Abstract
♦ Background: High serum concentrations of the protein-bound uremic retention solutes p-cresyl sulfate (PCS) and indoxyl sulfate (IndS) and inflammation are associated with increased cardiovascular morbidity and mortality in chronic kidney disease. Renal clearance contributes to up to 80% of the total clearance of PCS and IndS in peritoneal dialysis (PD) patients. Cross-sectional studies evaluating the impact of residual renal function (RRF) on serum concentrations of PCS, IndS, and circulating inflammatory markers have yielded conflicting results.
♦ Methods: To clarify this issue, we carried out a prospective observational cohort study in incident PD patients (n = 35; 19 men; mean age: 55 ± 17 years). Midday blood samples were collected and analyzed for total serum PCS, IndS, C-reactive protein, and high-sensitivity interleukin 6. Peritoneal and renal clearances were calculated from urine and dialysate collections, and RRF was calculated as the mean of renal urea nitrogen and creatinine clearances. Patients were assessed 1, 6, 12, and 24 months after PD start. Differences between time points were analyzed using linear mixed models (LMMs).
♦ Results: Residual renal function declined significantly over time (LMM p < 0.0001). Peritoneal clearances of both toxins tended to increase, but did not compensate for the declining renal clearances. Serum concentrations of PCS and IndS increased significantly over time (LMM p = 0.01; p = 0.0009). In contrast, total mass removal of both toxins remained stable. Circulating inflammatory markers did not change over time.
♦ Conclusions: Our data indicate that serum concentrations of PCS and IndS, but not inflammatory markers, increase in incident PD patients in parallel with loss of RRF.
Keywords: Indoxyl sulfate, p-cresyl sulfate, residual renal function, inflammation
Residual renal function (RRF) has a beneficial effect on survival in peritoneal dialysis (PD) patients (1,2). Several hypotheses have been formulated to explain that finding. In the presence of RRF, adequate fluid balance is better maintained, phosphorus control is superior, and renal endocrine functions are preserved (3-5). In addition, even a small amount of RRF can contribute significantly to the removal of uremic toxins, especially toxins relying on renal metabolism or tubular secretion, such as p-cresyl sulfate (PCS) and indoxyl sulfate (IndS). Renal clearance might contribute up to 80% of total clearance of PCS in PD patients (6). Surprisingly, in a recent cross-sectional study involving 34 PD patients, Pham et al. (7) reported that serum concentrations of PCS were similar in anuric and non-anuric patients and that serum concentrations of IndS were modestly increased in anuric patients only. The authors raised the hypothesis that lower PCS and IndS generation rates were obscuring the consequences of decreased renal clearance. Concentrations of uremic retention molecules (URMs) indeed reflect a dynamic balance of removal, generation, and exchange between intracellular and extracellular stores. Like the study by Pham and colleagues, a recent prospective study with 24 incident PD patients failed to demonstrate increasing PCS levels parallel with declining RRF (6). Those studies were, however, all hampered by either cross-sectional design or short follow-up.
Inflammation is highly prevalent (12% - 65%) in patients on dialysis (8). Loss of RRF has been associated with an increased inflammatory response as denoted by increased levels of C-reactive protein (CRP), soluble vascular cell adhesion molecules, and interleukin 6 (IL-6) in hemodialysis and PD patients alike (9-11). Decreased clearance of cytokines, fluid overload, and accumulation of compounds that trigger the inflammatory response—such as advanced glycation endproducts (12) and IndS and PCS (13,14)—have all been suggested as factors in the association between inflammation and RRF.
To establish the impact of RRF on serum concentrations of PCS and IndS and any resulting association with inflammation, we carried out a prospective study in incident PD patients.
Methods
Study Population
Our study recruited 35 incident PD patients from an ongoing prospective observational study (NCT01306149). To increase the homogeneity of the study population, only patients with a technique survival exceeding 2 years and no missing clinic visits were selected for inclusion. Most patients were started on a continuous ambulatory PD regimen with 4 exchanges of conventional lactate-buffered glucose solutions (Dianeal 1.36%: Baxter Healthcare, Lessines, Belgium), the so-called full-dose regimen. At a later stage, some patients were switched to a cycler, mainly because of personal preference. Patients were followed at the outpatient clinic at 6- to 8-week intervals. At each visit, clinical parameters, biochemistry, Kt/V, and creatinine clearance were assessed to guide dialytic and medical therapy. A total weekly Kt/V of 1.7 or more was aimed for (adequacy target), and normovolemia was targeted with the use of any one or a combination of loop diuretics, hypertonic glucose solutions (Dianeal 3.86%: Baxter Healthcare), and polyglucose icodextrin (Extraneal: Baxter Healthcare), as judged appropriate by the treating physician.
The study was approved by the ethics committee of the University Hospitals, Leuven, and informed consent was obtained from all patients.
Study Visits and Procedures
Patients were assessed 1, 6, 12, and 24 months after PD start. All patients were free of infectious complications at the time of evaluation. At each visit, urine and peritoneal effluent collected during the preceding 24-hour period were weighed and sampled, and a midday blood sample was obtained. All samples were stored at -80°C until analysis. Demographics and maintenance mineral metabolism therapy were recorded at all visits.
Analytical Techniques
As previously described (15), high-performance liquid chromatography (Alliance 2695 coupled to a Waters 2475 fluorescence detector: Waters, Zellik, Belgium) was used to quantify PCS and IndS. Free PCS and IndS concentrations were measured at 37°C in serum ultrafiltered with the use of 30 000-Da molecular cut-off filters (Centifree UF devices: Amicon, Beverly, MA, USA). Ultrafiltrates (600 μL) were concentrated using a vacuum concentrator (Christ rotary vacuum concentrator 2-18 and cool trap 2-50: Qlab, Vilvoorde, Belgium) at 30°C overnight. Dried ultrafiltrates were dissolved in 200 μL phosphate-buffered saline by sonication for 30 minutes. Limits of quantification were 3.2 μmol/L for IndS and 1.8 μmol/L for PCS. Recovery, tested in hemodialysis patients, was 105% for PCS and 102% for IndS. Total, within-run, between-run, and between-day imprecision values for PCS and IndS were less than 6%. Standard assays were used to measure creatinine, urea nitrogen, and CRP. High-sensitivity IL-6 (hsIL-6) was measured by enzyme-linked immunosorbent assay (eBioscience, San Diego, CA, USA).
Calculations and Definitions
Residual renal function was estimated by calculating the arithmetic mean of renal urea nitrogen and creatinine clearances, expressed in milliliters per minute per 1.73 m2 of body surface area. Anuria was defined as 24-hour urine output less than 100 mL or RRF less than 1 mL/min/1.73 m2. Peritoneal, renal, and total clearances normalized to 1.73 m2 of body surface area (liters per week per 1.73 m2) were calculated for creatinine and urea nitrogen by direct determination from dialysis effluent, urine, and midday serum solute concentrations. Values for peritoneal, renal, and total Kt/V were calculated only for urea nitrogen (Kt/VUN). The Du Bois and Du Bois method was used to estimate body surface area (16). The distribution volume of urea nitrogen (V) was assessed using the Watson formula for total body water (17). Values for the normalized protein equivalent of nitrogen appearance were calculated according to Bergström et al. (18).
Statistics
Data are expressed as mean ± standard deviation or median and interquartile range, as appropriate. Differences (month 1 vs month 23) were evaluated using the paired t-test or a nonparametric analog (as appropriate) for continuous data and a chi-square test of association for categorical data. The linear mixed model method was used to analyze the time course of solute concentrations and their clearances in PD patients. Dialysis vintage, defined as the number of months from the start of PD treatment, was introduced as a fixed effect. The model used random effects to allow for inter-individual variation of the intercept and slope of time courses. The linearity assumption, an essential condition for the use of linear mixed models, was checked for all variables. Nonlinear data were (natural) log-transformed for the linear mixed model analysis; to include all variables, the transformation was applied to renal clearance values plus 1. A two-sided p < 0.05 was considered statistically significant. The SAS software program (version 9.2: SAS Institute, Cary, NC, USA) was used for the statistical analysis.
Results
Patient Characteristics
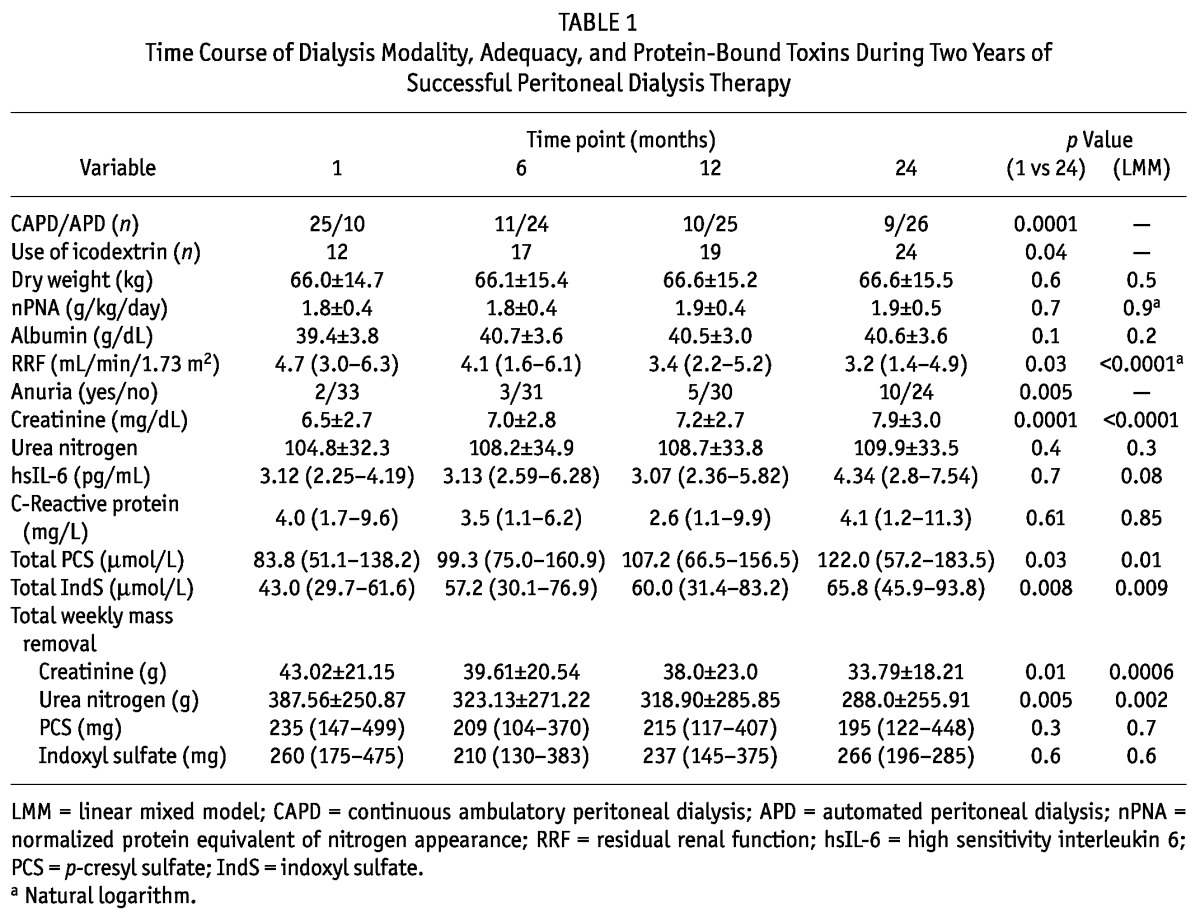
Table 1 summarizes the characteristics of the study group. Of the 35 patients, 19 were men. Causes of end-stage renal disease were diabetic nephropathy (n = 6), polycystic kidney disease or congenital kidney disease (n = 6), glomerular disease (n = 7), tubulointerstitial disease (n = 2), vascular disease (n = 2), miscellaneous (n = 1), and unknown (n = 11). At the start of PD therapy, mean age in the group was 57 ± 17 years. Residual renal function was 5.3 mL/min/m2 (range: 3.1 - 7.2 mL/min/m2) 1 month after PD start. The use of icodextrin increased significantly over time.
TABLE 1.
Time Course of Dialysis Modality, Adequacy, and Protein-Bound Toxins During Two Years of Successful Peritoneal Dialysis Therapy
Time Profiles of URM Serum Concentrations, Clearances, and Total Mass Removal
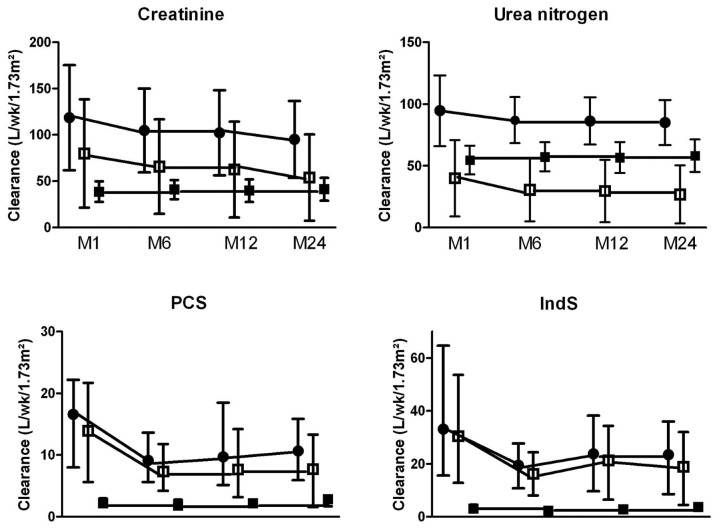
Table 1 shows a time profile of the serum concentration of URMs. Serum creatinine, PCS, and IndS, but not urea nitrogen, increased significantly over time. Figure 1 and Table 2 show the time course of renal, peritoneal, and total clearances. Total clearances of all URMs declined significantly over time. This decline can be attributed solely to the loss of RRF, because peritoneal clearances remained stable. The decrease in total weekly Kt/VUN was modest (to 2.1 from 2.3). Total Kt/VUN exceeded the target of 1.7 in more than 85% of the patients at all time points. At month 24, RRF contributed up to 87%, 75%, 48%, and 29% of total clearances of PCS, IndS, creatinine, and urea nitrogen respectively. Table 3 shows the serum concentration and total mass removal of protein-bound URMs at month 24 for anuric and non-anuric PD patients. Serum concentrations of IndS, but not PCS, were higher in anuric patients, whereas total mass removal of both toxins was lower in that group. Serum concentrations, clearances, and total mass removal of PCS and IndS were not different between continuous ambulatory PD and automated PD patients or between icodextrin users and non-users (data not shown).
Figure 1 —
Time course of renal (open squares), peritoneal (closed squares), and total (closed circles) clearances over time. PCS = p-cresyl sulfate; IndS = indoxyl sulfate.
TABLE 2.
Time Course of Renal, Peritoneal, and Total Clearances During Two Years of Peritoneal Dialysis Therapy
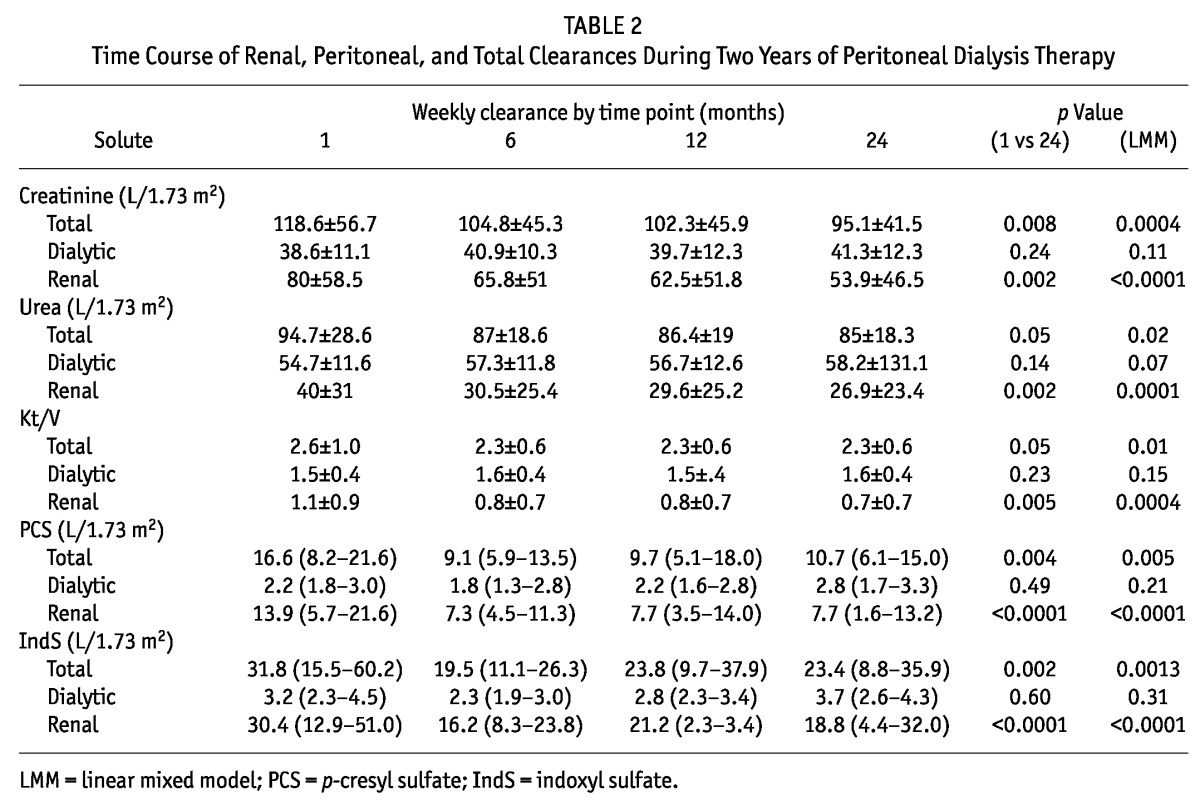
TABLE 3.
Total Weekly Mass Removal Depending on Residual Renal Function in Peritoneal Dialysis Patients After Two Years of Therapy
Time Profiles of Selected Markers of Inflammation
Serum levels of CRP and hsIL-6 remained stable in incident PD patients up to 2 years after dialysis initiation (Table 1). Serum levels of CRP and hsIL-6 did not differ significantly between anuric and non-anuric patients (Table 2), but hsIL-6 levels tended to be higher in anuric individuals. We observed no significant correlations between inflammatory markers, indices of RRF, and serum levels of PCS and IndS at any time (data not shown).
Discussion
The major finding of the present prospective observational study is that serum concentrations of PCS and IndS increased in incident PD patients in parallel with decline in RRF. Circulating inflammatory markers did not change over time.
p-Cresyl sulfate and IndS are both prototypic representatives of protein-bound URMs (19). Renal clearance of PCS and IndS occurs mainly through tubular secretion (20). The dialytic clearance of protein-bound URMs is limited (6,21) because only the free fraction is available for diffusion across the dialysis membrane (22). In contrast to prevailing beliefs, high-flux hemodialysis was shown to be better than PD at clearing PCS (23). Thus, especially in PD patients, removal of PCS and IndS depends largely on RRF. The present study confirms that dependence, showing that RRF accounts for more than 75% of total PCS and IndS clearance, even 2 years after dialysis initiation. Despite the paramount importance of RRF, investigators have so far failed to show increased serum levels of PCS and IndS in PD patients with declining or lost RRF (7,19). However, those studies were hampered by cross-sectional design or short follow-up. Our present longitudinal cohort study provides convincing evidence that, in incident PD patients, serum concentrations of PCS and IndS increase over time parallel with decline in RRF.
If we assume no net exchanges between the intracellular and extracellular compartments, the serum concentrations of URMs reflect the balance between generation and elimination. p-Cresyl sulfate and IndS are both endproducts of bacterial protein fermentation in the colon (24). In steady state, total mass removal may be assumed to equal the colonic generation rate. In the present study, the intestinal production rate, estimated as total mass removal, remained stable over time for both URMs, suggesting that the increased serum concentrations we observed are a unique reflection of impaired elimination. We observed that, compared with non-anuric PD patients, anuric PD patients experienced less total mass removal of both PCS and IndS, consistent with data in the literature (7). The greater mass removal of both toxins in non-anuric PD patients is most probably the result of higher protein intake, which is suggested by the greater mass removal of urea nitrogen in those patients. It is well established that a high protein diet fosters bacterial protein fermentation in the large intestine, resulting in increased generation of p-cresol and indole, the precursors of PCS and IndS (25-27). Our findings are consistent with data from a large cross-sectional study showing that RRF in continuous ambulatory PD patients is correlated with dietary protein intake, independent of dialysis adequacy (28). The apparent discrepancy between serum PCS and IndS in non-anuric and anuric patients confirms yet again that both toxins are endproducts of unrelated bacterial metabolic pathways. Decreased intestinal production was most pronounced for PCS and might explain why serum concentrations of PCS were comparable in both groups, despite markedly different RRF.
Several lines of evidence indicate that PCS and IndS are implicated in the pathogenesis of accelerated cardiovascular disease and adynamic bone disease. In vitro data show that PCS and IndS cause endothelial dysfunction and cell senescence by generating intracellular oxidative stress (29-37). Consistent with those in vitro data, high serum concentrations of PCS and IndS have repeatedly been associated with increased all-cause mortality and with cardiovascular mortality and morbidity in patients with chronic kidney disease (38-41).
Loss of RRF has been associated with increased inflammatory response in incident PD patients (42). Prospective studies addressing the issue are scarce. One small cohort had increased serum IL-6 levels after 1 year of PD therapy (11). The largest prospective study also failed to observe a significant increase after 1 year of PD therapy (43). Our data after 2 years of PD therapy further challenge the thesis that loss of RRF per se contributes to the uremic pro-inflammatory milieu.
Preservation of RRF is a well-established independent predictor of survival in hemodialysis (44,45) and PD patients (1,2) alike. Although preservation of RRF might simply represent a marker of better cardiovascular health, it is tempting to speculate on a causal relationship between RRF and cardiovascular outcomes (9). Residual renal function facilitates maintenance of adequate fluid balance, helps to maintain phosphorus homeostasis, and better preserves renal endocrine functions (3,4,46). Our data strengthen the thesis that conservation of renal elimination mechanisms other than glomerular filtration (tubular secretion, metabolism) also contributes to the inverse relationship between RRF and mortality.
We acknowledge that our study has several limitations. First, the number of patients was rather low. However, to the best of our knowledge, this is the largest prospective study, with the longest follow-up, involving incident PD patients. A type 2 statistical error with respect to the effect of RRF on inflammation cannot be excluded. At a minimum, our study challenges the independent role of RRF in inflammation and raises the need for additional data. Second, only patients with a technique survival of at least 2 years were enrolled, which could have introduced selection bias. When designing the study, we argued that a homogeneous study population would be most suited to the primary aim: that is, to evaluate the impact of RRF on serum PCS and IndS and on inflammation. We excluded patients experiencing technique failure within 2 years of the start of PD, which in many case is related to recurrent peritonitis, ultrafiltration failure, or mechanical problems—all potential confounders of the primary aim.
Conclusions
Serum concentrations of PCS and IndS increase in incident PD patients in parallel with loss of RRF. Increased serum PCS and IndS may be hypothesized to be on the causal pathway between RRF and mortality.
Disclosures
The authors have no financial conflicts of interest to declare.
Acknowledgments
Part of this work was presented at the 44th Annual American Society of Nephrology Conference; Philadelphia, PA, USA; 8 - 13 November 2011 (FR-PO1706).
References
- 1. Bargman JM, Thorpe KE, Churchill DN. on behalf of the CANUSA Peritoneal Dialysis Study Group. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001; 12:2158–62 [DOI] [PubMed] [Google Scholar]
- 2. Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, et al. on behalf of the Mexican Nephrology Collaborative Study Group. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 2002; 13:1307–20 [DOI] [PubMed] [Google Scholar]
- 3. Ateş K, Nergizoğlu G, Keven K, Sen A, Kutlay S, Ertürk S, et al. Effect of fluid and sodium removal on mortality in peritoneal dialysis patients. Kidney Int 2001; 60:767–76 [DOI] [PubMed] [Google Scholar]
- 4. Burkart JM. The ADEMEX study and PD adequacy. Blood Purif 2003; 21:37–41 [DOI] [PubMed] [Google Scholar]
- 5. Pecoits-Filho R, Gonçalves S, Barberato SH, Bignelli A, Lindholm B, Riella MC, et al. Impact of residual renal function on volume status in chronic renal failure. Blood Purif 2004; 22:285–92 [DOI] [PubMed] [Google Scholar]
- 6. Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y. Time profiles of peritoneal and renal clearances of different uremic solutes in incident peritoneal dialysis patients. Am J Kidney Dis 2005; 46:512–19 [DOI] [PubMed] [Google Scholar]
- 7. Pham NM, Recht NS, Hostetter TH, Meyer TW. Removal of the protein-bound solutes indican and p-cresol sulfate by peritoneal dialysis. Clin J Am Soc Nephrol 2008; 3:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stenvinkel P, Wanner C, Metzger T, et al. Inflammation and outcome in end-stage renal failure: does female gender constitute a survival advantage? Kidney Int 2002; 62:1791–8 [DOI] [PubMed] [Google Scholar]
- 9. Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int 2006; 69:1726–32 [DOI] [PubMed] [Google Scholar]
- 10. Shafi T, Jaar BG, Plantinga LC, Fink NE, Sadler JH, Parekh RS, et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis 2010; 56:348–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pecoits-Filho R, Carvalho MJ, Stenvinkel P, Lindholm B, Heimbürger O. Systemic and intraperitoneal interleukin-6 system during the first year of peritoneal dialysis. Perit Dial Int 2006; 26:53–63 [PubMed] [Google Scholar]
- 12. Miyata T, Ishiguro N, Yasuda Y, Ito T, Nangaku M, Iwata H, et al. Increased pentosidine, an advanced glycation end product, in plasma and synovial fluid from patients with rheumatoid arthritis and its relation with inflammatory markers. Biochem Biophys Res Commun 1998; 244:45–9 [DOI] [PubMed] [Google Scholar]
- 13. Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J 2010; 31:1771–9 [DOI] [PubMed] [Google Scholar]
- 14. Schepers E, Meert N, Glorieux G, Goeman J, Van der Eycken J, Vanholder R. p-Cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant 2007; 22:592–6 [DOI] [PubMed] [Google Scholar]
- 15. de Loor H, Meijers BK, Meyer TW, Bammens B, Verbeke K, Dehaen W, et al. Sodium octanoate to reverse indoxyl sulfate and p-cresyl sulfate albumin binding in uremic and normal serum during sample preparation followed by fluorescence liquid chromatography. J Chromatogr A 2009; 1216:4684–8 [DOI] [PubMed] [Google Scholar]
- 16. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989; 5:303–11 [PubMed] [Google Scholar]
- 17. Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 1980; 33:27–39 [DOI] [PubMed] [Google Scholar]
- 18. Bergström J, Heimbürger O, Lindholm B. Calculation of the protein equivalent of total nitrogen appearance from urea appearance. Which formulas should be used? Perit Dial Int 1998; 18:467–73 [PubMed] [Google Scholar]
- 19. Vanholder R, Meert N, Van Biesen W, Meyer T, Hostetter T, Dhondt A, et al. Why do patients on peritoneal dialysis have low blood levels of protein-bound solutes? Nat Clin Pract Nephrol 2009; 5:130–1 [DOI] [PubMed] [Google Scholar]
- 20. Enomoto A, Takeda M, Tojo A, Sekine T, Cha SH, Khamdang S, et al. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J Am Soc Nephrol 2002; 13:1711–20 [DOI] [PubMed] [Google Scholar]
- 21. Lesaffer G, De Smet R, D’Heuvaert T, Belpaire FM, Lameire N, Vanholder R. Comparative kinetics of the uremic toxin p-cresol versus creatinine in rats with and without renal failure. Kidney Int 2003; 64:1365–73 [DOI] [PubMed] [Google Scholar]
- 22. Meijers BK, Bammens B, Verbeke K, Evenepoel P. A review of albumin binding in CKD. Am J Kidney Dis 2008; 51:839–50 [DOI] [PubMed] [Google Scholar]
- 23. Evenepoel P, Bammens B, Verbeke K, Vanrenterghem Y. Superior dialytic clearance of β2-microglobulin and p-cresol by high-flux hemodialysis as compared to peritoneal dialysis. Kidney Int 2006; 70:794–9 [DOI] [PubMed] [Google Scholar]
- 24. Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, et al. Colonic contribution to uremic solutes. J Am Soc Nephrol 2011; 22:1769–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cummings JH, Hill MJ, Bone ES, Branch WJ, Jenkins DJ. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr 1979; 32:2094–101 [DOI] [PubMed] [Google Scholar]
- 26. Geypens B, Claus D, Evenepoel P, Hiele M, Maes B, Peeters M, et al. Influence of dietary protein supplements on the formation of bacterial metabolites in the colon. Gut 1997; 41:70–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel KP, Luo FJ, Plummer NS, Hostetter TH, Meyer TW. The production of p-cresol sulfate and indoxyl sulfate in vegetarians versus omnivores. Clin J Am Soc Nephrol 2012; 7:982–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang AY, Sea MM, Ip R, Law MC, Chow KM, Lui SF, et al. Independent effects of residual renal function and dialysis adequacy on actual dietary protein, calorie, and other nutrient intake in patients on continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 2001; 12:2450–7 [DOI] [PubMed] [Google Scholar]
- 29. Cerini C, Dou L, Anfosso F, Sabatier F, Moal V, Glorieux G, et al. p-Cresol, an uremic retention solute, alters the endothelial barrier function in vitro. Thromb Haemost 2004; 92:140–50 [DOI] [PubMed] [Google Scholar]
- 30. Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost 2007; 5:1302–8 [DOI] [PubMed] [Google Scholar]
- 31. Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, et al. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int 2004; 65:442–51 [DOI] [PubMed] [Google Scholar]
- 32. Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y, et al. Elevation of endothelial microparticles in chronic renal failure: role of uremic toxins [Abstract]. Nephrol Dial Transplant 2005; 20(Suppl 5):V63–64 [Google Scholar]
- 33. Ito S, Osaka M, Higuchi Y, Nishijima F, Ishii H, Yoshida M. Indoxyl sulfate induces leukocyte-endothelial interactions through up-regulation of E-selectin. J Biol Chem 2010; 285:38869–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Masai N, Tatebe J, Yoshino G, Morita T. Indoxyl sulfate stimulates monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells by inducing oxidative stress through activation of the NADPH oxidase-nuclear factor-κB pathway. Circ J 2010; 74:2216–24 [DOI] [PubMed] [Google Scholar]
- 35. Meijers BK, Van Kerckhoven S, Verbeke K, Dehaen W, Vanrenterghem Y, Hoylaerts MF, et al. The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis 2009; 54:891–901 [DOI] [PubMed] [Google Scholar]
- 36. Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T. Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-κB activation. Am J Nephrol 2010; 31:435–41 [DOI] [PubMed] [Google Scholar]
- 37. Yu M, Kim YJ, Kang DH. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol 2011; 6:30–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 2006; 69:1081–7 [DOI] [PubMed] [Google Scholar]
- 39. Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, et al. on behalf of the European Uraemic Toxin Work Group (EUTox). Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 2010; 25:1183–91 [DOI] [PubMed] [Google Scholar]
- 40. Meijers BK, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 2008; 73:1173–80 [DOI] [PubMed] [Google Scholar]
- 41. Meijers BK, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, et al. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol 2010; 5:1182–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chung SH, Heimbürger O, Stenvinkel P, Bergström J, Lindholm B. Association between inflammation and changes in residual renal function and peritoneal transport rate during the first year of dialysis. Nephrol Dial Transplant 2001; 16:2240–5 [DOI] [PubMed] [Google Scholar]
- 43. Cho JH, Hur IK, Kim CD, Park SH, Ryu HM, Yook JM, et al. Impact of systemic and local peritoneal inflammation on peritoneal solute transport rate in new peritoneal dialysis patients: a 1-year prospective study. Nephrol Dial Transplant 2010; 25:1964–73 [DOI] [PubMed] [Google Scholar]
- 44. Shemin D, Bostom AG, Laliberty P, Dworkin LD. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis 2001; 38:85–90 [DOI] [PubMed] [Google Scholar]
- 45. Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT. on behalf of the NECOSAD Study Group. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis 2003; 41:1293–302 [DOI] [PubMed] [Google Scholar]
- 46. Pecoits-Filho R, Heimbürger O, Bárány P, Suliman M, Fehrman-Ekholm I, Lindholm B, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis 2003; 41:1212–18 [DOI] [PubMed] [Google Scholar]