Abstract
♦ Introduction: Acutely decompensated heart failure (HF) in patients with diuretic resistance is often treated with extracorporeal ultrafiltration. Peritoneal ultrafiltration (PUF) has been proposed for the long-term management of severe HF after resolution of the acute episode. The aim of the present study was to evaluate the use of PUF in the treatment of chronic refractory HF in patients without end-stage renal disease.
♦ Methods: This multicenter (10 nephrology departments throughout Italy) retrospective observational study included patients with severe HF refractory to maximized drug treatment. The patients were proposed for PUF because they had experienced at least 3 hospital admissions in the preceding year for acutely decompensated HF requiring extracorporeal ultrafiltration.
♦ Results: Of the 48 study patients (39 men, 9 women; mean age 74 ± 9 years), 30 received 1 nocturnal icodextrin exchange, 5 required 2 daily exchanges, and 13 received 2 - 4 sessions per week of automated peritoneal dialysis. During the first year, renal function remained stable (initial: 20.8 ± 10.0 mL/min/1.73 m2; end: 22.0 ± 13.6 mL/min/1.73 m2), while pulmonary artery systolic pressure declined to 40 ± 6.09 mmHg from 45.5 ± 9.18 mmHg (p = 0.03), with a significant concomitant improvement in New York Heart Association functional status. Hospitalizations decreased to 11 ± 17 days/patient-year from 43 ± 33 days/patient-year before the start of PUF (p < 0.001). The incidence of peritonitis was 1 episode in 45 patient-months. Patient survival was 85% at 1 year and 56% at 2 years.
♦ Conclusions: This study confirms the satisfactory results of using PUF for chronic HF in elderly patients.
Keywords: Heart failure, peritoneal ultrafiltration, patient survival, ultrafiltration, sodium
Heart failure (HF) is a progressive and lethal disease with a high prevalence: in 2008, for example, it affected 2.4% of the entire U.S. population (1). A similar prevalence has been described in Europe (2). In Italy, the incidence is 170 000 new diagnoses per year, and the prevalence is 1 million patients (1.5% - 2%), with 150 000 hospital admissions annually (3). In elderly Italian patients aged 65 - 84 years, the overall prevalence of HF was recently found to be 7% (4). These patients face a very poor prognosis, even if properly treated (5).
Heart failure is often characterized by the presence of a vicious cycle of heart and kidney interaction. As a consequence of venous congestion and reduced renal perfusion, the glomerular filtration rate (GFR) declines and activity in the renin-angiotensin and sympathetic nervous systems increases. Sodium delivery to the distal tubule therefore declines, as do the effects of natriuretic peptides, leading to diuretic resistance and acute cardiac decompensation (6,7). Worsening of fluid overload may in turn negatively affect cardiac function and further reduce cardiac output (8).
For acutely decompensated HF with diuretic resistance, the standard treatment is fluid removal by continuous renal replacement therapy. Recovery of diuretic responsiveness with such therapy is usually associated with improved cardiac output—thanks to a more favorable lung-heart interaction (9)—and with a reduction of right and left ventricular diastolic filling pressures (10,11). Peritoneal dialysis (PD) has been proposed as a treatment for the home-based long-term management of severe congestive HF after resolution of the acute episode (12-14). However, even though this idea has been around for more than 60 years, only a few case series have been reported in the literature (15). Thus, in the absence of evidence-based indications, a variety of schedules for PD or peritoneal ultrafiltration (PUF) have been proposed in the past few years. Patients treated with PUF for congestive HF have not usually needed dialysis for creatinine or urea clearance; instead, the main indications have been reduction of chronic fluid overload and prevention of additional acute decompensation episodes. Those needs have led to the use of a variety of techniques: a single night-long exchange; two daily exchanges; or automated PD 2 - 4 nights per week. Criteria for starting PUF have also varied, but a frequent indication has been the number of hospital admissions for acute decompensated HF in the preceding year (15-17).
Even in the absence of strong evidence, guidelines, or long-term follow-up studies, most of the available reports indicate better survival for patients treated with PUF than for historical controls; PUF may therefore be considered a “last chance” therapy for refractory HF. However, the clinical characteristics of HF patients who may benefit most from PUF and the best protocols and procedures for delivering PUF are still poorly defined.
The aim of the present study was to evaluate the clinical indications and protocols for, and the long-term outcomes of, using PUF for the treatment of severe congestive HF in patients without end-stage renal disease at 10 nephrology departments throughout Italy.
Methods
Study Population
This multicenter retrospective study investigated the use of PUF as a therapy for severe HF, even among patients who did not need dialysis to maintain their level of GFR. Ten nephrology facilities cooperated in collecting the data. All consecutive patients with severe congestive HF who had been treated with PUF between 1 January 2006 and 31 December 2010 and followed for at least 6 months were included in the study.
Patients were referred to the nephrologist only after adequate cardiology therapy, including extracorporeal ultrafiltration for acutely decompensated HF, thereby resolving any possible diuretic resistance.
Patients needing PD for any indication other than chronic fluid overload (end-stage renal disease with GFR < 6 mL/min, uremic symptoms, severe hyperkalemia, oliguria, or acute HF) were excluded. Fluid overload was defined according to center practice, including clinical assessment, chest radiography, and echocardiography.
The study was approved by the ethics committees of the participating nephrology departments as a retrospective cohort study.
Study Protocol
These parameters were evaluated in all patients at baseline (before PUF initiation): clinical and laboratory characteristics, procedural techniques and protocols for PUF, and number of hospitalizations for congestive HF in the preceding year. During follow-up, patients were evaluated monthly, and their charts were reviewed 3, 6, and 12 months after they started PUF. At each step, the attending nephrologist collected these data: major adverse clinical events (death, need for hemodialysis or “full dose” PD), body weight, urine output, measured GFR, New York Heart Association (NYHA) class, serum albumin, hemoglobin, left ventricular ejection fraction (LVEF), pulmonary artery systolic pressure [PAPs (by echocardiography)], total days of hospitalization, and possible side effects related to treatment. After the first year of PUF, patients underwent surveillance according to nephrology facility protocol, and major adverse clinical events were recorded.
Statistical Analysis
All data are presented as mean ± standard deviation.
The Mann-Whitney test was used to analyze differences in continuous variables between the patient groups. Differences in proportions were determined by means of a one-way analysis of variance, and a general linear model was used to assess any repeated measurements of the same variable. Peritonitis rates and hospitalizations were calculated as episodes per patient-month at risk and as days per patient-year at risk. Patient survival was analyzed using an actuarial method and plotted as a Kaplan-Meyer graph.
All tests were two-tailed, and a p value less than 0.05 was required for statistical significance. All calculations were performed using the SAS software package (version 9.13: SAS Institute, Cary, NC, USA).
Results
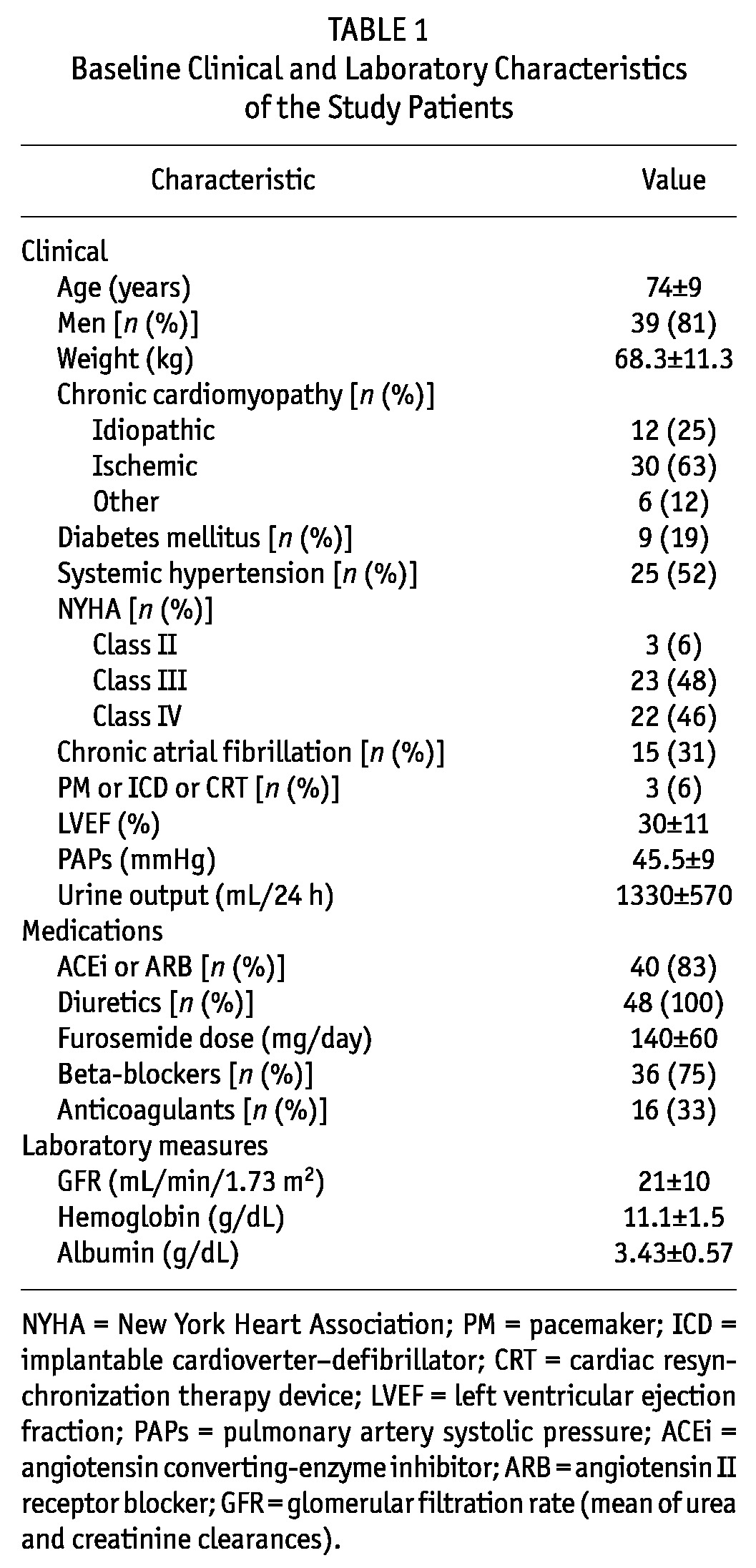
The study included 48 patients [39 men, 9 women; mean age: 74 ± 9 years (range: 40 - 90 years)]. Table 1 shows the baseline clinical characteristics of the study population. The cause of HF was ischemic cardiomyopathy in 63% of patients, idiopathy in 25%, and other forms of cardiomyopathy in 12%. Measured GFR was in the range 8 - 50 mL/min/1.73 m2 (mean: 21 ± 10.3 mL/min/1.73 m2). Only 1 patient had a GFR below 10 mL/min/1.73 m2, but that patient had no symptoms of end-stage renal disease or any other indication to start dialysis except fluid overload.
TABLE 1.
Baseline Clinical and Laboratory Characteristics of the Study Patients
Because of the multicenter nature of the study, the PUF schedules and protocols varied between the nephrology facilities. Tenckhoff peritoneal catheters were used in most cases (90%). In accordance with facility protocol, a surgical or semi-surgical procedure was used to place the catheter in the abdomen. The time between catheter placement and the start of PUF (break-in period) ranged from 2 to 4 weeks (mean: 20 ± 6 days).
The PUF modality was manual exchange in 35 patients (73%). In 30 of those patients (63%), a single exchange with icodextrin solution (8 - 10 hours overnight) was used; the other 5 (10%) required 2 exchanges daily [8 - 10 hours during the day with a glucose-based solution (1.36%, 2.27%, or 3.86% concentration) and 8 - 10 hours overnight with icodextrin]. In the remaining 13 patients (27%), a cycler was used for PUF overnight (2 - 4 nights each week).
During follow-up, mean body weight declined slightly during the first 3 months of treatment from 68.1 ± 11.3 kg to 67.2 ± 11.0 kg and then remained stable up to 12 months (67 ± 10.0 kg). During the study period, GFR and urine volume did not change, being 20.8 ± 10 mL/min/1.73 m2 and 1330 ± 570 mL/24 h respectively at the start, and 22 ± 13.6 mL/min/1.73 m2 and 1400 ± 660 mL/24 h at the end, with a slight decrease in the mean diuretic dose (to 120 ± 40 mg from 140 ± 60 mg daily). The patient whose GFR was less than 10 mL/min/1.73 m2 experienced a partial recovery of renal function: the 8 mL/min/1.73 m2 observed before the start of PUF rose to 15 mL/min/1.73 m2 at 3 months and then to 24 mL/min/1.73 m2 at 12 months. Interestingly, hemoglobin increased significantly to 12.2 ± 1.62 g/dL from 11.1 ± 1.54 g/dL (p = 0.02), while serum albumin remained stable (3.43 ± 0.57 g/dL at baseline, 3.50 ± 0.48 g/dL at 12 months, p = 0.90).
During PUF, most patients improved in NYHA functional class (Figure 1). At 12 months, a reduction by at least 1 NYHA class was observed in 41 patients (85%).
Figure 1 —
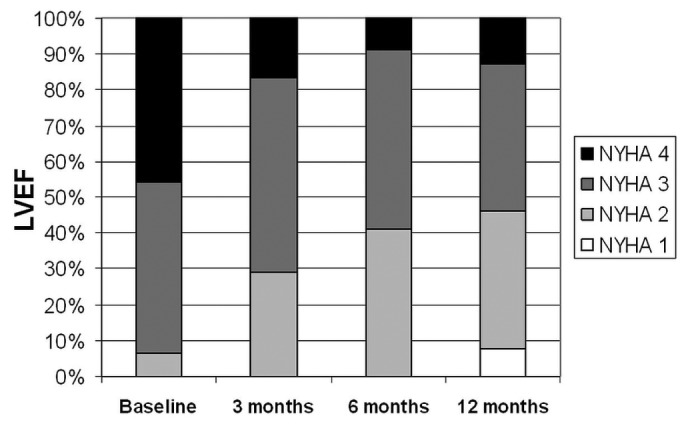
Evolution of the prevalence of the New York Heart Association (NYHA) functional classes during the first year after the start of peritoneal ultrafiltration. LVEF = left ventricular ejection fraction.
Figure 2 shows the changes in LVEF and PAPs during follow-up. Both parameters significantly improved during PUF, with p values of <0.01 and 0.03 respectively.
Figure 2 —
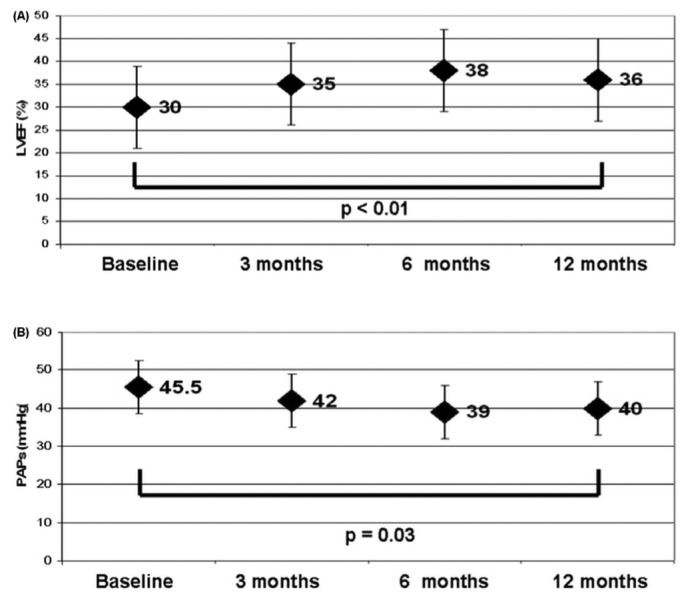
Echocardiography parameters of the study patients during the first year. (A) Left ventricular ejection fraction (LVEF). (B) Pulmonary artery systolic pressure (PAPs).
The number of days of hospitalization per patient was compared for two periods: the year preceding the beginning of PUF and the first year of treatment. Hospitalizations declined to 11 ± 17 days/patient-year from 43 ± 33 days/patient-year (p < 0.001, Figure 3).
Figure 3 —
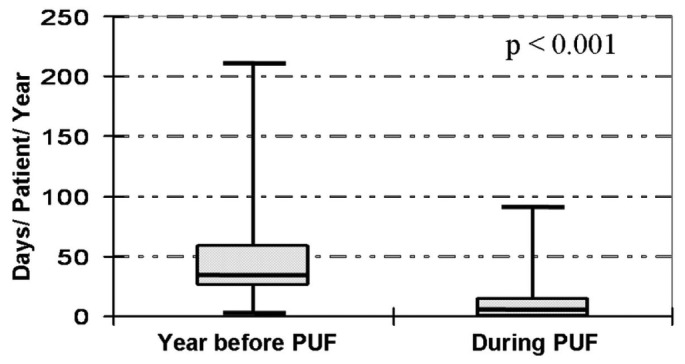
Morbidity: Hospitalization days per patient-year (median, interquartile range, and minimum-to-maximum range) before (left) and after (right) the start of peritoneal ultrafiltration (PUF).
When the study population was stratified by icodextrin use (1 overnight exchange) compared with other schedules, the LVEF and PAPs responses during follow-up were similar, despite a trend toward greater improvement in LVEF at 12 months in patients treated with icodextrin (Figure 4).
Figure 4 —
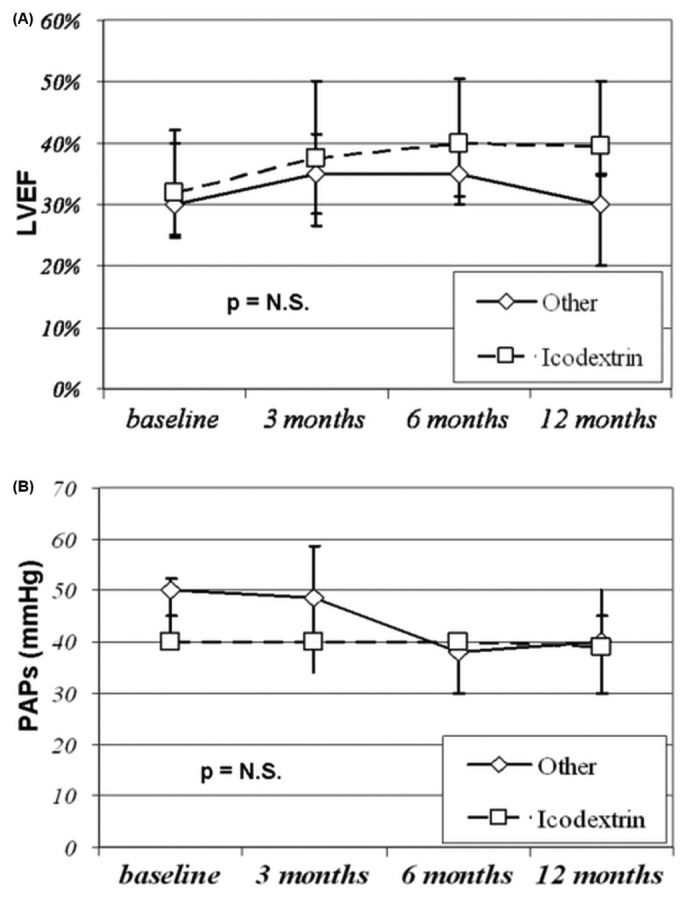
Comparison of (A) left ventricular ejection fraction (LVEF) and (B) pulmonary artery systolic pressure (PAPs) in patients treated or not treated with a single icodextrin dwell. N.S. = nonsignificant.
Peritonitis was the only complication associated with the PD technique: overall, 12 episodes were observed, for an average of 1 episode in 45 patient-months.
Within the first year of observation, 8 patients dropped out: 7 (15%) died (3 sudden deaths and 1 death each from sepsis, ischemic stroke, myocardial infarction, and cachexia), and 1 (2%) needed chronic hemodialysis.
Figure 5 shows the actuarial survival of the study population. Of the 48 patients in the initial cohort, 22 (46%) died within 24 months, with 1-year survival being 85%, and 2-year survival being 56%. Overall, 36 patients (75%) died at a mean of 24 ± 15 months from the start of PUF (range: 6 - 60 months). Of those 36 patients, 10 died from worsening cardiac failure; 9, from acute myocardial infarction; 5, from sudden death; 3, from ischemic stroke; 4, from sepsis; 2, from cancer; 2, from intestinal ischemia or perforation; and 1, from acute pulmonary embolism.
Figure 5 —
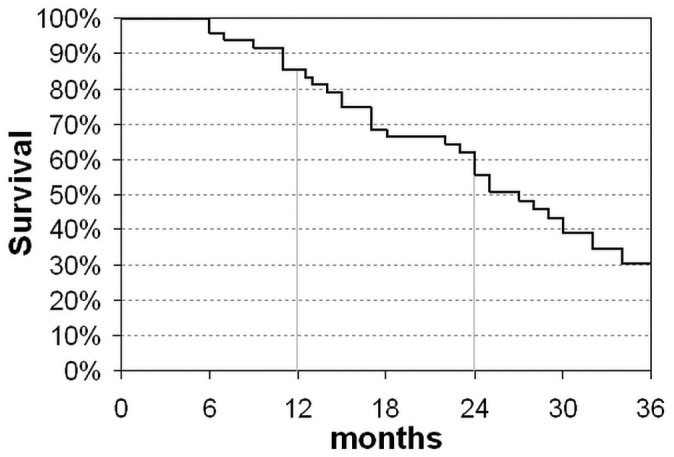
Cumulative actuarial patient survival. The analysis was stopped at 34 months because only 10 patients remained in the study.
Compared with patients who used other PUF schedules, those using a single icodextrin exchange showed a higher survival rate at 1 year (96% vs 70%, p = 0.04) and at 2 years (59% vs 38%, p = 0.21).
Overall technique survival was good if censored for patient death: 18 patients (37%) switched to “full dose” PD after 13 ± 10 months, and 3 of them then switched to hemodialysis after 3, 13, and 31 months. Another 3 patients switched from PUF directly to hemodialysis after 6, 14, and 47 months.
Discussion
Peritoneal ultrafiltration is a therapeutic strategy that numerous nephrology departments have been proposing for patients with severe congestive HF. Those patients are usually first managed by the cardiology team for acute decompensated HF and then evaluated by the nephrologist and proposed for “chronic” PUF treatment. Peritoneal ultrafiltration is a relatively simple choice for chronic water removal, and it appears to be a useful option for the management of patients with HF who develop severe edema, need frequent hospital admissions, and otherwise have a poor prognosis (14).
The PUF schedules used in the past have varied in terms of the kind and number of solutions used and the duration of dwells. In addition, patients with different clinical characteristics and HF severity have been considered for PUF treatment. Despite promising results from single-center studies with small populations, information is still lacking about the best clinical indications and procedural features for PUF.
In this multicenter study, we collected information on a cohort of patients larger than those in other series reported in the literature (15,16). In 2006, Mehrotra et al. (15) reviewed the literature on PD or PUF in HF, including 7 studies with a total of 68 patients (3 - 20 patients in each study). Since then, studies have been published by Sánchez et al. (18), Núñez et al. (19), and Nakayama et al. (20) involving, respectively, 17, 25, and 12 patients.
In our study, PUF was associated with a rapid and significant long-term improvement in clinical status, as demonstrated by increases in LVEF and hemoglobin, and reductions in NYHA class, PAPs, and diuretic dosage without any increase in body weight or worsening of renal function. The importance of elevated PAPs in HF patients was addressed by Cappola et al. (21), who identified PAPs as the most important hemodynamic predictor of death in patients undergoing right heart catheterization and myocardial biopsy. Hence, a reduction in PAPs should be considered a positive effect, possibly associated with better prognosis.
Our study also confirms that PUF has a positive impact on morbidity in patients with severe HF: total hospitalization days were significantly reduced during the treatment period compared with the preceding year. That finding accords with results in other studies. For instance, Gotloib et al. (16) showed a reduction in hospitalizations to a mean of 13 days from 157 days per patient-year, and the Sánchez et al. (18) study reported a reduction in hospitalizations to 11 ± 5 days from 62 ± 16 days per patient-year.
An important feature of PUF is that it is a home-based treatment for which the patient or caregiver requires little training. Clinically, it has the advantage of allowing for strict volume control. If the patient shows any sign of decompensated HF, fluid removal can easily be increased, and even if the patient is admitted for acutely decompensated HF, fluid removal through intense PUF can be performed with hyperosmolar fluids and without the need for extracorporeal ultrafiltration.
The present study showed a patient survival rate of 56% at 2 years. However, the prognosis of the enrolled population was expected to be poor because of severely impaired clinical status and multiple comorbidities. A 1-year survival of about 44% has been reported for other patients with similar clinical characteristics, and end-of-life options have been proposed (19). Taking that background into account, we note that our data are similar to those reported by Hébert et al. (14) and Sánchez et al. (18), despite the older age of our patients (74 years vs 52 years and 64 years respectively), suggesting a significant extension of survival compared with that in patients undergoing medical treatment only (22). Peritoneal ultrafiltration seems to provide a reasonable survival advantage or at least to markedly reduce the number, length, and associated costs of hospitalizations, suggesting that frequently hospitalized patients can be considered ideal candidates for this treatment.
Of the possible PUF schedules, a single nocturnal exchange with icodextrin solution has been suggested to be the best option (23,24). Indeed, that schedule reduces the risk of peritonitis and leads to slow and sustained water and sodium removal; furthermore, the procedure can easily be performed by the patient or a trained partner. Because of those potential advantages, most patients (63%) in our study were treated with icodextrin. Even though the icodextrin subgroup showed a slightly better improvement in LVEF at 12 months (and probably a better survival rate), our data do not allow us to reach definite conclusions about the strategy that is likely to be better.
Our study also shows that, throughout Italy, the approach to PUF is not uniform. We found some agreement on the most important patient selection criteria, in particular frequent hospitalizations; however, the causes of cardiomyopathy, degrees of kidney failure, and burdens of comorbidity were different for the members of the study population treated at each nephrology facility. Moreover, the PUF schedule may have varied not only for individual patients, but also according to the logistic and organizational models used at each facility.
Some unanswered questions remain for future studies. First, it remains unclear when a patient should be proposed for PUF. Currently, a patient with both cardiac failure and chronic kidney disease (CKD), receiving optimized medical treatment (diuretics, angiotensin converting-enzyme inhibitors, beta blockers, etc.), and experiencing several hospital admissions for HF (at least 3 in the preceding year) should be considered an ideal candidate. However, these inclusion criteria should be prospectively validated in HF patients.
Second, it is not clear if all kinds of HF can benefit from PUF. In most of the series reported in the literature, the patients had left ventricular systolic dysfunction. It would be interesting to determine whether patients with other conditions, such as severe right ventricular systolic or diastolic dysfunction, might benefit from daily PUF.
Our study showed that PUF allows for preservation of renal function, hemodynamic stability, and sodium and water balance without major electrolyte abnormalities. The mechanisms underlying the positive clinical effects of PUF remain uncertain, at least in part. The peritoneal membrane has a high capacity to eliminate some middle molecules, such as cytokines (interleukins 1 and 6 and tumor necrosis factor α), that have been implicated in the development and progression of HF (25,26). Thus, we can speculate that those molecules might be actively removed by PUF, resulting in a positive effect on myocardial contractility.
Moreover, because PUF appears to lead to consistent sodium removal of 130 - 150 mmol per liter of ultrafiltrate (27) compared with the 50 - 100 mmol per liter of urine achieved with diuretics, only a few exchanges are needed to keep the patient free from salt and fluid overload. The patient with HF and stage 4 CKD has a reduced nephronic mass, which may lead to altered sodium and water management. However, in patients with HF and stage 1 or 2 CKD, such alternations are less common.
Finally, we can hypothesize that maintenance of a more stable blood volume, without periods of expansion and dehydration, might reduce negative effects on cardiac and renal function, potentially leading to a more favorable environment for both the heart and the kidney (28, 29).
The main limitations of our study are its observational and retrospective nature and lack of comparisons with a control group. It should also be noted that patients starting PUF are followed more closely by nephrologists, especially during the first year of treatment, which could represent a possible bias. However, the clinical benefit observed in the present study should stimulate larger multicenter randomized controlled studies to confirm our preliminary data.
Conclusions
Peritoneal ultrafiltration appears to be a valid strategy for the treatment of severe congestive HF associated with CKD. Its clinical benefit—and, possibly, better survival—seems to be associated with the significant reduction observed in the number and length of hospitalizations. Future randomized clinical trials should confirm these preliminary results and provide more in-depth information on the best protocol for treatment.
Disclosures
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents. No extramural funding was used to support this work, and the authors have no financial conflicts of interest to declare.
References
- 1. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics 2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. on behalf of the ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33:1787–847 [Erratum in: Eur Heart J 2013; 34:158] [DOI] [PubMed] [Google Scholar]
- 3. Cacciatore P, Ceccolini C, Granella P, Lispi L, Boldrini R, Di Cesare M, et al. Analysis of Hospitalizations for Heart Failure in Italy: Years 2001 - 2003 [Italian]. Rome, Italy: Ministry of Health; 2007. [Available online at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_663_allegato.pdf; accessed 2 July 2012] [Google Scholar]
- 4. Mureddu GF, Agabiti N, Rizzello V, Forastiere F, Latini R, Cesaroni G, et al. on behalf of the PREDICTOR Study Group. Prevalence of preclinical and clinical heart failure in the elderly. A population-based study in Central Italy. Eur J Heart Fail 2012; 14:718–29 [DOI] [PubMed] [Google Scholar]
- 5. O’Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, et al. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J 2008; 156:662–73 [DOI] [PubMed] [Google Scholar]
- 6. Cadnapaphornchai MA, Gurevich AK, Weinberger HD, Schrier RW. Pathophysiology of sodium and water retention in heart failure. Cardiology 2001; 96:122–31 [DOI] [PubMed] [Google Scholar]
- 7. Gil P, Justo S, Castilla MA, Criado C, Caramelo C. Cardiorenal insufficiency: the search for management strategies. Curr Opin Nephrol Hypertens 2005;14:442–7 [DOI] [PubMed] [Google Scholar]
- 8. Chatterjee K. Neurohormonal activation in congestive heart failure and the role of vasopressin. Am J Cardiol 2005; 95:8B–13B [DOI] [PubMed] [Google Scholar]
- 9. Agostoni PG, Marenzi GC, Pepi M, Doria E, Salvioni A, Perego G, et al. Isolated ultrafiltration in moderate congestive heart failure. J Am Coll Cardiol 1993; 212:424–31 [DOI] [PubMed] [Google Scholar]
- 10. Bradley BA. Should ultrafiltration be used preferentially instead of diuretics for initial treatment of ADHF patients? Circ Heart Fail 2009; 2:499–504 19808381 [Google Scholar]
- 11. Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348:2007–18 [DOI] [PubMed] [Google Scholar]
- 12. Alpert MA, Hüting J, Twardowski ZJ, Khanna R, Nolph KD. Continuous ambulatory peritoneal dialysis and the heart. Perit Dial Int 1995; 15:6–11 [PubMed] [Google Scholar]
- 13. Takane H, Nakamoto H, Arima H, Shoda J, Moriwaki K, Ikeda N, et al. Continuous ambulatory peritoneal dialysis is effective for patients with severe congestive heart failure. Adv Perit Dial 2006; 22:141–6 [PubMed] [Google Scholar]
- 14. Hébert MJ, Falardeau M, Pichette V, Houde M, Nolin L, Cardinal J, et al. Continuous ambulatory peritoneal dialysis for patients with severe left ventricular systolic dysfunction and end-stage renal disease. Am J Kidney Dis 1995; 25:761–8 [DOI] [PubMed] [Google Scholar]
- 15. Mehrotra R, Kathiura P. Place of peritoneal dialysis in the management of treatment-resistant congestive heart-failure. Kidney Int Suppl 2006; 70:S67–71 [DOI] [PubMed] [Google Scholar]
- 16. Gotloib L, Fudin R, Yakubovich M, Vienken J. Peritoneal dialysis in refractory end-stage heart failure: a challenge facing a no-win situation. Nephrol Dial Transplant 2005; 20(Suppl 7):vii32–6 [DOI] [PubMed] [Google Scholar]
- 17. Ortiz AM, Acosta PA, Corbalan R, Jalil JE. Long-term automated peritoneal dialysis in patients with refractory congestive heart failure. Adv Perit Dial 2003; 19:77–80 [PubMed] [Google Scholar]
- 18. Sánchez JE, Ortega T, Rodríguez C, Díaz-Molina B, Martín M, Garcia-Cueto C, et al. Efficacy of peritoneal ultrafilil-tration in the treatment of refractory congestive heart failure. Nephrol Dial Transplant 2010; 25:605–10 [DOI] [PubMed] [Google Scholar]
- 19. Núñez J, González M, Miñana G, Garcia-Ramón R, Sanchis J, Bodí V, et al. Continuous ambulatory peritoneal dialysis as a therapeutic alternative in patients with advanced congestive heart failure. Eur J Heart Fail 2012; 14:540–8 [DOI] [PubMed] [Google Scholar]
- 20. Nakayama M, Nakano H, Nakayama M. Novel therapeutic option for refractory heart failure in elderly patients with chronic kidney disease by incremental peritoneal dialysis. J Cardiol 2010; 55:49–54 [DOI] [PubMed] [Google Scholar]
- 21. Cappola TP, Felker GM, Kao WH, Hare JM, Baughman KL, Kasper EK. Pulmonary hypertension and risk of death in cardiomyopathy: patients with myocarditis are at higher risk. Circulation 2002; 105:1663–8 [DOI] [PubMed] [Google Scholar]
- 22. Konstam MA. Progress in heart failure management? Lessons from the real world. Circulation 2000; 102:1076–8 [DOI] [PubMed] [Google Scholar]
- 23. Bertoli SV, Ciurlino D, Maccario M, Martino S, Bigatti G, Traversi L, et al. Home peritoneal ultrafiltration in patients with severe congestive heart failure without end stage renal disease. Adv Perit Dial 2005; 21:123–7 [PubMed] [Google Scholar]
- 24. Basile C, Chimienti D, Bruno A, Cocola S, Libutti P, Teutonico A, et al. Is intermittent peritoneal dialysis with icodextrin a valid option in the long-term treatment of refractory congestive heart failure? [Italian]. G Ital Nefrol 2009; 26(Suppl 46):44–9 [PubMed] [Google Scholar]
- 25. Diwan A, Tran T, Misra A, Mann DL. Inflammatory mediators and the failing heart: a translational approach. Curr Mol Med 2003; 3:161–82 [DOI] [PubMed] [Google Scholar]
- 26. Hörl WH, Riegel W. Cardiac depressant factors in renal disease. Circulation 1993; 87(Suppl 5):IV77–82 [PubMed] [Google Scholar]
- 27. Musetti C, Ciurlino D, Bertoli SV. Free water transport measured by double mini-PET may be increased by higher glucose exposure in peritoneal dialysis. Perit Dial Int 2012; 32:211–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin PY, Schrier RW. Sodium and water retention in heart failure: pathogenesis and treatment. Kidney Int Suppl 1997; 59:S57–61 [PubMed] [Google Scholar]
- 29. Andreoli TE. Pathogenesis of renal sodium retention in congestive heart failure. Miner Electrolyte Metab 1999; 25:11–20 [DOI] [PubMed] [Google Scholar]


