Abstract
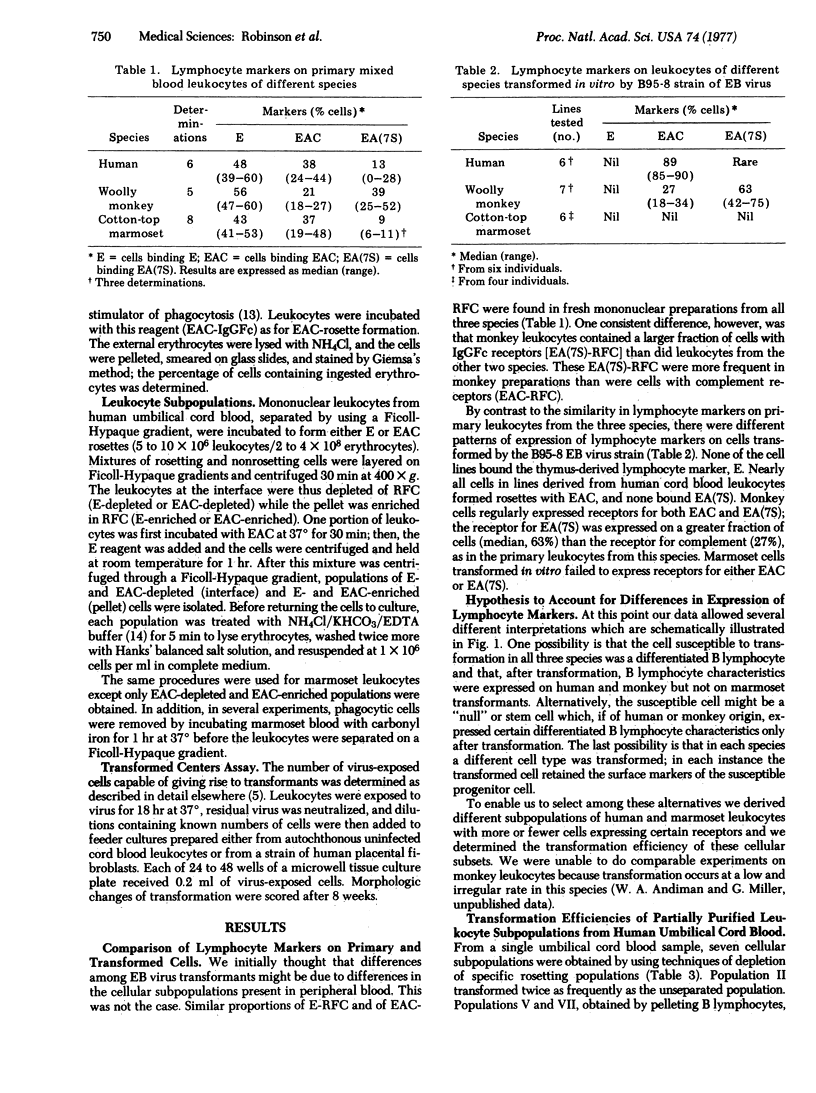
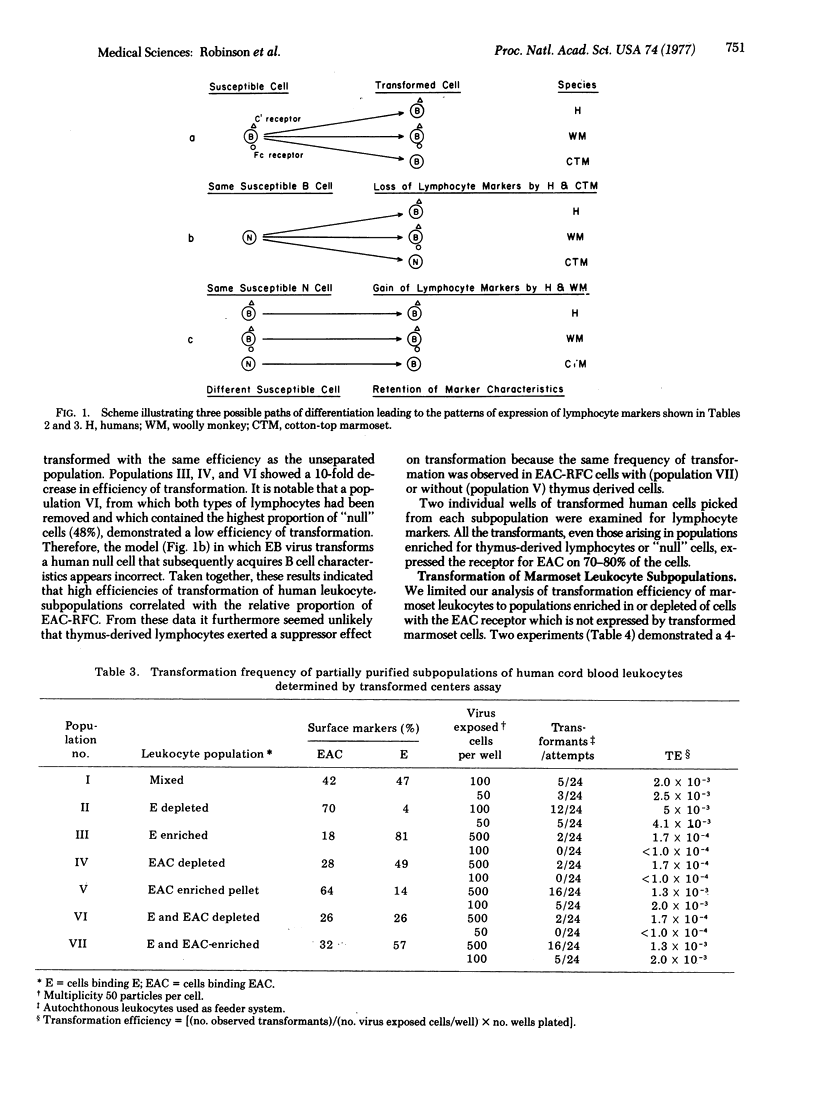
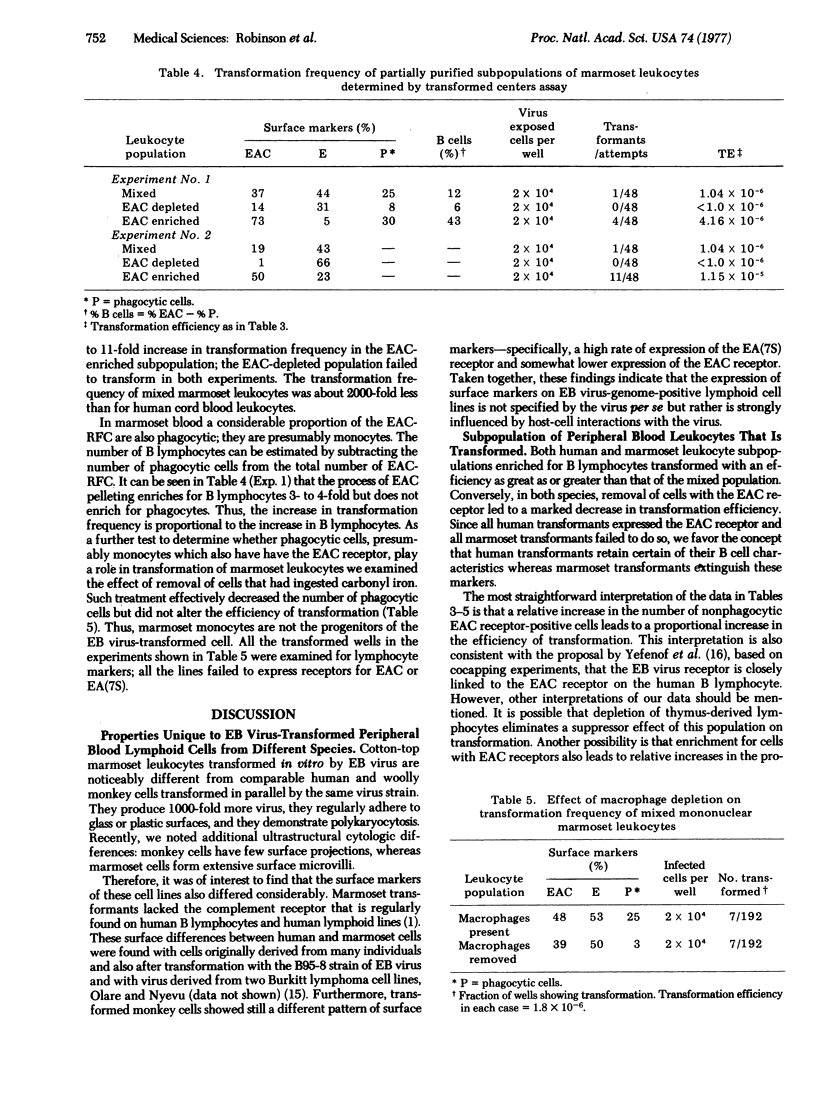
In an attempt to account for differences in the biologic behavior of Epstein-Barr virus in different primate species, we studied lymphocyte surface markers on primary and transformed cells. Among primary leukocytes, the distribution of cells with characteristics of bone-marrow-derived cells (B cells) was similar in humans, wooly monkeys, and cotton-top marmosets. However, after transformation by Epstein-Barr virus, cells from each species were characterically different. Transformed human umbilical cord cells expressed the complement receptor; monkey cells exhibited both this receptor and the receptor for IgG Fc (EA7S); and marmoset cells did not have either surface marker. We measured the transformation efficiency of human and marmoset leukocyte subpopulations enriched or depeleted in cells with the complement receptor. In both species the highest efficiencies of transformation were found in populations with the greatest numbers of cells with the receptor. The data therefore suggest that, in all species, a cell with the complement receptor is susceptible to transformation but that this receptor is not expressed on transformed marmoset cells. Thus, in Epstein-Barr virus-induced transformation it is necessary to distinguish between transformation of growth properties (immortalization) and transformation of cell surface properties.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Ehlenberger A. G., McWilliams M., Phillips-Quagliata J. M., Lamm M. E., Nussenzweig V. Immunoglobulin-bearing and complement-receptor lymphocytes constitute the same population in human peripheral blood. J Clin Invest. 1976 Jan;57(1):53–56. doi: 10.1172/JCI108268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein A. L., Henle W., Henle G., Hewetson J. F., Kaplan H. S. Surface marker characteristics and Epstein-Barr virus studies of two established North American Burkitt's lymphoma cell lines. Proc Natl Acad Sci U S A. 1976 Jan;73(1):228–232. doi: 10.1073/pnas.73.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A., Andiman W. A., Miller G. Epstein-Barr virus and nonhuman primates: natural and experimental infection. Adv Cancer Res. 1976;23:171–201. doi: 10.1016/s0065-230x(08)60546-1. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Epstein-Barr virus binding sites on lymphocyte subpopulations and the origin of lymphoblasts in cultured lymphoic cell lines and in the blood of patients with infectious mononucleosis. Clin Immunol Immunopathol. 1975 Mar;3(4):514–524. doi: 10.1016/0090-1229(75)90076-8. [DOI] [PubMed] [Google Scholar]
- Huber C., Sundström C., Nilsson K., Wigzell H. Surface receptors on human haematopoietic cell lines. Clin Exp Immunol. 1976 Sep;25(3):367–376. [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos J. A., Roos D. Ficoll-isopaque gradients for the determination of density distributions of human blood lymphocytes and other reticulo-endothelial cells. Exp Cell Res. 1974 Jun;86(2):333–341. doi: 10.1016/0014-4827(74)90721-6. [DOI] [PubMed] [Google Scholar]
- Menezes J., Jondal M., Leibold W., Dorval G. Epstein-Barr virus interactions with human lymphocyte subpopulations: virus adsorption, kinetics of expression of Epstein-Barr virus-associated nuclear antigen, and lymphocyte transformation. Infect Immun. 1976 Feb;13(2):303–310. doi: 10.1128/iai.13.2.303-310.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Coope D., Niederman J., Pagano J. Biological properties and viral surface antigens of Burkitt lymphoma- and mononucleosis- derived strains of Epstein-Barr virus released from transformed marmoset cells. J Virol. 1976 Jun;18(3):1071–1080. doi: 10.1128/jvi.18.3.1071-1080.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattengale P. K., Smith R. W., Gerber P. B-cell characteristics of human peripheral and cord blood lymphocytes transformed by Epstein-Barr virus. J Natl Cancer Inst. 1974 Apr;52(4):1081–1086. doi: 10.1093/jnci/52.4.1081. [DOI] [PubMed] [Google Scholar]
- Schneider U., zur Hausen H. Epstein-Barr virus-induced transformation of human leukocytes after cell fractionation. Int J Cancer. 1975 Jan 15;15(1):59–66. doi: 10.1002/ijc.2910150108. [DOI] [PubMed] [Google Scholar]
- Shope T., Dechairo D., Miller G. Malignant lymphoma in cottontop marmosets after inoculation with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2487–2491. doi: 10.1073/pnas.70.9.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Yata J., Desgranges C., Nakagawa T., Favre M. C., De-The G. Lymphoblastoid transformation and kinetics of appearance of viral nuclear antigen (EBNA) in cord-blood lymphocytes infected by Epstein-Barr Virus (EBV). Int J Cancer. 1975 Mar 15;15(3):377–384. doi: 10.1002/ijc.2910150303. [DOI] [PubMed] [Google Scholar]
- Yefenof E., Klein G., Jondal M., Oldstone M. B. Surface markers on human B and T-lymphocytes. IX. Two-color immunofluorescence studies on the association between ebv receptors and complement receptors on the surface of lymphoid cell lines. Int J Cancer. 1976 Jun 15;17(6):693–700. doi: 10.1002/ijc.2910170602. [DOI] [PubMed] [Google Scholar]



