Abstract
♦ Introduction: Spectral analysis of heart rate variability is a noninvasive method for evaluating autonomic cardiovascular dysfunction under various clinical conditions, such as in dialysis patients, in whom an imbalance between the sympathetic and parasympathetic nervous system appears to be an important risk factor for sudden cardiovascular death and arrhythmia.
♦ Objective: We compared the effect of icodextrin-based dialysis solution, an option that allows for better metabolic and fluid overload control, with that of glucose-based dialysis fluid on sympathetic and parasympathetic activity in the heart, as assessed by heart rate variability, in diabetic patients on peritoneal dialysis (PD).
♦ Methods: This secondary analysis uses data from a randomized controlled trial in diabetic PD patients with high or high-average peritoneal transport using icodextrin-based (ICO group, n = 30) or glucose-based (GLU group, n = 29) solutions for the long dwell. All patients underwent 24-hour electrocardiographic Holter monitoring at baseline, and at 6 and 12 months of follow-up.
♦ Results: We observed no significant differences between the groups in most of the variables analyzed, although values were, in general, below reference values. In the ICO group, total power and both low- and high-frequency power in normalized units increased, but the percentage of RR intervals with variation of more than 50 ms declined over time; in the GLU group, all those values declined. Plasma catecholamine levels were higher at baseline and declined over time.
♦ Conclusions: These results indicate a partial recovery of sympathetic activity in the ICO group, probably because of better extracellular fluid control and lower exposure to glucose with the use of icodextrin-based dialysis solutions.
Keywords: End-stage renal disease, diabetes, icodextrin, heart rate variability, high peritoneal transport type
Sudden cardiac death and arrhythmias are among the most frequent causes of cardiovascular mortality (1,2) in patients with end-stage renal disease (ESRD), in whom an imbalance between the sympathetic and parasympathetic nervous systems known as autonomic cardiovascular dysfunction may contribute to such complications (3-6). Sympathetic overactivity, which is commonly found in skeletal muscle in patients with ESRD, is thought to play a role in the pathophysiology of the autonomic cardiovascular dysfunction usually found in ESRD (7). Furthermore, a high level of plasma norepinephrine, another index of sympathetic activity, is an independent risk factor for mortality in hemodialysis (HD) patients (8). However, sympathetic overactivity may not be a systemic phenomenon, because it was not observed in skin (9). On the other hand, when evaluated by spectral analysis of heart rate variability (HRV), a noninvasive method that allows for evaluation of autonomic cardiovascular dysfunction under various clinical conditions (10-14), the most common finding has been decreased low- and high-frequency power of the decomposed spectrum of pulse interval variability, which are indices of sympathetic and parasympathetic activity respectively and have been associated with uremic neuropathy (5,13-15). The magnitude of those changes is even more pronounced in diabetic patients because of concomitant diabetic neuropathy (16,17).
In addition to uremic and diabetic neuropathy, other modifiable factors may influence HRV. The degree of reduction of HRV correlates inversely with dialysis adequacy and ultrafiltration (18), and baroreflex sensitivity declines with exposure to peritoneal dialysis (PD) solutions with high glucose concentrations (19). Inflammation has also been mentioned as a factor influencing HRV, probably through the interaction of cytokines with neurotransmitters (20). Currently available glucose-sparing PD solutions have been demonstrated to improve ultrafiltration and to reduce glucose exposure and absorption, blood pressure, left ventricular mass, and possibly the degree of peritoneal and systemic inflammation (21-23), all factors that influence HRV. However, the effect of glucose-sparing solutions on HRV has not been sufficiently studied. The aim of the present study was to determine if the use of icodextrin improves sympathetic and parasympathetic activity in the heart in diabetic patients on PD, as analyzed by HRV.
Methods
Study Design
This secondary analysis used data from an open, randomized controlled trial (registered under number 2004-36001-0004, Cochrane Renal Group CRG040600073) published previously (24,25) in which the main objective was to compare the effects of icodextrin-based PD solutions (ICO group) with those of glucose-based PD solutions (GLU group) on metabolic and extracellular fluid volume control in patients with type 2 diabetes on continuous ambulatory PD with high or high-average peritoneal transport. Here, we look at whether the effect on sympathetic and parasympathetic tone varies depending on treatment with icodextrin PD or glucose PD solutions.
Patients
The study included adult patients with type 2 diabetes and high-average or high peritoneal transport, prevalent on PD, with no previous exposure to icodextrin (30 in the ICO group: 18 women, 58.9 ± 7.9 years of age; 29 in the GLU group: 13 women, 60.5 ± 9.3 years of age). Patients were excluded if they had infections or were hospitalized in the month preceding the study. Patients received three 2-L exchanges of standard 1.5% dextrose for their daytime dwells. For the long dwell, patients in the GLU group received one 2-L bag of standard 2.5% dextrose, and patients in the ICO group received one 2-L bag of 7.5% icodextrin (Extraneal: Baxter México, México City, México). Liberal use of 2.5% or 4.25% dextrose solution was allowed in both groups to reach treatment targets and to maintain patients free of edema.
Procedures
Relevant clinical data were obtained from medical records, complemented by personal interviews with the patients, and basic biochemical data were recorded. At baseline and at 6 and 12 months of follow-up, all patients underwent 24-hour electrocardiographic Holter monitoring. Monitoring was performed using 3-channel recorders (Medilog FD5: Oxford Instruments, Manor Way, Old Woking, UK), bipolar chest leads (CM-5, CM-1), and a modified aVF lead. Registers were analyzed blind by a trained cardiologist using the Oxford Medilog Excel 3.0 device (Oxford Instruments). Cardiac autonomic function was assessed by analysis of the beat-to-beat (RR) interval in the frequency domain for the entire 24-hours, using the frequency range 0 - 0.4 Hz and a fast Fourier transform spectral analysis algorithm with a spectral resolution of 0.0005 Hz. The frequency-domain analyses used were: total power (≤0.4 Hz), very-low-frequency (VLF) band (0.003 - 0.04 Hz), low-frequency (LF) band (0.04 - 0.15 Hz), high-frequency (HF) band (0.15 - 0.4 Hz), and LF/HF ratio. The measurements of total power and of the VLF, LF, and HF power components were expressed as absolute values (milliseconds squared). The VLF is thought to be influenced by thermoregulation of vasomotor tone; LF is affected by the baroreceptor reflex and is thought to reflect sympathetic and parasympathetic tone; HF is influenced by respiratory frequency and is thought to reflect parasympathetic tone. The LF/HF ratio is an index of sympathovagal balance and thus of autonomic status (5,10,11).
The time-domain parameters used were mean RR (in milliseconds), standard deviation [that is, the square root of the variance of all normal-normal (SDNN) intervals], and pNN50 (the percentage difference between two consecutive NN intervals during 50 ms). In addition, the SDANN/5 min [the standard deviation of the mean (average) of the RR intervals, each mean being calculated over 5 min] and the mean square root of successive differences (RMSSD, corresponding to the square root of the mean differences in successive RR intervals) were calculated, but those values are not used in the current presentation.
Plasma norepinephrine (NE) for biochemical evaluations was measured by high-performance liquid chromatography from venous blood samples obtained at the time of the monitoring (26).
Statistical Analysis
The data are presented as means and standard deviations for continuous variables and as proportions for categorical variables. Chi-square tests, Student t-tests, or Mann-Whitney tests were used for comparisons between groups at baseline, depending on the variable type. Two-way analysis of variance was used to analyze differences between the groups during follow-up, considering p < 0.05 to be significant. The statistical analysis was performed using W/SPSS (version 15: SPSS, Chicago, IL, USA).
Results
Table 1 shows baseline demographic, clinical, and biochemical characteristics of the 59 patients. There were no significant differences between the ICO and GLU groups at baseline.
TABLE 1.
Baseline Clinical Characteristics and Laboratory Measurements in the Patient Groups
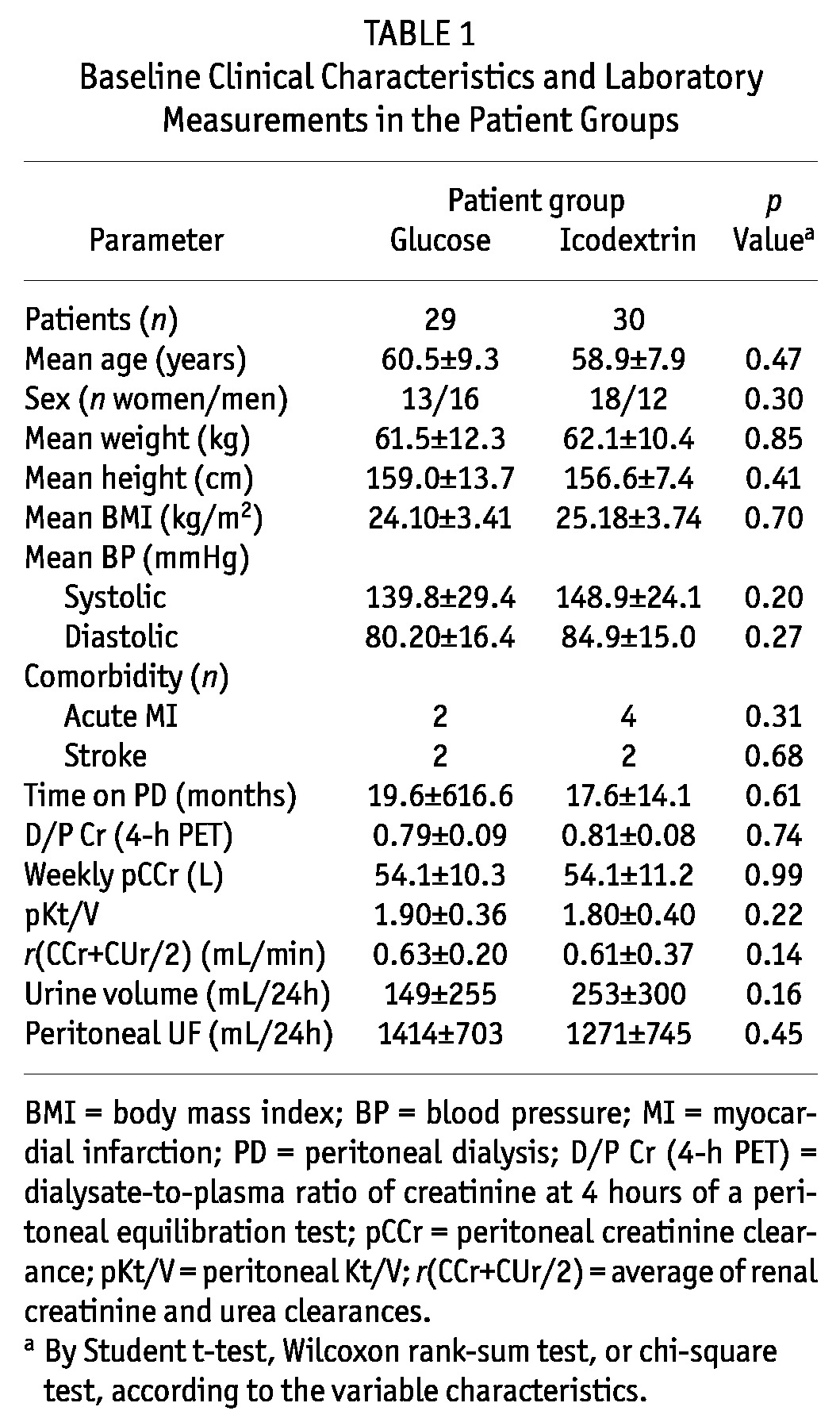
Table 2 shows basal values for HRV, mean values for the RR interval, total power, VLF, LF, HF, LF/HF ratio, SDNN, and pNN50. In general, measured values were below reference values for apparently healthy normal subjects (the far right column shows in-center reference values obtained from potential kidney donors). We observed no differences between the ICO and GLU groups except for the SDNN, which was at the limit of statistical significance (Mann-Whitney, p = 0.045).
TABLE 2.
Baseline Heart Rate Variability Parameters in the Study Patients and Reference Values in Healthy Subjects
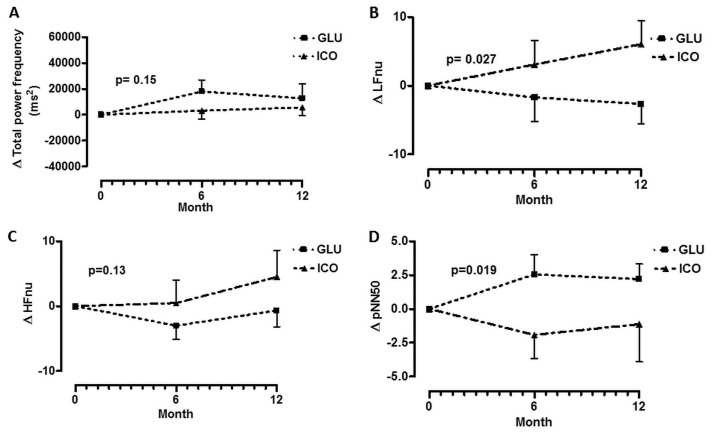
Figure 1 shows changes in the HRV parameters during follow-up in both groups. In the ICO group, total power [expressed in normalized units (nU)], LFnU, and HFnU increased, but pNN50 declined over time. In the GLU group, changes were nonsignificant. Panels B and D show changes in LFnU and pNN50 respectively; the differences between the groups were significant. The trends for total power and HFnU were similar; however, the differences between the groups did not reach statistical significance.
Figure 1 —
Changes (Δ) in parameters of heart rate variability during follow-up. (A) Change in total power frequency. (B) Change in low-frequency normalized units. (C) Change in high-frequency normalized units. (D) Change in pNN50 (percentage of RR intervals with more than 50 ms variation). GLU = patients using glucose dialysate; ICO = patients using icodextrin dialysate.
Figure 2 shows changes in plasma NE during follow-up. Levels declined over time, but there were no differences between the groups at any time of comparison. There were no correlations between change in plasma NE and change in HRV.
Figure 2 —
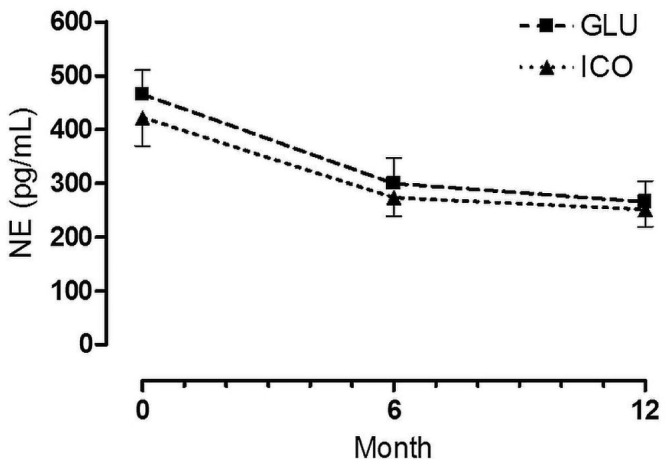
Plasma norepinephrine (NE) levels during follow-up. GLU = patients using glucose dialysate; ICO = patients using icodextrin dialysate.
Discussion and Conclusions
Our findings can be summarized as follows: Diabetic patients on PD showed a general decline in parameters of HRV analyzed in the domains of frequency and time. Compared with diabetic patients treated with glucose-based solutions alone, those treated with icodextrin-based solutions during the 1-year follow-up showed small but significant increments of LF power over baseline values and a decline in pNN50. Improvements might be related to the effects of icodextrin-based solutions on factors negatively influencing HRV and diabetic neuropathy—for example, fluid overload and increased glucose absorption (17-19).
At baseline, we observed no statistical differences between the groups in most of the analyzed variables, but the ICO group tended to contain more women, to have higher blood pressure, and to show a marginal statistical difference in SDNN. The study population did not differ from participant samples in several other studies of HRV in dialysis patients.
In general, HRV analyzed in both the time and frequency domains was below normal limits. Those changes could potentially be interpreted as visceral neuropathy, with additive effects from both diabetes and uremia. With or without dialysis, ESRD patients are known to experience significant reductions in HRV, and when those patients are diabetic, the changes are more pronounced in magnitude (16,17). Changes in HRV have clinical importance because they are associated with other important factors such as age, diabetes, and inflammation, and have also been identified as predictors of adverse cardiovascular and renal outcomes in non-dialysis patients (27).
Intervention by substitution of icodextrin-based solutions for glucose-based PD solutions during the long-dwell exchange induced slight but significant changes in some HRV-related variables. Thus, HRV in the frequency domain showed significant increments in LFnU in the ICO group, but decrements in the GLU group. In the time domain, HRV increased in the ICO group and showed a trend toward decrease in the GLU group. It is possible that those changes can be explained, at least in part, by the well-known glucose-sparing effect of icodextrin (28). In an earlier paper reporting on this study group, we showed a reduction of about 80 g of glucose absorption daily from baseline with the use of icodextrin (25). It has been demonstrated that high glucose content in PD solutions impairs baroreflex sensitivity (19), so that less exposure to glucose may improve nerve discharge.
Icodextrin provides better control of fluid overload (29), which may contribute to its effect on HRV. During the follow-up period, we showed significant and sustained differences of ultrafiltration (150 - 250 mL) in favor of the ICO group over the GLU group of study patients. Significant changes in extracellular fluid volume of 1.20 ± 0.34 L and 1.02 ± 0.44 L were seen in the ICO group at 6 and 12 months of treatment respectively. In comparison, changes in the GLU group were less than 0.2 L over the same period. Blood pressure showed a similar tendency. Systolic blood pressure declined more than 2 mmHg at 6 and 12 months, and diastolic blood pressure declined 16.6 ± 5.4 mmHg and 6.6 ± 6.4 mmHg in parallel (25). In other studies in HD patients, HD sessions were noted to improve HRV (30,31), an effect that can be interpreted as a consequence of better removal of uremic toxins, as well as better control of extracellular volume.
Another factor associated with icodextrin-based solutions that influences HRV is inflammation intensity. Experimental and clinical studies have demonstrated that the interleukin environment has important repercussions for HRV (32,33). In PD patients, we previously showed a direct correlation of extracellular fluid volume with C-reactive protein, and given that a reduction in extracellular volume would be expected to be followed by reduction in inflammation markers, those changes could explain, at least in part, the observed changes in HRV (34,35).
High circulating levels of NE, a biochemical index of sympathetic activity, are common in patients on HD and PD (36-38) and have been found to be an independent risk factor for cardiovascular mortality in this population (39). The mechanism that increases NE levels has not been completely identified. In PD patients, it has been suggested that dialytic removal of NE is substantially lower than normal renal removal. As a consequence, NE accumulates in plasma (40). Little or no attention has been paid to the effect of other factors that might possibly increase NE levels—among them, exposure to glucose load. In some studies in which the acute effects of glucose load were analyzed, NE increased after glucose challenge (41). On the other hand, after acute and severe ultrafiltration during HD, plasma NE declined significantly. In patients with good control of extracellular fluid volume, such as those in daily nocturnal dialysis programs, significant reductions in plasma NE have been documented (42).
Taken together, the foregoing findings suggest that glucose exposure and absorption and fluid overload both raise plasma NE. In the patients reported here, both groups showed indications of improved extracellular fluid volume and metabolic control; however, the control appeared better in the ICO group, presumably because more efficient ultrafiltration and glucose savings were achieved. As a consequence, the observed decline in NE levels from baseline was expected. Still, despite those results, it should be noted that other studies have mentioned that plasma NE does not necessarily reflect sympathetic activity in chronic kidney disease patients, because concentrations did not correlate with other validated methods (43). In the present study, electrophysiologic findings and plasma NE moved in the same way.
The present study has some limitations. Despite its value as a randomized controlled trial, the sample size was relatively small. Another important limitation derived from the sample size is an inability to properly analyze interactions between sex and icodextrin on HRV, which were not statistically significant in the available subjects. The current understanding is that women are more susceptible than men to left ventricular hypertrophy and arterial damage and that responsiveness to NE is one of the mechanisms involved in that difference. Another important limitation is the fact that we did not analyze the effect of the observed changes with respect to mortality. Nevertheless, we think that our results support the idea that improved metabolic and extracellular fluid volume control ameliorate the deleterious effect of chronic kidney disease on the cardiovascular neurologic balance.
Disclosures
The sponsors did not participate in study design, data collection, data analysis, data interpretation, or the writing of this report. The authors had no relationships, commercial or otherwise, with Baxter, S.A. de R.L., México. BL and EGL are employed by Baxter Novum at Karolinska Institutet, Stockholm, Sweden. The other authors are employees of the IMSS. The corresponding author had full access to all data and final responsibility for submitting for publication.
Acknowledgments
We thank Baxter, S.A. de R.L., México (grant 2005/23-585) and the Instituto Mexicano del Seguro Social (IMSS) for financial support. Baxter Novum is the result of a grant from Baxter Healthcare Corporation to the Karolinska Institutet. The authors also thank Ms. Susan Drier for her assistance in preparing the manuscript and Mr. Alejandro Hinojosa for database management.
References
- 1. Ritz E, Wanner C. The challenge of sudden death in dialysis patients. Clin J Am Soc Nephrol 2008; 3:920–9 [DOI] [PubMed] [Google Scholar]
- 2. Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation 2007; 116:85–97 [DOI] [PubMed] [Google Scholar]
- 3. Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, et al. Sympathetic nerve activity in end-stage renal disease. Circulation 2002; 106:1974–9 [DOI] [PubMed] [Google Scholar]
- 4. Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, et al. Sympathetic activation in chronic renal failure. J Am Soc Nephrol 2009; 20:933–9 [DOI] [PubMed] [Google Scholar]
- 5. Robinson TG, Carr SJ. Cardiovascular autonomic dysfunction in uremia. Kidney Int 2002; 62:1921–32 [DOI] [PubMed] [Google Scholar]
- 6. Grassi G, Arenare F, Pieruzzi F, Brambilla G, Mancia G. Sympathetic activation in cardiovascular and renal disease. J Nephrol 2009; 22:190–5 [PubMed] [Google Scholar]
- 7. Converse RL, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 1992; 327:1912–18 [DOI] [PubMed] [Google Scholar]
- 8. Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 2002; 105:1354–9 [Erratum in: Circulation 2002; 105:2230] [DOI] [PubMed] [Google Scholar]
- 9. Grassi G, Seravalle G, Arenare F, Buccianti G, Furiani S, Ilardo V, et al. Behaviour of regional adrenergic outflow in mild-to-moderate renal failure. J Hypertens 2009; 27:562–6 [DOI] [PubMed] [Google Scholar]
- 10. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996; 93:1043–65 [PubMed] [Google Scholar]
- 11. Kamath MV, Fallen EL. Power spectral analysis of heart rate variability: a noninvasive signature of cardiac autonomic function. Crit Rev Biomed Eng 1993; 21:245–311 [PubMed] [Google Scholar]
- 12. Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994; 90:1826–31 [DOI] [PubMed] [Google Scholar]
- 13. Axelrod S, Lishner M, Oz O, Bernheim J, Ravid M. Spectral analysis of fluctuations in heart rate: an objective evaluation of autonomic nervous control in chronic renal failure. Nephron 1987; 45:202–6 [DOI] [PubMed] [Google Scholar]
- 14. Cloarec-Blanchard L, Girard A, Houhou S, Grünfeld JP, Elghozi JL. Spectral analysis of short-term blood pressure and heart rate variability in uremic patients. Kidney Int Suppl 1992; 37:S14–18 [PubMed] [Google Scholar]
- 15. Takahashi H, Matsuo S, Toriyama T, Kawahara H, Hayano J. Autonomic dysfunction in hemodialysis patients with persistent hypotension. Nephron 1996; 72:418–23 [DOI] [PubMed] [Google Scholar]
- 16. Mylonopoulou M, Tentolouris N, Antonopoulos S, Mikros S, Katsaros K, Melidonis A, et al. Heart rate variability in advanced chronic kidney disease with or without diabetes: midterm effects of the initiation of chronic haemodialysis therapy. Nephrol Dial Transplant 2010; 25:3749–54 [DOI] [PubMed] [Google Scholar]
- 17. Yamanaka N, Aoyama T, Ikeda N, Higashihara M, Kamata K. Characteristics of heart rate variability entropy and blood pressure during hemodialysis in patients with end-stage renal disease. Hemodial Int 2005; 9:303–8 [DOI] [PubMed] [Google Scholar]
- 18. Tong YQ, Hou HM. Alteration of heart rate variability parameters in nondiabetic hemodialysis patients. Am J Nephrol 2007; 27:63–9 [DOI] [PubMed] [Google Scholar]
- 19. John SG, Selby NM, McIntyre CW. Effects of peritoneal dialysis fluid biocompatibility on baroreflex sensitivity. Kidney Int Suppl 2008; (108):S119–24 [DOI] [PubMed] [Google Scholar]
- 20. Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology 2008; 33:1305–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mistry CD, Gokal R, Peers E. A randomized multicenter clinical trial comparing isosmolar icodextrin with hyperosmolar glucose solutions in CAPD. MIDAS Study Group. Multicenter Investigation of Icodextrin in Ambulatory Peritoneal Dialysis. Kidney Int 1994; 46:496–503 [DOI] [PubMed] [Google Scholar]
- 22. Frampton J, Plosker G. Icodextrin: a review of its use in peritoneal dialysis. Drugs 2003; 63:2079–105 [DOI] [PubMed] [Google Scholar]
- 23. García-López E, Lindholm B, Davies S. An update on peritoneal dialysis solutions. Nat Rev Nephrol 2012; 8:224–33 [DOI] [PubMed] [Google Scholar]
- 24. Paniagua R, Orihuela O, Ventura MD, Avila-Díaz M, Cisneros A, Vicenté-Martínez M, et al. Echocardiographic, electrocardiographic and blood pressure changes induced by icodextrin solution in diabetic patients on peritoneal dialysis. Kidney Int Suppl 2008; (108):S125–30 [DOI] [PubMed] [Google Scholar]
- 25. Paniagua R, Ventura MD, Avila-Díaz M, Cisneros A, Vicenté-Martínez M, Furlong MD, et al. Icodextrin improves metabolic and fluid management in high and high-average transport diabetic patients. Perit Dial Int 2009; 29:422–32 [PubMed] [Google Scholar]
- 26. Hjemdahl P, Daleskog M, Kahan T. Determination of plasma catecholamines by high performance liquid chromatography with electrochemical detection: comparison with a radioenzymatic method. Life Sci 1979; 25:131–8 [DOI] [PubMed] [Google Scholar]
- 27. Chandra P, Sands RL, Gillespie BW, Levin NW, Kotanko P, Kiser M, et al. Predictors of heart rate variability and its prognostic significance in chronic kidney disease. Nephrol Dial Transplant 2012; 27:700–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Babazono T, Nakamoto H, Kasai K, Kuriyama S, Sugimoto T, Nakayama M, et al. Effects of icodextrin on glycemic and lipid profiles in diabetic patients undergoing peritoneal dialysis. Am J Nephrol 2007; 27:409–15 [DOI] [PubMed] [Google Scholar]
- 29. Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, et al. Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double-blind randomized controlled trial. J Am Soc Nephrol 2003; 14:2338–44 [DOI] [PubMed] [Google Scholar]
- 30. Giordano M, Manzella D, Paolisso G, Caliendo A, Varricchio M, Giordano C. Differences in heart rate variability parameters during the post-dialytic period in type II diabetic and non-diabetic ESRD patients. Nephrol Dial Transplant 2001; 16:566–73 [DOI] [PubMed] [Google Scholar]
- 31. Laaksonen S, Voipio-Pulkki L, Erkinjuntti M, Asola M, Falck B. Does dialysis therapy improve autonomic and peripheral nervous system abnormalities in chronic uraemia? J Intern Med 2000; 248:21–6 [DOI] [PubMed] [Google Scholar]
- 32. Psychari SN, Sinos L, Iatrou C, Liakos G, Apostolou TS. Relations of inflammatory markers to lipid levels and autonomic tone in patients with moderate and severe chronic kidney disease and in patients under maintenance hemodialysis. Clin Nephrol 2005; 64:419–27 [DOI] [PubMed] [Google Scholar]
- 33. Lanza GA, Pitocco D, Navarese EP, Sestito A, Sgueglia GA, Manto A, et al. Association between cardiac autonomic dysfunction and inflammation in type 1 diabetic patients: effect of beta-blockade. Eur Heart J 2007; 28:814–20 [DOI] [PubMed] [Google Scholar]
- 34. Vicenté-Martínez M, Martínez-Ramírez L, Muñoz R, Avila M, Ventura MD, Rodríguez E, et al. Inflammation in patients on peritoneal dialysis is associated with increased extracellular fluid volume. Arch Med Res 2004; 35:220–4 [DOI] [PubMed] [Google Scholar]
- 35. Avila-Díaz M, Ventura MD, Valle D, Vicenté-Martínez M, García-González Z, Cisneros A, et al. Inflammation and extracellular volume expansion are related to sodium and water removal in patients on peritoneal dialysis. Perit Dial Int 2006; 26:574–80 [PubMed] [Google Scholar]
- 36. Zabetakis PM, Kumar DN, Gleim GW, Gardenswartz MH, Agrawal M, Robinson AG, et al. Increased levels of plasma renin, aldosterone, catecholamines and vasopressin in chronic ambulatory peritoneal dialysis (CAPD) patients. Clin Nephrol 1987; 28:147–51 [PubMed] [Google Scholar]
- 37. Ratge D, Augustin R, Wisser H. Plasma catecholamines and alpha- and beta-adrenoceptors in circulating blood cells in patients on continuous ambulatory peritoneal dialysis. Clin Nephrol 1987; 28:15–21 [PubMed] [Google Scholar]
- 38. Zoccali C, Enia G, Tripepi G, Panuccio V, Mallamaci F. Clinical epidemiology of major nontraditional risk factors in peritoneal dialysis patients. Perit Dial Int 2005; 25(Suppl 3):S84–7 [PubMed] [Google Scholar]
- 39. Mallamaci F, Tripepi G, Maas R, Malatino L, Böger R, Zoccali C. Analysis of the relationship between norepinephrine and asymmetric dimethyl arginine levels among patients with end-stage renal disease. J Am Soc Nephrol 2004; 15:435–41 [DOI] [PubMed] [Google Scholar]
- 40. Vlachojannis JG, Tsakas S, Alexandri S, Petropoulou C, Goumenos DS. Continuous ambulatory peritoneal dialysis is responsible for an increase in plasma norepinephrine. Perit Dial Int 2000; 20:322–7 [PubMed] [Google Scholar]
- 41. Mujais S, Tapiawala SN, Yip P, Al-Rowaie F, Burdzy D, Bargman JM, et al. Glucoregulatory hormones and choice of osmotic agent in peritoneal dialysis. Perit Dial Int 2010; 30:626–32 [DOI] [PubMed] [Google Scholar]
- 42. Chan CT, Harvey PJ, Picton P, Pierratos A, Miller JA, Floras JS. Short-term blood pressure, noradrenergic, and vascular effects of nocturnal home hemodialysis. Hypertension 2003; 42:925–31 [DOI] [PubMed] [Google Scholar]
- 43. Porojan M, Costin S, Poantă L, Cerghizan A, Pop D, Dumitraşcu DL. Autonomic neuropathy and plasma catecholamine in patients with diabetes mellitus. Rom J Intern Med 2010; 48:341–5 [PubMed] [Google Scholar]