Abstract
♦ Background: End-stage renal disease patients have significant cardiovascular morbidity and mortality, but little is known about differences in coagulation profiles between patients on hemodialysis (HD) and on peritoneal dialysis (PD). Given their long-term exposure to glucose-based dialysate, patients on PD can experience metabolic derangements. Theoretically, that exposure should create a more prothrombotic environment than occurs in HD patients. The objective of the present study was to quantify potential differences in baseline coagulation between PD and HD patients.
♦ Methods: Our single-center cross-sectional study at a large academic health science center enrolled 50 age-, race-, and sex-matched subjects (10 control subjects, 20 HD patients, and 20 PD patients). Measurements included platelet function, platelet receptor distribution, and coagulation dynamics by thromboelastography and Hemodyne hemostasis assay (Hemodyne, Richmond, VA, USA).
♦ Results: Compared with healthy control subjects, patients on both forms of dialysis showed prothrombotic coagulation protein profiles. The tissue-factor pathway was markedly elevated in both groups, but PD was associated with significantly greater concentrations of tissue factor (p = 0.0056) and tissue-factor pathway inhibitor (p = 0.0138). Similarly, compared with patients receiving HD, patients on PD had greater concentrations of fibrinogen (p = 0.0325), which corresponded with platelet hyperfunction as measured by platelet contractile force and clot elastic modulus (p = 0.003 and 0.017 respectively, compared with values in HD patients). Platelet receptor distribution was similar between the groups.
♦ Conclusions: Compared with patients on HD, patients on PD appear to have a more prothrombotic profile. The clinical relevance of these findings needs to be studied in a prospective manner.
Keywords: Hemodialysis, coagulation
Cardiovascular disease (CVD) is a leading killer in chronic kidney disease (CKD) patients (1,2). The morbidity and mortality statistics are staggering: nearly half of all people with end-stage renal disease will develop CVD, and cardiac deaths account for approximately 40% of all mortality in these individuals (2). Furthermore, the cardiovascular mortality rate in hemodialysis (HD) patients has been estimated to be up to 20 times that reported for the age-matched general population (3). The presence of traditional cardiac risk factors, such as diabetes mellitus, hypertension, dyslipidemia, and advanced age certainly contribute to CVD and are well described. Other possible mediators may lie within the inflammation-coagulation axis as suggested by the presence of nontraditional cardiac risk factors such as C-reactive protein, hyperhomocysteinemia, and activation of the coagulation cascade (4,5). Defects in both coagulation initiation and fibrinolysis have been identified in CKD patients. The tissue-factor (TF) pathway has been found to be upregulated in CKD patients, suggesting that events taking place during clot initiation may mediate the prothrombotic state (6-8). At the same time, altered fibrin clot structure leading to increased resistance to fibrinolysis has also been demonstrated both in diabetic patients and in CKD patients requiring HD or PD. Patients with diabetes tend to produce denser clots that are less porous and more resistant to fibrinolysis (9-11). Similar increases in clot density and resistance to fibrinolysis have been found in long-term PD patients (12) and specifically in those experiencing cardiovascular death (3).
The data suggesting that, compared with HD patients, those on PD may have higher rates of CVD events and mortality are conflicting (13,14). The debate is far from over, but the continuous nature of PD, with the long-term concomitant exposure to glucose-based dialysate, can create significant metabolic derangements such as hyperinsulinemia, dyslipidemia, and metabolic syndrome, which is important because of the known links between metabolic syndrome, endothelial dysfunction, inflammation, and a prothrombotic tendency. In theory, those metabolic derangements should therefore create a more prothrombotic environment than is seen in patients receiving HD. To date, however, few data have been published (15).
The purpose of the present pilot study was to quantify potential differences between PD and HD with respect to platelet function, thrombin generation, platelet receptor distribution, and functional clotting dynamics.
Methods
Study Design, Setting, and Patient Selection
This single-center cross-sectional pilot study set out to characterize differences in biochemical, cellular, and functional coagulation parameters in patients receiving maintenance HD and PD. It enrolled 50 age-, race-, and sex-matched subjects: 10 healthy volunteers who served as a reference standard; 20 subjects receiving thrice-weekly maintenance HD; and 20 subjects receiving maintenance continuous cycling PD. The continuous cycling PD regimen consisted of four 2-hour exchanges nightly and one 6-hour dialysis exchange daily. Each HD patient received thrice-weekly 4-hour high-flux HD sessions using a Fresenius Optiflux 180 dialyzer (Fresenius Medical Care North America, Waltham, MA, USA). Of the 20 HD patients, 18 had arteriovenous grafts, and 2 had tunneled central venous catheters because of multiple arteriovenous graft failures.
The dialysis prescriptions in both treatment groups were tailored to achieve goal Kt/V. All subjects received recombinant human erythropoietin as standard-of-care anemia treatment. Subjects were excluded if they had any of recent trauma or surgery (<7 days), active bleeding or a known bleeding disorder (for example, von Willebrand disease, hemophilia), active thrombosis or known thrombotic tendency (for example, antithrombin III, protein C, or protein S deficiency), cirrhosis or other liver abnormality, active cancer, thrombocytopenia (platelets < 100×109/L), or concurrent use of fish oil or antiplatelet or antithrombotic medications. The Virginia Commonwealth University Institutional Review Board approved the study before subject enrollment, and the study itself was conducted in accordance with the Declaration of Helsinki. All subjects provided written informed consent before study commencement. Upon enrollment of subjects into the study, demographics, laboratory chemistry parameters, and coagulation parameters were recorded.
Blood Sampling and Processing
Blood (approximately 25 mL) was collected through a 15-gauge needle into a syringe; 5 mL was injected into each of four 3.2% sodium citrate tubes, and 5 mL was injected into a serum separator tube. In HD patients, the blood samples were drawn immediately before dialysis to avoid interference with heparin administration, and all sodium citrate tubes were treated with 180 μL heparinase before sample processing to avoid potential heparin contamination. All blood samples were assayed within 2 hours of collection.
Coagulation Proteins
Coagulation proteins—TF, TF pathway inhibitor (TFPI), and von Willebrand factor (vWf)—were assessed by ELISA using commercially available kits (Imubind Tissue Factor, Imubind Total Tissue Factor Pathway Inhibitor, vWF kit: American Diagnostica, Stamford, CT, USA). Prothrombin fragments 1+2 and thrombin-antithrombin III complex (TAT) were analyzed using standard ELISA techniques (Enzygnost F 1+2 (monoclonal) and TAT micro: Siemens Healthcare Diagnostics, Marburg, Germany). Fibrinogen, prothrombin time, activated partial thromboplastin time, factor VII coagulant activity, and factor X activity were performed using the standard one-stage clotting assay (STart4 Hemostasis Analyzer: Diagnostica Stago, Parsippany, NJ, USA). All assays were performed according to the manufacturer’s instructions and run in duplicate; the average of the duplicate runs is reported.
Platelet Receptor Detection
Flow cytometric analysis was performed using citrated whole blood according to current standards from the European Working Group on Cell Analysis (16). To identify platelets and their activation status, CD41a conjugated with PE-Cy5 (mouse anti-human: BD Pharmingen, Franklin Lakes, NJ, USA), PAC-1 conjugated with fluorescein isothiocyanate conjugate [FITC (BD Biosciences, San Jose, CA, USA)], and CD62p conjugated with phycoerythrin [PE (mouse anti-human: BD Pharmingen)] were used. Corresponding isotype-matched monoclonal antibodies PE-Mouse IgG1-K Isotype, FITC-Mouse IgM-K Isotype, and PE-Cy5-Mouse IgG1-K Isotype (BD Pharmingen) were used as negative controls. A portion of each whole-blood specimen was treated with 0.005 mL adenosine diphosphate as a marker of platelet activation. Results are expressed in mean fluorescence intensity units for CD41 and in percentages for other markers of activation.
Functional Platelet Testing
In vitro coagulation monitoring was performed to determine platelet function and the dynamics of blood viscoelasticity during clotting. The whole-blood clotting parameters platelet contractile force (PCF), clot elastic modulus (CEM), and force onset time (FOT) were measured using the Hemodyne Hemostasis Analysis System (Hemodyne, Richmond, VA, USA). The PCF is the force produced by platelets during clot retraction, and it is therefore a measure of platelet function during clotting. The PCF is sensitive to platelet number, platelet metabolic status, and glycoprotein IIb/IIIa status. The CEM is a measure of clot stiffness, and it is sensitive to fibrinogen concentration, platelet concentration, the rate of thrombin generation, and the force produced by platelets. The FOT is the time required for thrombin to be generated in the whole-blood sample (17). The normal values for PCF, CEM, and FOT are 4.8 - 9.5 Kdyn, 14.0 - 35.0 Kdyn/cm2, and 3.0 - 8.0 min respectively. Thromboelastography was performed using a TEG 5000 Thrombelastograph hemostasis analyzer system (Haemoscope, Niles, IL, USA), and the reaction time (measure of time to clot initiation), kinetics time (measure of clot propagation time), and maximal amplitude (measure of clot firmness) were reported. All analytic procedures were completed using methods previously described in the literature (18-20). Assays were run in duplicate, and the average of the runs is reported.
Statistical Analysis
Descriptive statistics—mean ± standard deviation or median and interquartile range—characterize subject demographics and continuous data. Continuous data were evaluated using analysis of variance or the non-parametric Kruskal-Wallis test. A Tukey or Wilcoxon test was used for post-hoc multiple-comparison testing as appropriate. Data were evaluated for normal distribution or skewness by visual inspection of normal quantile plots. All statistical analyses were performed using the JMP statistical software (version 10.0.0: SAS Institute, Cary, NC, USA). The level of significance for all statistical tests was p < 0.05.
Results
Table 1 presents demographic and biochemical data for the 50 enrolled subjects (20 on PD, 20 on HD, and 10 healthy controls). Diabetes and hypertension were present in similar proportions in all the groups, but more HD patients had a history of clinically documented CVD as determined by history of myocardial infarction, arrhythmia, angina, heart failure, or cardiac intervention. The Davies comorbidity scores were similar in all groups, as were the chemistry and complete blood count results. Serum ferritin levels were high in the PD and HD patients, probably reflecting high iron utilization and chronic inflammation. Serum albumin was consistent in all groups, and all patients were receiving adequate dialysis as represented by Kt/V.
TABLE 1.
Characteristicsa of the Study Subjects

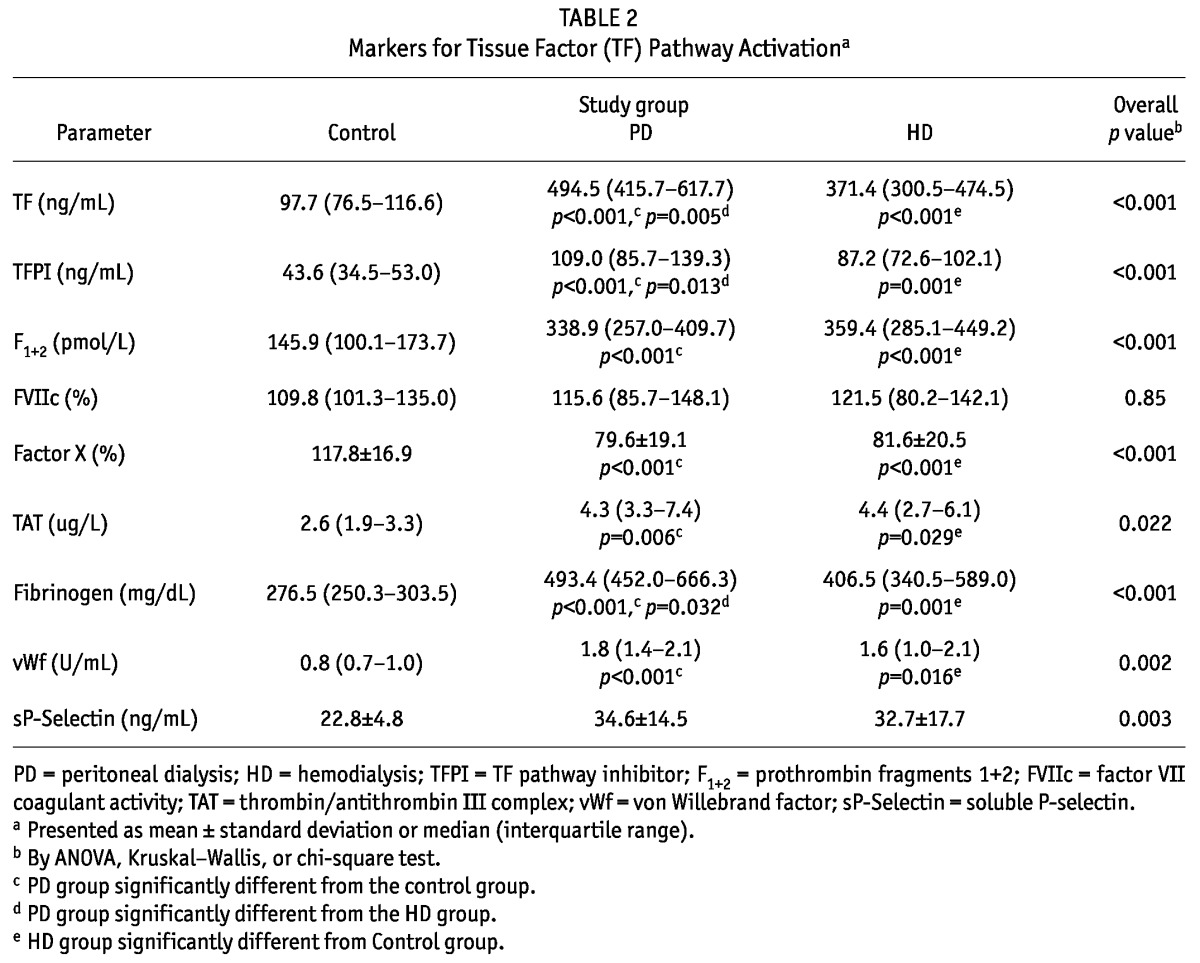
Table 2 summarizes the differences in coagulation proteins, thrombin generation markers, and inhibitors of coagulation and thrombin generation. Concentrations of TF and fibrinogen were higher in the PD and HD groups than in control subjects (p < 0.001), and the concentration of factor VII coagulant trended higher in the PD and HD groups. Similarly, relative to the control subjects, the PD and HD patients showed increased vWf and thrombin generation as evidenced by prothrombin fragments 1+2 (p < 0.0001). Levels of TFPI and TAT were significantly elevated in the PD and HD patients. Markers of endothelial activation (vWf and soluble P-selectin) were also significantly elevated in those groups.
TABLE 2.
Markers for Tissue Factor (TF) Pathway Activationa
The only platelet receptor distribution significantly different between groups was CD62P, which was higher in the HD group (mean: 7.7%) than in the PD group and the control subjects (mean: 5.7% and 3.4% respectively; p = 0.048). The percentage expression of PAC-1 was not different between the groups.
Compared with the HD patients and the control subjects, the PD patients made significantly firmer clots (denoted by PCF, CEM, and maximal amplitude; Table 3). In fact, in the PD group, the PCF and CEM were grossly above their normal target ranges and nearly 50% - 100% higher than values in the control subjects. The onset of clot initiation (denoted by FOT and reaction time) was not different between the groups, but the the PD and HD groups both had more rapid clot propagation (kinetics time), with the PD group having the fastest clot propagation (1.3 minutes).
TABLE 3.
Functional Platelet Activitya

Discussion
Coagulation involves complex interactions between platelets, endothelium, and coagulation proteins. It is well accepted that most contemporary dialysis patients have a prothrombotic tendency, as represented by their unacceptably high rates of CVD morbidity and mortality (1-3). The goal of the present study was to compare hemostatic profiles in patients receiving PD and HD, because the data suggesting that, compared with HD patients, PD patients have higher rates of CVD events and mortality are conflicting (13,14).
Our data clearly confirm that PD and HD patients both have a procoagulant profile, as denoted by hyperfibrinogenemia, enhanced inflammation, elevated markers of endothelial activation (TFPI, vWf, and soluble P-selectin), and upregulation of the TF pathway. Although TF and TFPI levels were higher in the PD group, the endproduct (prothrombin fragments 1+2) was similar in the PD and HD groups. It might therefore be expected that the intergroup TAT levels would be similar, which was indeed the case. There was also evidence of platelet activation, as measured by CD62P expression on the platelet surface in both groups. The natural anticoagulant TFPI was increased in both dialysis groups to counteract the prothrombotic milieu.
Given the presence of similar baseline characteristics, inherent cardiovascular risk factors, and the pro-coagulable milieu, it was expected that similar functional clot formation dynamics, such as clot stiffness and clot propagation, would be observed in both the PD and the HD groups. That was not the case. In fact, the PCF was 70% greater, and the CEM was 50% greater in the PD group than in the HD group, reflecting abnormally strong clot formation. The PD group also tended to have faster clot propagation, as reflected in the kinetics time. To our knowledge, our research report is the first to demonstrate this phenomenon.
One potential explanation of this finding is that the PD group had a slightly higher fibrinogen concentration than did the HD and control groups. When thrombin is generated, fibrinogen is converted to fibrin, which forms a cross-linked network around the platelet plug through factor XIII mediation. Fibrin fiber size and overall clot density are directly related to fibrinogen concentration. High fibrinogen levels have been shown to be directly linked to CVD in patients with CKD (21,22). Previous data from Sjøland et al. (12) elegantly show that PD patients make denser clots that are less susceptible to fibrinolysis. Undas et al. (3) also showed similar fibrinolysis patterns in a HD cohort.
Because PD patients are chronically exposed to glucose-based dialysate, they more readily develop advanced glycation endproducts. One potential hypothesis generated from our study is that, given the chronic inflammatory and oxidative stress in the PD population, it is possible that proteins such as fibrinogen become glycated and later oxidized through post-translational modifications, thereby leading to stiffer clots that become resistant to fibrinolysis. The hypothesis is supported in part by the fact diabetic patients tend to produce denser clots resistant to fibrinolysis (9-11) and in part by emerging data showing that advanced oxidative protein products in dialysis patients may in fact be oxidized fibrinogen (23-26). Moreover, recent data support the potential links between glycated endproducts, oxidative stress, and cardiovascular morbidity in PD patients (27). This hypothesis needs to be examined in a larger prospective study.
A logical follow-up question is “How do these parameters correlate with clinical status?” Our research group previously showed that PCF and CEM are significantly elevated in patients with coronary artery disease presenting to the emergency department with chest pain (28). In the present study, the PD group appeared to have a more prothrombotic profile than did the HD group, and yet only 1 patient in the group had documented CVD; the HD group contained 10 such patients. That finding is perplexing and might be a result of the PD group being slightly younger and perhaps more healthy, and of a broad definition for CVD being used in the study. For example, HD patients have risks for coronary events that are unrelated to the prothrombotic milieu: for example, wide swings in volume status during dialysis, a higher incidence of hyperkalemia leading to arrhythmias, heart failure, and a more rapid loss of residual renal function. Another possibility is that the PD patients might in fact have had CVD that was not manifested clinically during the study.
Our study was not without drawbacks. This relatively small cross-sectional pilot study was intended to generate hypotheses. Although the findings showed altered platelet function, clot rigidity, and thrombin generation, we were budgetarily constrained from performing more elaborate studies of platelet aggregation and fibrinolysis. However, what is already known in those two areas provides us with confidence in our results. Similarly, we could not assess more specific inflammatory markers in this study (interleukin 6 and C-reactive protein, for example), but given the marked elevations in ferritin and fibrinogen (two well-known acute-phase reactants), we are confident that interleukin 6 and C-reactive protein concentrations would have been elevated in our study population, as in earlier reports. Despite those potential limitations, our study is, to our knowledge, one of the few to report hemostatic differences between PD and HD using the complementary approach of coagulation protein concentrations, platelet receptor expression, and functional platelet assays.
Conclusions
This pilot study demonstrated that, although PD and HD patients both exhibit prothrombotic plasma milieus, PD patients tend to have hyperactive platelet function, resulting in stronger, firmer blood clots than those seen in HD patients. The clinical relevance of these findings needs to be studied in a prospective manner. Moreover, our data raise the question of whether more PD patients should receive primary and secondary CVD prevention with antiplatelet therapies.
Disclosures
The authors have no conflicts of interest, financial or other wise, to declare.
Acknowledgments
This study was sponsored by the A.D. Williams fund of Virginia Commonwealth University and was presented in abstract format at the American Society of Nephrology Annual Meeting; San Diego, California; 4 November 2012.
References
- 1. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Hamm LL, McCullough PA, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003; 42:1050–65 [DOI] [PubMed] [Google Scholar]
- 2. Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, et al. Cardiac diseases in maintenance hemodialysis patients: results on the HEMO study. Kidney Int 2004; 65:2380–9 [DOI] [PubMed] [Google Scholar]
- 3. Undas A, Kolarz M, Kopec G, Tracz W. Altered fibrin clot properties in patients on long-term haemodialysis: relation to cardiovascular mortality. Nephrol Dial Transplant 2008; 23:2010–15 [DOI] [PubMed] [Google Scholar]
- 4. Oksa A. Cardiovascular risk in patients with chronic kidney diseases: a time for new risk markers? Bratisl Lek Listy 2006; 107:314–19 [PubMed] [Google Scholar]
- 5. Parekh RS, Plantinga LC, Kao WHL, Meoni LA, Jaar BG, Fink NE, et al. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int 2008; 74:1335–42 [DOI] [PubMed] [Google Scholar]
- 6. Adams MJ, Irish AB, Watts GF, Oostryck R, Dogra GK. Hypercoagulability in chronic kidney disease is associated with coagulation activation but not endothelial function. Thromb Res 2008; 123:374–80 [DOI] [PubMed] [Google Scholar]
- 7. Mercier E, Branger B, Vecina F, Al-Sabadani B, Berlan J, Dauzat M, et al. Tissue factor coagulation pathway and blood cells activation state in renal insufficiency. Hematol J 2001; 2:18–25 [DOI] [PubMed] [Google Scholar]
- 8. Adams RLC, Bird RJ. Coagulation cascade and therapeutics update: relevance to nephrology. Part 1. Overview of coagulation, thrombophilias and history of anticoagulants. Nephrology 2009; 14:462–70 [DOI] [PubMed] [Google Scholar]
- 9. Dunn EJ, Phillippou H, Ariëns RA, Grant PJ. Molecular mechanisms involved in the resistance of fibrin to clot lysis by plasmin in subjects with type 2 diabetes. Diabetologia 2006; 49:1071–80 [DOI] [PubMed] [Google Scholar]
- 10. Dunn EJ, Ariëns RA, Grant PJ. The influence of type 2 diabetes on fibrin structure and function. Diabetologia 2005; 48:1198–206 [DOI] [PubMed] [Google Scholar]
- 11. Alzahrani SH, Ajjan RA. Coagulation and fibrinolysis in diabetes. Diab Vasc Dis Res 2010; 7:260–73 [DOI] [PubMed] [Google Scholar]
- 12. Sjøland JA, Sidelmann JJ, Brabrand M, Pedersen RS, Pedersen JH, Esbensen K, et al. Fibrin clot structure in patients with end-stage renal disease. Thromb Haemost 2007; 98:339–45 [PubMed] [Google Scholar]
- 13. Johnson DW, Dent H, Hawley CM, McDonald SP, Rosman JB, Brown FG, et al. Association of dialysis modality and cardiovascular mortality in incident dialysis patients. Clin J Am Soc Nephrol 2009; 4:1620–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vonesh EF, Snyder JJ, Foley RN, Collins AJ. Mortality studies comparing peritoneal dialysis and hemodialysis. What do they tell us? Kidney Int Suppl 2006; (103):S3–11 [DOI] [PubMed] [Google Scholar]
- 15. Kobayashi M, Yorioka N, Yamakido M. Hypercoagulability and secondary hyperfibrinolysis may be related to abnormal lipid metabolism in patients treated with continuous ambulatory peritoneal dialysis. Nephron 1997; 76:56–61 [DOI] [PubMed] [Google Scholar]
- 16. Schmitz G, Rothe G, Ruf A, Barlage S, Tschope D, Clementson KJ, et al. European working group on clinical cell analysis: Consensus protocol for the flow cytometric charaterisation of platelet function. Thromb Haemost 1998; 79:885–96 [PubMed] [Google Scholar]
- 17. Carr ME., Jr Development of platelet contractile force as a research and clinical measure of platelet function. Cell Biochem Biophys 2003; 38:55–78 [DOI] [PubMed] [Google Scholar]
- 18. Brophy DF, Martin EJ, Nolte ME, Kuhn J, Carr ME., Jr Effect of recombinant factor VIIa variant (NN1731) on platelet function, clot structure and force onset time in whole blood from healthy volunteers and haemophilia patients. Haemophilia 2007; 13:533–41 [DOI] [PubMed] [Google Scholar]
- 19. Brophy DF, Martin EJ, Carr SL, Kirschbaum B, Carr ME., Jr The effect of uremia on platelet contractile force, clot elastic modulus and bleeding time in hemodialysis patients. Thromb Res 2007; 119:723–9 [DOI] [PubMed] [Google Scholar]
- 20. Brophy DF, Martin EJ, Gehr TW, Best AM, Carr ME., Jr Thrombin generation time is a novel parameter for monitoring enoxaparin therapy in patients with end-stage renal disease. J Thromb Haemost 2006; 4:372–6 [DOI] [PubMed] [Google Scholar]
- 21. Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, et al. Inflammation and cardiovascular events in individuals with and without chronic kidney disease. Kidney Int 2008; 73:1406–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, et al. The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis 2008; 51:212–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Selmeci L, Szekely M, Soos P, Seres L, Klinga N, Geiger A, et al. Human blood plasma advanced oxidation protein products correlates with fibrinogen levels. Free Radic Res 2006; 40:952–8 [DOI] [PubMed] [Google Scholar]
- 24. Selmeci L. Advanced oxidation protein products (AOPP): novel uremic toxins, or components of the non-enzymatic antioxidant system of the plasma proteome? Free Radic Res 2011; 45:1115–23 [DOI] [PubMed] [Google Scholar]
- 25. Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 1996; 49:1304–13 [DOI] [PubMed] [Google Scholar]
- 26. Descamps-Latscha B, Wilko-Sarsat V, Nguyen-Khoa T, Nguyen AT, Gausson V, Mothu N, et al. Advanced oxidation protein products as risk factors for atherosclerotic cardiovascular events in nondiabetic predialysis patients. Am J Kidney Dis 2005; 45:39–47 [DOI] [PubMed] [Google Scholar]
- 27. Jiang J, Chen P, Chen J, Yu X, Xie D, Mei C, et al. Accumulation of tissue advanced glycation end products correlated with glucose exposure dose and associated with cardiovascular morbidity in patients on peritoneal dialysis. Atherosclerosis 2012; 224:187–94 [DOI] [PubMed] [Google Scholar]
- 28. Krishnaswami A, Carr ME, Jr, Jesse RL, Kontos MC, Minisi AJ, Ornato JP, et al. Patients with coronary artery disease who present with chest pain have significantly elevated platelet contractile force and clot elastic modulus. Thromb Haemost 2002; 88:739–44 [PubMed] [Google Scholar]


