definitive CChT/PBT patients and the preoperative patients. However, the definitively treated patients are not comparable to the preoperative group since patients that don’t go on to surgery often have negative prognostic factors of either being technically unresectable, have poor performance status, or have distant metastasis at preoperative evaluation.
The limitation of our study is the relatively short follow-up time (20.1 months) for survivors, so it is not possible to ascertain late toxicities for the majority of the patients. However, the strengths of this study is the relative homogeneity in the multidisciplinary approach of how each patient is managed, from the way radiation treatment is planned and delivered to the surgery team that manages these patients. This setting controls the quality of the treatment planning process and perioperative management of these patients, reducing the potential variability due to factors other than disease-related processes.
Summary
CChT/PBT holds good promise in the management of esophageal cancers. Acute treatment-related toxicities and perioperative morbidities are relatively low and the tumor response and disease related outcomes are encouraging. Although this is a non-randomized study and cannot be compared directly to photon-based radiation, our clinical experience demonstrates the feasibility and safety of this treatment modality and should set the stage for future trials comparing PBT to photon-based radiotherapy.
Supplementary Material
Acknowledgments
Research Support: Funding was provided by The University of Texas MD Anderson Cancer Center. Caimiao Wei’s work is partially supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A portion of this work has been presented in abstract form at the 93rd Annual Meeting of the American Radium Society, April 30–May 4, 2011, Palm Beach, Florida
Conflicts of Interest Notification: The authors declare no conflicts of interest.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer Journal for Clinicians. 60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ruderman AI, Kondratyeva AP, Ratner TG. Proton beam irradiation in esophageal cancer. Meditsinskaya Radiologiya. 1981;26:20–25. [PubMed] [Google Scholar]
- 3.Isacsson U, Lennernäs B, Grusell E, et al. Comparative treatment planning between proton and x-ray therapy in esophageal cancer. International Journal of Radiation Oncology Biology Physics. 1998;41:441–450. doi: 10.1016/s0360-3016(98)00047-9. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Zhao Kl, Guerrero TM, et al. Four-Dimensional Computed Tomography-Based Treatment Planning for Intensity-Modulated Radiation Therapy and Proton Therapy for Distal Esophageal Cancer. International Journal of Radiation Oncology Biology Physics. 2008;72:278–287. doi: 10.1016/j.ijrobp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugahara S, Tokuuye K, Okumura T, et al. Clinical results of proton beam therapy for cancer of the esophagus. International Journal of Radiation Oncology Biology Physics. 2005;61:76–84. doi: 10.1016/j.ijrobp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Mizumoto M, Sugahara S, Nakayama H, et al. Clinical results of proton-beam therapy for locoregionally advanced esophageal cancer. Strahlentherapie und Onkologie. 186:482–488. doi: 10.1007/s00066-010-2079-4. [DOI] [PubMed] [Google Scholar]
- 7.Moyers MF, Miller DW, Bush DA, et al. Methodologies and tools for proton beam design for lung tumors. International Journal of Radiation Oncology Biology Physics. 2001;49:1429–1438. doi: 10.1016/s0360-3016(00)01555-8. [DOI] [PubMed] [Google Scholar]
- 8.Urie M, Goitein M, Wagner M. Compensating for heterogeneities in proton radiation therapy. Physics in Medicine and Biology. 1984;29:553–566. doi: 10.1088/0031-9155/29/5/008. [DOI] [PubMed] [Google Scholar]
- 9.Nutting CM, Bedford JL, Cosgrove VP, et al. A comparison of conformal and intensitymodulated techniques for oesophageal radiotherapy. Radiotherapy and Oncology. 2001;61:157–163. doi: 10.1016/s0167-8140(01)00438-8. [DOI] [PubMed] [Google Scholar]
- 10.Fenkell L, Kaminsky I, Breen S, et al. Dosimetric comparison of IMRT vs. 3D conformal radiotherapy in the treatment of cancer of the cervical esophagus. Radiotherapy and Oncology. 2008;89:287–291. doi: 10.1016/j.radonc.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Chandra A, Guerrero TM, Liu HH, et al. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiotherapy and Oncology. 2005;77:247–253. doi: 10.1016/j.radonc.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T, Ishikawa H, Ebara T, et al. The role of intensity modulated radiotherapy (IMRT) for locally advanced esophageal cancer. Japanese Journal of Clinical Radiology. 55:1114–1120. [Google Scholar]
- 13.Terasawa T, Dvorak T, Ip S, et al. Systematic review: Charged-particle radiation therapy for cancer. Annals of Internal Medicine. 2009;151:556–565. doi: 10.7326/0003-4819-151-8-200910200-00145. [DOI] [PubMed] [Google Scholar]
- 14.Welsh J, Gomez D, Palmer MB, et al. Intensity-Modulated Proton Therapy Further Reduces Normal Tissue Exposure During Definitive Therapy for Locally Advanced Distal Esophageal Tumors: A Dosimetric Study. International Journal of Radiation Oncology, Biology, Physics. doi: 10.1016/j.ijrobp.2010.07.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibuya S, Takase Y, Watanabe M, et al. Usefulness of proton irradiation therapy as preoperative measure for esophageal cancer. Diseases of the Esophagus. 1989;2:99–104. [Google Scholar]
- 16.Koyama S, Tsujii H. Proton beam therapy with high-dose irradiation for superficial and advanced esophageal carcinomas. Clinical Cancer Research. 2003;9:3571–3577. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.