Abstract
♦ Objectives: Peritoneal dialysis (PD) is one of the first-line modalities of renal replacement therapy in patients with end-stage renal disease. Guidelines recommended a break-in period of at least 2 weeks before full PD start. However, the optimal duration of the break-in period is still unclear. In the present study, we investigated the effect of various break-in periods on short-term outcomes in patients on PD.
♦ Methods: All patients who underwent Tenckhoff catheter implantation and initiated PD in Renji Hospital, Shanghai Jiao Tong University School of Medicine, between 1 January 2001 and 31 December 2010 were included. Patients were grouped according to the duration of their break-in period: 7 days or less (BI≤7), 8 - 14 days (BI8-14), and more than 14 days (BI>14). Kaplan-Meier curves and log-rank tests were used to compare short-term outcomes in the various groups.
♦ Results: Our study enrolled 657 patients (44.5% men), of whom 344, 137, and 176 patients were in the respective break-in groups. Compared with BI>14 patients, BI≤7 patients had a lower estimated glomerular filtration rate (5.34 ± 1.86 mL/min/1.73 m2 vs 6.55 ± 1.71 mL/min/1.73 m2, p < 0.001) and lower serum albumin (33.29 ± 5.36 g/L vs 36.64 ± 5.40 g/L, p < 0.001). The incidence of mechanical complications during the first 6 months was significantly higher in BI≤7 patients than in BI>14 patients (8.4% vs 1.7%, p = 0.004). However, we observed no significant differences between the three groups with respect to the prevalence of catheter dysfunction requiring surgical intervention (p > 0.05). Logistic regression analysis showed that BI≤7 [relative risk: 4.322; 95% confidence interval (CI): 1.278 to 14.608; p = 0.019] was an independent predictor of catheter dysfunction, but not of catheter dysfunction requiring surgical intervention (p > 0.05). Catheter dysfunction [hazard ratio (HR): 20.087; 95% CI: 7.326 to 55.074; p < 0.001] and peritonitis (HR: 4.533; 95% CI: 1.748 to 11.751; p = 0.002) were risk factors for technique failure during the first 6 months, but BI≤7 was not correlated with technique failure.
♦ Conclusions: Patients starting PD with a break-in period of less than 1 week might experience a minor increased risk of mechanical complications, but no major effect on technique survival.
Keywords: Break-in period, catheter-related complications, peritonitis, technique survival
Peritoneal dialysis (PD) has become a well-established complementary alternative to hemodialysis (HD) as a first-line renal replacement modality for end-stage renal disease patients (1,2). A break-in period after catheter insertion is usually required to avoid catheter-related complications such as leaks (3-6). International guidelines recommended that catheter insertion should be performed at least 2 weeks before PD start (3,7). Some researchers suggest delaying PD for 4 - 6 weeks after catheter insertion to accelerate wound healing (8). However, quite a few patients have to start urgently on dialysis because of late referral or unexpected deterioration of residual renal function (9,10). Currently, the optimal duration of the break-in period is still unclear. We therefore investigated the effect of various break-in periods on short-term outcomes in patients starting on PD.
Methods
Patients
All patients who underwent Tenckhoff catheter implantation and initiated PD in Renji Hospital, Shanghai Jiao Tong University School of Medicine, between 1 January 2001 and 31 December 2010 were included in the study. Patients were grouped according to the duration of their break-in period (from catheter implantation and to dialysis initiation): 7 days or less (BI≤7), 8 - 14 days (BI8-14), and more than 14 days (BI>14).
All Tenckhoff catheter insertions at our center are performed by nephrologists using the laparotomy method and adhering to one protocol. The key points of the protocol for catheter insertion are
administration of prophylactic antibiotics at the time insertion. Intravenous cephalosporin or vancomycin is used at our center.
placement of the catheter in a downward direction, with the superficial cuff 2 - 3 cm from the exit site.
testing of catheter function by fill and drain of PD fluid before tunneling.
bowel preparation to avoid constipation before and after surgery.
appropriate care after insertion, including anchoring the catheter to immobilize the exit site and minimizing entry of bacteria into the tunnel tract.
During the study period, the choice of when to start dialysis after catheter implantation was made by the nephrologists based on the clinical condition of individual patients. For patients who needed to initiate PD urgently, a low intraperitoneal volume (0.75 - 1.2 L) was used, which was gradually increased to 2 L per exchange within 2 weeks after catheter insertion. For continuous ambulatory PD (CAPD) patients, 3 or 4 exchanges were performed daily, and for automated PD (APD) patients, 6 or 7 cycles were prescribed and converted to standard CAPD with a 2-L intraperitoneal volume within 2 weeks after catheter insertion. In patients with a break-in period of 2 weeks or longer, PD was usually started with 2 L, except in small patients who could not tolerate 2 L fluid in the peritoneal cavity. Exchanges were performed by PD nurses until the patients had finished their training. All patients were dialyzed using glucose-based PD solution (Dianeal: Baxter China, Shanghai, PR China).
When signs of catheter dysfunction occurred, the cause of catheter dysfunction was determined by some combination of physical examination, abdominal radiography, and peritoneography, as required. In patients who developed catheter dysfunction, conservative therapy was given initially: supine position and a lower infusion volume for leaks; abdominal massage, administration of aperients or enemas, or ambulation for malposition; clot dislodgement with heparin or urokinase for obstruction; and administration of aperients or enemas for omental wrap. If conservative treatment failed, surgical intervention or transfer to hemodialysis (HD) was considered and performed.
Data Collection
The data collected included patient demographics, comorbid diseases, laboratory parameters, and medical history. Data recorded at the time of PD initiation included age, sex, body weight, height, underlying cause of end-stage renal disease, presence of comorbid diseases such as diabetes and cardiovascular disease, estimated glomerular filtration rate, serum albumin, use of steroids, past history of abdominal surgery, date of catheter insertion, date of PD initiation, and initial modality of PD. To exclude the possibility that accumulated experience might affect outcomes, we allocated all enrolled patients to a treatment period (before or after 2005) based on date of catheter insertion.
The primary outcomes of the study were the incidences of catheter dysfunction, peritonitis, and technique survival during the 6 months after catheter insertion. Catheter dysfunction (episodes, type, intervention strategy, and outcome), peritonitis (number of episodes, date of first episode), and patient outcomes (death, transfer to HD, transplantation, or transfer to other centers) were carefully tracked and recorded. Causes of transfer to HD were grouped as peritonitis, catheter dysfunction, and other causes.
Statistical Analysis
All results are expressed as mean ± standard deviation for normally distributed data and frequencies and percentages for categorical data. Differences between the groups in patient demographics and clinical and laboratory parameters were evaluated by one-way analysis of variance. Comparisons of percentages between groups were performed using the chi-square test. Logistic regression analysis was used to determine the factors associated with catheter dysfunction. Covariates in the logistic regression analyses included age, sex, comorbidities, and variables with a p value of less than 0.2 in the bivariate analysis for catheter dysfunction.
Actuarial cumulative technique survival curves were generated by the Kaplan-Meier method and compared using the log-rank test. A backward stepwise elimination multivariate Cox modeling analysis was performed to determine the independent predictors of patient outcomes, and only covariates that remained significant (p < 0.05) were kept in the model. Data for patient outcomes were censored at death (except in the patient survival analysis), switch to HD (except in the technique survival analysis), renal transplantation, and transfer to another center. Covariates in the multivariate Cox models included age, sex, comorbidities, and variables with a p value of less than 0.2 in the bivariate analysis for technique failure or death.
All statistical analyses were performed using SPSS for Windows (version 16.0: SPSS, Chicago, IL, USA). A p value less than 0.05 was considered statistically significant.
Results
Demographic and Clinical Characteristics
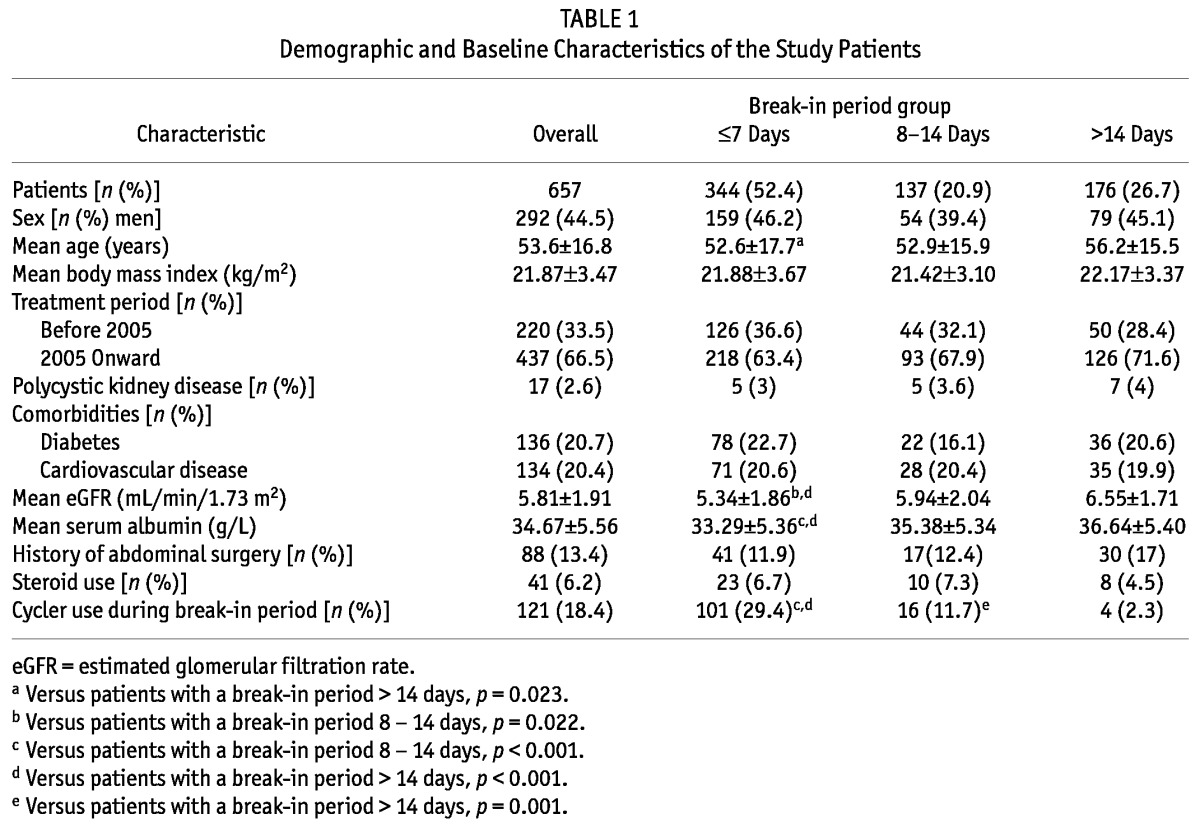
Our study enrolled 657 patients (44.5% men), including 344 (52.4%) in the BI≤7 group, 137 (20.9%) in the BI8-14 group, and 176 (26.7%) in the BI>14 group. There were no significant differences between the groups with respect to sex, body mass index, treatment period, prevalence of polycystic kidney disease, comorbidities, past history of abdominal surgery, or use of steroids (all p > 0.05).
Compared with BI>14 patients, BI≤7 patients were younger (52.6 ± 17.7 years vs 56.2 ± 15.5 years, p = 0.023) and had a lower estimated glomerular filtration rate (5.34 ± 1.86 mL/min/1.73 m2 vs 6.55 ± 1.71 mL/min/1.73 m2, p < 0.001) and a lower serum albumin (33.29 ± 5.36 g/L vs 36.64 ± 5.40 g/L, p < 0.001). Table 1 presents baseline demographic and clinical characteristics of the study populations.
TABLE 1.
Demographic and Baseline Characteristics of the Study Patients
Catheter Dysfunction
As Table 2 shows, the incidence of catheter dysfunction was significantly higher in BI≤7 patients than in BI>14 patients (8.4% vs 1.7%, p = 0.004). Episodes of catheter dysfunction were not significantly different between BI8-14 patients and BI>14 patients (p > 0.05). Patients in the BI≤7 group were more likely to experience catheter malposition than were patients in the BI8-14 group (3.5% vs 0.7%, p = 0.006); they also seemed to more often experience catheter malposition, but the difference in that variable did not reach statistical significance (3.5% vs 1.1% in BI>14 patients, p = 0.089). We observed no significant differences between the groups with respect to leaks, omental wrap, and obstruction (all p > 0.05).
TABLE 2.
Catheter-Related Complications in the Study Groups During a Six-Month Period
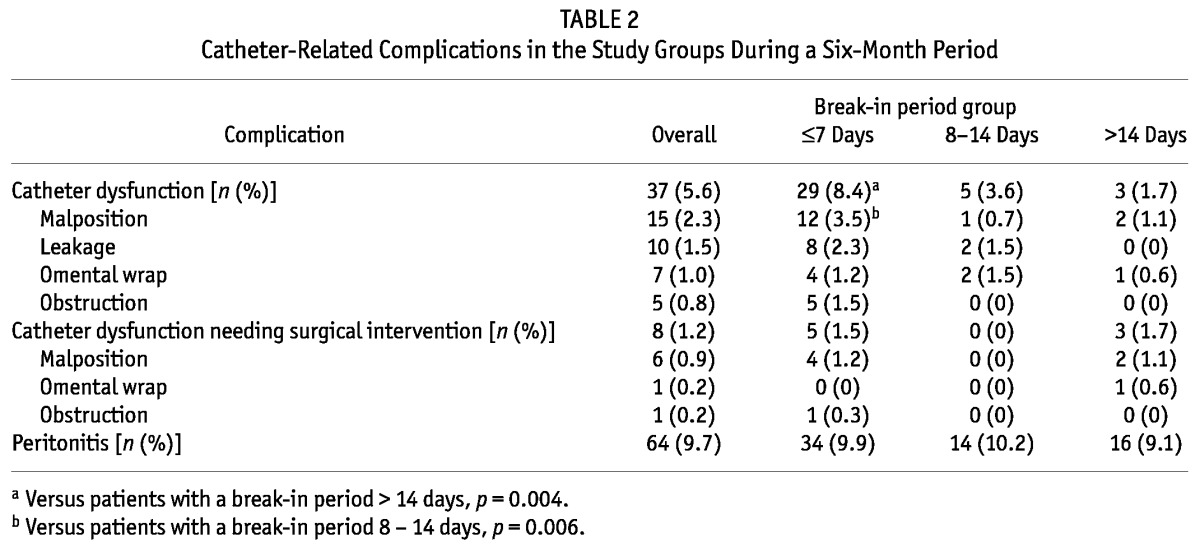
Of the 37 patients who developed catheter dysfunction, 17 recovered completely after conservative therapy, 12 ultimately transferred to HD, and 8 underwent surgical intervention. Occurrences of catheter dysfunction that required surgical intervention were not significantly different between the groups (p > 0.05, Table 2). The causes of catheter dysfunction requiring surgical intervention were malposition (n = 6), obstruction (n = 1), and omental wrap (n = 1).
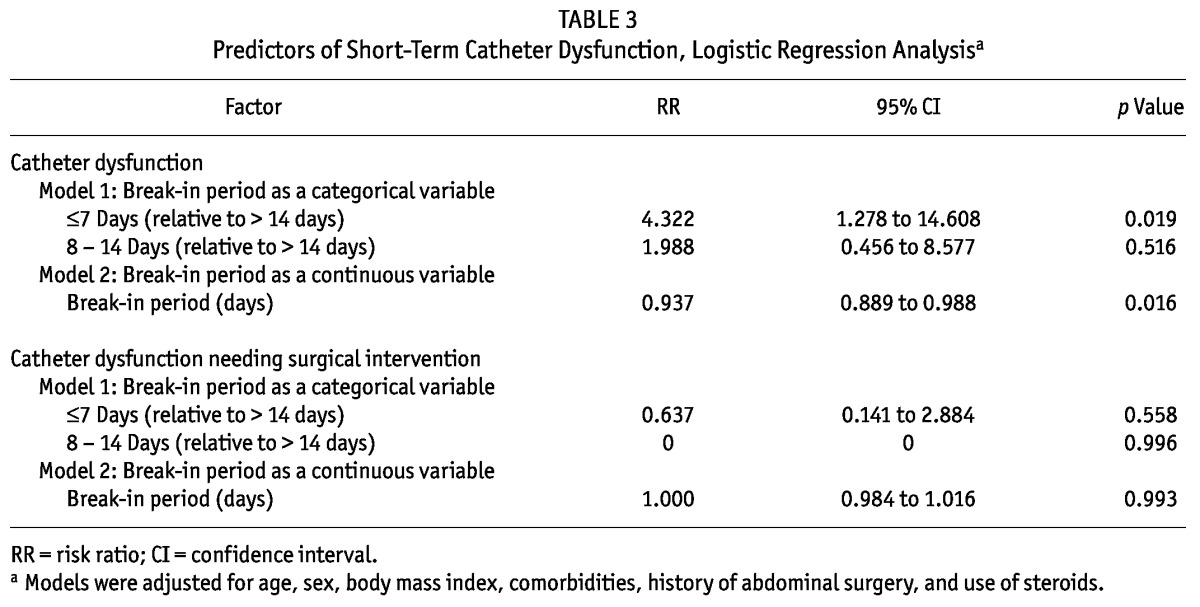
After adjustment for age, sex, body mass index, comorbidities, history of abdominal surgery, and use of steroids, logistic regression showed that a break-in period of 7 days or less was an independent risk factor for developing catheter dysfunction [relative risk (RR): 4.322; 95% confidence interval (CI): 1.278 to 14.608; p = 0.019]. When break-in period was analyzed as a continuous variable, a shorter break-in period was an independent predictor of catheter dysfunction (RR: 0.937; 95% CI: 0.889 to 0.988; p = 0.016). However, after adjustment for patient characteristics, neither a break-in period of 7 days or less nor a shorter break-in period was associated with catheter dysfunction requiring surgical intervention (p > 0.05, Table 3).
TABLE 3.
Predictors of Short-Term Catheter Dysfunction, Logistic Regression Analysisa
Peritonitis
During the study period, 34 BI≤7 patients (9.9%), 14 BI8-14 patients (10.2%), and 16 BI>14 patients (9.1%) developed peritonitis. No difference with respect to number of peritonitis episodes was observed between the groups (p > 0.05, Table 2). There was also no significant difference in peritonitis-free survival (p > 0.05). The multivariate Cox model showed no significant associations between break-in period and peritonitis.
Technique Survival
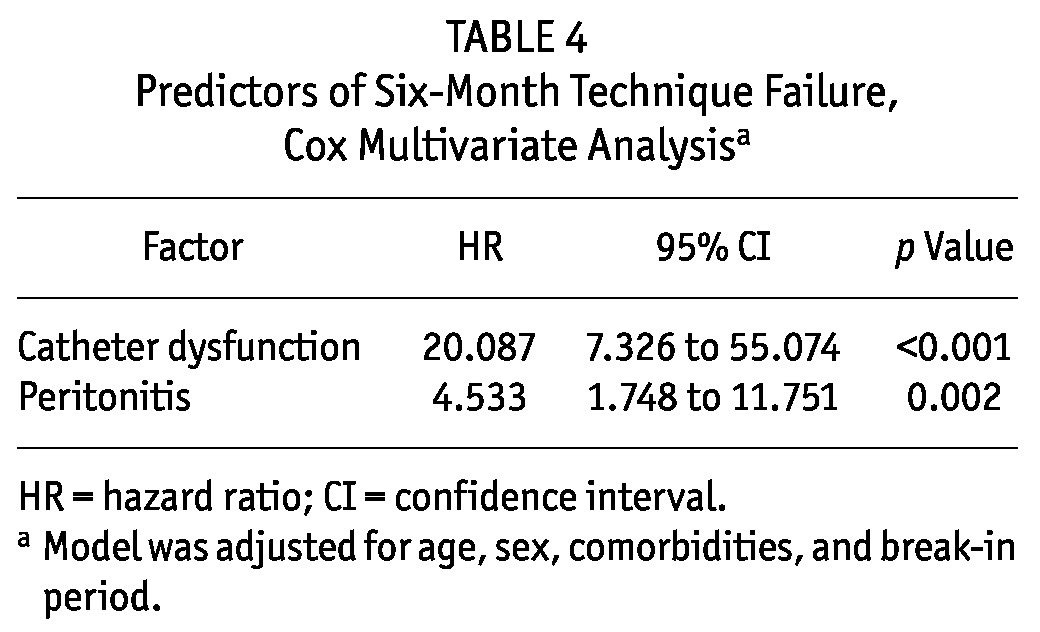
During the first 6 months after catheter insertion, 21 patients were transferred to HD. In that group, catheter dysfunction (n = 12) was the leading cause of transfer, followed by peritonitis (n = 8) and other causes (n = 1). Catheter dysfunction leading to transfer to HD was higher in BI≤7 patients (n = 11) than in BI>14 patients (3.2% vs 0%, p = 0.020). Crude technique survival at 6 months was 94% for BI≤7 patients, 99% for BI8-14 patients, and 98% for BI>14 patients. Technique survival was lower among BI≤7 patients than among BI8-14 patients (log rank: 5.438; p = 0.020) and BI>14 patients (log rank: 3.980; p = 0.046; Figure 1), but after adjustment for age, sex, and comorbidities, only catheter dysfunction [hazard ratio (HR): 20.087; 95% CI: 7.326 to 55.074; p < 0.001] and peritonitis (HR: 4.533; 95% CI: 1.748 to 11.751; p = 0.002) were independent risk factors for technique failure. A shorter break-in period was not associated with technique failure (p > 0.05, Table 4).
Figure 1 —
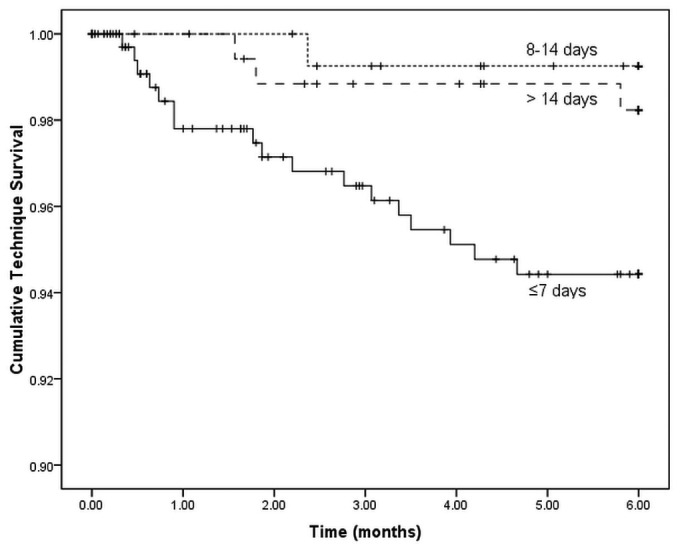
Technique survival curves for patients with different break-in periods. Technique survival was significantly lower for patients with a break-in period of 7 days or less than for patients with a break-in period of 8 - 14 days (log rank: 5.438; p = 0.020) or of more than 14 days (log rank: 3.980; p = 0.046).
TABLE 4.
Predictors of Six-Month Technique Failure, Cox Multivariate Analysisa
Patient Survival
In each group, the crude rates of patient survival at 6 months were 94% for BI≤7 patients, 95% for BI8-14 patients, and 99% for BI>14 patients. After adjustment for baseline characteristics, older age (HR: 1.027; 95% CI: 1.001 to 1.053; p = 0.043) and lower serum albumin (HR: 0.908; 95% CI: 0.842 to 0.980; p = 0.014) were both risk factors for 6-month patient mortality, but a break-in period of 7 days or less did not predict patient mortality during the first 6 months.
Discussion
To our knowledge, this study is the first with a large sample to investigate the effect of various break-in periods on short-term outcomes in patients newly started on PD. Our results suggest that patients who started PD with a break-in period of 7 days or less experienced a slightly higher risk of catheter dysfunction, but no major effect on technique survival.
A shorter break-in period has previously been reported to be associated with more catheter-related complications. Diaz-Buxo (5) reported that catheter leaks occurred most frequently during the immediate postoperative period and were seen in 7% - 24% of patients if PD was initiated early after catheter insertion. An adequate break-in period from catheter insertion to PD start is therefore usually required to permit wound healing and might reduce the risk of catheter-related complications. International guidelines recommend that catheter insertion should be performed at least 2 weeks before PD start (3,7); however, recent studies have demonstrated that PD might be a feasible and safe modality—and a complementary alternative to HD—not only in the chronic setting, but also in the acute setting (11,12). Povlsen and Ivarsen (13) compared short-term (3-month) outcomes and dialysis-related complications in a group of patients (n = 52) started acutely on chronic PD (<24 hours) and in an unmatched group (n = 52) undergoing planned start on chronic PD (>12 days). They found no differences in short-term PD technique survival and peritonitis-free survival between the groups, but mechanical complications were significantly higher in the acute group (28.9%) than in the planned-start group (7.7%). Lobbedez et al. (14) reported that only 2 of 34 unplanned PD patients (median break-in period: 4 days) experienced a peritoneal leak. Sharma et al. (15) showed that, after using a technique to more tightly secure the PD catheter during insertion (n = 48), the overall incidence of pericatheter leak remained low despite a shorter break-in period (<7 days). Similarly, a recent study from Taiwan suggested that early PD initiation (<14 days) in patients undergoing surgical implantation of a Tenckhoff catheter was not associated with an increased number of complications (16). However, our study showed that patients who started PD with a break-in period of 7 days or less experienced more catheter dysfunction.
A shorter break-in period was an independent risk factor for catheter dysfunction in our study. Several factors might have contributed to that observation. One possible factor is that urgent initiation of PD soon after catheter insertion might induce flotation of the catheter and a rise in peritoneal cavity pressure, resulting in catheter displacement and leakage. Urgent-start PD had adverse effects for wound healing, which might increase the risk of catheter leakage or other catheter-related complications. In addition, patients who need urgent dialysis start are often complicated by hypoalbuminemia, which has negative effect on wound healing. Early-start patients also usually have less-sufficient preparation and education before catheter insertion, which might also lead to increased risk for catheter dysfunction. Early use of the PD catheter might also place more demands on the catheter, with catheter dysfunction more likely to be noticed in such a group than in patients who wait to start dialysis. However, in our study, the prevalence of catheter dysfunction requiring surgical intervention was similar in all break-in groups. That result indicates that urgent-start PD right after catheter insertion might be associated with only a minor increased risk of mechanical complications.
Contrary to our previous report and other reports with long-term observation (17,18), the present study showed that catheter dysfunction was the leading cause of technique failure (followed by peritonitis) in patients starting PD soon after catheter insertion. The incidence of catheter dysfunction requiring transfer to HD was higher in BI≤7 patients than in BI>14 patients. However, after adjustment for patient characteristics, catheter dysfunction and peritonitis were the only independent predictors of technique failure; a shorter break-in period was not associated with technique failure. Those results suggest that urgent-start PD immediately after catheter insertion might have no major effect on technique survival. Early initiation of PD might therefore be a safe and feasible alternative to the use of a temporary catheter for HD in patients who need dialysis immediately, given that the latter access type has been linked with increased risks for septicemia, thrombosis, and stenosis (19,20).
Overall technique survival in our study was superior to that in other reports (14,21). The fact that our patient population was young and had a low incidence of comorbidities might be one of the explanations for the high technique survival observed in our study. On the other hand, center-related factors likely also contribute to achieving good catheter outcomes. Huisman et al. (22) reported that, in centers managing fewer than 20 PD patients or having a small fraction of patients on PD, the PD patients are at increased risk of technique failure. Our center has a big PD program, and the high patient volume certainly relies on the availability of special medical expertise and extensive experience in practicing PD. The techniques of catheter insertion and postoperative care are the most important contributors to a functional catheter. Some studies showed that PD catheter insertion can be safely and successfully performed by nephrologists, reporting excellent catheter outcome data. Because of their better understanding of renal patients and the intricacies of the disease process, nephrologists are ideally suited to perform catheter insertions (23-25). In our study, all catheter insertions were performed by skilled and experienced nephrologists who adhered to one standard protocol, which might be a primary explanation for the remarkably low incidence of catheter-related complications and the high technique survival observed in our study.
Patients who started PD with a break-in period of 7 days or less had worse residual renal function and lower serum albumin, meaning that those patients were sicker than their counterparts who initiated PD after more than 7 days. It has been reported that residual renal function and serum albumin are both important predictors for patient survival (26-30). On the other hand, our urgent-start patients were also younger, and so the risk of death was partly offset. After adjustment for baseline characteristics, age and serum albumin were independent predictors of patient mortality, but a shorter break-in period was not.
Our study has several limitations. It was a single-center, retrospective study, and patients were not randomized, nor was there any standardization in the treatment protocol. The initial PD procedures were not unique in the three groups, and so it might be difficult to assess the impact of the break-in period per se on outcomes in the PD patients. The single-center nature of the study also limits generalizability of the results. The break-in period was decided by the individual nephrologists based on patient condition, and although all the nephrologists practicing PD are experienced doctors and would typically make the same decision, individual bias cannot be completely excluded. We have no data about the number of late-referred patients, inflammation, and severity of cardiovascular diseases in the groups. We therefore do not know whether the incidence of those factors was increased in the urgent-start group, thus possibly having an impact on outcomes. Other unknown confounders might also exist. In addition, we had no data about other potentially important endpoints such as the rate of exit-site infection, the number of hospitalizations, and so on. Clearly, prospective randomized controlled trials are needed to definitively demonstrate the optimal break-in period in PD patients.
Conclusions
Our study suggests that a break-in period of less than 1 week might result in a minor increased risk of mechanical complications, but might have no major effect on technique survival in PD patients.
Disclosures
The authors have no financial conflicts of interest to declare.
Acknowledgments
This study was funded by a grant from the Science and Technology Commission of Shanghai Municipality (07QA14040, 08dz1900500, 114119a5900).
References
- 1. Van Biesen W, Vanholder R, Lameire N. The role of peritoneal dialysis as the first-line renal replacement modality. Perit Dial Int 2000; 20:375–83 [PubMed] [Google Scholar]
- 2. Gokal R, Mallick N. Peritoneal dialysis. Lancet 1999; 353:823–8 [DOI] [PubMed] [Google Scholar]
- 3. Figueiredo A, Goh BL, Jenkins S, Johnson DW, Mactier R, Ramalakshmi S, et al. Clinical practice guidelines for peritoneal access. Perit Dial Int 2010; 30:424–9 [DOI] [PubMed] [Google Scholar]
- 4. Gokal R, Alexander S, Ash S, Chen TW, Danielson A, Holmes C, et al. Peritoneal catheters and exit-site practices toward optimum peritoneal access: 1998 update. Perit Dial Int 1998; 18:11–33 [PubMed] [Google Scholar]
- 5. Diaz-Buxo JA. Mechanical complications of chronic peritoneal dialysis catheters. Semin Dial 1991; 4:106–11 [Google Scholar]
- 6. Tzamaloukas AH, Gibel LJ, Eisenberg B, Goldman RS, Kanig SP, Zager PG, et al. Early and late peritoneal dialysate leaks in patients on CAPD. Adv Perit Dial 1990; 6:64–71 [PubMed] [Google Scholar]
- 7. Dombros N, Dratwa M, Feriani M, Gokal R, Heimbürger O, Krediet R, et al. European best practice guidelines for peritoneal dialysis. 3 Peritoneal access. Nephrol Dial Transplant 2005; 20(Suppl 9):ix8–12 [DOI] [PubMed] [Google Scholar]
- 8. Banli O, Altun H, Oztemel A. Early start of CAPD with the Seldinger technique. Perit Dial Int 2005; 25:556–9 [PubMed] [Google Scholar]
- 9. Heatley SA. Optimal referral to pre-dialysis services: one center’s experience. Perit Dial Int 2009; 29(Suppl 2):S115–16 [PubMed] [Google Scholar]
- 10. Sprangers B, Evenepoel P, Vanrenterghem Y. Late referral of patients with chronic kidney disease: no time to waste. Mayo Clin Proc 2006; 81:1487–94 [DOI] [PubMed] [Google Scholar]
- 11. Jo YI, Shin SK, Lee JH, Song JO, Park JH. Immediate initiation of CAPD following percutaneous catheter placement without break-in procedure. Perit Dial Int 2007; 27:179–83 [PubMed] [Google Scholar]
- 12. Stegmayr BG. Three purse-string sutures allow immediate start of peritoneal dialysis with a low incidence of leakage. Semin Dial 2003; 16:346–8 [DOI] [PubMed] [Google Scholar]
- 13. Povlsen JV, Ivarsen P. How to start the late referred ESRD patient urgently on chronic APD. Nephrol Dial Transplant 2006; 21(Suppl 2):ii56–9 [DOI] [PubMed] [Google Scholar]
- 14. Lobbedez T, Lecouf A, Ficheux M, Henri P, Hurault de Ligny B, Ryckelynck JP. Is rapid initiation of peritoneal dialysis feasible in unplanned dialysis patients? A single-centre experience. Nephrol Dial Transplant 2008; 23:3290–4 [DOI] [PubMed] [Google Scholar]
- 15. Sharma AP, Mandhani A, Daniel SP, Filler G. Shorter break-in period is a viable option with tighter PD catheter securing during the insertion. Nephrology (Carlton) 2008; 13:672–6 [DOI] [PubMed] [Google Scholar]
- 16. Yang YF, Wang HJ, Yeh CC, Lin HH, Huang CC. Early initiation of continuous ambulatory peritoneal dialysis in patients undergoing surgical implantation of Tenckhoff catheters. Perit Dial Int 2011; 31:551–7 [DOI] [PubMed] [Google Scholar]
- 17. Mujais S, Story K. Peritoneal dialysis in the US: evaluation of outcomes in contemporary cohorts. Kidney Int Suppl 2006; (103):S21–6 [DOI] [PubMed] [Google Scholar]
- 18. Fang W, Qian J, Lin A, Rowaie F, Ni Z, Yao Q, et al. Comparison of peritoneal dialysis practice patterns and outcomes between a Canadian and a Chinese centre. Nephrol Dial Transplant 2008; 23:4021–8 [DOI] [PubMed] [Google Scholar]
- 19. Oliver MJ, Rothwell DM, Fung K, Hux JE, Lok CE. Late creation of vascular access for hemodialysis and increased risk of sepsis. J Am Soc Nephrol 2004; 15:1936–42 [DOI] [PubMed] [Google Scholar]
- 20. Xue H, Ix JH, Wang W, Brunelli SM, Lazarus M, Hakim R, et al. Hemodialysis access usage patterns in the incidence dialysis year and associated catheter-related complications. Am J Kidney Dis 2013; 61:123–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han SH, Lee JE, Kim DK, Moon SJ, Kim HW, Chang JH, et al. Long-term clinical outcomes of peritoneal dialysis patients: single center experience from Korea. Perit Dial Int 2008; 28(Suppl 3):S21–6 [PubMed] [Google Scholar]
- 22. Huisman RM, Nieuwenhuizen MG, Th de Charro F. Patient-related and centre-related factors influencing technique survival of peritoneal dialysis in the Netherlands. Nephrol Dial Transplant 2002; 17:1655–60 [DOI] [PubMed] [Google Scholar]
- 23. Alvarez AC, Salman L. Peritoneal dialysis catheter insertion by interventional nephrologists. Adv Chronic Kidney Dis 2009; 16:378–85 [DOI] [PubMed] [Google Scholar]
- 24. United States, Department of Health and Human Services, Centers for Medicare and Medicaid Services (CMS). Physician/Supplier Procedure Summary Master File. Baltimore, MD:, CMS; 2007. [Google Scholar]
- 25. Crabtree JH. Who should place peritoneal dialysis catheters? Perit Dial Int 2010; 30:142–50 [DOI] [PubMed] [Google Scholar]
- 26. Sesso R, Belasco AG. Late diagnosis of chronic renal failure and mortality on maintenance dialysis. Nephrol Dial Transplant 1996; 11:2417–20 [DOI] [PubMed] [Google Scholar]
- 27. Jungers P, Zingraff J, Albouze G, Chauveau P, Page B, Hannedouche T, et al. Late referral to maintenance dialysis: detrimental consequences. Nephrol Dial Transplant 1993; 8:1089–93 [PubMed] [Google Scholar]
- 28. Avram MM, Mittman N, Bonomini L, Chattopadhyay J, Fein P. Markers for survival in dialysis: a seven-year prospective study. Am J Kidney Dis 1995; 26:209–19 [DOI] [PubMed] [Google Scholar]
- 29. Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis 1999; 33:523–34 [DOI] [PubMed] [Google Scholar]
- 30. Friedman AN, Fadem SZ. Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol 2010; 21:223–30 [DOI] [PubMed] [Google Scholar]


