Abstract
♦ Background and Objectives: Peritoneal dialysis catheter (PDC) complications are an important barrier to peritoneal dialysis (PD) utilization. Practice guidelines for PDC placement exist, but it is unknown if these recommendations are followed. We performed a quality improvement study to investigate this issue.
♦ Methods: A prospective observational study involving 46 new patients at a regional US PD center was performed in collaboration with a nephrology fellowship program. Patients completed a questionnaire derived from the International Society for Peritoneal Dialysis (ISPD) catheter guidelines and were followed for early complications.
♦ Results: Approximately 30% of patients reported not being evaluated for hernias, not being asked to visualize their exit site, or not receiving catheter location marking before placement. After insertion, 20% of patients reported not being given instructions for follow-up care, and 46% reported not being taught the warning signs of PDC infection. Directions to manage constipation (57%), immobilize the PDC (68%), or leave the dressing undisturbed (61%) after insertion were not consistently reported. Nearly 40% of patients reported that their PDC education was inadequate. In 41% of patients, a complication developed, with 30% of patients experiencing a catheter or exit-site problem, 11% developing infection, 13% needing PDC revision, and 11% requiring unplanned transfer to hemodialysis because of catheter-related problems.
♦ Conclusions: There were numerous deviations from the ISPD guidelines for PDC placement in the community. Patient satisfaction with education was suboptimal, and complications were frequent. Improving patient education and care coordination for PDC placement were identified as specific quality improvement needs.
Keywords: Complications, patient education, peritoneal dialysis catheter, practice guidelines, quality improvement
Skilled peritoneal dialysis catheter (PDC) placement is vital to the success of any peritoneal dialysis (PD) program (1-9). The PDC is the lifeline for PD patients and an important target for quality improvement efforts (1-5,8,10-13). Malfunction of the PDC and related complications can lead to patient discomfort, infection, a need for revision, and technique failure (3,4,6,12-15). These events can be quite frustrating for patients and providers, increasing health care costs and potentially contributing to reduced utilization of PD (6,7,12,13,15-17). Although the technical competence of operators is vital, successful PDC placement involves more than just the insertion procedure; it relies on adherence to established protocols and care before, during, and after surgery (1,2,4-6,13,16,18-22). Expert bodies have established clinical practice guidelines providing specific recommendations for each step involved in PDC placement (1-5).
Although some evidence is inconclusive, strong consensus has developed about most components of PDC care (1-5,23). Evaluation for proper catheter and exit-site location and detection of hernias are recommended during the preoperative assessment (1-3,6,19,24). Screening for nasal carriage of methicillin-resistant Staphylococcus aureus and antibiotic prophylaxis during insertion are supported by evidence (1-5). Strict post-operative exit-site and bowel care are essential to prevent complications (1-5).
Despite the existence of best-practice guidelines, concerns have been raised that these recommendations are not followed by practitioners (11,12,21,22,25). Furthermore, deviation from recommendations may be an underappreciated contributor to PD technique failure (11,12,21,22).
Quality improvement for PDC placement may be challenging because of deficiencies in physician and nursing knowledge, lack of established protocols, and inconsistent practice patterns (10,12,21,22,26). In particular, the education and care patients receive related to PDC placement may be difficult to assess because of a lack of available metrics. Additionally, the domain of patient experience and perspective about their dialysis care is poorly studied, but has gained increasing recognition as a relevant and necessary measure of quality (10,27).
At a large regional PD center, we devised a quality improvement project in conjunction with a nephrology fellowship program to examine PDC placement in the community. We sought to determine the extent of PDC placement care from the patients’ perspective and to measure early PDC complications. We gathered this data to identify targets for quality improvement and to provide systems-based education to nephrology fellows (28).
Methods
This prospective observational study of PD patients was conducted at the Northwest Kidney Centers, a regional PD program serving the greater urban Seattle community and a mixed base of private and academic nephrologists. The study took place between January and December 2010 and was designed as a quality improvement study by University of Washington nephrology fellows under the guidance of the PD medical director and nursing leadership. Fellow participation was authorized by the fellowship training program director. The study protocol was approved by the Institutional Review Board of the University of Washington. All patients provided written informed consent.
Study Population
All patients presenting for PD-related services were evaluated. Patients less than 18 years of age and those who were pregnant, who left PD before recruitment, or whose nephrologist declined participation in the study were not eligible for enrollment. Only English- or Spanish-speaking patients were eligible. The study population was divided into new and existing patients. “New patient” was defined as any incident PD patient or any patient transferring from hemodialysis (HD) or a failed transplant with a new PDC. Initiation of PD was defined as the first day that Medicare or private insurance was billed for treatment; new patients were typically enrolled within 30 days of that date. “Existing patient” was defined as any patient enrolled who had already been on PD for more than 30 days.
Data Collection
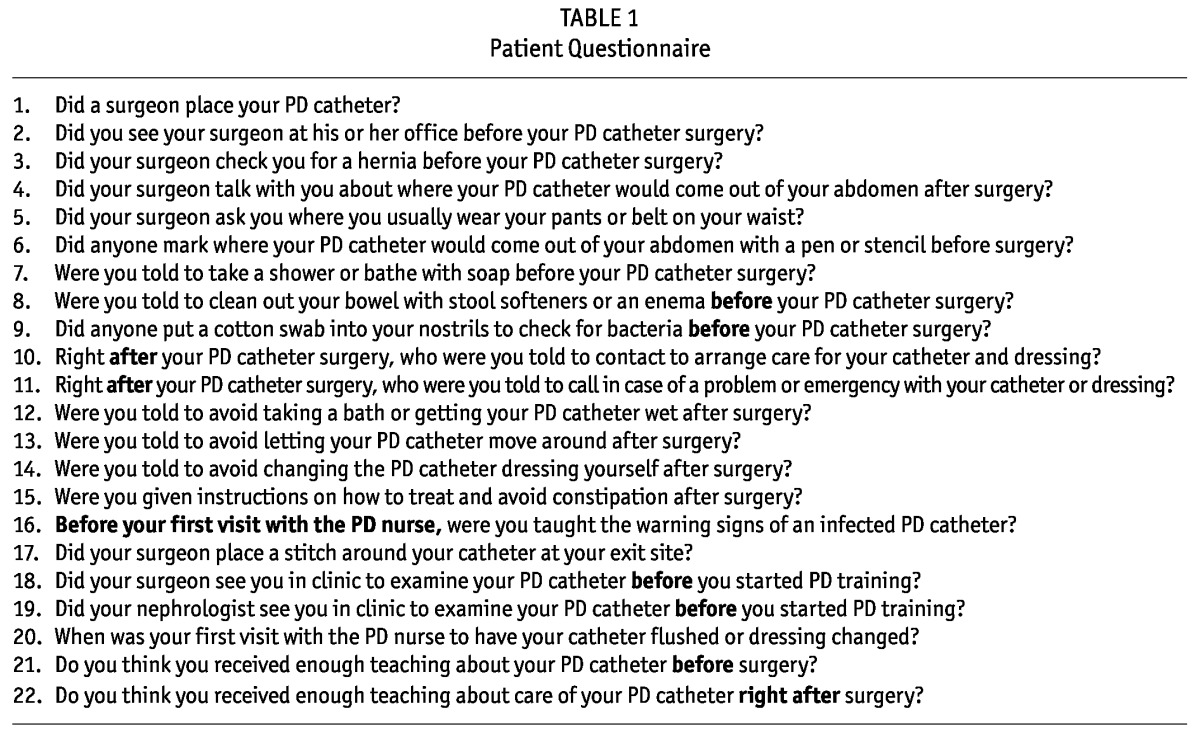
Based on the 2005 and 1998 catheter guidelines and the best practice recommendations published by the International Society for Peritoneal Dialysis (3,4,6), investigators and PD nurses developed a questionnaire about preoperative and postoperative PDC patient education and care (Table 1). The questionnaire was given to all patients after written consent had been obtained. Information on age, ethnicity, sex, history of previous dialysis, body mass index, comorbidities, and education level was obtained. Local concerns communicated to investigators during study design suggested that linking complications to specific providers would discourage study participation, and so explicit assurances were issued that no physician-specific data would be gathered.
TABLE 1.
Patient Questionnaire
Because of concerns about recall bias affecting the responses of patients less proximate to their PDC placement, the responses of existing patients were recorded for information purposes only. New PD patients who completed the questionnaire were followed prospectively for complications from study entry to 90 days after PD initiation, including the time period before PD training. The investigators and PD nurses met regularly to review study enrollment and data collection, and to adjudicate reported complications. Patients who failed to complete the questionnaire or for whom significant clinical data were missing were subsequently excluded.
Study Endpoints
Questionnaire responses were grouped into two categories: preoperative PDC preparation and post-operative PDC care. Only “yes” and “no” responses were included in the study. Patients who answered “I don’t remember” or who did not respond to a particular question were not included in the denominator for that analysis. Patients were followed prospectively for these categories of complications:
Infection (peritonitis or exit-site infection)
Catheter or exit-site problem (hardware problem, tip migration, kinking, flow problems significant enough to interrupt therapy, exit-site suture, upward-facing exit site, or cuff extrusion)
Anatomic problems (hernia, dialysate leak, omental wrapping, or hydrothorax)
Need for intervention (catheter revision or replacement)
Unplanned transfer to HD (temporary or permanent) resulting from catheter-related problems
Need for intervention or HD were counted as separate events in addition to any underlying complications. Events were reported by the nursing staff to investigators using a standardized tracking form and were verified from medical records by investigator adjudication. Patient characteristics were compared between groups with and without complications.
Statistical Analysis
Continuous variables are expressed as means ± standard deviation or medians with interquartile range (or the full range). Categorical variables are expressed as proportions. Between-group differences were analyzed using the Student t-test, the Mann-Whitney test, the chi-square test, or the Fisher exact test. We considered two-tailed p values less than 0.05 to be statistically significant. All statistical analyses were performed using the SAS software application (version 9.3: SAS Institute, Cary, NC, USA).
Results
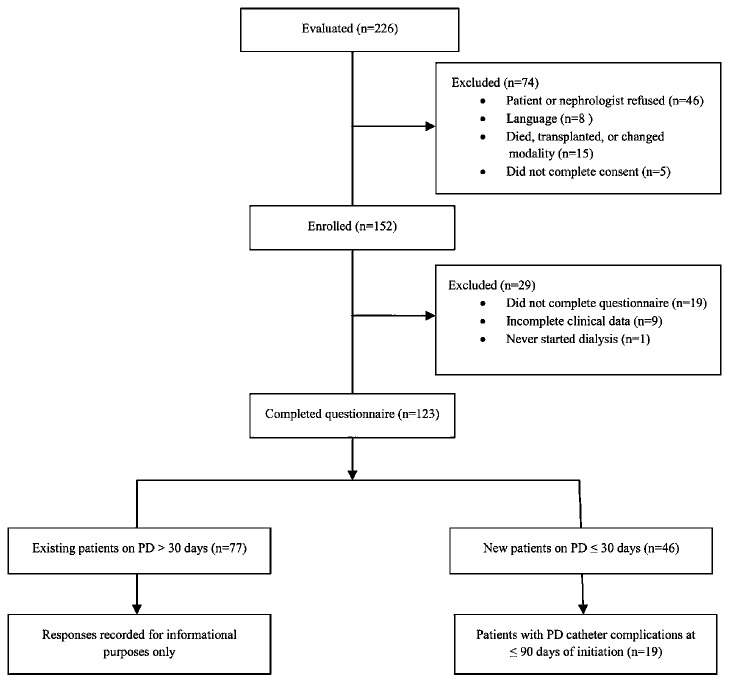
Figure 1 shows patient selection for the study. Of 226 potential participants, 74 were excluded, largely because of patient or nephrologist refusal to participate. Of 152 patients enrolled, 29 were subsequently excluded after failing to complete the questionnaire or for incomplete clinical data. The characteristics and responses from 77 existing PD patients were recorded for information purposes only (Tables 2, 3, 4). The 46 new PD patients who completed the questionnaire were followed prospectively until 90 days after PD initiation. Of those patients, 19 (41%) developed a complication during the study.
Figure 1 —
Flow diagram of patient selection. PD = peritoneal dialysis.
TABLE 2.
Characteristics of Existing and New Patients
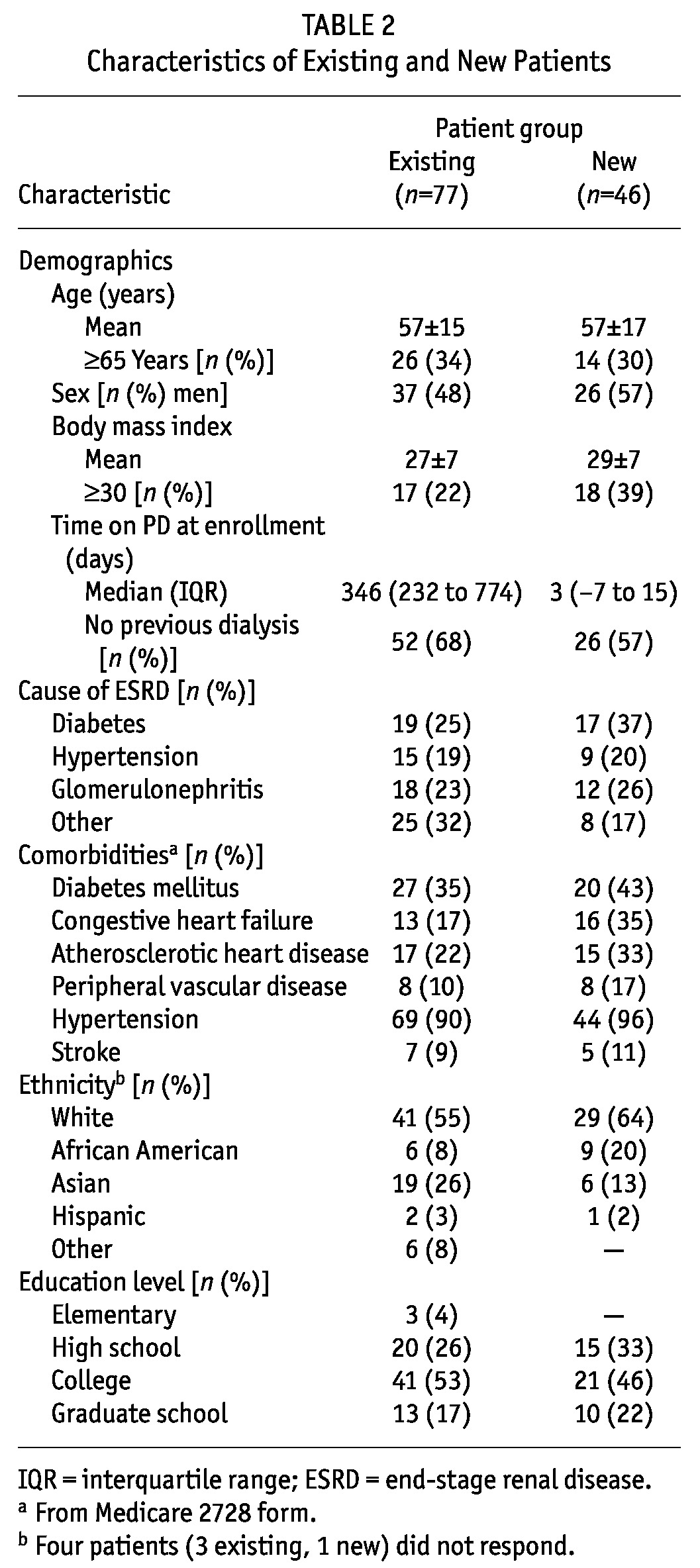
TABLE 3.
Preoperative Peritoneal Dialysis (PD) Catheter Education and Care: Existing Patients and New Patients
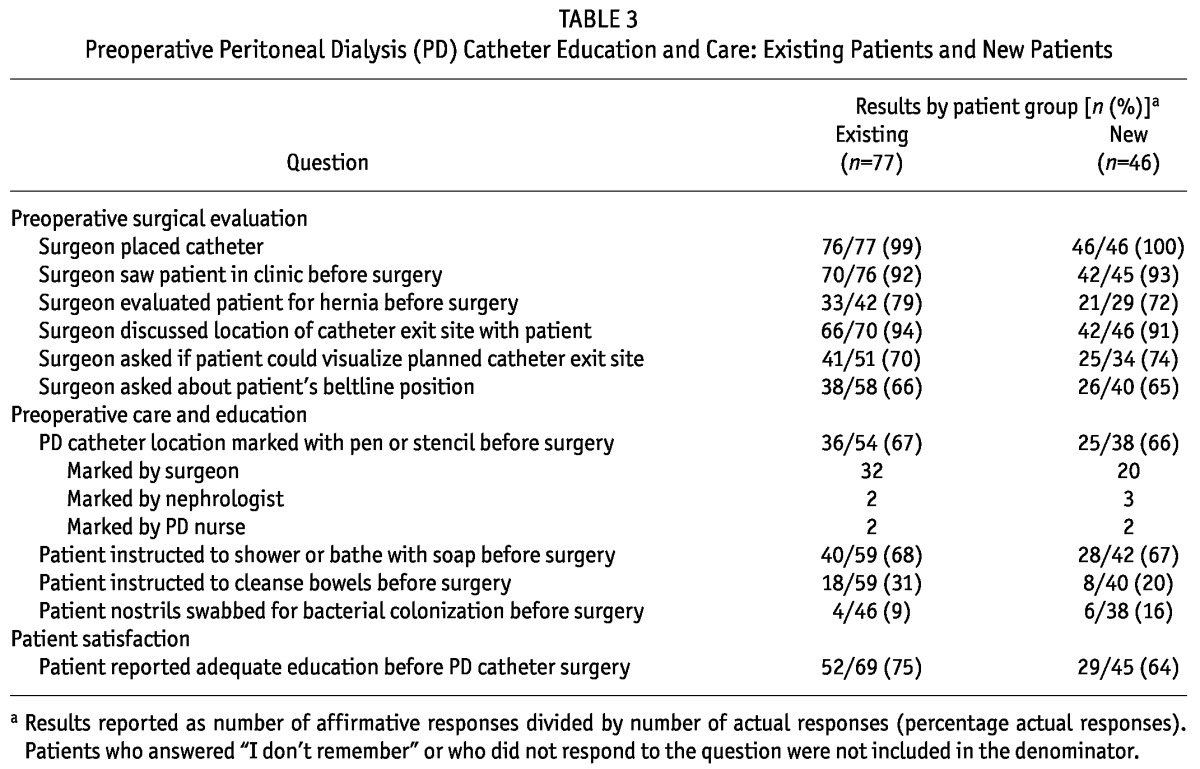
TABLE 4.
Postoperative Peritoneal Dialysis (PD) Catheter Education and Care: Existing Patients and New Patients
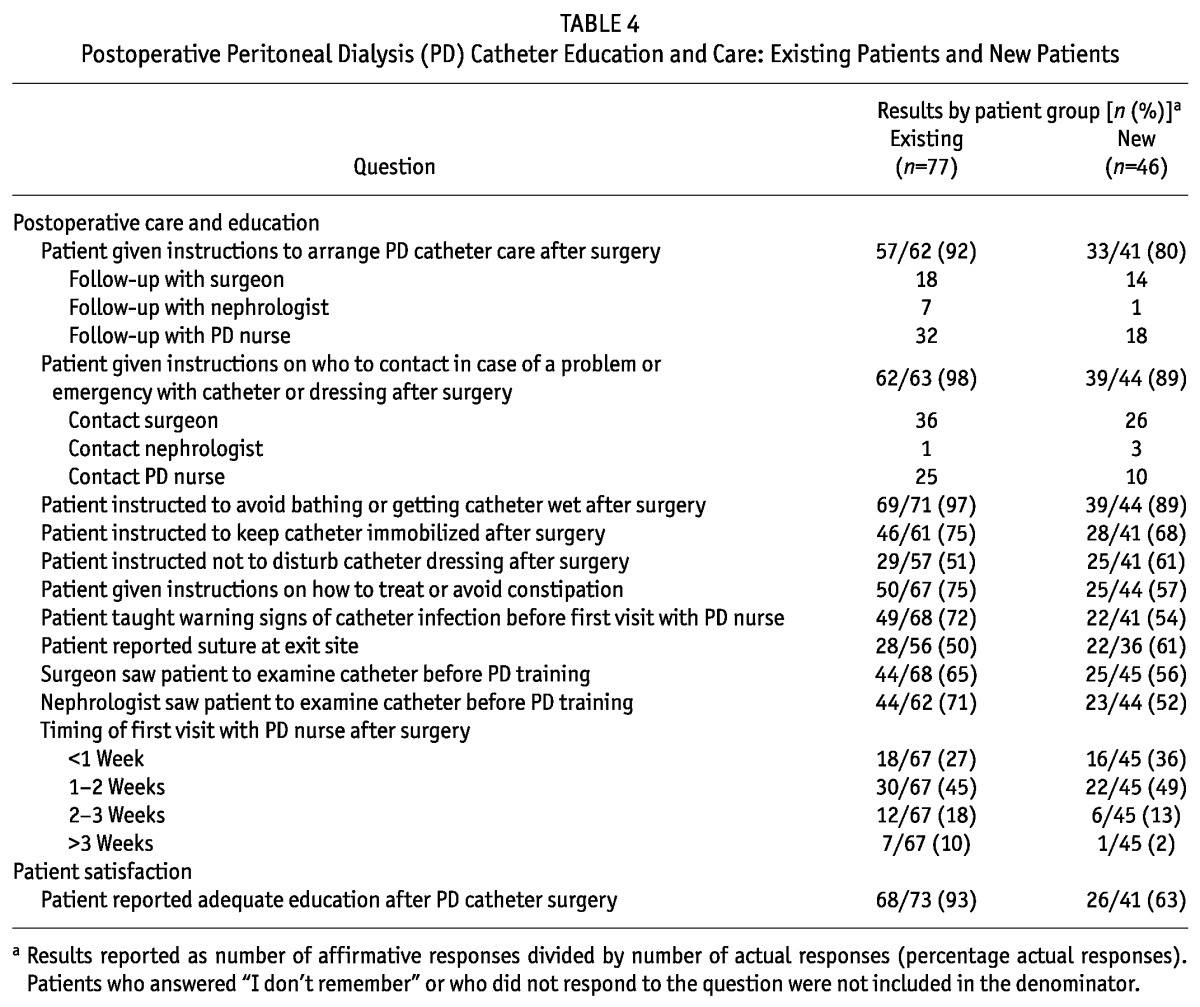
Table 5 shows the characteristics of the new patients. Mean age was 57 years, with 30% of patients being 65 years of age or older. Men constituted 57% of the study population, and 39% of the patients were obese (body mass index > 30). Median time on PD at enrollment was 3 days. A sizeable proportion of the patients (43%) had transferred from HD. Hypertension (96%), diabetes (43%), congestive heart failure (35%), and atherosclerotic heart disease (33%) were common comorbidities. Most patients were white (64%) and educated, with 68% having attended college or graduate school. Patients with complications were more likely to be obese (p = 0.03) or to have peripheral vascular disease (p = 0.05). We observed no other significant differences between patients with and without complications.
TABLE 5.
Patient Characteristics
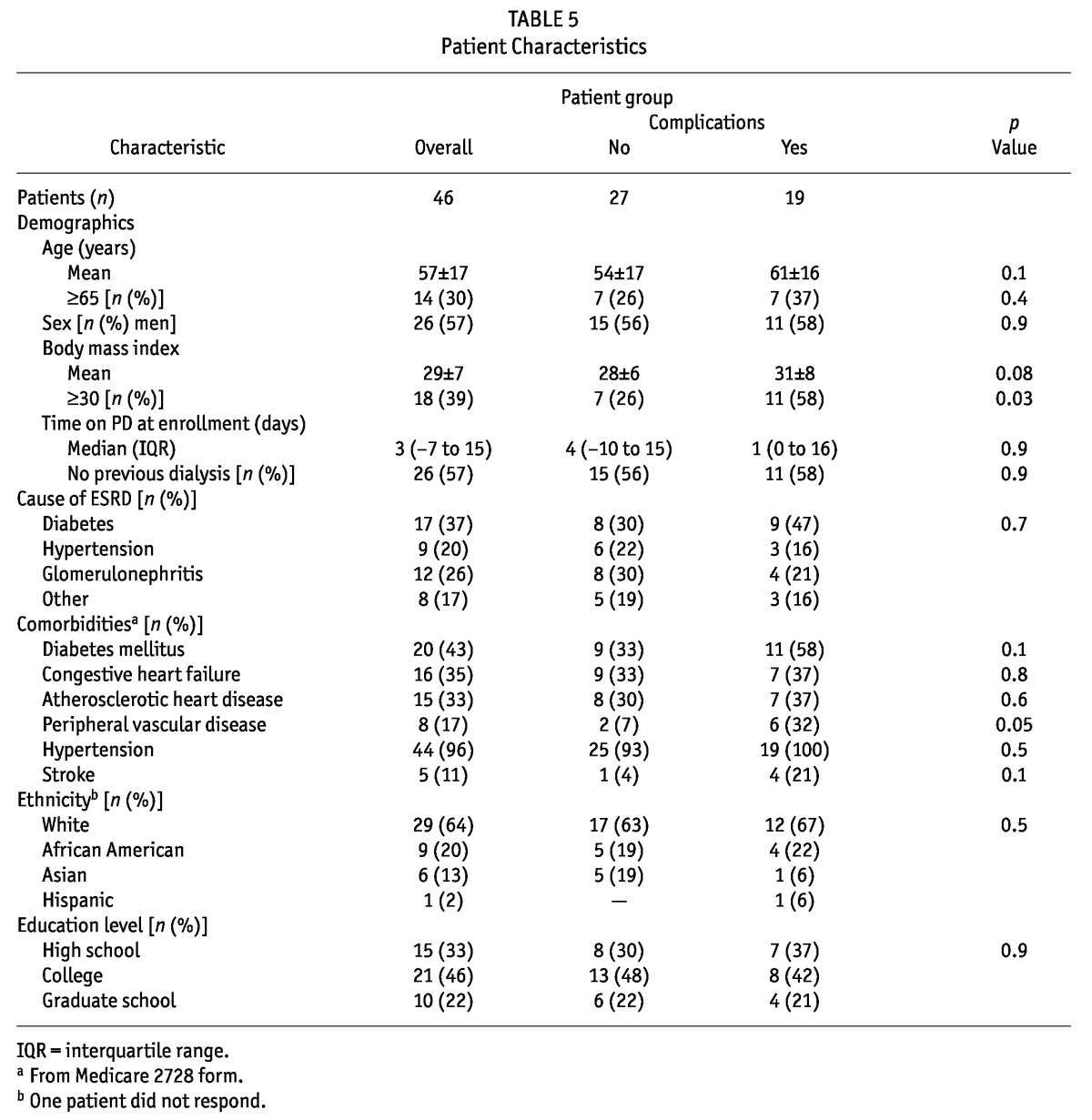
Table 6 shows responses by patients about preoperative PDC preparation. All PDCs were placed by surgeons. Most patients reported being seen by the surgeon before PDC placement. Among the responding patients, 72% reported being evaluated for a hernia, though 37% of the patients did not remember an evaluation or did not answer the question. Only 74% reported their surgeon asking if they could visualize their exit site, and 65% reported being asked about beltline location. Only 66% of patients reported having their PDC location marked preoperatively. Instructions to bathe before surgery were reported by 67% of patients. Only 20% of patients reported being directed to cleanse their bowels before surgery. Nasal swabbing for S. aureus was rarely reported. Among responding patients, 64% reported receiving adequate education about their catheter before surgery. Responses did not differ between patients with and without complications.
TABLE 6.
Preoperative Peritoneal Dialysis (PD) Catheter Education and Care
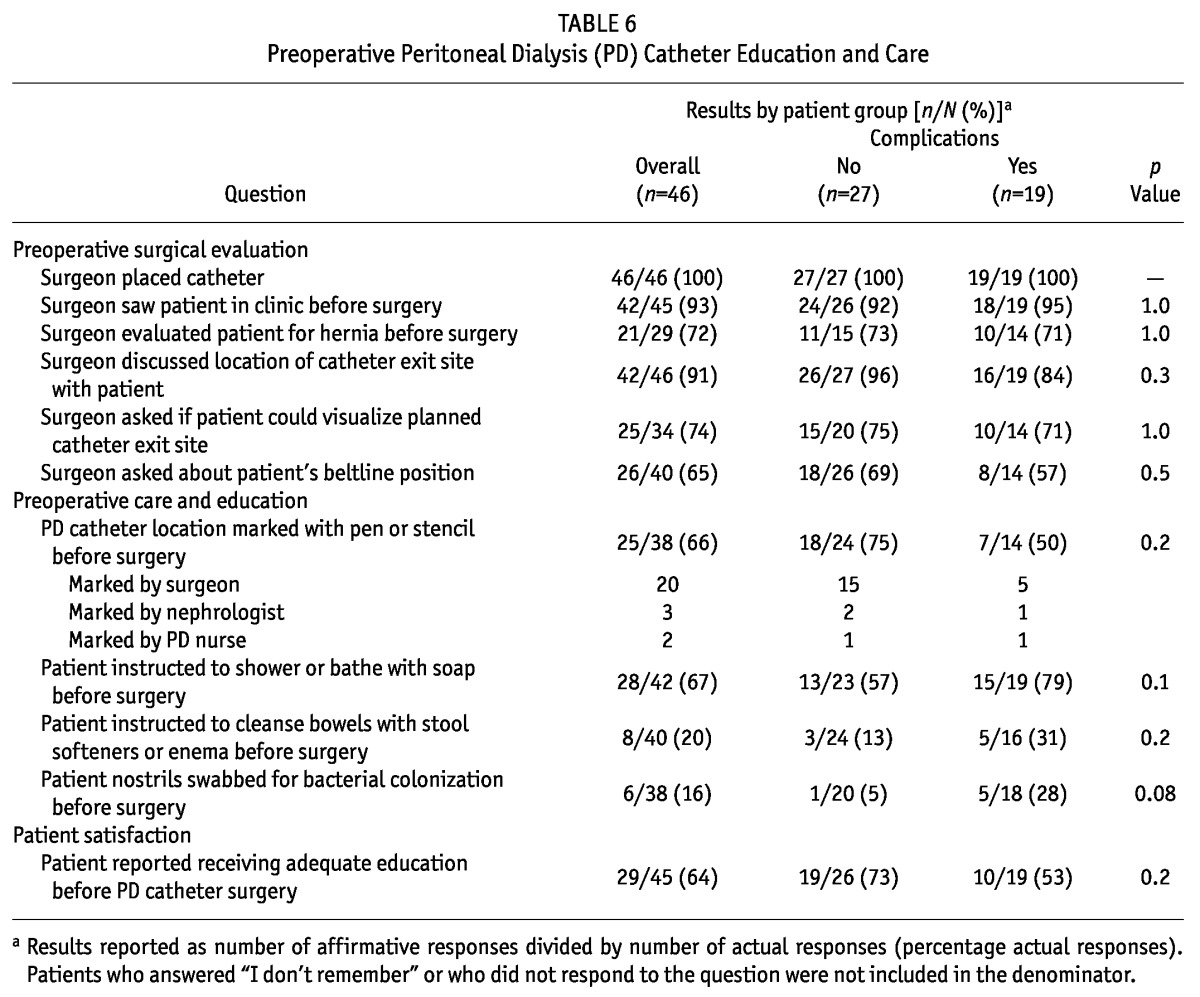
Table 7 shows responses related to postoperative PDC care. Of responding patients, 80% reported being given instructions to arrange for PDC care after surgery. Patients chiefly reported being told to visit the PD nurse or the surgeon for this care. Not being given instructions about whom to contact in case of a problem or emergency after surgery was reported by 11% of patients. Postoperative instructions to avoid bathing or getting the PDC wet (89%), to keep the PDC immobilized (68%), or to leave the PDC dressing undisturbed (61%) were not consistently reported. Directions about how to treat and avoid constipation—a preventable cause of PDC malfunction—were reported by only 57% of patients. Only 54% of patients reported being taught the warning signs of PDC infection before their first visit with the PD nurse. Although 85% of patients reported being seen by the PD nurse within 2 weeks of surgery, only about half reported seeing either their surgeon or nephrologist in the interval between surgery and PD training. A suture at the exit site was reported by 61% of patients. Only 63% of patients reported receiving adequate PDC education after surgery. We observed no differences between the responses of patients with and without complications.
TABLE 7.
Postoperative Peritoneal Dialysis (PD) Catheter Education and Care
Figure 2 categorizes 33 PDC events observed in 19 patients. As Table 8 shows, 11% of patients developed an infection, and 30% experienced a catheter or exit-site problem. Most problems involving the PDC or exit site were detected by the PD nurses.
Figure 2 —
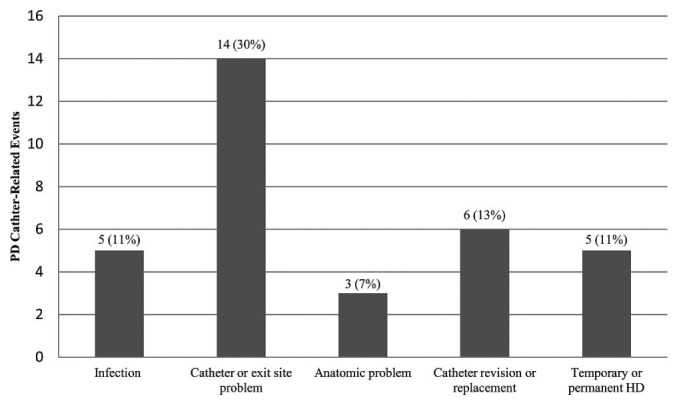
Peritoneal dialysis (PD) catheter-related complications by category [n (%) new patients]. HD = hemodialysis.
TABLE 8.
Timing of Peritoneal Dialysis (PD) Catheter-Related Complications
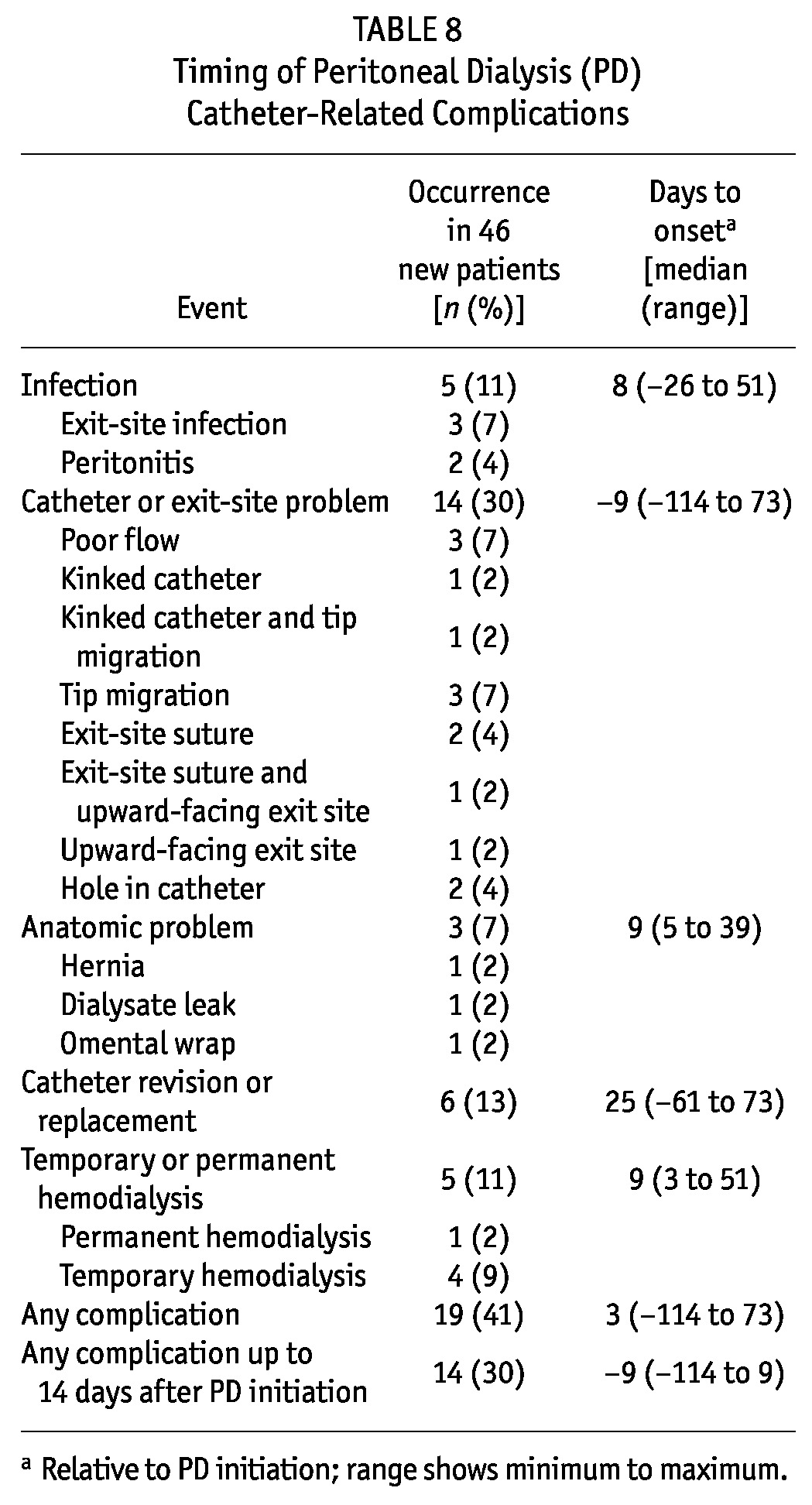
Overall, 13% of patients required intervention, and 11% required unplanned HD—some of whom had multiple concurrent PDC issues. Reasons for intervention included poor flow, hole in the PDC (2 patients), hernia with kinked catheter and tip migration, exit-site infection and tip migration, and tip migration alone. All interventions were performed by surgeons. The PDC-related reasons for unplanned HD included dialysate leak, tip migration, hernia with kinked catheter and tip migration, exit-site infection and tip migration, and omental wrap (resulting in permanent HD).
As seen in Table 8, 19 of 46 patients (41%) experienced a complication within 90 days of PD start (median onset: 3 days), with 14 of the 19 (74%) experiencing a complication before being on PD more than 14 days (median onset: 9 days). Overall, 30% of new PD patients experienced a catheter-related complication before being on PD more than 2 weeks. Ten patients (22% overall) experienced more than 1 complication. Given the small sample size, we did not perform any further analysis of outcomes.
Discussion
A fundamental requirement for successful PDC placement is a team approach involving nephrologists, PD nurses, surgeons, and increasingly, interventional nephrologists and radiologists (1-5,12,13,29-32). Each member of this access team must understand and appreciate the details involved in PDC placement (1-5). Our study suggested care deficiencies at all stages of the PDC placement process.
Of responding patients, 7% reported seeing their surgeon only at the time of surgery. Inconsistent reports of preoperative hernia evaluation and exit-site marking reinforced concerns about operator knowledge of best demonstrated practices (6,16,17). Simple instructions such as bathing before surgery and bowel preparation in particular were not always reported. Surveillance for S. aureus did not appear to be widely practiced. We did not ask patients about prophylactic antibiotic use because of an inability to verify administration and the possibility that the question might be misinterpreted.
Our patients reported that basic precautions such as catheter immobilization, an undisturbed dressing, and who to contact for problems were not consistently communicated. Although the onus is on the operator to ensure that adequate postoperative care is given, a gap may occur if the operator does not or chooses not to provide that care. Invariably, the PD nurse assumes this responsibility, but may be greatly disadvantaged if there is inadequate handoff or communication from the operator, who may not appreciate the strict attention to detail needed for PDC care (personal observation). Poor understanding of this role by some operators may stem from inadequate training in PDC placement, a prevalent issue in the United States (17). This lack of effective coordination between caregivers may explain why so many patients rated their PDC-related education as inadequate.
Defining PDC complications is not always straight-forward or consistent (1-5,12,29,31,33). A strict classification of PDC complications includes only those resulting in intervention or technique failure, including death (31). To capture the entire spectrum of PDC problems encountered, we set a lower threshold for defining complications. Even if a complication does not lead to an intervention, the increased morbidity adds to the psychological burden of PD, which may contribute to patient burnout (6,15-17). Although some complications (such as upward-facing exit sites and exit-site sutures) may not result in discontinuation of PD, they are completely avoidable and attributable solely to poor operator technique. These potential targets for quality improvement might be missed with a higher threshold for classification. In the present report, the incidence of serious complications requiring intervention or unplanned HD appeared high compared with those in other series (13,29,31,33). Although only 1 patient experienced permanent technique failure, the situation could have been worse were it not for the experienced staff of a large regional PD center.
Our study has several strengths. The reported experience represents a realistic practice environment and illustrates the challenge of addressing PDC complications in a diverse community of surgeons and nephrologists. Delivery of PD care can be hindered if the various providers are not aligned with respect to best practices, coordination of care, and communication (27). Our experience may be valuable for other PD programs in the United States to review, because PD access may be one of the factors limiting PD growth in their communities (7,9). Because we examined patient experiences with their PDC care, rather than survey providers, our study is an example of patient-centered quality improvement (10,27). Education content is important, but the perception and retention of that content by patients largely determines its effectiveness. Those concepts should not be neglected, because experience and satisfaction of patients with their modality education may influence outcomes by affecting their behavior and feelings about PD (10,27). We did not observe a difference in complications for patients who rated their teaching as inadequate, but that finding does not disprove the impact of education. The next step in the quality improvement process would be to act by implementing education measures and measuring the results, repeating the cycle as necessary until the desired results are achieved (27,34).
A primary tenet of quality improvement is that outcomes should be studied and root causes identified to help drive process change (27,34-36). Although we assured referring nephrologists that neither they nor their surgeons would be identified, a number of providers declined participation. Those choices may reflect reluctance by physicians in general to address negative patient outcomes. Unfortunately, many physicians lack training in quality improvement, and few detailed examples of PD quality improvement are available (8,10,11,27). Although our goal was to improve nephrology fellowship training, it is imperative that all stakeholders involved in PDC placement—dialysis nurses, nephrologists, surgeons, interventional nephrologists, and interventional radiologists—be educated about best practices and participate in quality improvement (1,2,6,8,27). One step would be for institutions to recognize this need and devote resources to ensure adequate training of operators (16,17).
Limitations of our study include its observational design, questionnaire validity, lack of physician-specific data, and sample size. Patient responses were subject to recall bias, and we could not validate whether a particular intervention was actually performed or not. Non-responses could have affected the results. For example, approximately 40% of patients did not remember being checked for a hernia or did not respond to the question. It is possible that underreporting of care occurred if patients did not understand or realize that care was being delivered to them. Measurement error and response bias may have occurred. For example, 61% of patients reported an exit-site suture, but a suture was detected in only 7% on exam. A number of patients were not permitted to enroll, which might have resulted in non-response bias. It is possible that the participants, many of whom were highly educated, had higher-than-average expectations about their teaching. We did not attempt to compare placement techniques, PDC types, or PD modalities. Because all catheters were placed surgically, we had no data about interventional nephrology or radiology practices. We were not permitted to gather physician-specific data and did not study patterns of operator PDC placement.
Conclusions
The recommended patient education and care for PDC placement at a large US regional PD program appeared inconsistent and suboptimal. Many patients reported their PDC education as inadequate, and catheter-related complications were significant in number. Quality improvement efforts should aim to increase physician awareness of International Society for Peritoneal Dialysis catheter guidelines, to improve patient education, and to develop better care processes by stakeholders to ensure a more coordinated approach to PDC placement. Whether such interventions will affect outcomes is not known, but they seem prudent based on current standards.
Disclosures
There were no sources of funding or author financial conflicts of interest related to this study.
Acknowledgments
The authors thank the PD staff of Northwest Kidney Centers and Dr. Rudolph Rodriguez, VA Puget Sound Health Care System, for their support. These data were presented as an abstract at the American Society of Nephrology Renal Week; Denver, Colorado; 16-21 November 2010.
References
- 1. Figueiredo A, Goh BL, Jenkins S, Johnson DW, Mactier R, Ramalakshmi S, et al. Clinical practice guidelines for peritoneal access. Perit Dial Int 2010; 30:424–9 [DOI] [PubMed] [Google Scholar]
- 2. UK Renal Association. Home > Clinical > Guidelines > Peritoneal Access [Web page]. Petersfield, UK: Renal Association; 2009. [Available online at: http://www.renal.org/Clinical/GuidelinesSection/PeritonealAccess.aspx; accessed 14 May 2012] [Google Scholar]
- 3. Flanigan M, Gokal R. Peritoneal catheters and exit-site practices toward optimum peritoneal access: a review of current developments. Perit Dial Int 2005; 25:132–9 [PubMed] [Google Scholar]
- 4. Gokal R, Alexander S, Ash S, Chen TW, Danielson A, Holmes C, et al. Peritoneal dialysis catheters and exit-site practices toward optimum peritoneal access: 1998 update. (Official report from the International Society for Peritoneal Dialysis). Perit Dial Int 1998; 18:11–33 [PubMed] [Google Scholar]
- 5. Dombros N, Dratwa M, Feriani M, Gokal R, Heimbürger O, Krediet R, et al. on behalf of the EBPG Expert Group on Peritoneal Dialysis. European best practice guidelines for peritoneal dialysis. 3 Peritoneal access. Nephrol Dial Transplant 2005; 20(Suppl 9):ix8–12 [DOI] [PubMed] [Google Scholar]
- 6. Crabtree JH. Selected best demonstrated practices in peritoneal dialysis access. Kidney Int Suppl 2006; (103):S27–37 [DOI] [PubMed] [Google Scholar]
- 7. Chaudhary K, Sangha H, Khanna R. Peritoneal dialysis first: rationale. Clin J Am Soc Nephrol 2011; 6:447–56 [DOI] [PubMed] [Google Scholar]
- 8. Finkelstein FO. Structural requirements for a successful chronic peritoneal dialysis program. Kidney Int Suppl 2006; (103):S118–21 [DOI] [PubMed] [Google Scholar]
- 9. Gadallah MF, Ramdeen G, Torres-Rivera C, Ibrahim ME, Myrick S, Andrews G, et al. Changing the trend: a prospective study on factors contributing to the growth rate of peritoneal dialysis programs. Adv Perit Dial 2001; 17:122–6 [PubMed] [Google Scholar]
- 10. Peritoneal Dialysis Working Group. Clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis 2006; 48(Suppl 1):S98–129 [DOI] [PubMed] [Google Scholar]
- 11. Jose MD, Johnson DW, Mudge DW, Tranæus A, Voss D, Walker R, et al. Peritoneal dialysis practice in Australia and New Zealand: a call to action. Nephrology (Carlton) 2011; 16:19–29 [DOI] [PubMed] [Google Scholar]
- 12. Taro Y, Yoshimoto A, Kawakita M, Ueta H, Toda N, Utsunomiya N, et al. Impact of the inclusion of a nephrologist on the surgical team for peritoneal catheter insertion. Perit Dial Int 2012; 32:346–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brunier G, Hiller JA, Drayton S, Pugash RA, Tobe SW. A change to radiological peritoneal dialysis catheter insertion: three-month outcomes. Perit Dial Int 2010; 30:528–33 [DOI] [PubMed] [Google Scholar]
- 14. Mujais S, Story K. Peritoneal dialysis in the US: evaluation of outcomes in contemporary cohorts. Kidney Int Suppl 2006; (103):S21–6 [DOI] [PubMed] [Google Scholar]
- 15. Diaz-Buxo JA. Management of peritoneal catheter malfunction. Perit Dial Int 1998; 18:256–9 [PubMed] [Google Scholar]
- 16. Crabtree JH. Who should place peritoneal dialysis catheters? Perit Dial Int 2010; 30:142–50 [DOI] [PubMed] [Google Scholar]
- 17. Wong LP, Liebman SE, Wakefield KA, Messing S. Training of surgeons in peritoneal dialysis catheter placement in the United States: a national survey. Clin J Am Soc Nephrol 2010; 5:1439–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang H, Bernardini J, Piraino B. Placement of peritoneal dialysis catheters on an outpatient basis. Perit Dial Int 2002; 22:616–18 [PubMed] [Google Scholar]
- 19. Crabtree JH, Fishman A. A laparoscopic method for optimal peritoneal dialysis access. Am Surg 2005; 71:135–43 [DOI] [PubMed] [Google Scholar]
- 20. Crabtree JH, Fishman A, Siddiqi RA, Hadnott LL. The risk of infection and peritoneal catheter loss from implant procedure exit-site trauma. Perit Dial Int 1999; 19:366–71 [PubMed] [Google Scholar]
- 21. Castro MJ, Vijt D, Endall G, Elseviers M, Lindley E. on behalf of the EDTNA/ERCA Research Board. Post insertion catheter care in peritoneal dialysis centers across Europe: results of the Post Insertion Project of the Research Board. EDTNA ERCA J 2004; 30:42–7 [DOI] [PubMed] [Google Scholar]
- 22. Vijt D, Castro MJ, Endall G, Lindley E, Elseviers M. on behalf of the EDTNA/ERCA Research Board. Post insertion catheter care in peritoneal dialysis (PD) centres across Europe—part 2: complication rates and individual patient outcomes. EDTNA ERCA J 2004; 30:91–6 [DOI] [PubMed] [Google Scholar]
- 23. Strippoli GF, Tong A, Johnson D, Schena FP, Craig JC. Catheter-related interventions to prevent peritonitis in peritoneal dialysis: a systematic review of randomized, controlled trials. J Am Soc Nephrol 2004; 15:2735–46 [DOI] [PubMed] [Google Scholar]
- 24. Crabtree JH. Construction and use of stencils in planning for peritoneal dialysis catheter implantation. Perit Dial Int 2003; 23:395–8 [PubMed] [Google Scholar]
- 25. Wilkie M, Wild J. Peritoneal dialysis access—results from a UK Survey. Perit Dial Int 2009; 29:355–7 [PubMed] [Google Scholar]
- 26. Crabtree JH. The use of the laparoscope for dialysis catheter implantation: valuable carry-on or excess baggage? Perit Dial Int 2009; 29:394–406 [PubMed] [Google Scholar]
- 27. Leung DK. Monitoring clinical standards in a chronic peritoneal dialysis program. Perit Dial Int 2009; 29(Suppl 2):S72–3 [PubMed] [Google Scholar]
- 28. Parker MG. Nephrology training in the 21st century: toward outcomes-based education. Am J Kidney Dis 2010; 56:132–42 [DOI] [PubMed] [Google Scholar]
- 29. Moon JY, Song S, Jung KH, Park M, Lee SH, Ihm CG, et al. Fluoroscopically guided peritoneal dialysis catheter placement: long-term results from a single center. Perit Dial Int 2008; 28:163–9 [PubMed] [Google Scholar]
- 30. Zaman F. Peritoneal dialysis catheter placement by nephrologist. Perit Dial Int 2008; 28:138–41 [PubMed] [Google Scholar]
- 31. Chow KM, Szeto CC, Leung CB, Kwan BC, Pang WF, Li PK. Tenckhoff catheter insertion by nephrologists: open dissection technique. Perit Dial Int 2010; 30:524–7 [DOI] [PubMed] [Google Scholar]
- 32. Crabtree JH. Fluoroscopic placement of peritoneal dialysis catheters: a harvest of low-hanging fruits. Perit Dial Int 2008; 28:134–7 [PubMed] [Google Scholar]
- 33. Liu WJ, Hooi LS. Complications after Tenckhoff catheter insertion: a single-center experience using multiple operators over four years. Perit Dial Int 2010; 30:509–12 [DOI] [PubMed] [Google Scholar]
- 34. Nicolay CR, Purkayastha S, Greenhalgh A, Benn J, Chaturvedi S, Phillips N, et al. Systematic review of the application of quality improvement methodologies from the manufacturing industry to surgical healthcare. Br J Surg 2012; 99:324–35 [DOI] [PubMed] [Google Scholar]
- 35. DeOreo PB. The medical directorship of renal dialysis facilities under the new Medicare conditions for coverage: challenges and opportunities. Blood Purif 2009; 27:16–21 [DOI] [PubMed] [Google Scholar]
- 36. Wish JB. What is expected of a medical director in the Centers for Medicare and Medicaid Services Conditions of Coverage? Blood Purif 2011; 31:61–5 [DOI] [PubMed] [Google Scholar]