Editor:
Although uncommon, hydrothorax is a well-recognized complication of peritoneal dialysis (PD). The incidence of this complication ranges from 1.6% to 10% in the adult population (1-3); in pediatric patients, an incidence of up to 3% has been reported (4). The postulated mechanisms of its pathogenesis are pleural-peritoneal communication (2,5), congenital or acquired anatomic defects of the diaphragm causing a track between the peritoneal cavity and the pleural space (6-8), and defective lymphatic drainage resulting from a difference in pressure gradient between the peritoneal cavity and the pleural cavity (9).
Various tools for diagnosing hydrothorax have been evaluated. Chest radiography is the easiest and quickest way to demonstrate the presence of pleural effusion. Thoracentesis is used to differentiate between transudative and exudative pleural fluid. However, those methods cannot establish the provenance of the fluid beyond a doubt. Transudative pleural effusion may have other causes: pneumonia, fluid overload, heart failure, or hypoalbuminemia, for example. High glucose content in the pleural fluid has been associated with the peritoneal cavity (10), but those values are not always precise, and a consensus has not been reached on cut-off values. For many years, scintigraphy has been used to demonstrate peritoneal leaks, and it has effectively demonstrated the direction of the fluid (2,11,12). However, description of its use in infancy has been elusive.
Case Description
Our patient was 6 months old at the time of admission to the pediatric floor. This little girl had developed hypoxic ischemic encephalopathy secondary to a presumed placental abruption. She also sustained acute kidney injury from hypoxia and bilateral renal venous thrombosis. Peritoneal dialysis was initiated at 4 days of life and had to be continued despite fibrinolytic therapy.
At 6 months of age, this infant presented with feeding intolerance and had to be admitted. Eighteen days into the admission, she developed respiratory symptoms, including intercostal retractions and tachypnea. Chest radiography showed the presence of bilateral pleural effusions (Figure 1). Because the infant was generally edematous (although her weight had increased to only 6.6 kg from 6.5 kg), the pleural effusions were thought to be secondary to fluid overload. The PD prescription was adjusted to increase ultrafiltration [identical volume, but changed to 3.86% Physioneal from 2.27% (Baxter International, Mississauga, Canada)], but despite the increased concentration, the infant retained her dialysate.
Figure 1 —
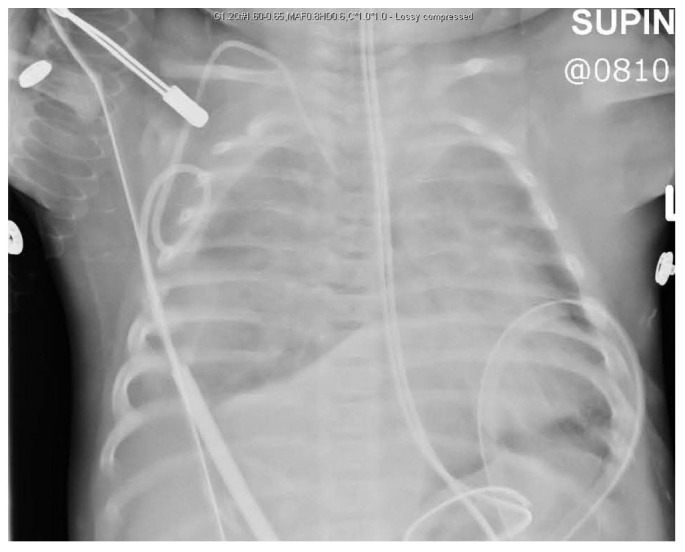
Chest radiograph at initial presentation of respiratory symptoms.
She developed severe respiratory distress and desaturation and had to be transferred to the pediatric intensive care unit, where she was treated with continuous positive airway pressure. The possibility that her hydrothorax was caused by PD fluid was entertained, and the PD catheter was left to drain. The patient then rapidly improved and required only low-flow oxygen after 12 hours. Repeat chest radiography (Figure 2) and chest ultrasonography (Figure 3) showed a large collection of fluid in the chest.
Figure 2 —
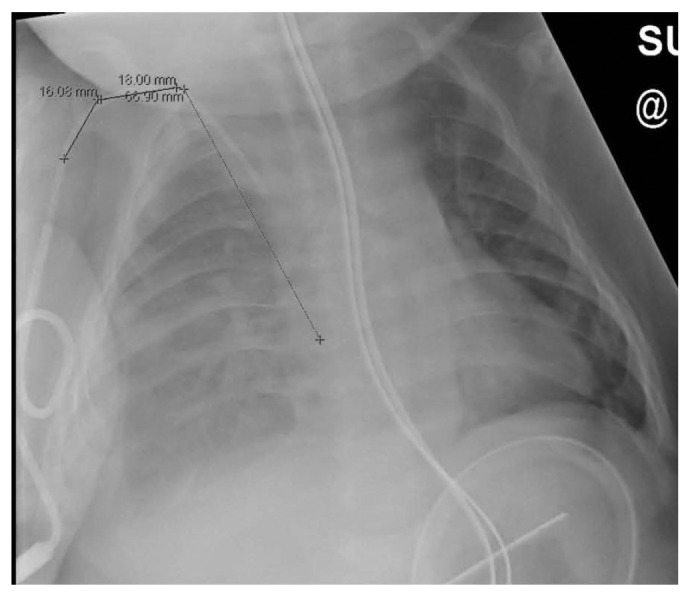
Second radiograph, after development of symptoms. The fluid collection in the entire pleural space on the right is clearly visible.
Figure 3 —
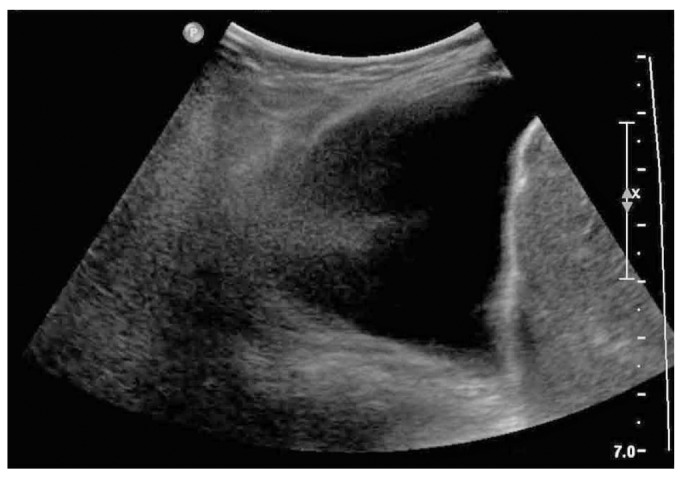
Ultrasonography of chest demonstrates the extent of hydrothorax (sagittal view of right thorax).
The patient underwent radionuclide scintigraphy to assess the possibility of PD-associated hydrothorax (Figure 4). The examination involved infusion of Physioneal PD fluid, spiked with 115 MBq (standard pediatric dose) 99Tc diethylenetriamine pentaacetic acid, into the peritoneal cavity through the existing peritoneal access and observation of the PD fluid leaking into the pleural cavity. The scintigrams showed predominantly right-sided leakage and minor accumulation in the left pleural cavity. The diagnosis of PD-associated hydrothorax was confirmed. Peritoneal dialysis was discontinued after that finding, and the patient was converted to hemodialysis, which resulted in stable respiratory status immediately after the first treatment. The parents opted against a new challenge with PD after 3 months, and the patient is continuing hemodialysis until a suitable donor for transplantation can be found.
Figure 4 —
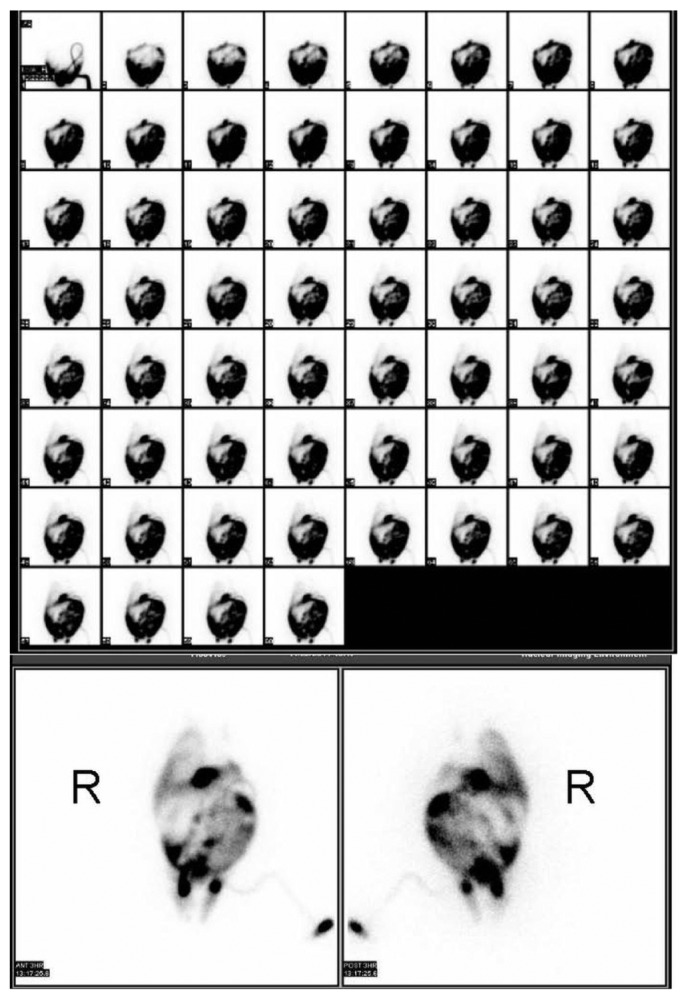
Peritoneal scintigraphy demonstrates leakage of peritoneal fluid into the right pleural cavity and also minimal drainage into the left pleural space. Early images (top) were taken at 1-minute intervals. The later images were taken at 3 hours after infusion. R = patient’s right side.
Discussion
The diagnosis of pleural effusion is made on the basis of chest radiography and confirmed by ultrasonography or computed tomography imaging. However, those investigations do not indicate the origin of the fluid (13), and exposure to radiation from the computed tomography imaging is considerable.
Biomarkers from pleural fluid analysis, such as high glucose content, can be indicative of peritoneal fluid in the pleural cavity. However, the exact cut-off levels have not been completely defined and may depend on the glucose concentration of the dialysate (10). Moreover, obtaining pleural fluid is an invasive procedure with significant risks in an infant on dialysis with respiratory distress. Infusion of methylene blue into the peritoneal cavity is not a sensitive enough test and may have the serious side effect of chemical peritonitis (13,14).
Several case reports have demonstrated the usefulness of peritoneal scintigraphy in showing a peritoneal-pleural leak. Most of the case reports involved adult patients; only a few involved children (4,13,15-18). Peritoneal scintigraphy was considered to have the best sensitivity and specificity for the diagnosis of PD-associated hydrothorax (14,19), although in other series, the diagnostic sensitivity was lower (20). In one study, Juergensen et al. (11) showed the value of scintigraphy: 1 patient of 4 with end-stage renal failure on PD was found to have a leak through that diagnostic test; the other 3 patients responded to peritoneal ultrafiltration and thus were unlikely to have had a leak. In another study, researchers speculated that, in addition to offering diagnostic value in hydrothorax, scintigraphy is useful for diagnosing hernias, leaks, and catheter malfunction (21).
Re-challenging the peritoneum after 3 - 6 months is an option, although success rates may be lower with bilateral leakage, as in our case (Blake P. Personal communication, May 2012). Other therapeutic options for treating hydrothorax usually involve surgical procedures, such as pleurodesis, or discontinuation of PD. Recently, protocols for initiating fill volumes were evaluated with the aim of preventing complications such as peritoneal leaks and PD-associated hydrothorax (22), but such protocols have not been validated in children.
Our patient currently remains on hemodialysis awaiting a renal graft.
Conclusions
Peritoneal dialysis-associated hydrothorax is an uncommon, yet problematic complication of therapy for end-stage renal failure. Its presentation can be very severe and life-threatening, as demonstrated in this case report. A variety of methods for diagnosing hydrothorax are currently undergoing evaluation. Timely and rapid diagnosis is imperative and has therapeutic implications. Peritoneal scintigraphy is a rapid and adequate method for showing peritoneal leakage, and it can also be used to demonstrate areas of leakage in the pleural cavity, even in infants. Further research will involve not only the therapeutic value of this diagnostic test, but also the development of ways to prevent PD-associated hydrothorax altogether.
Disclosures
The authors have no relevant affiliations or financial involvements—including contracts, consultancy, advisory boards, memberships, or grants—with any organizations with a financial interest in or conflict with the subject matter discussed in this manuscript.
References
- 1. Nomoto Y, Suga T, Nakajima K, Sakai H, Osawa G, Ota K, et al. Acute hydrothorax in continuous ambulatory peritoneal dialysis—a collaborative study of 161 centers. Am J Nephrol 1989; 9:363–7 [DOI] [PubMed] [Google Scholar]
- 2. Szeto CC, Chow KM. Pathogenesis and management of hydrothorax complicating peritoneal dialysis. Curr Opin Pulm Med 2004; 10:315–19 [DOI] [PubMed] [Google Scholar]
- 3. Chow CC, Sung JY, Cheung CK, Hamilton-Wood C, Lai KN. Massive hydrothorax in continuous ambulatory peritoneal dialysis: diagnosis, management and review of the literature. N Z Med J 1988; 101:475–7 [PubMed] [Google Scholar]
- 4. Kawaguchi AL, Dunn JC, Fonkalsrud EW. Management of peritoneal dialysis-induced hydrothorax in children. Am Surg 1996; 62:820–4 [PubMed] [Google Scholar]
- 5. Lew SQ. Hydrothorax: pleural effusion associated with peritoneal dialysis. Perit Dial Int 2010; 30:13–18 [DOI] [PubMed] [Google Scholar]
- 6. Yim AP, Lee TW, Wan IY, Ng C. Images in cardiothoracic surgery. Pleuroperitoneal fistula. Ann Thorac Surg 2002; 73:1327 [DOI] [PubMed] [Google Scholar]
- 7. Gagnon RF, Thirlweil M, Arzoumanian A, Mehio A. Systemic amyloidosis involving the diaphragm and acute massive hydrothorax during peritoneal dialysis. Clin Nephrol 2002; 57:474–9 [DOI] [PubMed] [Google Scholar]
- 8. Van Dijk CM, Ledesma SG, Teitelbaum I. Patient characteristics associated with defects of the peritoneal cavity boundary. Perit Dial Int 2005; 25:367–73 [PubMed] [Google Scholar]
- 9. Saillen P, Mosimann F, Wauters JP. Hydrothorax and end-stage chronic renal failure. Chest 1991; 99:1010–1 [DOI] [PubMed] [Google Scholar]
- 10. Chow KM, Szeto CC, Wong TY, Li PK. Hydrothorax complicating peritoneal dialysis: diagnostic value of glucose concentration in pleural fluid aspirate. Perit Dial Int 2002; 22:525–8 [PubMed] [Google Scholar]
- 11. Juergensen PH, Rizvi H, Caride VJ, Kliger AS, Finkelstein FO. Value of scintigraphy in chronic peritoneal dialysis patients. Kidney Int 1999; 55:1111–19 [DOI] [PubMed] [Google Scholar]
- 12. Mestas D, Wauquier JP, Escande G, Baguet JC, Veyr A. Diagnosis of hydrothorax complicating CAPD and demonstration of successful therapy by scintigraphy. Perit Dial Int 1991; 11:283–4 [PubMed] [Google Scholar]
- 13. Cho Y, D’Intini V, Ranganathan D. Acute hydrothorax complicating peritoneal dialysis: a case report. J Med Case Rep 2010; 4:355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang S, Chui WH, Tang AW, Li FK, Chau WS, Ho YW, et al. Video-assisted thoracoscopic talc pleurodesis is effective for maintenance of peritoneal dialysis in acute hydrothorax complicating peritoneal dialysis. Nephrol Dial Transplant 2003; 18:804–8 [DOI] [PubMed] [Google Scholar]
- 15. Kechrid MC, Malik GH, Shaikh JF, Al-Mohaya S, Al-Wakeel JS, El Gamal H. Acute hydrothorax complicating continuous ambulatory peritoneal dialysis: a case report and review of literature. Saudi J Kidney Dis Transpl 1999; 10:163–6 [PubMed] [Google Scholar]
- 16. Chow KM, Szeto CC, Li PK. Management options for hydrothorax complicating peritoneal dialysis. Semin Dial 2003; 16:389–94 [DOI] [PubMed] [Google Scholar]
- 17. Gibbons GD, Baumert J. Unilateral hydrothorax complicating peritoneal dialysis. Use of radionuclide imaging. Clin Nucl Med 1983; 8:83–4 [DOI] [PubMed] [Google Scholar]
- 18. Rajnish A, Ahmad M, Kumar P. Peritoneal scintigraphy in the diagnosis of complications associated with continuous ambulatory peritoneal dialysis. Clin Nucl Med 2003; 28:70–1 [DOI] [PubMed] [Google Scholar]
- 19. Pankaj P, Pathak V, Sen IB, Verma R, Bhalla AK, Marwaha A, et al. Use of radionuclide peritoneography in the diagnosis of pleuroperitoneal communication as a complication of continuous ambulatory peritoneal dialysis. Ind J Nucl Med 2005; 20:4–8 [Google Scholar]
- 20. Goh AS, Lee GS, Kee SG, Ang ES, Sundram FX. Radionuclide detection of dialysate leakage in patients on continuous ambulatory peritoneal dialysis. Ann Acad Med Singapore 1994; 23:315–18 [PubMed] [Google Scholar]
- 21. Huang JJ, Wu JS, Chi WC, Lan RR, Yang LF, Chiu NT. Hydrothorax in continuous ambulatory peritoneal dialysis: therapeutic implications of Tc-99m MAA peritoneal scintigraphy. Nephrol Dial Transplant 1999; 14:992–7 [DOI] [PubMed] [Google Scholar]
- 22. Krishnan RG, Ognjanovic MV, Crosier J, Coulthard MG. Acute hydrothorax complicating peritoneal dialysis. Perit Dial Int 2007; 27:296–9 [PubMed] [Google Scholar]


