Abstract
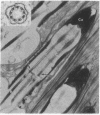
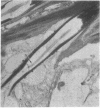
Combined high-voltage electron-microscopic and electrophysiological studies strongly suggest that cilia play an active role in sensory transduction in the grasshopper proximal femoral chordotonal organ (FCO) a ciliated mechanoreceptor. The FCO of pro- and mesothoracic legs of Melanoplus bivittatus contains a group of several hundred chorodontal sensilla arranged in a near-parallel bundle and slung between the proximal femur and the knee joint. Both flexion and extension of the tibia stimulate the FCO, which appears to measure the femoro-tibial angle. The FCO's U-shaped response curve indicates that progressive flexion or extension from the resting joint angle of 90 degrees increases the response frequency of individual receptors and recruits additional units as well. Since the FCO is a purely tonic mechanoreceptor, it is possible to fix FCOs during maximum and minimum states of stimulation and electron-microscopically observed changes in the receptor's fine structure. The most conspicuous change is the production of a pronounced bend at the base of the sensory cilia in chordotonal sensilla of maximally stimulated femoral chordotonal organs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns M. D. Structure and physiology of the locust femoral chordotonal organ. J Insect Physiol. 1974 Jul;20(7):1319–1339. doi: 10.1016/0022-1910(74)90236-4. [DOI] [PubMed] [Google Scholar]
- Chapman K. M., Duckrow R. B., Moran D. T. Form and role of deformation in excitation of an insect mechanoreceptor. Nature. 1973 Aug 17;244(5416):453–454. doi: 10.1038/244453a0. [DOI] [PubMed] [Google Scholar]
- GRAY E. G., PUMPHREY R. J. Ultrastructure of the insect ear. Nature. 1958 Mar 1;181(4609):618–618. doi: 10.1038/181618a0. [DOI] [PubMed] [Google Scholar]
- Gilula N. B., Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972 May;53(2):494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran D. T., Chapman K. M., Ellis R. A. The fine structure of cockroach campaniform sensilla. J Cell Biol. 1971 Jan;48(1):155–173. doi: 10.1083/jcb.48.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran D. T., Rowley J. C., 3rd The fine structure of the cockroach subgenual organ. Tissue Cell. 1975;7(1):91–105. doi: 10.1016/s0040-8166(75)80009-7. [DOI] [PubMed] [Google Scholar]
- Moran D. T., Rowley J. C., 3rd, Varela F. G. Ultrastructure of the grasshopper proximal femoral chordotonal organ. Cell Tissue Res. 1975 Aug 27;161(4):445–457. doi: 10.1007/BF00224135. [DOI] [PubMed] [Google Scholar]
- Moran D. T., Rowley J. C., 3rd, Zill S. N., Varela F. G. The mechanism of sensory transduction in a mechanoreceptor. Functional stages in campaniform sensilla during the molting cycle. J Cell Biol. 1976 Dec;71(3):832–847. doi: 10.1083/jcb.71.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran D. T., Rowley J. C. High voltage and scanning electron microscopy of the site of stimulus reception of an insect mechanoreceptor. J Ultrastruct Res. 1975 Jan;50(1):38–46. doi: 10.1016/s0022-5320(75)90006-4. [DOI] [PubMed] [Google Scholar]
- Moran D. T., Varela F. G. Microtubules and sensory transduction. Proc Natl Acad Sci U S A. 1971 Apr;68(4):757–760. doi: 10.1073/pnas.68.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. C., 3rd, Moran D. T. A simple procedure for mounting wrinkle-free sections on formvar-coated slot grids. Ultramicroscopy. 1975 Dec;1(2):151–155. doi: 10.1016/s0304-3991(75)80018-0. [DOI] [PubMed] [Google Scholar]
- Satir P. How cilia move. Sci Am. 1974 Oct;231(4):44–52. doi: 10.1038/scientificamerican1074-44. [DOI] [PubMed] [Google Scholar]
- Satir P. Studies on cilia. 3. Further studies on the cilium tip and a "sliding filament" model of ciliary motility. J Cell Biol. 1968 Oct;39(1):77–94. doi: 10.1083/jcb.39.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifer E. H., Sekhon S. S. The femoral chordotonal organs of a grasshopper, Orthoptera, Acrididae. J Neurocytol. 1975 Aug;4(4):419–438. doi: 10.1007/BF01261373. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Warner F. D., Satir P. The structural basis of ciliary bend formation. Radial spoke positional changes accompanying microtubule sliding. J Cell Biol. 1974 Oct;63(1):35–63. doi: 10.1083/jcb.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold M. L. Mechanosensory transduction in "sensory" and "motile" cilia. Annu Rev Biophys Bioeng. 1976;5:39–62. doi: 10.1146/annurev.bb.05.060176.000351. [DOI] [PubMed] [Google Scholar]