Abstract
Peritoneal dialysis (PD) is associated with functional and structural changes of the peritoneal membrane, also known as peritoneal remodeling. The peritoneal membrane is affected by many endogenous and exogenous factors such as cytokines, PD fluids, and therapeutic interventions. Here, we present an overview of various studies that have investigated pharmacologic interventions aimed at regression of peritoneal damage and prolongation of PD treatment.
Keywords: Therapeutic interventions
Long-term exposure to peritoneal dialysis (PD) fluid results in morphologic and functional changes of the peritoneal membrane, also known as peritoneal remodeling. These changes to the peritoneal membrane ultimately contribute to ultrafiltration failure, resulting in technique failure and discontinuation of PD therapy (1). The prevalence of ultrafiltration failure is high in long-term PD, and it is associated with an enlargement of the vascular surface area, fibrotic changes in the peritoneal membrane, impaired channel-mediated water transport, and reduced mesothelial cell (MC) mass (2). Consequently, the most important challenge currently faced in PD is the long-term preservation of peritoneal membrane structure and function.
Morphologic changes of the peritoneum are already seen in pre-dialysis uremic patients. In the largest peritoneal biopsy study to date, the measured submesothelial thickness in normal patients was 50 μm (3). In pre-dialysis uremic patients, it was similar to that for biopsies obtained from hemodialysis patients (140 μm and 150 μm respectively), implying that uremia itself may induce change in the peritoneal membrane. In patients undergoing PD, the submesothelial thickness was 270 μm. That value increased to 700 μm in patients who had been on PD for more than 8 years, indicating a fibrotic response of the peritoneal membrane to PD. In the study, Williams et al. (3) also identified vascular changes, including progressive subendothelial hyalinization, which led to luminal narrowing and obliteration (vasculopathy). A positive correlation was found between those vascular changes and fibrosis, suggesting that vasculopathy may lead to ischemia, exacerbating the development of fibrosis. Similar findings about hyalinization of the media of venules, accompanied by degradation of the smooth muscle cells, were described by Honda et al. (4,5) in patients on PD for 3 years. Those investigators linked the degree of vasculopathy to loss of ultrafiltration. Mateijsen et al. (6) also showed that peritoneal fibrosis, angiogenesis, and vasculopathy increase with time on PD. Plum et al. (7) confirmed those findings and identified an association between increased submesothelial thickness and increased solute transport. That observation was important, because high solute transport increases with time on PD and predicts technique failure and death (1).
Apart from being uremia-induced, peritoneal remodeling is also caused by factors associated with PD treatment. Those factors include the presence of the catheter and instillation of PD fluids, and the entire process can be aggravated by peritonitis episodes. Various components of PD fluid—including buffer, low pH, glucose concentration, and glucose degradation products (GDPs) generated during heat sterilization—can affect the peritoneal membrane (8). In nonuremic rats, injection of lactate buffer without glucose or GDPs resulted in increased cell influx, mesothelial regeneration, angiogenesis, and an increased number of milky spots, although it did not significantly enhance fibrosis (9,10). Addition of glucose to the buffer enhanced angiogenesis, mesothelial regeneration, and duplication of the basement membranes of omental capillaries. It also induced fibrosis and cell influx (9-12). The presence of GDPs further enhanced all the mentioned peritoneal changes apart from cell influx and mesothelial regeneration (9-15). In addition to producing local effects, GDPs also enter the systemic circulation and lead to deterioration of residual renal function (10). Advanced glycation end-products (AGEs), which are formed by the heating of glucose, also contribute to the toxicity of PD fluid and impair host defense mechanisms (5,16).
On a molecular level, important cytokines and growth factors related to angiogenesis and fibrosis are, respectively, vascular endothelial growth factor (VEGF) and transforming growth factor β (TGF-β). Glucose can induce the production of VEGF in peritoneal vascular endothelial cells and vascular smooth muscle cells (17). The GDPs and AGEs are also able to upregulate VEGF expression and subsequently induce angiogenesis. In addition, it has been suggested the GDPs and AGEs mediate the deposition of collagen and laminin in the peritoneal membrane and induce TGF-β expression, leading to fibrosis (5,18). Furthermore, peritoneal MCs undergo a transition from an epithelial phenotype to a mesenchymal phenotype (EMT) within 6 months after initiation of PD, with progressive loss of epithelial morphology (19). Transforming growth factor β and VEGF play a central role in the process of EMT.
The awareness that conventional PD fluids are bioincompatible prompted an intensive effort to develop alternative, more biocompatible PD fluids. At present, in vitro and animal data suggest a potential benefit for more biocompatible PD fluids, but consistent clinical data are lacking. Because glucose and its end-products increasingly seem to be harmful to the peritoneum, several substances have been examined as alternative osmotic agents. Only two agents—icodextrin and amino acids—have been successfully used clinically. Despite their proven benefit in certain clinical conditions, both solutions can be used for only 1 dwell daily. Icodextrin use is limited because of an accumulation of icodextrin metabolites, resulting in a fall in serum sodium (20); and amino acids, because of a risk for the development of acidosis and azotemia (21).
Recently, a study using l-carnitine as an alternative but similarly effective osmotic agent in 4 PD patients was published (22), but further studies on safety and efficacy are needed. In the recently published balANZ study, 185 incident patients were randomized to receive either low-GDP or conventional PD fluids. After 2 years, there appeared to be a delay in the onset of anuria and a reduction in the incidence of peritonitis in the group being treated with more biocompatible fluids (23).
Attention has been directed not only to the development of more physiologic PD fluids and to the search for glucose alternatives, but also to the potential beneficial effects of pharmacologic agents. Recently González-Mateo (24) published a very short review on the latter option, focusing on peroxisome proliferator-activated receptor γ (PPARG) agonists, cyclooxygenase 2 (COX-2) inhibitors, angiotensin converting-enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and bone morphogenetic protein 7 (BMP-7). The objective of the present article is to provide a more detailed overview of pharmacologic intervention strategies aimed at peritoneal damage regression and PD treatment prolongation, and of the mechanisms involved.
Potentially Beneficial Pharmacologic Agents
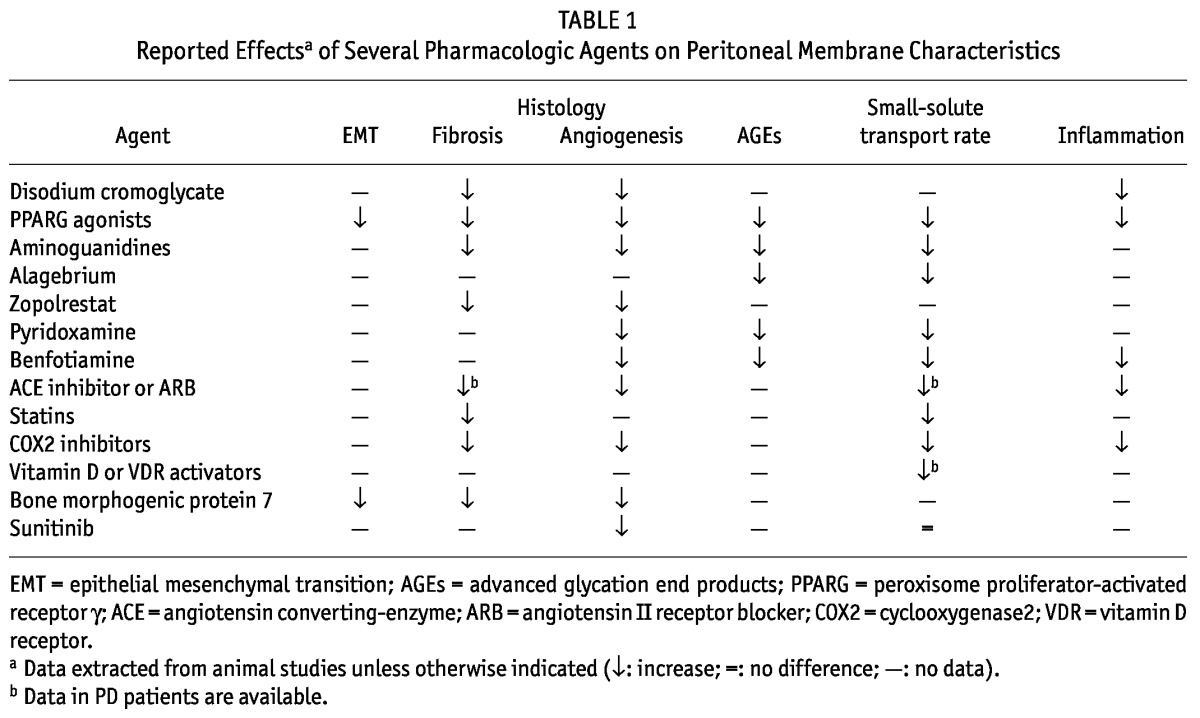
Evidence that a number of cytokines, growth factors, and prostaglandins play a key role in regulating and sustaining peritoneal remodeling is increasing (25,26). To gain more insight into processes in the peritoneal cavity upon instillation of PD fluid, several animal models for PD have been described (27). Studies have investigated whether therapeutic interventions can influence those processes. Pharmacologic agents might interfere in several ways. First, they can play a direct role in lessening chronic inflammation. Second, they can act on several mediators such as cytokines, growth factors, and hormones that play a key role in PD-mediated tissue remodeling. Third, they can up- or downregulate cellular signaling pathways induced by the activation of peritoneal cells during PD therapy, including MCs and recruited leucocytes (Table 1).
TABLE 1.
Reported Effectsa of Several Pharmacologic Agents on Peritoneal Membrane Characteristics
Agents Targeting Chronic Inflammation
Oral Disodium Cromoglycate: Activated mast cells can produce and release potent angiogenic factors such as VEGF, fibroblast growth factor 2 (FGF-2), tumor necrosis factor α, and interleukin 8 (28,29). Zareie et al. (30) showed that omental changes such as milky spots and angiogenesis are significantly reduced in rats deficient in mast cells. In the same study, wild-type Wister rats were exposed to conventional PD fluid with and without oral disodium cromoglycate, a mast cell stabilizer. That intervention yielded exactly the same results that were found in the mast cell-deficient rats.
Mast cell numbers are upregulated in the peritoneum of PD patients; the same holds for patients with other inflammatory diseases. Patients with encapsulating peritoneal sclerosis appear not to have an increased number of mast cells (31); however, in another study, the exact opposite was found: fewer mast cells in PD patients, suggesting no critical role for mast cells in the process of PD-related changes of the peritoneum (32). Nonetheless, it should be noted that, especially in situ, mast cells increase in number after PD, but they disappear in effluent, which might explain the differences in study findings. Because of these still-not-fully-explained findings concerning the role of mast cells in peritoneal tissue remodeling, disodium cromoglycate is so far not used as a therapeutic agent; however, further investigation is warranted.
Agents Targeting Ages
Rosiglitazone: The PPARG agonists are used to treat type 2 diabetes by improving insulin sensitivity, and they were also mentioned by González-Mateo (24). Besides their glucose-lowering effects, PPARG agonists have anti-inflammatory properties. In experimental models of both diabetic and nondiabetic chronic kidney disease, they have demonstrated antifibrotic effects (33,34). Oral administration of rosiglitazone to mice treated with PD fluid reduced AGE formation, peritoneal thickness, and angiogenesis; preserved the mesothelium; and resulted in less expression of myofibroblasts and in improved peritoneal function (35). In a small randomized crossover trial of continuous ambulatory PD patients treated with pioglitazone, Li et al. (36) found improvement of insulin resistance and reduced levels of C-reactive protein, indicating that pioglitazone might diminish inflammation in PD patients. In clinical practice, PPARG agonists are not easily used because of several side effects in patients with end-stage renal disease, such as increased risks of myocardial infarction, heart failure, bone fractures, hypoglycemia, and rise in cholesterol (37).
Benfotiamine: Benfotiamine prevents high-glucose-induced tissue damage in vitro and in vivo by activation of the enzyme transketolase, which in turn activates the pentose phosphate pathway. Benfotiamine is thought to act through several mechanisms, including a reduction in the accumulation of AGEs and direct antioxidant properties (38). In a uremic rat model of PD, treatment with benfotiamine reduced AGE accumulation, new vessel formation, and staining for VEGF in peritoneum. In addition, benfotiamine treatment lowered interleukin 6 and resulted in a slower rate of small-solute transport (38). In vitro experiments with human peritoneal MCs incubated with PD fluid and benfotiamine led to enhanced transketolase activity and decreased AGEs, which is in line with the results from rat experiments, suggesting that benfotiamine protects the peritoneal membrane in animals. These promising results need further investigation in humans.
Aminoguanidine: Evidence suggests that aminoguanidine can prevent PD fluid-induced peritoneal changes. Aminoguanidine is a diamine oxidase and nitric oxide synthase inhibitor. It acts by lowering the levels of AGEs through interaction with 3-deoxyglucosone, thus showing an ability to reduce vascular proliferation by modulating the expression of growth factors (16). In an in vitro study by Lamb et al. that used effluent from PD patients, AGE formation was shown to be inhibited by the addition of aminoguanidine to dialysate (39). Oral administration of aminoguanidine to rats also resulted in inhibition of peritoneal AGE accumulation and mesothelial denudation, as well as a lower peritoneal small-solute transport rate (40). In a rat model of PD, addition of aminoguanidine-bicarbonate to PD fluid effectively scavenged GDPs and was associated with reduced leucocyte rolling in mesenteric venules; fibrosis and vessel density in omentum and parietal peritoneum were also reduced (41). However, no clinical trials have been performed using either oral or intraperitoneal (IP) administration of aminoguanidine, possibly because oral use of high concentrations of aminoguanidine was found to be toxic in diabetic patients, causing neurotoxic complications and vitamin B6 deficiency (42).
Alagebrium: Administration of aminoguanidine to rats inhibits AGE formation and protein crosslinking. However, aminoguanidine cannot cleave or remove preformed tissue AGEs (40). Alagebrium, an AGE crosslink breaker, has been reported to reverse a number of diseases, presumably because of cleavage of tissue AGEs (43). In a study comparing IP alagebrium with IP aminoguanidine in rats on PD, it was confirmed that aminoguanidine cannot cleave and remove preformed AGEs from tissue. In contrast, alagebrium reduced the amount of preformed AGEs in capillaries and MCs of parietal peritoneum, and improved the peritoneal small-solute transport rate after 12 weeks of PD (43). Tested in humans, alagebrium reduced arterial stiffness and enhanced endothelial function in peripheral arteries (44). So far, no clinical data on the use of alagebrium in PD have been developed.
Zopolrestat: Another substance that prevents enhancement of the formation of AGEs is zopolrestat, an inhibitor of aldose reductase activity. In the polyol pathway (important for intracellular glucose metabolism), glucose is reduced to sorbitol by aldose reductase, with the eventual formation of AGEs (45). Administration of either oral or IP zopolrestat in a rat model of PD was effective in preventing the development of peritoneal fibrosis and angiogenesis (46). No clinical studies have so far been performed with zopolrestat in PD.
Pyridoxamine: Pyridoxamine is an inhibitor of AGEs and “carbonyl stress,” but the inhibitory mechanism remains elusive. Reactive carbonyl compounds, originating from the uremic circulation and from glucose-based PD fluid, initiate the modification of AGEs and accelerate the structural modification of peritoneal membrane proteins by AGEs (“peritoneal carbonyl stress”) (47). Oral administration of pyridoxamine to uremic rats on PD resulted in lower small-solute transport rates, decreased blood vessel density, and reduction in AGE accumulation and angiogenic cytokine expression of VEGF and FGF-2 (47).
Summary: Animal data show less peritoneal damage from PD with pharmacologic intervention against AGEs. Nevertheless, no available data currently demonstrate that any of the foregoing agents might be beneficial for PD patients.
Agents Targeting Angiotensin II
Peritoneal fibrosis can result from MCs secreting extracellular matrix macromolecules: collagen, fibronectin, laminin, proteoglycans, and cytokines including VEGF, TGF-β1, and interleukin 1 (48). Angiotensin II is an important factor in the development of renal fibrosis (49), and its stimulatory effects on the synthesis of extracellular matrix are probably mediated by TGF-β1 (50,51).
An in vitro study with human MCs incubated with any of captopril, enalapril (both ACE inhibitors), or losartan (an ARB) showed less production of tumor necrosis factor α and interleukin 1. That finding was associated with reduced basic VEGF production by mesothelial cells (52). Captopril also showed a concentration-dependent attenuation of VEGF that could not be shown for enalapril and losartan. That effect might be a result of captopril’s early phosphorylation effects. Duman et al. (48) investigated the effect of enalapril on peritoneal damage in nonuremic rats on PD. Vascularity, fibrosis and inflammatory cell infiltration were less in the enalapril-treated group than in rats treated only with PD. Although production of TGF-β1 was partially inhibited, peritoneal thickness was nevertheless more prominent in the enalapril group. A similar study yielding similar results was performed in nonuremic rats on PD using either enalapril or valsartan, an ARB (53).
In patients, Jing et al. (54) demonstrated an advantage of ACE inhibitor or ARB treatment for 12 months. Concentrations of TGF-β1, fibronectin, and VEGF in dialysate effluent were significantly higher in the group that did not use ACE inhibitor or ARB than in patients that did, suggesting that ACE inhibitor or ARB may protect the peritoneal membrane.
Kolesnyk et al. (55,56) focused on the long-term effects in PD patients of the use of either ACE inhibitor or ARB. The patient group that used such agents experienced decreased small-solute transport after 3 years. In controls, an increase of small-solute transport was seen with time on PD treatment, suggesting inhibition of peritoneal angiogenesis by additional ACE inhibitor or ARB treatment. In a larger prospective observational study with patients from the Netherlands Cooperative Study on the Adequacy of Dialysis, those results were confirmed (57). The 24-hour dialysate-to-plasma creatinine of two groups were compared after a follow-up of 4 years. Patients who had received ACE inhibitor or ARB during the 4 years showed stable creatinine transport; the untreated group showed an increase in creatinine transport.
Apart from the renin-angiotensin system, attention has been focused on aldosterone. In a rat model of peritoneal fibrosis induced by mechanical scraping of the peritoneum, resulting in a nonbacterial form of peritonitis, oral spironolactone treatment reduced peritoneal thickening, the number of macrophages in the peritoneum, and expression of monocyte chemoattractant protein 1 and TGF-β in the peritoneum (58). No data on mineralo-corticoid blockade in PD patients are available.
To summarize, animal data show improved ultrafiltration and less fibrosis with the use of ACE inhibitor or ARB. In humans, different findings are observed with respect to the effects of ACE inhibitor or ARB on residual renal function. These agents seem to preserve the function and morphology of the peritoneal membrane, but the long-term data are as yet insufficient to make recommendations.
Agents Targeting Fibrinolytic Systems
Statins: Statins inhibit the enzyme 3-hydroxy-3-methylglutaryl-CoA reductase and were designed to lower cholesterol. However, beneficial effects of statins unrelated to their lipid-lowering capacity, such as effects protective against atherothrombosis, have also been reported (59). Simvastatin increases synthesis of the fibrinolytic enzyme tissue-type plasminogen activator and decreases plasminogen activator inhibitor type 1 in human MCs (60). Simvastatin also suppresses tissue factor expression and increases fibrinolytic activity in tumor necrosis factor α-activated human peritoneal MCs (61). This action of simvastatin on the peritoneal fibrinolytic system might represent a mechanism for removing fibrin deposition in the peritoneal membrane and thereby reversing peritoneal thickening.
In an ischemic rat model, IP injection of statins has been shown to increase mRNA expression of tissue-type plasminogen activator and to reduce postoperative adhesions (62). Statins have also shown to prevent EMT in tubular epithelial cells in vitro (63). Adding atorvastatin to the drinking water of nonuremic rats on PD preserved ultrafiltration and resulted in less thickening of the peritoneal membrane (64). Clinical studies are needed to investigate the potential benefits of statin use for the prevention of peritoneal membrane alterations.
Agents Targeting Prostaglandins
Celecoxib and Indomethacin: Besides cytokines and growth factors, prostaglandins play an important role in the process of interstitial fibrosis, alteration of the mesothelial layer, and new vessel formation in PD. The COX enzymes catalyze the rate-limiting step in prostaglandin synthesis from arachidonic acid. Their metabolites play a crucial role in many physiologic and pathophysiologic processes. Expression of COX-2 has been demonstrated in several tissues and cells under both inflammatory and proliferative conditions (65), and this enzyme mediates angiogenesis through several mechanisms, including VEGF production and enhanced endothelial cell survival, among others (66).
In several in vitro assays and in vivo cancer models, celecoxib has been shown to inhibit angiogenesis (67). In rats receiving oral celecoxib treatment while on PD, ultrafiltration failure was completely prevented. Fibrosis was reduced in the parietal peritoneum. In addition, the angiogenic process was slower, and new lymphatic vessel formation was also prevented (68). In a study with mice receiving instilled PD fluid, celecoxib treatment also led to less fibrosis and partial recovery of ultrafiltration. Those outcomes were associated with a reduction of inflammatory cells (69).
Because inflammation is an early response of the peritoneum to PD fluid instillation, the beneficial effect of celecoxib could be a consequence of its antiinflammatory properties. In vitro exposure of cultured human peritoneal MCs to a selective COX-2 inhibitor (sc58236) showed a reduction of collagen I secretion and TGF-β1 expression, suggesting a role for this agent in ameliorating the course of progressive peritoneal fibrosis (70). Even though COX-2 was upregulated during EMT of MCs in vitro, COX-2 inhibition did not prevent EMT (69). In the clinical setting, COX-2 inhibitors have been associated with an increased risk of cardiac failure, making them difficult to use (71). At present, no data are available on the use of COX-2 inhibitors in humans on PD.
The role of locally produced prostaglandins in the regulation of peritoneal membrane characteristics during stable PD in humans has been investigated intraperitoneally using the prostaglandin inhibitor indomethacin, which inhibits 6-keto-prostaglandin F1α and thromboxane B2 intraperitoneally (72). A decrease in the release of these prostaglandins did not result in alterations of transport kinetics. During peritonitis, treatment with indomethacin resulted in less increased intrinsic permeability, but had no impact on effective surface area (73). Targeting prostaglandins might be beneficial in PD, but further research into the effects and safety of prostaglandin-targeting drugs is necessary.
Agents Targeting Biomarkers
Vitamin D: Vitamin D3 was originally identified as a key regulator of bone metabolism and calcium homeostasis. However, from experimental studies, it has become clear that vitamin D3 may play a direct role in the pathophysiology of inflammation, angiogenesis, and differentiation of many cell types.
Vitamin D3 has anti-inflammatory, anti-angiogenic and anti-proliferative effects (74). Zhang et al. (75) demonstrated that losartan treatment eliminates the difference in obstruction-induced interstitial fibrosis between unilateral uretal-obstructed vitamin D receptor (VDR) knockout mice and wild-type mice, a finding similar to that seen with inhibition of the renin-angiotensin-aldosterone system using ACE inhibitor or ARB and consistent with evidence that renin expression is suppressed by VDR activation (76). Moreover, Tan et al. (77) showed less renal interstitial fibrosis and less inflammation in rats receiving active vitamin D and ACE inhibitor. In addition, diabetic VDR knockout mice develop more severe albuminuria, glomerulosclerosis because of increased glomerular basement membrane and podocyte effacement, and higher levels of TGF-β1 and ACE. In vitro, 1,25-dihydroxy-vitamin D suppressed high-glucose-induced activation of the renin-angiotensin system and TGF-β1 in mesangial and juxtaglomerular cells derived from diabetic VDR knockout mice (78). Altogether, this evidence suggests that hyperglycemia and vitamin D deficiency both seem to play an important role in the pathway that leads to activation of the renin-angiotensin system and, in turn, stimulation of TGF-β1, which results in fibrosis (79).
In recent years, research has aimed to develop VDR activators that retain many of the anti-inflammatory properties of 1,25-dihydroxy-vitamin D, the bioactive form of vitamin D3, but with less intestinal calcium absorption (80,81). Treatment of uremic rats with Escherichia coli lipopolysaccharide induced inflammation; oral paricalcitol, a synthetic VDR activator, or calcitriol reduced the associated cytokine levels. Paricalcitol was more effective than calcitriol in lowering plasma levels of tumor necrosis factor α and monocyte chemoattractant protein 1 (82). Further, calcitriol increased lipopolysaccharide-induced vascular calcification, but paricalcitol did not. Those findings show that vitamin D influences inflammation, but suggest that not all vitamin D analogs have the same properties. Schilte et al. investigated whether oral paricalcitol affects peritoneal remodeling and immune parameters in rats on PD (unpublished data). Paricalcitol treatment resulted in restoration of ultrafiltration and decreased angiogenesis and fibrosis. In addition, upon VDR activation, a reduction of hyaluronic acid and an increase of monocyte chemoattractant protein 1 and cell numbers were found.
The effects of vitamin D3 on peritoneal remodeling in PD patients are not yet known. It can be hypothesized that vitamin D exerts its effects through multiple cell types involved in peritoneal remodeling because VDR is widely expressed throughout the body—in fibroblasts, MCs, vessel endothelium, and inflammatory cells, among others (83). Coronel et al. (84) recently showed less proteinuria and an increase in ultrafiltration in PD patients treated with paricalcitol compared with those who received no form of vitamin D. Further studies will be necessary to provide more insight concerning the effects of vitamin D in PD.
Bone Morphogenic Protein 7: The strong profibrotic cytokine TGF-β1 plays an important role in peritoneal structural and functional deterioration. It is among the factors that induce EMT (85). Bone morphogenic protein 7 (BMP-7) antagonizes TGF-β1, maintaining a delicate balance. The BMP-7 protects against and reverses fibrosis and negatively regulates EMT (19). In vitro addition of exogenous recombinant BMP-7 to human MCs completely blocks TGF-induced EMT of MCs. Administration of IP recombinant BMP-7 to rats exposed to PD fluid resulted in preservation of the MC monolayer and reduction of EMT, fibrosis, and angiogenesis in peritoneum. Recombinant BMP-7 had no effect on inflammatory and regenerative responses (86). It could not restore ultrafiltration, nor reduce angiogenesis in omentum and mesentery. So far, BMP-7 has not been used in humans to preserve peritoneal integrity during PD.
Sunitinib: The formation of new blood vessels is thought to be one of the major causes of ultrafiltration failure and technique failure in PD (87). Sunitinib inhibits many receptor tyrosine kinases, including the VEGF and platelet-derived growth factor receptors. Inhibition of receptor tyrosine kinases blocks signal transduction, thereby affecting many of the processes involved in tumor growth, progression, metastasis, and angiogenesis (88). The VEGF and platelet-derived growth factor signaling pathways are critical in the transduction of extracellular signals. Administration of sunitinib to rats on PD resulted in prevention of angiogenesis in omentum and mesentery; however, ultrafiltration did not improve (89). Moreover, other anti-VEGF agents such as octreotide can also improve peritoneal vascular alterations (90). Still, caution is needed so as not to provoke undesirable side effects when using anti-VEGF therapy, because this growth factor is involved in wound healing and tissue repair (91).
Conclusions
This review shows that, over the years, many therapeutic interventions have been investigated with the aim of preventing complicated processes such as the inflammation, fibrosis, and angiogenesis involved in peritoneal remodeling. Those processes can in part be prevented by medication. Drugs that lower chronic inflammation still have unexplained effects. Side effects of pharmacologic treatments should be further investigated, as should the clinical importance of these drugs for preserving the integrity of the peritoneal membrane for PD patients.
Future studies are needed to develop specific treatments to prevent peritoneal remodeling during chronic PD. The use of agents targeting AGEs, the fibrinolytic system, and prostaglandins result in less damage to the peritoneum. But most studies are performed in vitro or in animals. Less is known about the effects that these pharmacologic interventions may have on human peritoneum. The ACE inhibitors or ARBs seem to preserve the morphology of peritoneal membrane in humans, but further studies are needed. Accumulating experimental and clinical evidence indicates a renoprotective role of vitamin D in chronic renal disease. Activation of the VDR seems promising in PD, but there are not yet enough long-term data.
In our opinion, l-carnitine as an alternative osmotic agent in PD fluids seems promising, but very long trials involving large numbers of patients will be needed to confirm the results from animal studies. However, because developing a new kind of PD fluid is a costly process, drugs might be a less costly alternative. González-Mateo (24) made an interesting distinction concerning the way in which future drugs should be deployed. They propose using new pharmacologic agents in specific circumstances such as during peritonitis, to protect the peritoneal cavity. Our review has presented a more complete overview of the drugs that might potentially partly reverse the damaging effects of long-term exposure to PD and that therefore might have strong clinical applications in the future to prolong the course of patients on PD in a cost-effective way.
Disclosures
All the authors declared no competing interests.
References
- 1. Davies SJ, Phillips L, Griffiths AM, Russell LH, Naish PF, Russell GI. What really happens to people on long-term peritoneal dialysis? Kidney Int 1998; 54:2207–17 [DOI] [PubMed] [Google Scholar]
- 2. Smit W, Schouten N, van den Berg N, Langedijk MJ, Struijk DG, Krediet RT. on behalf of the Netherlands Ultrafiltration Failure Study Group. Analysis of the prevalence and causes of ultrafiltration failure during long-term peritoneal dialysis: a cross-sectional study. Perit Dial Int 2004; 24:562–70 [PubMed] [Google Scholar]
- 3. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. on behalf of the Peritoneal Biopsy Study Group. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 2002; 13:470–9 [DOI] [PubMed] [Google Scholar]
- 4. Honda K, Nitta K, Horita S, Yumura W, Nihei H. Morphological changes in the peritoneal vasculature of patients on CAPD with ultrafiltration failure. Nephron 1996; 72:171–6 [DOI] [PubMed] [Google Scholar]
- 5. Honda K, Nitta K, Horita S, Yumura W, Nihei H, Nagai R, et al. Accumulation of advanced glycation end products in the peritoneal vasculature of continuous ambulatory peritoneal dialysis patients with low ultra-filtration. Nephrol Dial Transplant 1999; 14:1541–9 [DOI] [PubMed] [Google Scholar]
- 6. Mateijsen MA, van der Wal AC, Hendriks PM, Zweers MM, Mulder J, Struijk DG, et al. Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int 1999; 19:517–25 [PubMed] [Google Scholar]
- 7. Plum J, Hermann S, Fusshöller A, Schoenicke G, Donner A, Röhrborn A, et al. Peritoneal sclerosis in peritoneal dialysis patients related to dialysis settings and peritoneal transport properties. Kidney Int Suppl 2001; 78:S42–7 [DOI] [PubMed] [Google Scholar]
- 8. Perl J, Nessim SJ, Bargman JM. The biocompatibility of neutral pH, low-GDP peritoneal dialysis solutions: benefit at bench, bedside, or both? Kidney Int 2011; 79:814–24 [DOI] [PubMed] [Google Scholar]
- 9. Zareie M, Keuning ED, ter Wee PM, Schalkwijk CG, Beelen RH, van den Born J. Improved biocompatibility of bicarbonate/lactate-buffered PDF is not related to pH. Nephrol Dial Transplant 2006; 21:208–16 [DOI] [PubMed] [Google Scholar]
- 10. Zareie M, Hekking LH, Welten AG, Driesprong BA, Schadee-Eestermans IL, Faict D, et al. Contribution of lactate buffer, glucose and glucose degradation products to peritoneal injury in vivo. Nephrol Dial Transplant 2003; 18:2629–37 [DOI] [PubMed] [Google Scholar]
- 11. Chan TM, Yung S. Studying the effects of new peritoneal dialysis solutions on the peritoneum. Perit Dial Int 2007; 27(Suppl 2):S87–93 [PubMed] [Google Scholar]
- 12. Zweers MM, Splint LJ, Krediet RT, Struijk DG. Ultrastructure of basement membranes of peritoneal capillaries in a chronic peritoneal infusion model in the rat. Nephrol Dial Transplant 2001; 16:651–4 [DOI] [PubMed] [Google Scholar]
- 13. Wieczorowska-Tobis K, Polubinska A, Schaub TP, Schilling H, Wisniewska J, Witowski J, et al. Influence of neutral-pH dialysis solutions on the peritoneal membrane: a long-term investigation in rats. Perit Dial Int 2001; 21(Suppl 3):S108–13 [PubMed] [Google Scholar]
- 14. Mortier S, Faict D, Lameire NH, De Vriese AS. Benefits of switching from a conventional to a low-GDP bicarbonate/lactate-buffered dialysis solution in a rat model. Kidney Int 2005; 67:1559–65 [DOI] [PubMed] [Google Scholar]
- 15. Musi B, Braide M, Carlsson O, Wieslander A, Albrektsson A, Ketteler M, et al. Biocompatibility of peritoneal dialysis fluids: long-term exposure of nonuremic rats. Perit Dial Int 2004; 24:37–47 [PubMed] [Google Scholar]
- 16. Miyata T, Devuyst O, Kurokawa K, van Ypersele de Strihou C. Toward better dialysis compatibility: advances in the biochemistry and pathophysiology of the peritoneal membranes. Kidney Int 2002; 61:375–86 [DOI] [PubMed] [Google Scholar]
- 17. Seo MJ, Oh SJ, Kim SI, Cho KW, Jo I, Schaub T, et al. High glucose dialysis solutions increase synthesis of vascular endothelial growth factors by peritoneal vascular endothelial cells. Perit Dial Int 2001; 21(Suppl 3):S35–40 [PubMed] [Google Scholar]
- 18. Ha H, Cha MK, Choi HN, Lee HB. Effects of peritoneal dialysis solutions on the secretion of growth factors and extracellular matrix proteins by human peritoneal mesothelial cells. Perit Dial Int 2002; 22:171–7 [PubMed] [Google Scholar]
- 19. Yáñez-Mó M, Lara-Pezzi E, Selgas R, Ramírez-Huesca M, Domínguez-Jiménez C, Jiménez-Heffernan JA, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Eng J Med 2003; 348:403–13 [Erratum in: N Engl J Med 2005; 353:2827] [DOI] [PubMed] [Google Scholar]
- 20. Posthuma N, ter Wee PM, Donker AJ, Oe PL, Peers EM, Verbrugh HA. Assessment of the effectiveness, safety, and biocompatibility of icodextrin in automated peritoneal dialysis. The Dextrin in APD in Amsterdam (DIANA) Group. Perit Dial Int 2000; 20(Suppl 2):S106–13 [PubMed] [Google Scholar]
- 21. Jones M, Hagen T, Boyle CA, Vonesh E, Hamburger R, Charytan C, et al. Treatment of malnutrition with 1.1% amino acid peritoneal dialysis solution: results of a multicenter outpatient study. Am J Kidney Dis 1998; 32:761–9 [DOI] [PubMed] [Google Scholar]
- 22. Bonomini M, Pandolfi A, Di Liberato L, Di Silvestre S, Cnops Y, Di Tomo P, et al. l-Carnitine is an osmotic agent suitable for peritoneal dialysis. Kidney Int 2011; 80:645–54 [DOI] [PubMed] [Google Scholar]
- 23. Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol 2012; 23:1097–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. González-Mateo GT, Aroeira LS, López-Cabrera M, Ruiz-Ortega M, Ortiz A, Selgas R. Pharmacological modulation of peritoneal injury induced by dialysis fluids: is it an option? Nephrol Dial Transplant 2012; 27:478–81 [DOI] [PubMed] [Google Scholar]
- 25. Oh KH, Margetts PJ. Cytokines and growth factors involved in peritoneal fibrosis of peritoneal dialysis patients. Int J Artif Organs 2005; 28:129–34 [DOI] [PubMed] [Google Scholar]
- 26. Jacobs P, Glorieux G, Vanholder R. Interleukin/cytokine profiles in haemodialysis and in continuous peritoneal dialysis. Nephrol Dial Transplant 2004; 19(Suppl 5):V41–5 [DOI] [PubMed] [Google Scholar]
- 27. Lameire N, Van Biesen W, Van Landschoot M, Wang T, Heimbürger O, Bergström J, et al. Experimental models in peritoneal dialysis: a European experience. Kidney Int 1998; 54:2194–206 [DOI] [PubMed] [Google Scholar]
- 28. Katsanos GS, Anogeianaki A, Orso C, Tetè S, Salini V, Antinolfi PL, et al. Mast cells and chemokines. J Biol Regul Homeost Agents 2008; 22:145–51 [PubMed] [Google Scholar]
- 29. Schilte MN, Celie JW, Wee PM, Beelen RH, van den Born J. Factors contributing to peritoneal tissue remodeling in peritoneal dialysis. Perit Dial Int 2009; 29:605–17 [PubMed] [Google Scholar]
- 30. Zareie M, Fabbrini P, Hekking LH, Keuning ED, Ter Wee PM, Beelen RH, et al. Novel role for mast cells in omental tissue remodeling and cell recruitment in experimental peritoneal dialysis. J Am Soc Nephrol 2006; 17:3447–57 [DOI] [PubMed] [Google Scholar]
- 31. Alscher DM, Braun N, Biegger D, Fritz P. Peritoneal mast cells in peritoneal dialysis patients, particularly in encapsulating peritoneal sclerosis patients. Am J Kidney Dis 2007; 49:452–61 [DOI] [PubMed] [Google Scholar]
- 32. Jiménez-Heffernan JA, Bajo MA, Perna C, del Peso G, Larrubia JR, Gamallo C, et al. Mast cell quantification in normal peritoneum and during peritoneal dialysis treatment. Arch Pathol Lab Med 2006; 130:1188–92 [DOI] [PubMed] [Google Scholar]
- 33. Kawai T, Masaki T, Doi S, Arakawa T, Yokoyama Y, Doi T, et al. PPAR-γ agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-β. Lab Invest 2009; 89:47–58 [DOI] [PubMed] [Google Scholar]
- 34. Toblli JE, Ferrini MG, Cao G, Vernet D, Angerosa M, Gonzalez-Cadavid NF. Antifibrotic effects of pioglitazone on the kidney in a rat model of type 2 diabetes mellitus. Nephrol Dial Transplant 2009; 24:2384–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sandoval P, Loureiro J, González-Mateo G, Pérez-Lozano ML, Maldonado-Rodríguez A, Sánchez-Tomero JA, et al. PPAR-γ agonist rosiglitazone protects peritoneal membrane from dialysis fluid-induced damage. Lab Invest 2010; 90:1517–32 [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Xie QH, You HZ, Tian J, Hao CM, Lin SY, et al. Twelve weeks of pioglitazone therapy significantly attenuates dysmetabolism and reduces inflammation in continuous ambulatory peritoneal dialysis patients—a randomized crossover trial. Perit Dial Int 2012; 32:507–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rizos CV, Elisaf MS, Mikhailidis DP, Liberopoulos EN. How safe is the use of thiazolidinediones in clinical practice? Expert Opin Drug Saf 2009; 8:15–32 [DOI] [PubMed] [Google Scholar]
- 38. Kihm LP, Müller-Krebs S, Klein J, Ehrlich G, Mertes L, Gross ML, et al. Benfotiamine protects against peritoneal and kidney damage in peritoneal dialysis. J Am Soc Nephrol 2011; 22:914–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lamb EJ, Cattell WR, Dawnay AB. In vitro formation of advanced glycation end products in peritoneal dialysis fluid. Kidney Int 1995; 47:1768–74 [DOI] [PubMed] [Google Scholar]
- 40. Lee EA, Oh JH, Lee HA, Kim SI, Park EW, Park KB, et al. Structural and functional alterations of the peritoneum after prolonged exposure to dialysis solutions: role of aminoguanidine. Perit Dial Int 2001; 21:245–53 [PubMed] [Google Scholar]
- 41. Zareie M, Tangelder GJ, ter Wee PM, Hekking LH, van Lambalgen AA, Keuning ED, et al. Beneficial effects of aminoguanidine on peritoneal microcirculation and tissue remodelling in a rat model of PD. Nephrol Dial Transplant 2005; 20:2783–92 [DOI] [PubMed] [Google Scholar]
- 42. Miyoshi H, Taguchi T, Sugiura M, Takeuchi M, Yanagisawa K, Watanabe Y, et al. Aminoguanidine pyridoxal adduct is superior to aminoguanidine for preventing diabetic nephropathy in mice. Horm Metab Res 2002; 34:371–7 [DOI] [PubMed] [Google Scholar]
- 43. Lee YK, Lee JY, Kim JS, Won KB, Kang HJ, Jang TJ, et al. The breakdown of preformed peritoneal advanced glycation end products by intraperitoneal alagebrium. J Korean Med Sci 2009; 24(Suppl):S189–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zieman SJ, Melenovsky V, Clattenburg L, Corretti MC, Capriotti A, Gerstenblith G, et al. Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. J Hypertens 2007; 25:577–83 [DOI] [PubMed] [Google Scholar]
- 45. Cameron NE, Cotter MA. Metabolic and vascular factors in the pathogenesis of diabetic neuropathy. Diabetes 1997; 46(Suppl 2):S31–7 [DOI] [PubMed] [Google Scholar]
- 46. van Westrhenen R, Aten J, Aberra M, Dragt CA, Deira G, Krediet RT. Effects of inhibition of the polyol pathway during chronic peritoneal exposure to a dialysis solution. Perit Dial Int 2005; 25(Suppl 3):S18–21 [PubMed] [Google Scholar]
- 47. Kakuta T, Tanaka R, Satoh Y, Izuhara Y, Inagi R, Nangaku M, et al. Pyridoxamine improves functional, structural, and biochemical alterations of peritoneal membranes in uremic peritoneal dialysis rats. Kidney Int 2005; 68:1326–36 [DOI] [PubMed] [Google Scholar]
- 48. Duman S, Günal AI, Sen S, Asçi G, Ozkahya M, Terzioglu E, et al. Does enalapril prevent peritoneal fibrosis induced by hypertonic (3.86%) peritoneal dialysis solution? Perit Dial Int 2001; 21:219–24 [PubMed] [Google Scholar]
- 49. Wolf G, Neilson EG. Angiotensin II as a renal growth factor. J Am Soc Nephrol 1993; 3:1531–40 [DOI] [PubMed] [Google Scholar]
- 50. Fern RJ, Yesko CM, Thornhill BA, Kim HS, Smithies O, Chevalier RL. Reduced angiotensinogen expression attenuates renal interstitial fibrosis in obstructive nephropathy in mice. J Clin Invest 1999; 103:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-β expression in rat glomerular mesangial cells. J Clin Invest 1994; 93:2431–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sauter M, Cohen CD, Wörnle M, Mussack T, Ladurner R, Sitter T. ACE inhibitor and AT1-receptor blocker attenuate the production of VEGF in mesothelial cells. Perit Dial Int 2007; 27:167–72 [PubMed] [Google Scholar]
- 53. Duman S, Sen S, Duman C, Oreopoulos DG. Effect of valsartan versus lisinopril on peritoneal sclerosis in rats. Int J Artif Organs 2005; 28:156–63 [DOI] [PubMed] [Google Scholar]
- 54. Jing S, Kezhou Y, Hong Z, Qun W, Rong W. Effect of renin-angiotensin system inhibitors on prevention of peritoneal fibrosis in peritoneal dialysis patients. Nephrology (Carlton) 2010; 15:27–32 [DOI] [PubMed] [Google Scholar]
- 55. Kolesnyk I, Noordzij M, Dekker FW, Boeschoten EW, Krediet RT. Treatment with angiotensin II inhibitors and residual renal function in peritoneal dialysis patients. Perit Dial Int 2011; 31:53–9 [DOI] [PubMed] [Google Scholar]
- 56. Kolesnyk I, Dekker FW, Noordzij M, le Cessie S, Struijk DG, Krediet RT. Impact of ACE inhibitors and AII receptor blockers on peritoneal membrane transport characteristics in long-term peritoneal dialysis patients. Perit Dial Int 2007; 27:446–53 [PubMed] [Google Scholar]
- 57. Kolesnyk I, Noordzij M, Dekker FW, Boeschoten EW, Krediet RT. A positive effect of AII inhibitors on peritoneal membrane function in long-term PD patients. Nephrol Dial Transplant 2009; 24:272–7 [DOI] [PubMed] [Google Scholar]
- 58. Nishimura H, Ito Y, Mizuno M, Tanaka A, Morita Y, Maruyama S, et al. Mineralocorticoid receptor blockade ameliorates peritoneal fibrosis in new rat peritonitis model. Am J Physiol Renal Physiol 2008; 294:F1084–93 [DOI] [PubMed] [Google Scholar]
- 59. Heimbürger O. Lipid disorders, statins and the peritoneal membrane. Contrib Nephrol 2009; 163:177–82 [DOI] [PubMed] [Google Scholar]
- 60. Haslinger B, Goedde MF, Toet KH, Kooistra T. Simvastatin increases fibrinolytic activity in human peritoneal mesothelial cells independent of cholesterol lowering. Kidney Int 2002; 62:1611–19 [DOI] [PubMed] [Google Scholar]
- 61. Haslinger B, Kleemann R, Toet KH, Kooistra T. Simvastatin suppresses tissue factor expression and increases fibrinolytic activity in tumor necrosis factor-α-activated human peritoneal mesothelial cells. Kidney Int 2003; 63:2065–74 [DOI] [PubMed] [Google Scholar]
- 62. Aarons CB, Cohen PA, Gower A, Reed KL, Leeman SE, Stucchi AF, et al. Statins (HMG-CoA reductase inhibitors) decrease postoperative adhesions by increasing peritoneal fibrinolytic activity. Ann Surg 2007; 245:176–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Patel S, Mason RM, Suzuki J, Imaizumi A, Kamimura T, Zhang Z. Inhibitory effect of statins on renal epithelial-to-mesenchymal transition. Am J Nephrol 2006; 26:381–7 [DOI] [PubMed] [Google Scholar]
- 64. Duman S, Sen S, Sozmen EY, Oreopoulos DG. Atorvastatin improves peritoneal sclerosis induced by hypertonic PD solution in rats. Int J Artif Organs 2005; 28:170–6 [DOI] [PubMed] [Google Scholar]
- 65. Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999; 18:7908–16 [DOI] [PubMed] [Google Scholar]
- 66. Gately S, Li WW. Multiple roles of COX-2 in tumor angiogenesis: a target for antiangiogenic therapy. Semin Oncol 2004; 31(Suppl 7):2–11 [DOI] [PubMed] [Google Scholar]
- 67. Iñiguez MA, Rodríguez A, Volpert OV, Fresno M, Redondo JM. Cyclooxygenase-2: a therapeutic target in angiogenesis. Trends Mol Med 2003; 9:73–8 [DOI] [PubMed] [Google Scholar]
- 68. Fabbrini P, Schilte MN, Zareie M, ter Wee PM, Keuning ED, Beelen RH, et al. Celecoxib treatment reduces peritoneal fibrosis and angiogenesis and prevents ultrafiltration failure in experimental peritoneal dialysis. Nephrol Dial Transplant 2009; 24:3669–76 [DOI] [PubMed] [Google Scholar]
- 69. Aroeira LS, Lara-Pezzi E, Loureiro J, Aguilera A, Ramírez-Huesca M, González-Mateo G, et al. Cyclooxygenase-2 mediates dialysate-induced alterations of the peritoneal membrane. J Am Soc Nephrol 2009; 20:582–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu H, Peng Y, Liu F, Li J, Chen X, Liu Y, et al. A selective cyclooxygenase-2 inhibitor decreases transforming growth factor-β1 synthesis and matrix production in human peritoneal mesothelial cells. Cell Biol Int 2007; 31:508–15 [DOI] [PubMed] [Google Scholar]
- 71. Andersohn F, Suissa S, Garbe E. Use of first- and second-generation cyclooxygenase-2-selective nonsteroidal antiinflammatory drugs and risk of acute myocardial infarction. Circulation 2006; 113:1950–7 [DOI] [PubMed] [Google Scholar]
- 72. Douma CE, de Waart DR, Zemel D, Struijk DG, Krediet RT. Prostaglandin inhibition by intraperitoneal indomethacin has no effect on peritoneal permeability during stable CAPD. Nephrol Dial Transplant 2001; 16:803–8 [DOI] [PubMed] [Google Scholar]
- 73. Zemel D, Struijk DG, Dinkla C, Stolk LM, ten Berge IJ, Krediet RT. Effects of intraperitoneal cyclooxygenase inhibition on inflammatory mediators in dialysate and peritoneal membrane characteristics during peritonitis in continuous ambulatory peritoneal dialysis. J Lab Clin Med 1995; 126:204–15 [PubMed] [Google Scholar]
- 74. Levin A, Li YC. Vitamin D and its analogues: do they protect against cardiovascular disease in patients with kidney disease? Kidney Int 2005; 68:1973–81 [DOI] [PubMed] [Google Scholar]
- 75. Zhang Y, Kong J, Deb DK, Chang A, Li YC. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol 2010; 21:966–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cunningham J, Zehnder D. New vitamin D analogs and changing therapeutic paradigms. Kidney Int 2011; 79:702–7 [DOI] [PubMed] [Google Scholar]
- 77. Tan X, He W, Liu Y. Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy. Kidney Int 2009; 76:1248–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang Z, Sun L, Wang Y, Ning G, Minto AW, Kong J, et al. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int 2008; 73:163–71 [DOI] [PubMed] [Google Scholar]
- 79. Klaus G. Renoprotection with vitamin D: specific for diabetic nephropathy? Kidney Int 2008; 73:141–3 [DOI] [PubMed] [Google Scholar]
- 80. Brown AJ, Slatopolsky E. Vitamin D analogs: perspectives for treatment. Miner Electrolyte Metab 1999; 25:337–41 [DOI] [PubMed] [Google Scholar]
- 81. Adorini L. Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell Immunol 2005; 233:115–24 [DOI] [PubMed] [Google Scholar]
- 82. Guerrero F, Montes de Oca A, Aguilera-Tejero E, Zafra R, Rodríguez M, López I. The effect of vitamin D derivatives on vascular calcification associated with inflammation. Nephrol Dial Transplant 2012; 27:2206–12 [DOI] [PubMed] [Google Scholar]
- 83. Braun N, Fritz P, Biegger D, Kimmel M, Reimold F, Ulmer C, et al. Difference in the expression of hormone receptors and fibrotic markers in the human peritoneum—implications for therapeutic targets to prevent encapsulating peritoneal sclerosis. Perit Dial Int 2011; 31:291–300 [DOI] [PubMed] [Google Scholar]
- 84. Coronel F, Cigarran S, Gomis A, Rodríguez-Cubillo B, Herrero JA, Delgado P, et al. Changes in peritoneal membrane permeability and proteinuria in patients on peritoneal dialysis after treatment with paricalcitol: a preliminary study. Clin Nephrol 2012; 78:93–9 [DOI] [PubMed] [Google Scholar]
- 85. Wang S, Chen Q, Simon TC, Strebeck F, Chaudhary L, Morrissey J, et al. Bone morphogenic protein-7 (BMP-7), a novel therapy for diabetic nephropathy. Kidney Int 2003; 63:2037–49 [DOI] [PubMed] [Google Scholar]
- 86. Loureiro J, Schilte M, Aguilera A, Albar-Vizcaíno P, Ramírez-Huesca M, Pérez-Lozano ML, et al. BMP-7 blocks mesenchymal conversion of mesothelial cells and prevents peritoneal damage induced by dialysis fluid exposure. Nephrol Dial Transplant 2010; 25:1098–108 [DOI] [PubMed] [Google Scholar]
- 87. Krediet RT, Lindholm B, Rippe B. Pathophysiology of peritoneal membrane failure. Perit Dial Int 2000; 20(Suppl 4):S22–42 [PubMed] [Google Scholar]
- 88. Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol 2007; 25:884–96 [DOI] [PubMed] [Google Scholar]
- 89. Stavenuiter AW, Schilte MN, Ter Wee PM, Beelen RH. Angiogenesis in peritoneal dialysis. Kidney Blood Press Res 2011; 34:245–52 [DOI] [PubMed] [Google Scholar]
- 90. Günal AI, Celiker H, Akpolat N, Ustündag B, Duman S, Akcicek F. By reducing production of vascular endothelial growth factor octreotide improves the peritoneal vascular alterations induced by hypertonic peritoneal dialysis solution. Perit Dial Int 2002; 22:301–6 [PubMed] [Google Scholar]
- 91. Rippe B. Peritoneal angiogenesis in response to dialysis fluid. Contrib Nephrol 2009; 163:60–6 [DOI] [PubMed] [Google Scholar]


