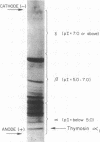
Abstract
The amino acid sequence of a biologically active polypeptide isolated from calf thymus, termed thymosin alpha1, has been determined. Thymosin alpha1 is a heat stable, highly acidic molecule composed of 28 amino acid residues. This peptide is one of several present in thymosin fraction 5 that may participate in the regulation, differentiation and function of thymus-dependent lymphocytes (T cells). A nomenclature for the family of polypeptides present in thymosin fraction 5 is suggested.
Full text
PDF
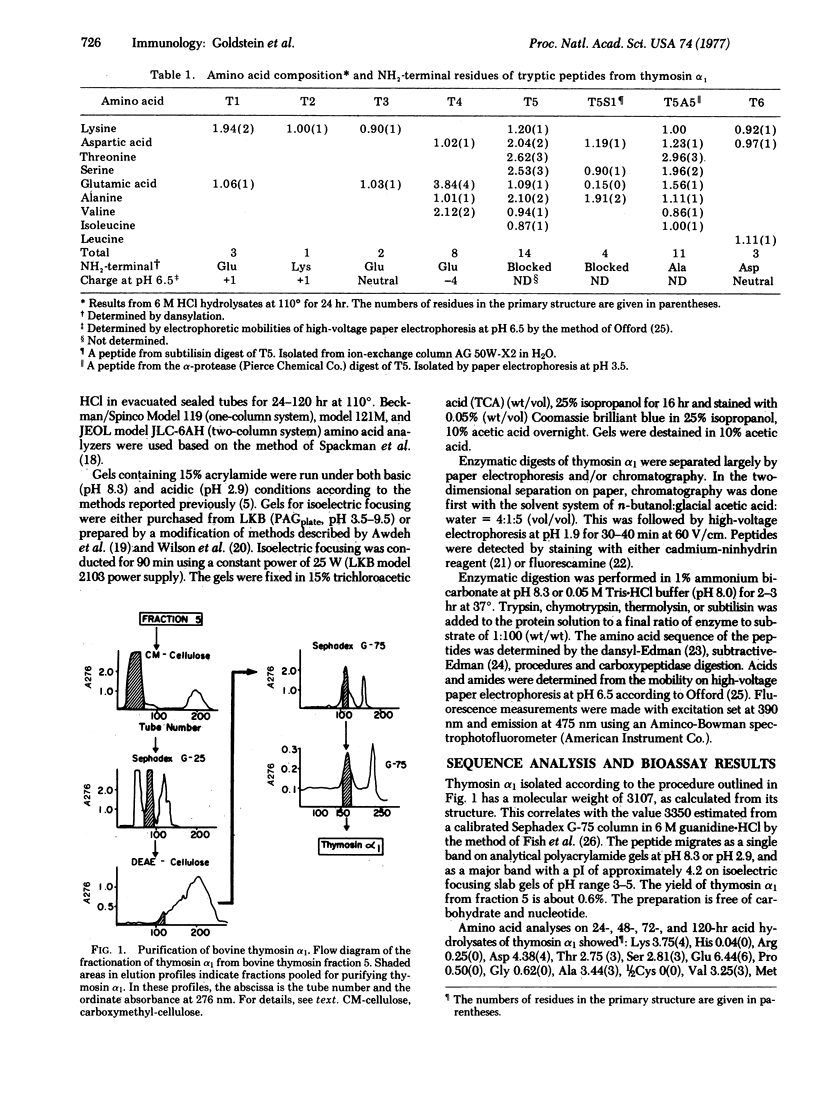
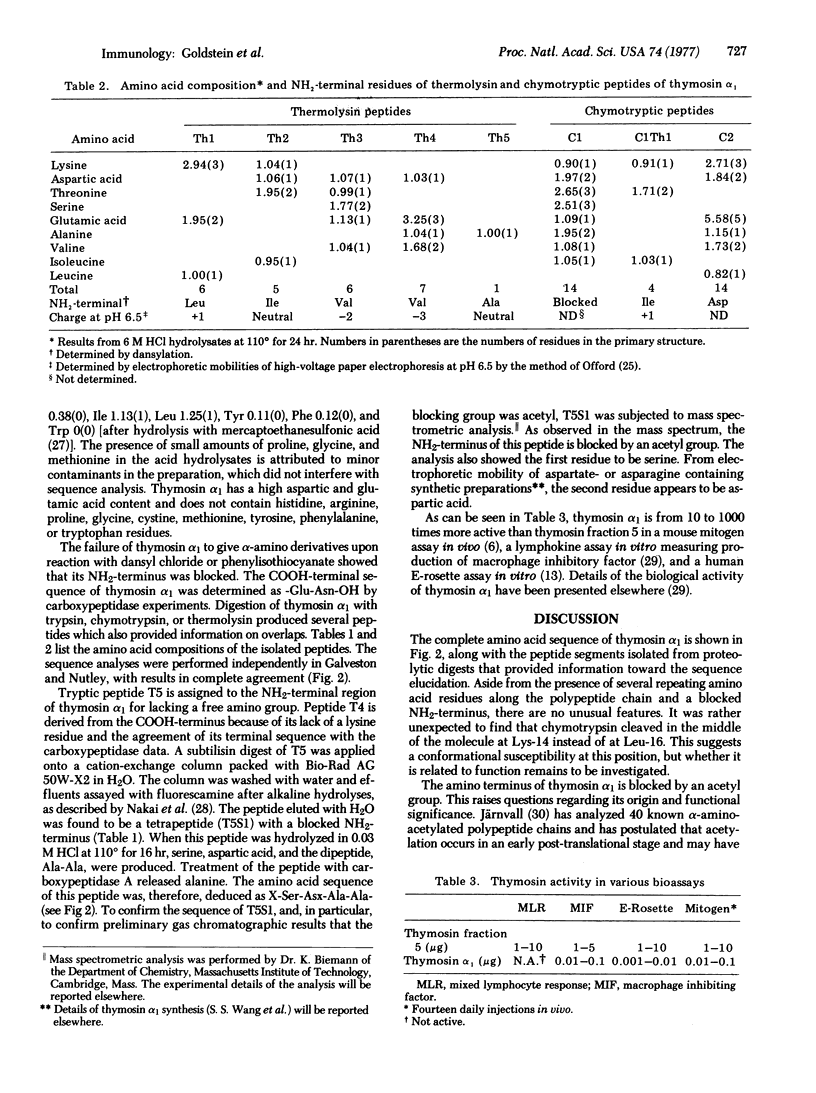


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awdeh Z. L., Williamson A. R., Askonas B. A. Isoelectric focusing in polyacrylamide gel and its application to immunoglobulins. Nature. 1968 Jul 6;219(5149):66–67. doi: 10.1038/219066a0. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Dardenne M., Goldstein A. L., Guha A., White A. Appearance of T-cell markers in bone marrow rosette-forming cells after incubation with thymosin, a thymic hormone. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2734–2738. doi: 10.1073/pnas.68.11.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Dauphinee M. J., Talal N., Goldstein A. L., White A. Thymosin corrects the abnormal DNA synthetic response of NZB mouse thymocytes. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2637–2641. doi: 10.1073/pnas.71.7.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish W. W., Mann K. G., Tanford C. The estimation of polypeptide chain molecular weights by gel filtration in 6 M guanidine hydrochloride. J Biol Chem. 1969 Sep 25;244(18):4989–4994. [PubMed] [Google Scholar]
- Goldstein A. L., Cohen G. H., Rossio J. L., Thurman G. B., Brown C. N., Ulrich J. T. Use of thymosin in the treatment of primary immunodeficiency diseases and cancer. Med Clin North Am. 1976 May;60(3):591–606. doi: 10.1016/s0025-7125(16)31900-9. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., Guha A., Zatz M. M., Hardy M. A., White A. Purification and biological activity of thymosin, a hormone of the thymus gland. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1800–1803. doi: 10.1073/pnas.69.7.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Slater F. D., White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proc Natl Acad Sci U S A. 1966 Sep;56(3):1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M. A., Zisblatt M., Levine N., Goldstein A. L., Lilly F., White A. Reversal by thymosin of increased susceptibility of immunosuppressed mice to Moloney sarcoma virus. Transplant Proc. 1971 Mar;3(1):926–928. [PubMed] [Google Scholar]
- Hooper J. A., McDaniel M. C., Thurman G. B., Cohen G. H., Schulof R. S., Goldstein A. L. Purification and properties of bovine thymosin. Ann N Y Acad Sci. 1975 Feb 28;249:125–144. doi: 10.1111/j.1749-6632.1975.tb29063.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H. Acetylation of Protein N-terminal amino groups structural observations on alpha-amino acetylated proteins. J Theor Biol. 1975 Nov;55(1):1–12. doi: 10.1016/s0022-5193(75)80105-6. [DOI] [PubMed] [Google Scholar]
- Khaw B. A., Rule A. H. Immunotherapy of the Dunning leukaemia with thymic extracts. Br J Cancer. 1973 Oct;28(4):288–292. doi: 10.1038/bjc.1973.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Reaction of peptides with fluorescamine on paper after chromatography or electrophoresis. Anal Biochem. 1975 May 12;65(1-2):281–292. doi: 10.1016/0003-2697(75)90511-4. [DOI] [PubMed] [Google Scholar]
- Nakai N., Lai C. Y., Horecker B. L. Use of fluorescamine in the chromatographic analysis of peptides from proteins. Anal Biochem. 1974 Apr;58(2):563–570. doi: 10.1016/0003-2697(74)90225-5. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Penke B., Ferenczi R., Kovács K. A new acid hydrolysis method for determining tryptophan in peptides and proteins. Anal Biochem. 1974 Jul;60(1):45–50. doi: 10.1016/0003-2697(74)90129-8. [DOI] [PubMed] [Google Scholar]
- Schafer L. A., Goldstein A. L., Gutterman J. U., Hersh E. M. In vitro and in vivo studies with thymosin in cancer patients. Ann N Y Acad Sci. 1976;277(00):609–620. doi: 10.1111/j.1749-6632.1976.tb41733.x. [DOI] [PubMed] [Google Scholar]
- Scheinberg M. A., Goldstein A. L., Cathcart E. S. Thymosin restores T cell function and reduces the incidence of amyloid disease in casein-treated mice. J Immunol. 1976 Jan;116(1):156–158. [PubMed] [Google Scholar]
- Schlesinger D. H., Goldstein G., Niall H. D. The complete amino acid sequence of ubiquitin, an adenylate cyclase stimulating polypeptide probably universal in living cells. Biochemistry. 1975 May 20;14(10):2214–2218. doi: 10.1021/bi00681a026. [DOI] [PubMed] [Google Scholar]
- Schlesinger D. H., Goldstein G. The amino acid sequence of thymopoietin II. Cell. 1975 Aug;5(4):361–365. doi: 10.1016/0092-8674(75)90054-9. [DOI] [PubMed] [Google Scholar]
- Wara D. W., Ammann A. J. Editorial: Thymic cells and humoral factors as therapeutic agents. Pediatrics. 1976 May;57(5):643–646. [PubMed] [Google Scholar]
- Wara D. W., Goldstein A. L., Doyle N. E., Ammann A. J. Thymosin activity in patients with cellular immunodeficiency. N Engl J Med. 1975 Jan 9;292(2):70–74. doi: 10.1056/NEJM197501092920204. [DOI] [PubMed] [Google Scholar]
- Wilson G. B., Jahn T. L., Fonseca J. R. Demonstration of serum protein differences in cystic fibrosis by isoelectric focusing in thin-layer polyacrylamide gels. Clin Chim Acta. 1973 Nov 23;49(1):79–91. doi: 10.1016/0009-8981(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Zisblatt M., Goldstein A. L., Lilly F., White A. Acceleration by thymosin of the development of resistance to murine sarcoma virus-induced tumor in mice. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1170–1174. doi: 10.1073/pnas.66.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]