Abstract
Using olfactory molecular specificity, we examined the inheritance of parental traumatic exposure, a phenomenon that has been frequently observed, but not understood. We subjected F0 mice to odor fear conditioning before conception and found that subsequently conceived F1 and F2 generations had an increased behavioral sensitivity to the F0-conditioned odor, but not to other odors. When an odor (acetophenone) that activates a known odorant receptor (Olfr151) was used to condition F0 mice, the behavioral sensitivity of the F1 and F2 generations to acetophenone was complemented by an enhanced neuroanatomical representation of the Olfr151 pathway. Bisulfite sequencing of sperm DNA from conditioned F0 males and F1 naive offspring revealed CpG hypomethylation in the Olfr151 gene. In addition, in vitro fertilization, F2 inheritance and cross-fostering revealed that these transgenerational effects are inherited via parental gametes. Our findings provide a framework for addressing how environmental information may be inherited transgenerationally at behavioral, neuroanatomical and epigenetic levels.
Responding to environmental stimuli is crucial to the survival of organisms and often manifests as alterations in the structure and function of the nervous system. When and how information from the environment results in experience-dependent alteration of nervous system structure and function are fundamental questions in behavioral neuroscience.
An important, but often ignored, factor that influences adult nervous systems is exposure of parents to salient environmental stimuli before the conception of their offspring. Such information transfer would be an efficient way for parents to ‘inform’ their offspring about the importance of specific environmental features that they are likely to encounter in their future environments. However, this would necessitate the transgenerational inheritance of environmental information via the germ line by offspring not even conceived at the time. Although our understanding of such non-Mendelian modes of inheritance is continually being revised in terms of the epigenetic inheritance of traits1, empirical data to support transgenerational epigenetic inheritance of behavioral traits in mammals are beginning to accumulate at the level of morphological, behavioral and metabolic traits2–15.
We used olfactory fear conditioning to address when and how the olfactory experience of a parent might influence their offspring. Specifically, we focused on the olfactory system, given its well-understood molecular biology and neuroanatomy16–18, the ability to differentially target odorant-receptor pairs in the same modality for differential and well-controlled behavioral studies, and previous findings that experience-dependent alterations occur in olfactory neuroanatomy and behavior following olfactory conditioning19.
We examined how specific features of the parental sensory environment before conception can influence sensory nervous system structure and function in a cue-specific manner in subsequently conceived F1 and F2 generations. Bisulfite sequencing of olfactory receptor genes in the sperm of the F0 and F1 generations revealed differences in methylation that may mark the specific olfactory receptor gene for enhanced transcription in the subsequent generation. Finally, using in vitro fertilization (IVF), F2 and cross-fostering studies, we found that the behavior and structural alterations were inherited and were not socially transmitted from the F0 generation.
RESULTS
Olfactory fear conditioning to study descendant generations
We examined whether olfactory fear conditioning of the F0 generation leads subsequently conceived adult F1, F2 and IVF-derived generations to exhibit F0-like behavioral sensitivity toward the F0 conditioned odor, and whether there were neuroanatomical changes at the level of the main olfactory epithelium (MOE) and olfactory bulb in these generations (Supplementary Fig. 1). The odors that we used were chosen on the basis of prior work demonstrating that the M71 odorant receptors (encoded by the Olfr151 gene) expressed by olfactory sensory neurons (OSNs) in the MOE are activated by acetophenone20. The use of a chemical mixture that contained compounds very similar to propanol did not elicit any responses from M71 cells, suggesting that propanol does not activate M71 receptors. In addition, glomerular activity patterns elicited by acetophenone or propanol (http://gara.bio.uci.edu) are different and non-overlapping, suggesting that a different population of OSNs primarily responds to each odor.
In this procedure, 2-month-old sexually inexperienced and odor naive C57Bl/6J male mice or homozygous M71-LacZ transgenic male mice were left in their home cage (F0-Home) or conditioned with acetophenone (F0-Ace) or propanol (F0-Prop). Subsequently conceived adult male offspring (F1) belonged to three groups: F1-Home, F1-Ace and F1-Prop (Online Methods). It is important to note that no F0 males were excluded from the study after training and that all of them were mated with naive females. Thus, any findings that we obtained were not the results of extreme phenotype biasing or a previously existing genetic sensitivity. Both C57Bl/6J and M71-LacZ mice possess the M71 odorant receptor in their olfactory epithelium21 and both can consequently detect acetophenone. The main difference between the strains is that the OSNs of the M71-LacZ mice produce β-galactosidase in M71-expressing neurons and can therefore be visualized22. This procedure allowed us to examine a seldom studied factor that might markedly influence the nervous systems of adults; namely, the experience of the F0 generation before conception.
Transgenerational olfactory sensitivity after F0 conditioning
Fear-potentiated startle (FPS) is a behavioral test to assay for fear learning23. FPS manifests as an augmented startle response in the presence of the aversive conditioned cue. In our case, to assay for behavioral sensitivity to an odor, we used a modified FPS protocol that consists of odor presentation before the startle stimuli. An odor-potentiated startle (OPS) score is computed, in which an enhanced OPS reflects a greater startle to the odor relative to control, when the odor is paired with the startle stimulus. Traditionally, FPS tests have been used to query the emotional state of the animal and the valence of the stimulus paired with the startle. It is important to note that we did not use this test as a measure of valence of the odor, but rather as a readout of the sensitivity toward that odor, similar to FPS tests that have been used to test the sensitivity of mice to natural odors such as fox urine24. Enhanced OPS to acetophenone in our experiment would be interpreted as an enhanced behavioral sensitivity to acetophenone, not necessarily an increase in fear to acetophenone. Making any statements about valence specificity and the emotional value of the odor would necessitate subjecting the F0 generation to an appetitive odor-conditioning task.
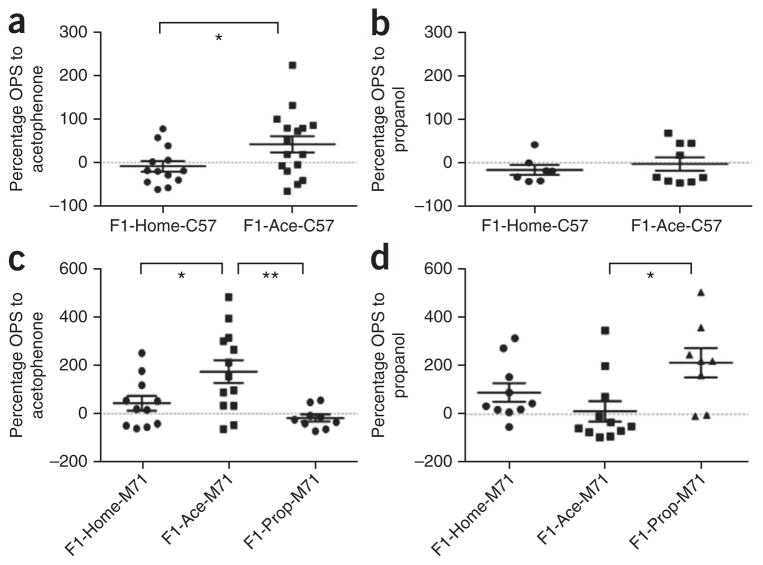
In the F0 generation, we previously reported that olfactory fear conditioning adult males to acetophenone increases FPS when the startle stimuli are paired with acetophenone presentation19. In the F1 generation, we found that C57Bl/6J F1-Ace mice (F1-Ace-C57) showed enhanced OPS (unconditioned) to acetophenone compared with C57Bl/6J F1-Home mice (F1-Home-C57) (Fig. 1a). No differences between groups were found when propanol was paired with the startle, indicating that the response was specific to acetophenone (Fig. 1b). Similarly, F1-Ace-M71 showed enhanced OPS to acetophenone, but not to propanol, compared with F1-Home-M71 and F1-Prop-M71 (Fig. 1c,d). In contrast, F1-Prop-M71 showed enhanced OPS to propanol, but not to acetophenone (Fig. 1c,d). These data suggest a double dissociation and specificity of the odor association, along with the inheritance of a behavioral sensitivity that is specific to the F0-conditioned odor.
Figure 1.
Behavioral sensitivity to odor is specific to the paternally conditioned odor. (a,b) Responses of individual C57Bl/6J F1 male offspring conceived after the F0 male was fear conditioned with acetophenone. F1-Ace-C57 mice had an enhanced sensitivity to acetophenone (a), but not to propanol (control odor, b) compared with F1-Home-C57 mice (F1-Ace-C57, n = 16; F1-Home-C57, n = 13; t test, P = 0.043, t27 = 2.123). (c,d) Responses of M71-LacZ F1 male offspring conceived after the F0 male was fear conditioned with acetophenone or propanol. F1-Ace-M71 mice had an enhanced sensitivity to acetophenone (c), but not to propanol (d), compared with F1-Home-M71, and F1-Prop-M71 mice. In contrast, F1-Prop-M71 mice had an enhanced sensitivity to propanol (d), but not acetophenone (c) (F1-Home-M71, n = 11; F1-Ace-M71, n = 13; F1-Prop-M71, n = 9; OPS to acetophenone: ANOVA, P = 0.003, F2,30 = 6.874; F1-Home-M71 versus F1-Ace-M71, P < 0.05; F1-Ace-M71 versus F1-Prop-M71, P < 0.01; OPS to propanol: ANOVA, P = 0.020, F2,26 = 4.541; F1-Ace-M71 versus F1-Prop-M71, P < 0.05). Data are presented as mean ± s.e.m. *P < 0.05, **P < 0.01.
To further corroborate the enhanced behavioral sensitivity to the F0-conditioned odor, we conducted an independent behavioral assay that directly probes behavioral sensitivity using an odor concentration curve and the association time of the mice with these concentrations. We found that F1-Ace males were able to detect acetophenone at lower concentrations than F1-Prop males, whereas F1-Prop males detected propanol at lower concentrations than F1-Ace males (Fig. 2a,b), further suggesting an enhanced detection sensitivity that is specific to the F0-conditioned odor. Although we make a case for both the OPS and association time assays testing for behavioral sensitivity, we used OPS in our subsequent experiments because of our ability to carefully calibrate odor presentation and removal, parameters that might influence habituation to odors and skew experimental results.
Figure 2.
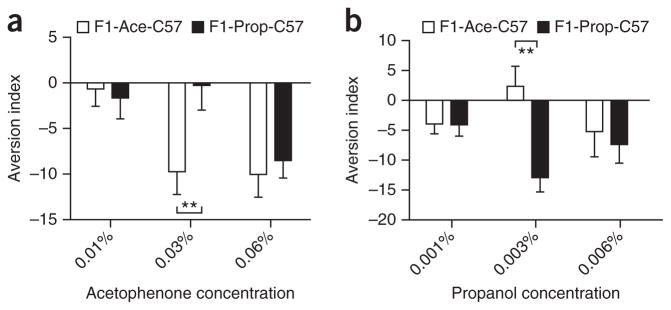
Sensitivity of F1 males toward F0-conditioned odor. Association time with either the concentration of odor on the x axis or an empty chamber was recorded. An aversion index was computed by subtracting the amount of time spent in the open chamber from the time spent in the odor chamber. (a) When tested with acetophenone, F1-Ace mice detected acetophenone at a lower concentration (0.03%) than F1-Prop mice, with both groups eventually showing equal aversion at the 0.06% concentration (P = 0.005 with Bonferroni correction for multiple comparisons). (b) When tested with propanol, F1-Prop mice detected propanol at a lower concentration (0.003%) than F1-Ace mice, with both groups eventually showing equal aversion at the 0.006% concentration (P = 0.0005 with Bonferroni correction for multiple comparisons) (F1-Ace-C57, n = 16; F1-Prop-C57, n = 16). Data are presented as mean ± s.e.m. (**P < 0.01).
Most noteworthy for these data is the fact that the naive F1 mice had never been exposed to any of the odors with which they were tested. Taken together, these data indicate that the behavioral sensitivity to an odor in adult offspring is specific to the odor that the F0 male was conditioned to, as shown across two different odorants and two different strains of mice. Furthermore, given the fact that both F0-Ace and F0-Prop mice received shocks during conditioning, these data suggest that these training-specific effects do not occur simply as a result of paternal history of the stress of shock exposure or conditioning to odors in general.
Studies that have examined the effect of parental stress after conception, either in utero or postnatally, have often found an anxiogenic phenotype in the offspring25,26. Using an elevated plus maze to assay for anxiety-like behavior, we found that prior, rather limited foot shock conditioning of the F0 generation, did not extend to generalized anxiety-like behavior in the F1 generation (Supplementary Fig. 2a,b).
To test the idea that olfactory fear conditioning of the F0 generation results in offspring that might be generally deficient in processing sensory cues and in learning and memory processes, we sought to examine whether auditory fear conditioning was affected in our experimental groups. Across all experimental groups, adult male F1 offspring subjected to auditory fear conditioning acquired, consolidated and extinguished fear similarly (Supplementary Fig. 3a–c).
F0 olfactory experience affects F1 neuroanatomy
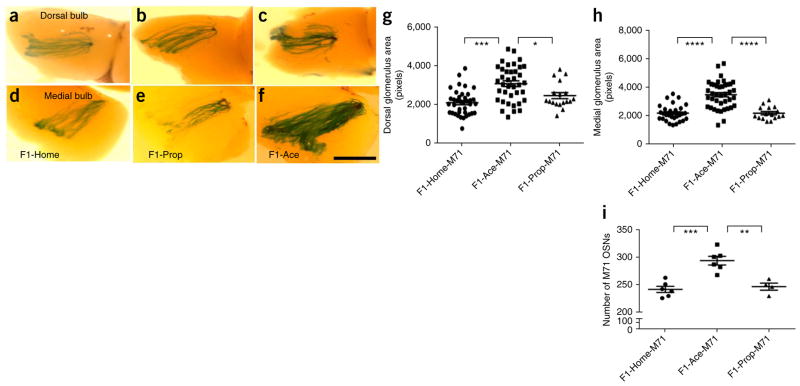
Previously19, we reported that the behavioral response (increased FPS to acetophenone) of F0-Ace conditioned males is complemented by an increase in the number of acetophenone–responsive M71-expressing OSNs in the MOE and M71 glomerular area in the olfactory bulbs. To examine whether alterations in the neuroanatomical representation of the conditioned odor accompanied the behavioral sensitivity reported above, we used standard β-galactosidase staining in naive M71-LacZ F1 males that had neither been behaviorally tested with, nor exposed to, any of the conditioned odors. We found that the dorsal and medial M71-specific glomeruli in the olfactory bulb of F1 offspring of acetophenone-trained F0 males (F1-Ace-M71) were significantly increased in size compared with those of the offspring of home cage or propanol-trained F0 males (F1-Home-M71 and F1-Prop-M71, respectively) (ANOVA, P < 0.0001 for dorsal and medial glomeruli; Fig. 3a–h). This increase in M71 glomerular area was accompanied by a significant increase in the numbers of M71 OSNs in the MOE (ANOVA, P < 0.0001; Fig. 3i).
Figure 3.
Neuroanatomical characteristics of the olfactory system in F1 males after paternal F0 olfactory fear conditioning. (a–f) β-galactosidase staining revealed that offspring of F0 males trained to acetophenone (F1-Ace-M71) had larger dorsal and medial acetophenone-responding glomeruli (M71 glomeruli) in the olfactory bulb compared with F1-Prop-M71 and F1-Home-M71 mice. Scale bar represents 1 mm. (g) Dorsal M71 glomerular area in F1 generation (M71-LacZ: F1-Home, n = 38; F1-Ace, n = 38; F1-Prop, n = 18; ANOVA, P < 0.0001, F2,91 = 15.53; F1-Home-M71 versus F1-Ace-M71, P < 0.0001; F1-Ace-M71 versus F1-Prop-M71, P < 0.05). (h) Medial M71 glomerular area in F1 generation (M71-LacZ: F1-Home, n = 31; F1-Ace, n = 40; F1-Prop, n = 16; ANOVA, P < 0.0001, F2,84 = 31.68; F1-Home-M71 versus F1-Ace-M71, P < 0.0001; F1-Ace-M71 versus F1-Prop-M71, P < 0.0001). (i) F1-Ace-M71 mice had a larger number of M71 OSNs in the MOE than F1-Prop-M71 and F1-Home-M71 mice (M71-LacZ: F1-Home, n = 6; F1-Ace, n = 6; F1-Prop, n = 4; ANOVA, P = 0.0001, F2,13 = 18.80; F1-Home-M71 versus F1-Ace-M71, P < 0.001; F1-Ace-M71 versus F1-Prop-M71, P < 0.01). Data are presented as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
These data suggest that the effect of paternal olfactory fear conditioning on neuroanatomy is associated with increased numbers of OSNs and increased glomerular area, both specific for the F0-conditioned odor. We posit that this increased structural representation in the main olfactory epithelium and olfactory bulb may underlie the specific enhanced olfactory sensitivity that we observed in the behavioral experiments (Figs. 1 and 2). We were concerned that performing behavior would make the offspring no longer odor naive, and thereby potentially confound the interpretation of the neuroanatomical results. Thus, all of the neuroanatomy data were generated using animal cohorts independent of any behavior data. Correlations between behavior and neuroanatomy within and between generations present an interesting and important future direction for research.
Inheritance of effects in the F2 and IVF-derived generations
Two mechanisms could explain how information about the F0- conditioned odor could be transferred to the subsequently conceived male offspring: inheritance via the gametes or transmission via a social route that is reminiscent of the transmission of maternal care in rodents27. To begin to dissociate these two possibilities, we conducted experiments with the F2 generation and with IVF-derived mice. Naive F1 males (F1-Ace, F1-Prop) were mated with naive females to generate F2 adults (F2-Ace, F2-Prop) whose F0 ancestors had been conditioned with either acetophenone or propanol. For the IVF experiment, sperm from F0 males was collected 10 d after the last conditioning day, and IVF was performed by the Transgenic Mouse Facility at Emory University in a location independent of our laboratory at Yerkes where we conducted all of the other studies reported. Subsequently conceived IVF offspring (F1-Ace-IVF and F1-Prop-IVF) were raised to adulthood and tissue was collected in this facility.
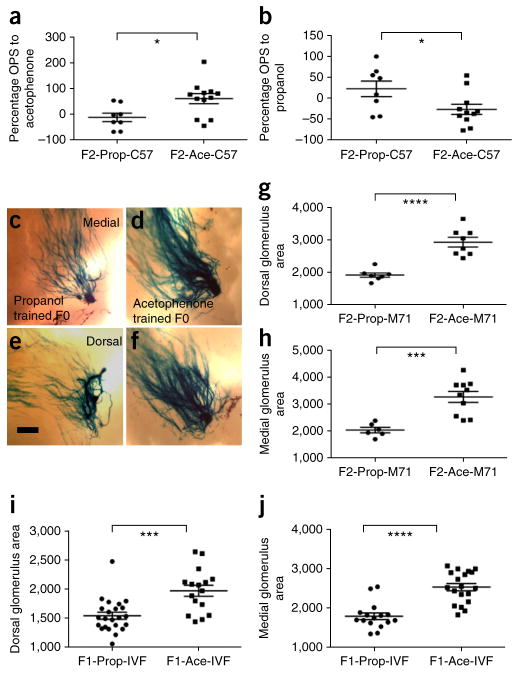
When tested in our behavioral assay, F2-Ace-C57 mice exposed to odors for the first time showed increased OPS to acetophenone compared with F2-Prop-C57 mice, whereas F2-Prop-C57 mice showed an increased OPS to propanol (Fig. 4a,b). This persistent behavioral sensitivity to the F0-conditioned odor was accompanied by corresponding increases in glomerular size in an independent set of F2 M71-LacZ mice that had no previous exposure to the odors used. The dorsal and medial M71-specific glomeruli in the olfactory bulbs of F2-Ace-M71 mice were significantly increased in size compared with those of F2-Prop-M71 mice (Fig. 4c–h). Similar results were obtained in our IVF study, using sperm from F0-Ace and F0-Prop males to generate offspring. We found that F1 offspring generated with sperm from acetophenone-trained F0 males (F1-Ace-IVF) had significantly larger dorsal and medial M71-specific glomeruli in the olfactory bulb, as compared with offspring generated with sperm from propanol-trained F0 males (F1-Prop-IVF) (t test, P < 0.001 for dorsal and medial glomeruli; Fig. 4i,j). We could not perform behavioral analyses on IVF-generated offspring because of animal quarantine issues. These data indicate that behavioral sensitivity and neuroanatomical alterations in the nervous system are specific to the F0- conditioned odor and persist until at least the F2 generation, as well as in the IVF-derived F1 generation, thereby pointing to an inheritance of these effects.
Figure 4.
Behavioral sensitivity and neuroanatomical changes are inherited in F2 and IVF-derived generations. (a,b) Responses of F2-C57Bl/6J males revealed that F2-Ace-C57 mice had an enhanced sensitivity to acetophenone compared with F2-Prop-C57 mice (a). In contrast, F2-Prop-C57 mice had an enhanced sensitivity to propanol compared with F2-Ace-C57 mice (b; F2-Prop-C57, n = 8; F2-Ace-C57, n = 12; OPS to acetophenone: t test, P = 0.0158, t18 = 2.664; OPS to propanol: t test, P = 0.0343, t17 = 2.302). (c–f). F2-Ace-M71 mice whose F0 generation male had been conditioned to acetophenone had larger dorsal and medial M71 glomeruli in the olfactory bulb than F2-Prop-M71 mice whose F0 generation had been conditioned to propanol. Scale bar represents 200 μm. (g) Dorsal M71 glomerular area in F2 generation (M71-LacZ: F2-Prop, n = 7; F2-Ace, n = 8; t test, P < 0.0001, t13 = 5.926). (h) Medial M71 glomerular area in F2 generation (M71-LacZ: F2-Prop, n = 6; F2-Ace, n = 10; t test, P = 0.0006, t14 = 4.44). (i) Dorsal M71 glomerular area in IVF offspring (F1-Prop-IVF, n = 23; F1-Ace-IVF, n = 16; t test, P < 0.001, t37 = 4.083). (j) Medial M71 glomerular area in IVF offspring (F1-Prop-IVF, n = 16; F1-Ace-IVF, n = 19; t test, P < 0.001, t33 = 5.880). Data are presented as mean ± s.e.m.*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Cross-fostering supports inheritance of information
Our observations of the behavioral and structural changes specific to the F0-conditioned odor being retained in the F2 generation, and the persistence of the structural effects after IVF, argue against social transmission and make a strong case for transgenerational inheritance. Notably, our results are highly specific in the olfactory sensory modality toward the F0-conditioned odor, and both F0-Ace and F0-Prop males were subjected to the same shock training conditions that might be deemed stressful and potentially conveyed to the mother. This argues against the idea that our results might merely be the transmission of a stressful paternal experience to the mother during the time of co-habitation. To ensure that our experimental groups were balanced for both odor and shock exposure, many of our experiments utilized F0-Prop as the control group rather than F0-Home.
To further address this issue, and to address potential maternal transmission, we conducted a cross-fostering study. Sexually naive female mice were conditioned with acetophenone or left in their home cage. They were then mated with odor-naive males for 10 d, after which the male was removed. Subsequent offspring were then divided into the following groups: offspring of home cage mothers (F1-Home), offspring of acetophenone-conditioned mothers (F1-Ace), offspring of home cage mothers cross-fostered starting at postnatal day 1 by mothers conditioned to acetophenone (F1-Home(fostered)), and offspring of acetophenone conditioned mothers cross-fostered by home cage mothers (F1-Ace(fostered)) (Supplementary Fig. 4). Notably, the females were only exposed to the conditioning odor before mating, and never while pregnant, precluding the possibility that offspring were directly exposed to any odor-related fear and in utero learning. We conducted this cross-fostering study in females for two main purposes. First, we sought to examine whether these effects were specific to paternal conditioning or could also be inherited via the female germ line. Second, given the possibility that mating with the F0 conditioned male in some way altered maternal behavior toward subsequently born offspring, we wanted to account for any differences in maternal investment or information transfer about the conditioned odor that might result from our conditioning protocol.
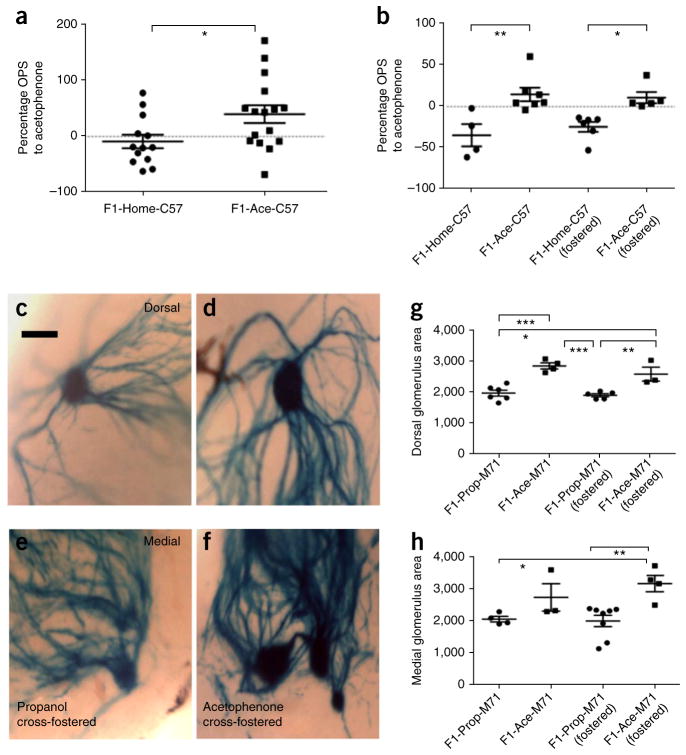
We found that, similar to the situation in which the F0 male (father) was conditioned to acetophenone, F1-Ace mice in this maternally trained experiment had an enhanced OPS to acetophenone compared with F1-Home controls (Fig. 5a). If our behavioral findings were a result of a ‘social transmission’ mode of information transfer, we would have predicted a reversal of the above result. Instead, we found that the F1-Ace-C57(fostered) male offspring still had a higher OPS to acetophenone than F1-Home-C57(fostered) offspring (Fig. 5b), suggesting a biological, rather than social, mode of inheritance.
Figure 5.
Behavioral sensitivity and neuroanatomical changes persist after cross-fostering. (a) F1 offspring of mothers that had been fear conditioned with acetophenone (F1-Ace-C57) showed enhanced sensitivity to acetophenone compared with F1-Home-C57 controls (F1-Home-C57, n = 13; F1-Ace-C57, n = 16; t test, P = 0.0256, t27 = 2.362). (b) Cross-fostering behavior. F1-Ace-C57 males had higher OPS to acetophenone than F1-Home-C57 males (P < 0.01). F1-Ace- C57(fostered) males still had higher OPS to acetophenone than F1-Home-C57(fostered) males (P < 0.05) (ANOVA, P = 0.0011, F3,18 = 6.874, planned post hoc comparisons). (c–f) Cross-fostering neuroanatomy. F1-Ace- M71 males cross-fostered by mothers conditioned to propanol (F1-Ace-M71(fostered)) continued to have larger M71 glomeruli than F1-Prop-M71 males cross-fostered by mothers conditioned to acetophenone (F1-Prop-M71(fostered)). Scale bar represents 100 μm. (g) Dorsal M71 glomerular area in F1 cross-fostered generation (M71-LacZ: F1-Prop, n = 6; F1-Ace, n = 4; F1- Prop(fostered), n = 5; F1-Ace(fostered), n = 3; ANOVA, P < 0.0001, F3,14 = 17.52; F1-Prop versus F1-Ace, P < 0.001; F1-Prop(fostered) versus F1-Ace(fostered), P < 0.01). (h) Medial M71 glomerular area in F1 cross-fostered generation (M71-LacZ: F1-Prop, n = 4; F1-Ace, n = 3; F1-Prop(fostered), n = 8; F1-Ace(fostered), n = 4; ANOVA, P < 0.01, F3,15 = 5.933; F1-Prop (fostered) versus F1-Ace(fostered), P < 0.01). Data are presented as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
For the equivalent experiment to visualize neuroanatomy, we performed a similar cross-fostering experiment using M71-LacZ females, and used female mice conditioned to propanol as our control group (offspring labeled as F1-Prop). We found that the increased dorsal and medial glomerular area persisted in F1-Ace mice even after they were cross-fostered by mothers conditioned to propanol (F1-Ace-M71(fostered)). In contrast, F1-Prop mice cross-fostered by mothers conditioned to acetophenone (F1-Prop-M71(fostered)) did not show any increases in M71 glomerular area (Fig. 5c–h). In summary, these cross-fostering results, taken together with our IVF and F2 studies, strongly suggest that our behavioral and structural data are a consequence of biological inheritance.
Altered epigenetic signature at Olfr151 (M71) locus in sperm
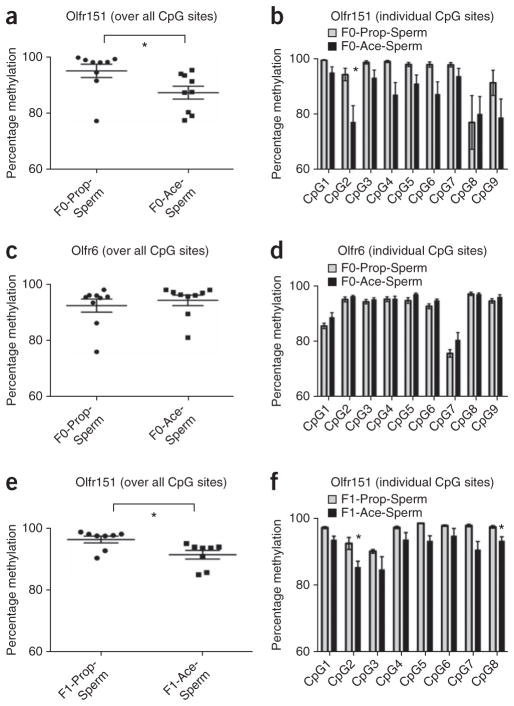
Given that our data suggests a biological inheritance of our behavioral and structural effects via parental gametes, we sought to examine sperm of the F0 generation males for epigenetic clues that might explain an enhanced representation for the M71 receptor (Fig. 6). CpG methylation is one mechanism by which a particular genetic locus can be marked for altered transcription, with less CpG di-nucleotide methylation typically being associated with more transcription. Bisulfite sequencing around the Olfr151 (M71) locus and the non-acetophenone–responsive Olfr6 locus (Supplementary Fig. 5) was conducted by Active Motif on DNA extracted from sperm of F0-Prop and F0-Ace mice. Olfr6 converges at a glomerular space that is distinct from glomerular activity patterns elicited by acetophenone or propanol (http://gara.bio.uci.edu) and we therefore used it as a control odorant receptor for bisulfite sequencing studies. We found that the Olfr151 (P = 0.0323; Fig. 6a), but not Olfr6 (P = 0.54; Fig. 6c), locus was significantly less methylated in sperm from F0-Ace males compared with F0-Prop males. In addition, after correcting for multiple comparisons, one particular CpG di-nucleotide at the 3′ end of Olfr151 was significantly less methylated in F0-Ace males than in F0-Prop males (P = 0.003; Fig. 6b,d).
Figure 6.
Methylation of odorant receptor genes in sperm DNA from conditioned F0 and odor naive F1 males. (a) Bisulfite sequencing of CpG di-nucleotides in the Olfr151 (M71) gene in F0 sperm revealed that F0-Ace mouse DNA (n = 12) was hypomethylated compared with that of F0-Prop mice (n = 10) (t test, P = 0.0323, t16 = 2.344). (b) A particular CpG di-nucleotide in the Olfr151 (M71) gene in F0 sperm was hypomethylated in F0-Ace mice (n = 12) compared with F0-Prop mice (n = 10) (P = 0.003, Bonferroni corrected). (c) We found no differences in methylation between F0-Ace (n = 12) and F0-Prop (n = 10) mice across all of the CpG di-nucleotides queried in the Olfr6 gene in F0 sperm (P > 0.05). (d) Across specific CpG di-nucleotides in the Olfr6 gene, we found no differences in methylation between F0-Ace (n = 12) and F0-Prop (n = 10) mice (Bonferroni corrected). (e) Bisulfite sequencing of the Olfr151 (M71) gene in F1 sperm revealed that F1-Ace mouse DNA (n = 4) was hypomethylated compared with that of F1-Prop mice (n = 4) (t test, P = 0.0153, t14 = 2.763). (f) Bisulfite sequencing of CpG di-nucleotides in the Olfr151 (M71) gene in F1 sperm revealed that two particular CpG di-nucleotides in the Olfr151 (M71) gene were hypomethylated in F1-Ace mice (n = 4) compared with F1-Prop mice (n = 4) (P = 0.002, Bonferroni corrected). Data are presented as mean ± s.e.m. *P < 0.05 after correction.
These findings led us to hypothesize that relative hypomethylation of Olfr151 in F0 sperm may lead to inheritance of the hypomethylated Olfr151 in F1 MOE and F1 sperm, creating an inheritance cascade. A related idea would be that, during the stochastic odorant receptor choice process in the MOE18,28, Olfr151 (M71) would be more likely to be expressed in the next generation as a consequence of the epigenetic signature around that locus in the sperm. When bisulfite-converted DNA from sperm of the F1 generation was sequenced, we found that, similar to the F0 scenario, the Olfr151 locus was hypomethylated in F1-Ace sperm compared with F1-Prop controls (Fig. 6e). In addition, after correcting for multiple comparisons, two particular CpG di-nucleotides in Olfr151 were significantly less methylated in F1-Ace sperm compared with F1-Prop sperm (P = 0.002; Fig. 6f). These data suggest that inheritance of an epigenetic signature around a salient genetic locus accompanies our transgenerational effects. At the level of the MOE, we did not find any differences in the methylation at the Olfr151 locus of either the F1 or F2 generations (Supplementary Fig. 6). This is perhaps unsurprising given that other modes of epigenetic modifications have been implicated in the marking of olfactory receptor loci in the MOE29, and mandates future investigation. For example, DNA methylation and histone modifications are known to be dependent on each other30, and changes in the methylation pattern in Olfr151 in sperm DNA that we observe may potentially result in histone modifications around Olfr151 in MOE DNA.
Published data also support the idea of epigenetic marking in sperm by indicating that sperm-associated histones are retained with chromatin of the paternal genome at the one-cell embryo stage31,32. To investigate the possibility that histone modifications mark the Olfr151 (M71) locus, we collected sperm from F0-Ace and F0-Prop males 10 d after the last day of conditioning and performed native-chromatin immunoprecipitation (N-ChIP) on the sperm chromatin. Briefly, chromatin was extracted from sperm and immunoprecipitated with antibodies that recognize histone modifications, after which quantitative PCR was performed for the Olfr151 gene. We did not observe any differences in histone-mediated epigenetic signatures around the M71 locus when chromatin was immunoprecipitated with antibodies that recognize histone modifications that either permit (acetylated H3) or repress (H3trimethyl K27) to transcription (Supplementary Fig. 7). The fact that the M71 locus was not epigenetically marked via histones in the F0 sperm could indicate that we did not immunoprecipitate with the relevant histone-modification antibody or that the epigenetic basis of this inheritance might not be histone based, instead relying on other mechanisms, such as DNA methylation (as reported above) or non-coding RNA, as has been demonstrated for the Kit locus33.
DISCUSSION
Focusing on classical conditioning in an F0 generation before conception and using specific odors as the conditioned stimuli allowed us to tag a specific olfactory experience and follow the salience of that experience at the level of behavior and neuroanatomy through subsequent generations. We found that the F1 and F2 generations were extremely sensitive to the specific odors used to condition F0 mice. Using a transgenic mouse in which OSNs expressing a specific odorant receptor can be visualized, we found that the behavioral sensitivity was accompanied by an altered olfactory neuroanatomy for the conditioned odor. The fact that these changes persisted after IVF, cross-fostering and across two generations is indicative of biological inheritance. Finally, we observed that the sperm of the F0 and F1 generation males bear epigenetic marks that could be the basis for such inheritance.
There have been other studies that examined the transmission of stimulus-specific behavioral and structural adaptations in the nervous system from parents to their offspring, albeit with substantial differences from our experimental design. For example, in utero taste aversion learning affects the offspring’s preference and avoidance of flavors and odors in the mother’s diet during gestation34. In addition, quality of maternal care is transmitted across generations in rodents27. Furthermore, fetal origins of diseases have been proposed for a multitude of disorders as having their roots in the experience of the fetus to the parental environment while in utero35. From a chemosensory perspective, anti-predatory behavior is transmitted from gravid female crickets to their offspring when the females are exposed to a high density of a predator36. Finally, indirectly related to our study is a report that supplementing the mouse maternal diet with acetophenone at various stages of gestation increases M71 glomerular area and preference for acetophenone in adolescent offspring37. This last study exemplifies how the olfactory sensitivity and neuroanatomy of offspring can bear imprints of parental experience.
However, it is imperative to realize that all of the aforementioned manipulations of the parental condition have occurred when the pups or embryos are in utero, thereby assaying behavior and neuroanatomy in animals that are extremely different from those conceived after perturbation to the parent. In other words, the fetuses in the cited studies were directly exposed to the environmental perturbation. This important point about perturbation of the parental (F0) environment affecting the F1 embryo directly, as well as the F2 germ line, has been used to argue that true transgenerational inheritance should manifest itself in the F3 generation38. It is important to note that the F2 mice that we tested are a full and complete generation removed from the environmental perturbation of their parent; as such, our observations suggest a transgenerational phenomenon. Our IVF data complement this point further.
Most recently, several studies have factored paternal effects and transgenerational inheritance of behavior and metabolic states into their experimental design. First, paternal diet has been shown to have marked effects on the metabolic physiology of offspring conceived after the father’s diet had been manipulated7. Second, exposure to the anti-androgenic endocrine disruptor vinclozolin during embryonic gonadal sex determination affects fertility and behavior in at least four subsequent generations, and it is associated with epigenetic changes in the sperm of descendant male offspring2,9,39. A recent study used a social defeat procedure in mice and found paternal transmission of depressive-like behavior in subsequently conceived adult offspring. These authors found an (epi)genetic inheritance of depression-like behavior in the forced swim test using IVF with sperm from socially defeated fathers, indicating that behavior in offspring can be affected by paternal experience even if the offspring have not been conceived at the time of paternal trauma5. There was also a recent report of epigenetic inheritance of a cocaine-resistance phenotype in rats sired by F0 males that self-administered cocaine40. Finally, behavioral and epigenetic changes have been shown in generations of normally raised offspring whose parents had been subjected to maternal separation procedures4,41. These data, including ours, emphasize that transgenerational epigenetic inheritance does occur in mammals, supporting findings of such inheritance in organisms ranging from flies to worms42–44.
How olfactory stimulation in the F0 generation comes to be linked to sperm is an intriguing question for which we can only offer speculation. Evidence exists for blood-borne odorants activating odorant receptors in the nose45. Thus, it is also conceivable that the odorants used in the F0 fear conditioning protocol enter the circulatory stream and activate odorant receptors that are expressed on sperm46. With mouse spermatogenesis occurring over, on average, 26 d (ref. 47), we reasoned that the interval of 13 d between the first conditioning day and breeding would be enough time for any mature sperm to be cleared from the system and for any information to be inherited by sperm precursors that were 13 d into the maturation process. However, at this point, we cannot and do not claim to know what fraction of sperm precursors, and consequently mature sperm, transferred to the female carry the pertinent information. Future studies would be well served by examining such information storage across spermatogenesis using irradiation-based approaches.
Behavioral sensitivity to odors might be linked to the olfactory topography in the MOE and bulb. For example, animals that have 95% of their OSN population dominated by the M71 receptor show deficits in odor detection48 as well as increased anxiety49. We hypothesize that a substantial increase in the number of M71 neurons in the MOE (but not nearly to the extent of the ‘monoclonal nose’ animals referred to above), and the subsequent enlarged M71-specific glomeruli, are a direct structural mechanism for the enhanced olfactory sensitivity phenotype. We chose to query the overall unconditioned sensitivity of the F1 and F2 generations to the F0 conditioned odor. It would be equally interesting to examine how the F1 and F2 generations respond to direct conditioning with the F0 conditioned odor. However, we felt that this nuance would be better addressed after the parameters and mechanisms underlying any unconditioned responses were appreciated.
In summary, we have begun to explore an under-appreciated influence on adult behavior—ancestral experience before conception. From a translational perspective, our results allow us to appreciate how the experiences of a parent, before even conceiving offspring, markedly influence both structure and function in the nervous system of subsequent generations. Such a phenomenon may contribute to the etiology and potential intergenerational transmission of risk for neuropsychiatric disorders, such as phobias, anxiety and post-traumatic stress disorder50. To conclude, we interpret these results as highlighting how generations can inherit information about the salience of specific stimuli in ancestral environments so that their behavior and neuroanatomy are altered to allow for appropriate stimulus-specific responses.
ONLINE METHODS
Mice
All experiments on adult offspring were conducted with 2-month-old male mice. When F0 males and F0 females were fear conditioned with odor, 2-month-old sexually inexperienced and odor-inexperienced mice were used. C57Bl/6J mice (parents) were procured from Jackson Laboratory. M71-IRES-tauLacZ mice (parents) maintained in mixed 129/Sv × C57Bl/6J background were bred in the Yerkes Neuroscience animal facility. Mice were housed on a 12-h light/dark cycle in standard groups cages (≤5/cage) with ad libitum access to food and water, with all experiments conducted during the light half of the cycle. All procedures were approved by the Institutional Animal Care and Use Committee of Emory University, and followed guidelines set by the US National Institutes of Health.
Behavior
All behavior was performed in a double-blind manner and data acquired using automated computer software programs. We are grateful to S. Banerjee, R. Andero-Gali, D. Choi, J. Goodman and F. Morrison for help with ensuring double-blindness of data acquisition and analysis.
Elevated plus maze
The elevated-plus maze consists of an elevated platform with two walled, closed arms and two non-walled, open arms connected by an open center. The mice were placed onto the center between the plus maze arms and were recorded exploring the plus maze for 5 min. The amount of time spent in the closed and open arms is viewed as a measure of anxiety.
Olfactory fear conditioning of parents
Mice were trained to associate acetophenone or propanol presentation with mild foot shocks. For this purpose, the Startle-Response system (SR-LAB, San Diego Instruments) was modified to deliver discrete odor stimuli as previously described19. The mice were trained on 3 consecutive days, with each training day consisting of 5 trials of odor presentation for 10-s co-terminating with a 0.25-s 0.4-mA foot shock with an average inter-trial interval of 120 s. Both acetophenone and propanol (from Sigma) were used at a 10% concentration diluted with propylene glycol.
OPS of adult offspring
Mice were habituated to the startle chambers for 5–10 min on three separate days. On the day of testing, mice were first exposed to 15 startle-alone (105-dB noise burst) trials (leaders), before being presented with ten odor + startle trials randomly intermingled with ten startle-alone trials. The odor + startle trials consisted of a 10-s odor presentation co-terminating with a 50-ms, 105-dB noise burst. For each mouse, an OPS score was computed by subtracting the startle response in the first odor + startle trial from the startle response in the last startle-alone leader. This OPS score was then divided by the last startle-alone leader and multiplied by 100 to yield the percent OPS score (% OPS) reported in the results. Mice were exposed to the acetophenone-potentiated startle (acetophe-none + startle) and propanol-potentiated startle (propanol + startle) procedures on independent days in a counter-balanced fashion.
Auditory fear conditioning
Mice were pre-exposed to sound attenuated conditioning chambers (San Diego Instruments) for three consecutive days before training. On the day of auditory fear conditioning, mice received five conditioned-unconditioned stimulus pairings (conditioned stimulus: 30-s, 6-kHz, 75-dB tone; unconditioned stimulus: 500-ms, 0.6-mA foot shock) with a 5-min inter-trial interval. The percentage of time spent freezing to the tones was measured by SR-LAB software (San Diego Instruments). The consolidation of fear memory was tested 24 h after fear conditioning in a novel context (modular test chambers, Med Associates) when mice were exposed to 15 conditioned stimulus tones with a 1.5-min inter-trial interval. Freezing during the tone presentations was measured with FreezeView software (Coulbourn Instruments). The extinction retention test occurred 24 h after extinction training and consisted of 30 conditioned stimulus tone presentations to the mice.
Odor sensitivity
Mice were placed in a three-chambered box and allowed to explore between all three chambers for 10 min. A particular concentration of odor (Fig. 2, either acetophenone or propanol) contained in a 1.5-ml Eppendorf tube was placed in one of the chambers with the middle chamber empty and an empty Eppendorf tube in the farthest chamber. Association time with either the odor or the empty chamber was recorded. An aversion index was computed by subtracting the amount of time spent in the open chamber from the time spent in the odor chamber. Pilot experiments on independent mice revealed an increasing aversion for either acetophenone or propanol as the concentration increased. Independent mice were used in the acetophenone and propanol experiments (F1-Ace-C57, n = 16; F1-Prop-C57, n = 16).
IVF
IVF was carried out by the Emory Transgenic Mouse Facility (TMF) located in a different building from our colony (http://med.emory.edu/research/core_labs/transgenic_mouse/) across the Emory Campus. Briefly, F0-M71 males were fear conditioned either to acetophenone or propanol as outlined above. 10 d after fear conditioning, sperm was collected from the caudal epididymis and vas deferens of these males in our facility, and then transported to the TMF wherein IVF was conducted by TMF personnel blinded to the experimental conditions of the sperm samples according to protocols followed by Jackson Laboratory51. In vitro fertilization culture medium, Mouse Vitro Fert (MVF, Cook Medical), was used for sperm isolation, IVF and zygote culture. Superovulated C57BL/6 female mice were used as oocyte donors. Sperm were co-incubated with oocytes in MVF for 4 h in a 5% CO2 incubator, the presumptive zygotes were washed, and were cultured overnight in a 150-μl MVF drop in the incubator. In the second morning, two-cell embryos were scored and washed in MVF drop; pseudopregnant CD-1 female mice of 9–13 weeks of age were used as embryo recipients. 15–20 embryos were transferred into one oviduct of each female. Pups were born after 19 d and weaned from their foster moms at 3.5 weeks of age, they were reared to 2 months of age and then tissue was collected in the TMF facility for β-galactosidase staining on the MOE and olfactory bulb.
β-galactosidase staining, quantitation of MOE OSN number and glomerular area in bulb
The MOE and olfactory bulbs of 2-month-old M71-LacZ mice were processed for β-galactosidase staining, and then M71 OSN number and glomerular area were quantitated using previously published protocols19. Briefly, lateral whole-mount MOE and brains were rapidly dissected and placed into 4% paraformaldehyde (wt/vol) for 10 min at ~23 °C, after which they were washed three times in 1× phosphate-buffered saline (PBS) for 5 min. M71-LacZ was stained for β-galactosidase using 45 mg of X-gal (1 mg ml−1) dissolved in 600 μl of DMSO and 45 ml of a solution of 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide and 2 mM MgCl in 1× PBS, and incubated at 37 °C for 3 h.
Quantitation of MOE M71-positive OSN number
The lateral whole mount MOE was imaged using a microscope-mounted digital camera. β-galactosidase– stained blue OSNs were manually counted by an experimenter blinded to the experimental groups.
Measurement of glomerular area in the olfactory bulb
A microscope-mounted digital camera was used to capture high-resolution images of the β-galactosidase– stained M71 glomeruli at 40× magnification. Images were converted to grayscale and equalized for background brightness. The distribution of pixel brightness was exported in ImageJ as gray levels from 0 = black to 255 = white. X-gal–labeled glomerular area was quantified as pixels, less than a set threshold gray level of 150 (optimized for axon versus background). Each glomerulus was traced using the lasso tool in Photoshop and the area was recorded from the histogram tool. This quantitation was conducted by two experimenters both blinded to the experimental groups.
N-ChIP on sperm
N-ChIP was conducted on sperm chromatin using previously described procedures52. Briefly, the cauda epidydymis was dissected into 1 ml of M2 medium (Sigma), and sperm were allowed to swim into the medium for 1 h at 37 °C. Five epidydymis were used per sample, and each experimental group had three samples. At least 3 × 106 sperm were used for each ChIP. Sperm were then collected by centrifugation at 4 °C for 10 min at 500g, and resuspended in 1× PBS, 1 mM PMSF. Sperm were then lysed on ice for 10 min in 1× PBS, 1 mM PMSF, 0.5% Triton X-100 (vol/vol). Nuclei were pelleted by centrifugation at 4 °C for 10 min at 371g. The pellet was then suspended in 1× PBS, 1 mM PMSF, 10 mM DTT, and incubated at 37 °C for 30 min, before the addition of 0.6 mM CaCl2 and MNase (Sigma) to yield mono-, di- and tri-nucleosomal chromatin. Immunoprecipitation was then carried out as described for the MOE and followed the previously established protocol52. The antibodies were used at 1:1,000 and were specific to H3 trimethyl lysine-27 (07-449) and acetyl histone H3 (06-599) from Upstate. Immunoprecipitated DNA was isolated by phenol-chloroform extraction and ethanol precipitation and used in quantitative PCR reactions on an ABI 7900 Real-Time PCR machine. 5 mM sodium butyrate was added to all buffers and wash solutions to inhibit histone deacetylases. Primers for the control genes were the same as those used in ref. 32. ChIP on sperm was conducted on two independent sets of samples with similar results.
NGS analysis of bisulfite PCR amplicons from sperm and MOE DNA
F0-C57 males were fear conditioned to either acetophenone (F0-Ace-Sperm, n = 12) or propanol (F0-Prop-Sperm, n = 10) as outlined above. 10 d after fear conditioning, sperm was collected from these males in our facility. As outlined above, a separate group of F0-Ace-C57 and F0-Prop-C57 males sired F1 offspring. At 2 months of age, sperm and MOE were collected from F1-Ace-C57 and F1-Prop-C57 mice (n = 4 each). In yet another independent experiment, F1-Ace or F1-Prop males sired F2-Ace-C57 and F2-Prop-C57 mice, respectively. MOE from this F2 generation (F2-Ace-C57, and F2-Prop-C57) were collected at 2 months of age. All samples were shipped to Active Motif (http://www.activemotif.com) for genomic DNA extraction, bisulfite conversion, PCR-based library generation and sequencing. We coded the samples, and personnel at Active Motif were blinded to this code.
PCR primers to the target regions were designed with the MethPrimer software (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi) (Supplementary Fig. 5). For the F1 sperm, F1 MOE and F2 MOE samples, a shorter amplicon was generated, and we queried eight CpG sites in this analysis, compared with nine sites in the F0 generation. The queried CpG sites 1–8 were the same in the F0 and F1 generations. Genomic DNA was isolated from the sperm samples using Quick-gDNA miniprep kit (Zymo Research), and bisulfite-converted using MethylDetector (Active Motif). PCR reactions (40–55 cycles) were performed using Invitrogen’s Platinum PCR supermix.
DNA samples containing approximately the same amounts of two (or four) bisulfite PCR products (~300 ng DNA total) were treated with T4 DNA polymerase, Klenow large fragment and T4 polynucleotide kinase to generate 5′-phophorylated blunt ends. After concatemerization with T4 DNA ligase, the sample was sonicated to an average fragment length of 150–300 bp using a Misonix cuphorn sonicator 3000. Libraries were generated from these sonicated DNA samples using the standard Illumina protocol. The 8 (16) samples were indexed with 6-bp barcodes (independent Illumina index read). Sequencing on Hi-Seq generated ~5–10 million reads per sample. Reads were aligned to chr7 and chr9 reference sequences (mm9 assembly) using the Bismark software (version 0.7.7)53. Alignment and methylation information was captured in BAM files, and percentage methylation and read coverage at each CpG site was determined by running the appropriate Bismark scripts. Alignments to the strands and genomic locations not expected to be present in the PCR products were filtered out using a combination of SAMtools54 and standard UNIX commands.
Supplementary Material
Acknowledgments
We would like to thank the animal care staff in the Yerkes Neuroscience Vivarium for assistance with animal husbandry. A. Magklara, S. Lomvardas, B. Carone, O. Rando and A.F.H.M. Peters provided invaluable input on the ChIP experiments. We would like to thank H. Zhang and the staff of the Emory Transgenic Mouse/Gene Targeting Core Facility for assistance with IVF studies. Bisulfite conversion of sperm DNA and sequencing was carried out by Active Motif and we especially thank P. Labhart for addressing our data interpretation queries. Finally, we are grateful to S. Banerjee, R. Andero-Gali, D. Choi, J. Goodman and F. Morrison for help with ensuring double-blindness of data acquisition and analysis, and members of the Ressler laboratory, S. Gourley and M. Davis for helpful feedback on the manuscript. Funding for this study was provided by the Howard Hughes Medical Institute and the Burroughs Wellcome Fund to K.J.R., and a US National Institutes of Health NCRR base grant (P51RR00-0165) to Yerkes National Primate Research Center.
Footnotes
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTIONS
B.G.D. conceived of the project, designed and performed experiments, analyzed the data, and wrote the paper. K.J.R. obtained funds, designed experiments, analyzed the data, wrote the paper and supervised the project.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anway MD. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver ICG. Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: let’s call the whole thing off. Epigenetics. 2007;2:22–28. doi: 10.4161/epi.2.1.3881. [DOI] [PubMed] [Google Scholar]
- 4.Franklin TB, et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Dietz DM, et al. Paternal transmission of stress-induced pathologies. Biol Psychiatry. 2011;70:408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23:314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 7.Carone BR, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng SF, et al. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature. 2010;467:963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 9.Crews D, et al. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA. 2007;104:5942–5946. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur J Hum Genet. 2002;10:682–688. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- 11.Rakyan VK, et al. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc Natl Acad Sci USA. 2003;100:2538–2543. doi: 10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31:11748–11755. doi: 10.1523/JNEUROSCI.1887-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13:269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 17.Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- 18.Mombaerts P. Molecular biology of odorant receptors in vertebrates. Annu Rev Neurosci. 1999;22:487–509. doi: 10.1146/annurev.neuro.22.1.487. [DOI] [PubMed] [Google Scholar]
- 19.Jones SV, Choi DC, Davis M, Ressler KJ. Learning-dependent structural plasticity in the adult olfactory pathway. J Neurosci. 2008;28:13106–13111. doi: 10.1523/JNEUROSCI.4465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bozza T, Feinstein P, Zheng C, Mombaerts P. Odorant receptor expression defines functional units in the mouse olfactory system. J Neurosci. 2002;22:3033–3043. doi: 10.1523/JNEUROSCI.22-08-03033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 22.Vassalli A, Rothman A, Feinstein P, Zapotocky M, Mombaerts P. Minigenes impart odorant receptor-specific axon guidance in the olfactory bulb. Neuron. 2002;35:681–696. doi: 10.1016/s0896-6273(02)00793-6. [DOI] [PubMed] [Google Scholar]
- 23.Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- 24.Hebb ALO, Zacharko RM, Gauthier M, Drolet G. Exposure of mice to a predator odor increases acoustic startle but does not disrupt the rewarding properties of VTA intracranial self-stimulation. Brain Res. 2003;982:195–210. doi: 10.1016/s0006-8993(03)03008-7. [DOI] [PubMed] [Google Scholar]
- 25.Weaver ICG, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstock M. Intrauterine factors as determinants of depressive disorder. Isr J Psychiatry Relat Sci. 2010;47:36–45. [PubMed] [Google Scholar]
- 27.Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59:1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Lomvardas S, et al. Interchromosomal interactions and olfactory receptor choice. Cell. 2006;126:403–413. doi: 10.1016/j.cell.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Magklara A, et al. An epigenetic signature for monoallelic olfactory receptor expression. Cell. 2011;145:555–570. doi: 10.1016/j.cell.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 31.van der Heijden GW, et al. Sperm-derived histones contribute to zygotic chromatin in humans. BMC Dev Biol. 2008;8:34. doi: 10.1186/1471-213X-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brykczynska U, et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol. 2010;17:679–687. doi: 10.1038/nsmb.1821. [DOI] [PubMed] [Google Scholar]
- 33.Rassoulzadegan M, et al. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature. 2006;441:469–474. doi: 10.1038/nature04674. [DOI] [PubMed] [Google Scholar]
- 34.Stickrod G, Kimble DP, Smotherman WP. In utero taste/odor aversion conditioning in the rat. Physiol Behav. 1982;28:5–7. doi: 10.1016/0031-9384(82)90093-2. [DOI] [PubMed] [Google Scholar]
- 35.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 36.Storm JJ, Lima SL. Mothers forewarn offspring about predators: a transgenerational maternal effect on behavior. Am Nat. 2010;175:382–390. doi: 10.1086/650443. [DOI] [PubMed] [Google Scholar]
- 37.Todrank J, Heth G, Restrepo D. Effects of in utero odorant exposure on neuroanatomical development of the olfactory bulb and odour preferences. Proc Biol Sci. 2011;278:1949–1955. doi: 10.1098/rspb.2010.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod Toxicol. 2008;25:2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE. 2010;5:e13100. doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2012;16:42–47. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF Gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greer EL, et al. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479:365–371. doi: 10.1038/nature10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rechavi O, Minevich G, Hobert O. Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans. Cell. 2011;147:1248–1256. doi: 10.1016/j.cell.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavalli G, Paro R. Epigenetic inheritance of active chromatin after removal of the main transactivator. Science. 1999;286:955–958. doi: 10.1126/science.286.5441.955. [DOI] [PubMed] [Google Scholar]
- 45.Maruniak JA, Silver WL, Moulton DG. Olfactory receptors respond to blood-borne odorants. Brain Res. 1983;265:312–316. doi: 10.1016/0006-8993(83)90348-7. [DOI] [PubMed] [Google Scholar]
- 46.Vanderhaeghen P, Schurmans S, Vassart G, Parmentier M. Specific repertoire of olfactory receptor genes in the male germ cells of several mammalian species. Genomics. 1997;39:239–246. doi: 10.1006/geno.1996.4490. [DOI] [PubMed] [Google Scholar]
- 47.Oakberg EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat. 1956;99:507–516. doi: 10.1002/aja.1000990307. [DOI] [PubMed] [Google Scholar]
- 48.Fleischmann A, et al. Mice with a “monoclonal nose”: perturbations in an olfactory map impair odor discrimination. Neuron. 2008;60:1068–1081. doi: 10.1016/j.neuron.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glinka ME, et al. Olfactory deficits cause anxiety-like behaviors in mice. J Neurosci. 2012;32:6718–6725. doi: 10.1523/JNEUROSCI.4287-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jovanovic T, et al. Physiological markers of anxiety are increased in children of abused mothers. J Child Psychol Psychiatry. 2011;52:844–852. doi: 10.1111/j.1469-7610.2011.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ostermeier GC, Wiles MV, Farley JS, Taft RA. Conserving, distributing and managing genetically modified mouse lines by sperm cryopreservation. PLoS ONE. 2008;3:e2792. doi: 10.1371/journal.pone.0002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Umlauf D, Goto Y, Feil R. Site-specific analysis of histone methylation and acetylation. Methods Mol Biol. 2004;287:99–120. doi: 10.1385/1-59259-828-5:099. [DOI] [PubMed] [Google Scholar]
- 53.Krueger F, Andrews SR. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27:1571–1572. doi: 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, et al. 1000 Genome Project Data Processing Subgroup. The sequence alignment/map (SAM) format and SAMtools. Bioinformatics. 2009;52:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.