Abstract
Although it is well established that regions in the medial temporal lobes are critical for explicit memory, recent work has suggested that one medial temporal lobe subregion – the perirhinal cortex (PRC) – may also support conceptual priming, a form of implicit memory. Here, we sought to investigate whether activity reductions in PRC, previously linked to familiarity-based recognition, might also support conceptual implicit memory retrieval. Using a free association priming task, the current study tested the prediction that PRC indexes conceptual priming independent of contributions from perceptual and response repetition. Participants first completed an incidental semantic encoding task outside of the MRI scanner. Next, they were scanned during performance of a free association priming task, followed by a recognition memory test. Results indicated successful conceptual priming was associated with decreased PRC activity, and that an overlapping region within the PRC also exhibited activity reductions that covaried with familiarity during the recognition memory test. Our results demonstrate that the PRC contributes to both conceptual priming and familiarity-based recognition, which may reflect a common role of this region in implicit and explicit memory retrieval.
Keywords: perirhinal cortex, hippocampus, implicit memory, conceptual priming, recognition memory, familiarity, recollection
1. Introduction
Conceptual implicit memory reflects the process by which associative or semantic cues prime the automatic retrieval of recently encountered information without any intentional recollection of the study event (Roediger & McDermott, 1993; Schacter, 1987). What differentiates conceptual implicit memory from other forms of implicit memory is that it is an enhancement in the speed or fluency of the processing of an item based on prior processing of its conceptual features. Whereas perceptual priming often requires a partial or complete re-instantiation of the studied stimulus (e.g., study octopus; complete word fragment o-t-pu- at test), conceptual priming can instead be elicited by simply providing a conceptually-related cue to a previously studied target, such as in free association priming (e.g., study dolphin; generate associate of porpoise at test). Elucidating the neural underpinnings of conceptual priming has been the focus of considerable work over the past decade.
In most behavioral studies, conceptual implicit memory tasks rely on the generation of studied items based on conceptual cues, but most fMRI studies have used conceptual repetition priming tasks in which semantic judgments (e.g., is turtle bigger than a shoebox) are performed on repeated (e.g., turtle) and unstudied (i.e., baseline) items. In these paradigms, behavioral priming manifests in the form of faster reaction times for repeated semantic judgments on studied items relative to semantic judgments on baseline items. Some fMRI studies have found that activity in perirhinal cortex (PRC) – a region within the medial temporal lobes (MTL) – is reduced during performance of repeated semantic decisions relative to novel semantic decisions (Heusser, Awipi, & Davachi, 2013; O'Kane, Insler, & Wagner, 2005; Voss, Hauner, & Paller, 2009; Voss, Federmeier, & Paller, 2012), suggesting the possibility that PRC may be involved in conceptual priming (for a review, see Dew & Cabeza, 2011).
However, whether repetition-related deactivations observed in these studies reflect conceptual priming per se, remains unclear. Priming for repeated semantic judgments can reflect fluent conceptual processing, but can also be influenced by response and perceptual repetition. It has in fact been proposed that activity reductions elicited during repeated semantic judgments (i.e., neural priming) may index response priming rather than conceptual priming (for a review, see Schacter, Dobbins, & Schnyer, 2004). For example, reversing semantic decisions for studied items, but not changing the semantic dimension that is being queried (e.g., is turtle smaller than a shoebox), disrupts behavioral and neural priming relative to repeated semantic decisions, indicating that these effects may reflect facilitated retrieval of stimulus-response associations, rather than concepts (Dobbins, Schnyer, Verfaellie, & Schacter, 2004; Horner & Henson, 2008). Furthermore, recent work has also observed neural priming in PRC to perceptual repetitions (e.g., Greene & Soto, 2012; for a review, see Ranganath & Ritchey, 2012), and more broadly, the PRC is considered to play an important role in perceptual processing (for reviews, see Bussey & Saksida, 2007; Graham, Barense, & Lee, 2010; Murray & Richmond, 2001). Accordingly, to clearly demonstrate a role for PRC in conceptual implicit retrieval, it is critical to measure conceptual priming separately from any response and perceptual repetition.
One recent study reported direct evidence for PRC involvement in conceptual priming without perceptual and response repetition (Wang, Lazzara, Ranganath, Knight, & Yonelinas, 2010). In that study, participants studied items (e.g., squid) and then at time of test were required to generate exemplars of various different categories (e.g., sea creatures). Relative to both age-matched controls and amnesic patients with relatively selective hippocampal damage, amnesic patients with damage that included PRC were significantly impaired on this task (i.e., they were no more likely to produce studied than nonstudied items). A parallel fMRI experiment in healthy young adults revealed that PRC activation was higher during encoding of words that were subsequently produced on the exemplar generation test (i.e., ‘subsequently primed’ items) than during encoding of words that were not produced on the priming test (i.e., ‘subsequently unprimed’ items). Furthermore, across participants, the magnitude of this subsequent priming effect correlated with behavioral measures of conceptual priming. This experiment revealed that PRC activity during initial encoding is critical for later conceptual implicit memory, but does not indicate whether the PRC is sensitive to conceptual priming during retrieval. That is, it is possible that the PRC may be involved in the initial elaborative processing that is necessary for conceptual priming to occur, but it may not be involved in the process of retrieval.
Although interest in the contribution of PRC to conceptual implicit memory has emerged recently, a large body of work has implicated the PRC in explicit memory retrieval, and in particular, familiarity-based recognition (for reviews, see Brown & Aggleton, 2001; Diana, Yonelinas, & Ranganath, 2007; Eichenbaum, Yonelinas, & Ranganath, 2007). Familiarity reflects item retrieval based on acontextual memory strength – in contrast to recollection, which reflects the qualitative retrieval of context or source information (for a review, see Yonelinas, 2002). There is evidence that damage to PRC selectively impairs familiarity-based recognition (Bowles et al., 2007), and consistent with the conceptual repetition priming studies described above, numerous studies have shown that PRC exhibits repetition-related deactivations during recognition memory retrieval. The presentation of studied items at test – compared to unstudied items -is often accompanied by reductions in PRC activity (e.g., Henson, Cansino, Herron, Robb, & Rugg, 2003; Brozinsky, Yonelinas, Kroll, & Ranganath, 2005; for a review, see Wais, 2008), and furthermore, it has also been demonstrated that increases in the familiarity, or memory strength, of items during recognition tests is related to decreases in PRC activity (e.g., Daselaar, Fleck, & Cabeza, 2006; Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006; Gonsalves, Kahn, Curran, Norman, & Wagner, 2005; Montaldi, Spencer, Roberts, & Mayes, 2006; but see Yonelinas, Otten, Shaw, & Rugg, 2005). Based on this literature, PRC deactivations during recognition tasks have been interpreted to reflect fluent processing of previously studied items that supports familiarity-based recognition discriminations (Fernandez & Tendolkar, 2006). This is in contrast to the hippocampus, which is thought to be critical for recollection-based recognition, but unnecessary for both familiarity-based recognition and conceptual priming (for reviews, see Dew & Cabeza, 2011, Ranganath & Ritchey, 2012).
Given that activity reductions are observed in PRC during both conceptual repetition priming and familiarity-based recognition judgments, it is possible that the same PRC-mediated process facilitates both conceptual implicit memory and familiarity-based recognition. Both forms of memory are sensitive to many of the same behavioral manipulations (for reviews, see Henke, 2010; Yonelinas, 2002), and direct comparisons across participants has indicated that familiarity and conceptual implicit memory are correlated (Wang & Yonelinas, 2012b). However, evidence from fMRI studies that have directly compared recognition and conceptual implicit memory is more mixed. Some did not report PRC involvement in either retrieval task (Donaldson, Petersen, & Buckner, 2001; Voss, Reber, Mesulam, Parrish, & Paller, 2008), and one other study observed activity reductions in PRC related to conceptual repetition priming, but none related to familiarity-based recognition (Voss et al., 2012).
Here, we sought to determine if the PRC was involved in supporting conceptual implicit memory at the time of retrieval, and to clarify the role of PRC in supporting both conceptual priming and familiarity-based recognition. As schematized in Figure 1a, at study, participants incidentally encoded a list of words, half of which served as studied items in the subsequent free association priming task, and the other half served as studied items in the subsequent recognition memory test. Following encoding, participants first completed the free association task in the MRI scanner where they were presented with a series of word cues and asked to respond with the first word that came to mind for each cue. Unbeknownst to participants, some of the cues were selected to be semantically associated with previously studied words. This allowed us to examine PRC activity in the instances when they were conceptually primed to produce a previously studied word. We expected that, if PRC indexes conceptual priming, activity should be decreased on trials that were associated with successful priming (i.e., generation of a studied target in response to an associated cue), relative to both unprimed (i.e., when an associated cue did not prime the generation of a studied target) and baseline (i.e., cues that were unrelated to studied items) trials. Following the free association priming task, we also examined recognition memory confidence and aimed to determine whether PRC would show evidence for decreased activity as a function of recognition confidence. Anatomical region of interest (ROI) and voxel-based analyses of the PRC were conducted for both retrieval tasks, the former of which provided more anatomical precision at the individual participant level, and the latter of which allowed us to determine whether regions within the PRC were sensitive to both conceptual priming and familiarity. Parallel ROI- and voxel-based analyses were conducted on the hippocampus, which was expected to be sensitive to recollection-based recognition (i.e., activity increases during high confident responses; for a review, see Diana et al., 2007), but neither familiarity-based recognition nor conceptual priming.
Figure 1.
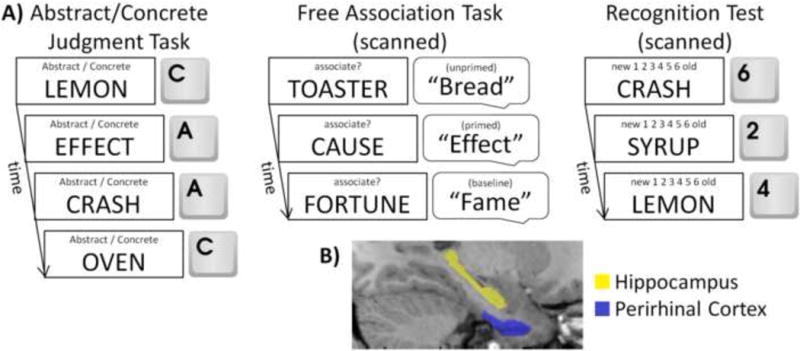
Experimental procedure and ROI tracings. (A) Schematic depiction of the experimental procedure. Participants first incidentally encoded words by completing an abstract/concrete judgment task. Half of these words served as studied targets in a subsequent scanned free association task and the other half served as studied words in a subsequent scanned recognition memory test. (B) Example of individually-defined ROI tracings of PRC and hippocampus from a representative participant.
2. Methods
2.1. Participants
All procedures were approved by the University of California, Davis institutional review board. Twenty five native English speakers from the University of California, Davis and surrounding communities were recruited and paid for their participation. Two participants were excluded from all analyses; one did not respond on 20% of trials during both retrieval tasks and the other was unable to be scanned following the study phase due to a miscommunication regarding the MRI scanner schedule. Additionally, of the remaining 23 participants, two were excluded from the priming analysis because of a recording malfunction and three others were excluded from the recognition analysis as they did not follow instructions to spread out their confidence responses and thus had no trials in certain confidence bins. Thus, 21 participants were included in the priming analysis (age M = 25.76, SD = 3.32; 8 females) and 20 in the recognition analysis (age M = 25.75, SD = 3.23, 8 females), with 18 in both analyses (age M = 25.78, SD = 3.30; 7 females).
2.2. Materials
Materials were identical to those used in Wang & Yonelinas (2012b) and consisted of 320 cue-target word pairs with a mean forward association strength of .38 (SD = 0.11). The word pairs were compiled from the Nelson, McEvoy, and Schreiber (2004) database and counterbalanced across participants.
2.3. Procedure and design
Following informed consent, participants incidentally encoded 192 target words outside of the scanner by judging whether they were abstract (e.g., effect) or concrete (e.g., lemon). Each word was presented for 1,200 ms with a 500 ms fixation interstimulus interval. Participants then entered the MRI scanner to perform two memory retrieval tasks.
Participants first completed a free association priming task across two functional runs. They were presented with 160 cue words across the two blocks. Ninety-six cues were paired with targets studied in the abstract/concrete task (e.g., cause-effect). The other 64 were paired with unstudied targets and served as a baseline measure of priming (e.g., fortune-fame). Participants were not informed about the relationship between the studied targets and their paired cues. For each cue, they responded verbally with the first strongly-associated word that came to mind. Cues were presented one at a time for 1,500 ms each, with a 2,500-6,500 ms fixation interstimulus interval. Prior to scanning, participants were instructed and trained to withhold their response for approximately 2 s following the onset of each cue, during the silent period between acquisitions of functional volumes. This sequence allowed for the minimization of both speech-related movements and scanner-related noise (e.g., Henson, Shallice, Josephs, & Dolan, 2002; Okada, Vilberg, & Rugg, 2012; also see fMRI acquisition and analysis below for further details). Responses were digitally recorded and later transcribed.
Following the free association priming task, participants were administered a surprise recognition memory test for the remaining 96 studied (e.g., lemon) and 64 unstudied (e.g., clay) targets. Each word was presented for 1,500 ms with a 500-6,500 ms fixation interstimlus interval. Participants responded on a 6-point confidence scale, from ‘1’ (sure new) to ‘6’ (sure old) and were encouraged to spread out their responses. Prior to debriefing, they completed an awareness questionnaire to assess whether they utilized intentional retrieval strategies during the free association task (e.g., Wang et al., 2010).
2.4. Image acquisition and analysis
Imaging data were collected on a 3T Siemens Skyra scanner with a 32-channel head coil. Functional images were acquired with a gradient echo-planar imaging (EPI) sequence (TA = 1,940 ms, TE = 25 ms, FOV = 205 mm, flip angle = 90°, matrix size = 64 × 64). EPI volumes consisted of 34 axial slices with a 3.2 mm isotropic voxel size. Each free association block consisted of 139 volumes with a TR of 4,000 ms. This allowed for a 2,060 ms silent period following the presentation of each cue and prior to the acquisition of the next functional volume during which participants overtly responded with the generated associate. The recognition block consisted of 274 volumes with a TR of 2,000 ms. All trial sequences and timings were optimized for fMRI using the optseq2 algorithm (http://surfer.nmr.mgh.harvard.edu/optseq/). Additionally, 1 mm isotropic high resolution T1 weighted coplanar structural MPRAGE images were acquired for each participant.
The data were preprocessed and analyzed with Statistical Parametric Mapping 8 (SPM8) software (Wellcome Trust Centre for Neuroimaging). After the first two scans of each functional block were discarded, the EPI data for each participant were slice-time corrected using sinc interpolation, realigned using a six-parameter, rigid-body transformation, and coregistered to their respective MPRAGE images, which were previously coregistered to the Montreal Neuroimaging Institute (MNI) T1 template. The MPRAGEs were segmented into separate grey and white matter images that were used with the DARTEL toolbox (Ashburner, 2007) to create average grey and white matter templates. Along with each participants' deformation flow field, these templates were used to normalize the EPIs and MPRAGEs into MNI space. The spatially-normalized EPI data were then resliced into 3 mm isotropic voxels. From this point, separate processing streams were established for the analysis of data from anatomically-defined ROIs and voxel-based statistical analyses. The spatially normalized, but unsmoothed EPIs were utilized in the ROI-based analysis, whereas the normalized EPI data were spatially smoothed with a 6 mm isotropic full-width at half-maximum Gaussian filter for the voxel-based analyses.
Statistical analyses were performed in SPM8 using the general linear model (GLM). A high-pass filter of 128 s and grand mean scaling were applied to the data and serial correlations in the time series were accounted for using the autoregressive model (AR[1]). Smoothed and unsmoothed EPIs were separately modeled with a first-level fixed-effects analysis using a canonical hemodynamic response function with the stimulus onsets serving as event onsets. Primed, unprimed, and baseline trials were modeled as conditions of interest in the priming analysis, and the six confidence responses, collapsed across old and new items, were modeled as conditions of interest in the recognition analysis (for frequency of responses per condition of interest, see Supplemental Table 1). Additional covariates of no interest included the six motion parameters estimated during realignment, baseline and session effects, and global mean and motion outliers obtained from the Artifact Detection Toolbox (http://www.nitrc.org/projects/artifact_detect/).
To assess the role of PRC during conceptual implicit and recognition memory retrieval we first conducted an anatomical ROI-based analysis. The left and right PRC were manually traced on each participant's normalized MPRAGE based on previously established criteria (Figure 1b; Franko, Insausti, Artacho-Perula, Insausti, & Chavoix, 2012; Insausti et al., 1998). For this ROI-based approach, mean parameter estimates from the conditions of interest of each retrieval task were extracted from voxels corresponding to these PRC ROIs within the unsmoothed GLMs and analyzed in the SPSS software package (http://ibm.com/software/analytics/spss/) to assess PRC activity during conceptual implicit and recognition memory, respectively. For the voxel-based approach, second-level random-effects analyses were conducted in SPM8 on the smoothed GLMs and masked with a PRC mask created from the union of every participant's manually-traced PRC, constrained within a whole-brain grey matter mask derived from the mean normalized structural image. The family-wise error rate was corrected for multiple comparisons to p < .05 using Monte Carlo simulations with the 3dClustSim program in the AFNI software package (http://afni.nimh.nih.gov/afni/) using a voxel-wide threshold of p < .005 and a cluster size of no less than nine contiguous voxels.
Furthermore, we also ran parallel ROI- and voxel-based analyses on the hippocampus. Left and right hippocampi, manually traced from each participant's normalized MPRAGE (Figure 1b), based on previously established criteria (Duvernoy, 2005), were used to conduct the ROI-based analysis, and a hippocampal mask created from the union of every participant's manually-traced hippocampus, constrained within the grey matter mask, was used to mask its corresponding voxel-based analysis. The family-wise error rate within the hippocampus was corrected for multiple comparisons to p < .05 using a voxel-wide threshold of p < .005 and a cluster size of no less than 10 contiguous voxels.
Lastly, we also reported results from whole-brain voxel-based analyses of conceptual implicit and recognition memory. These contrasts were masked by the grey matter mask and corrected for multiple comparisons to p <. 05 using a voxel-wide threshold of p < .001 and a cluster size of no less than 20 contiguous voxels. Significant clusters from the whole-brain conceptual priming, familiarity, and recollection contrasts are listed in Supplemental Tables 2, 3, and 4, respectively.
3. Result
3.1. Behavioral results
The proportion of studied (i.e., primed) and unstudied (i.e., baseline) targets produced during the free association priming task is illustrated in Figure 2a. Conceptual implicit memory was measured as the difference between these two proportions. Participants generated significantly more primed than baseline targets (t[20] = 7.73, p < .0001), indicating that the study manipulation yielded robust conceptual priming. Recognition memory was assessed by plotting receiver operating characteristics (ROCs) using the confidence responses (Figure 2b). Hits and false alarms were plotted as a function of recognition confidence, and these points were fitted to the dual-process signal detection (DPSD) model by minimizing the sum of squared error (Yonelinas, 1994; 1999; 2001). This allowed us to estimate the contributions of both recollection (M = .25, SEM = .03) and familiarity (M = .87, SEM = .07) to recognition. Importantly, both recollection and familiarity estimates were significantly above zero (ts[19] ≥ 8.34, ps < .0001) indicating that both processes contributed to recognition performance.
Figure 2.
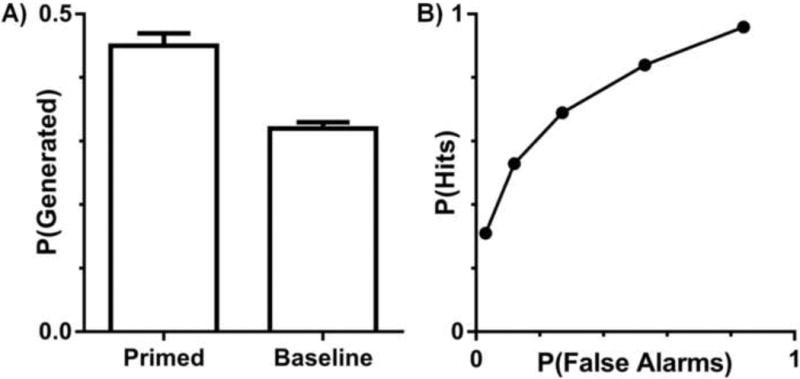
Memory performance. (A) Proportion of targets generated for studied (primed) and unstudied (baseline) cues. Error bars reflect the SEM. (B) Aggregate receiver operating characteristic curves from the recognition task.
3.2. fMRI results
3.2.1. Conceptual implicit memory and the PRC
To determine the role of PRC in conceptual implicit retrieval, we first conducted an anatomical ROI-based analysis. Mean parameter estimates for primed, unprimed, and baseline trials were extracted from each participant's individually-traced left and right PRCs and used in a two-way repeated-measures ANOVA (Condition×Hemisphere) to assess the effects of conceptual priming on PRC activity (Figure 3a). We found no significant effect of Hemisphere (F[1,20] < 1) and no interaction between Condition and Hemisphere (F[2,40] = 2.48, p > .05), indicating that the pattern of activity in left and right PRC was similar. Most importantly however, there was a significant main effect of Condition (F[2,40] = 5.40, p < .01), wherein there was less PRC activity for primed targets relative to both unprimed (t[20] = -2.48, p < .05) and baseline (t[20] = -2.90, p < .01) targets. To further examine the relationship between PRC activity and conceptual priming, we also assessed the correlation between behavioral and neural priming (Figure 3b). This analysis revealed that the difference in PRC activity between primed and unprimed targets was tightly coupled with individual differences in the magnitude of conceptual priming on the free association task in both left (r = -.44, p < .05) and right (r = -.63, p < .005) PRC.
Figure 3.
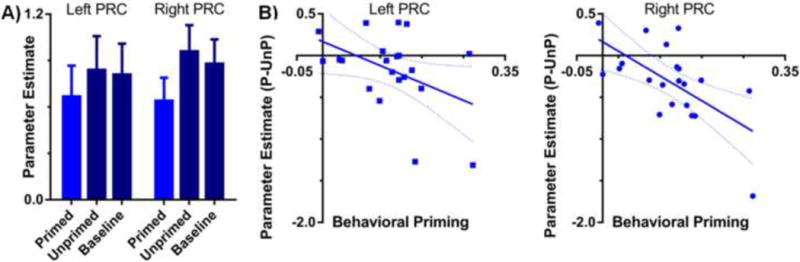
ROI-based analysis of PRC activity related to conceptual implicit memory. (A) Average parameter estimates from the PRC ROIs for primed, unprimed, and baseline trials from the free association priming task. There were activity reductions in the PRC for primed relative to both unprimed and baseline targets (ps < .05). Error bars reflect the SEM. (B) The relationship between the difference in activity for primed (P) and unprimed (UnP) targets in the PRC ROIs and the magnitude of the behavioral priming effects (i.e., the proportion of baseline targets produced relative to the proportion of studied targets produced) in the free association task. Neural priming in PRC was correlated with greater conceptual priming in both hemispheres (ps < 0.05). Dashed lines reflect the 95% CI.
The ROI results above indicated that activity in PRC was related to conceptual priming, but it did not reveal which regions within the PRC were most critical. To address this question, we conducted a voxel-based analysis within a PRC mask derived from the individually-traced PRCs. Consistent with the ROI analysis, we identified significant clusters in an anterior portion of left (peak MNI coordinates: x = -30, y = -6, z = -45; Cluster Size [CS] = 14; Z score = 3.34) and right (MNI: x = 24, y = -9, z = -45, CS = 13; Z = 3.83) PRC that exhibited less activity during primed trials relative to unprimed and baseline trials (i.e., Unprimed + Baseline > Primed; Figure 4a). Furthermore, both clusters, across participants, also exhibited a higher activation difference between primed and unprimed targets as a function of increasing behavioral priming (rs ≤ -.47, ps < .05; Figure 4b). No suprathreshold clusters exhibited more activity for primed trials relative to unprimed and baseline trials.
Figure 4.

Voxel-based analysis of PRC activity sensitive to conceptual implicit memory. (A) Bilateral clusters that exhibited less activity for primed trials relative to unprimed and baseline trials. Error bars reflect the SEM. (B) The difference in activity between primed (P) and unprimed (UnP) trials was significantly correlated with behavioral priming in both clusters (ps < .05). Dashed lines reflect the 95% CI.
3.2.2 Familiarity-based recognition and the PRC
To assess the role of PRC in familiarity-based recognition, we looked to see whether the PRC exhibited a linear decrease in activity as a function of increasing confidence, or familiarity (i.e., 1 > 2 > 3 > 4 > 5), collapsed across studied and unstudied items. The high confident ‘6’ responses, which were not included because they were expected to contain a high proportion of recollected trials, were examined separately below. As in the priming analysis, we first examined mean parameter estimates extracted from the anatomical PRC ROIs. For this ROI analysis we performed a bidirectional linear trend contrast which revealed a nonsignificant familiarity effect in left (F[1,19] = 2.36, p = .14) and right (F[1,19] < 1) PRC. To determine whether specific regions within PRC indexed familiarity-based recognition, we then conducted a voxel-wide analysis, constrained within the PRC, on the effects of recognition confidence. As illustrated in Figure 5a, a cluster in an anterior portion of right PRC exhibited a negative linear relationship (i.e., 1 > 2 > 3 > 4 > 5) with recognition confidence (MNI: x = 21, y = -3, z = -42; CS = 9; Z = 3.95). No suprathreshold clusters exhibited increasing activity from ‘1’ to ‘5’ responses.
Figure 5.
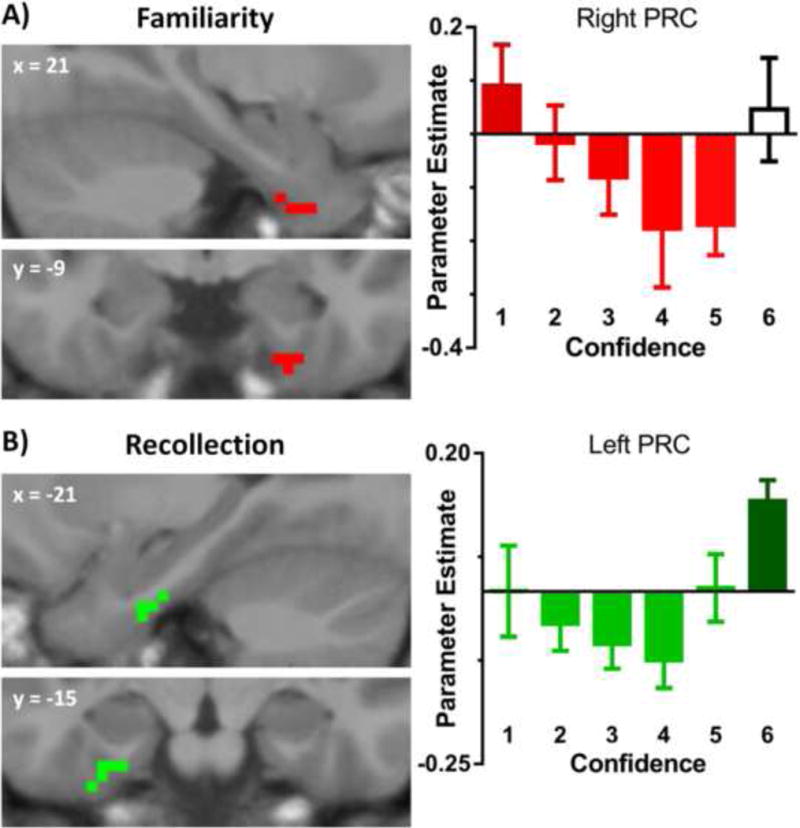
Voxel-based analysis of PRC activity sensitive to recognition memory. (A) Right PRC cluster that exhibited a linear decrease in activity as a function of increasing memory strength from ‘1’ to ‘5’ (excluding ‘6’ responses). Error bars reflect the SEM. (B) Left PRC cluster that exhibited more activity for high confidence ‘6’ responses relative to the average of all other responses. Error bars reflect the SEM.
3.2.3. Recollection-based recognition and the PRC
In a secondary analysis, we looked to see if the PRC exhibited activity indicative of recollection (i.e., more activity for the ‘6’ responses than the average of all other responses). To do this, we assessed whether there were activity differences between ‘6’ responses and ‘1-5’ responses within mean parameter estimates extracted from the PRC ROIs. Unexpectedly, we found significantly more activity for high confident ‘6’ responses relative to all other responses within right PRC (t[19] = 2.23, p < .05), and a marginally significant effect in left PRC (t[19] = 1.79; p = .09). In addition, the voxel-based analysis (i.e., 6 > 1-5) yielded one suprathreshold cluster in a posterior portion of left PRC (MNI: x = -24, y = -15, z = -33; CS = 15; Z = 4.59; Figure 5b). No suprathreshold clusters displayed the opposite pattern.
3.2.4. Overlap of PRC clusters related to conceptual priming, familiarity, and recollection
Figure 6 illustrates the spatial overlap between PRC regions involved in conceptual priming and involved in recognition. As mentioned above, a visual examination of these regions indicated that clusters sensitive to conceptual priming or familiarity were primarily limited to anterior PRC, and recollection-related activity was restricted to posterior PRC. The recollection cluster did not overlap with any PRC regions involved in conceptual priming or familiarity (Figure 6a), whereas regions sensitive to both priming and familiarity overlapped in an anterior portion of right PRC (3 voxels; Figure 6b). Note that although the region showing a significant recollection effect was in the left hemisphere whereas the familiarity effect was in the right, an informal inspection revealed that the same patterns of results were observed in both hemispheres at a more relaxed statistical threshold, indicating that there were no qualitative differences between left and right PRC. At this same relaxed threshold, PRC regions related to conceptual priming and familiarity were still situated more anteriorly than PRC regions related to recollection.
Figure 6.
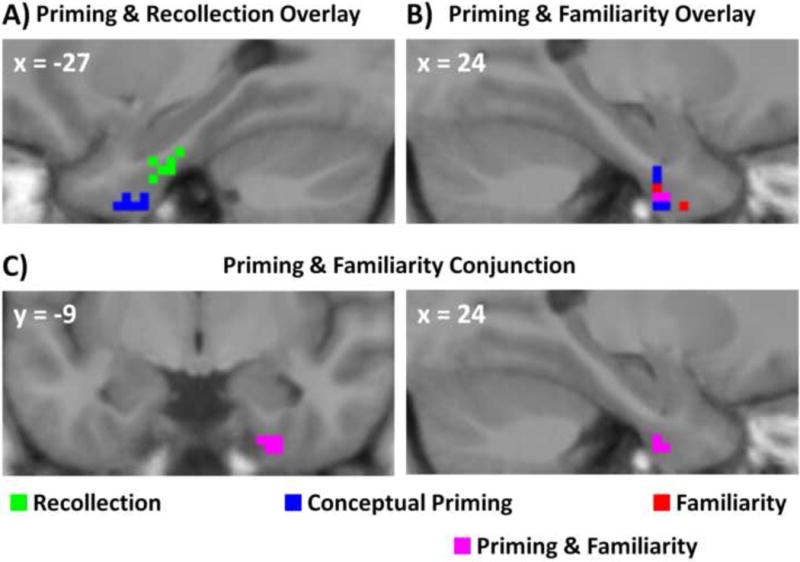
Overlay and conjunction images of significant clusters in the (A) left and (B) right hemispheres from the conceptual priming, familiarity, and recollection analyses. The only overlap was in right PRC between voxels sensitive to both conceptual priming and familiarity. (C) Right PRC cluster that exhibited significant conceptual priming and familiarity effects as revealed by a conjunction analysis.
A more formal analysis of the conjunction between these three forms of memory retrieval was conducted by assessing PRC regions involved in conceptual priming masked by either the familiarity contrast (i.e., priming and familiarity conjunction) or the recollection contrast (i.e., priming and recollection conjunction), and assessing PRC regions involved in familiarity masked by the recollection contrast (i.e., familiarity and recollection conjunction). These contrasts were thresholded at an uncorrected threshold of p < .01 leading to a joint probability of p < .0005 (Fisher, 1990). This analysis yielded one suprathreshold cluster of 8 voxels in the priming and familiarity conjunction (Figure 6c), and none in the priming and recollection or the familiarity and recollection conjunctions.
3.2.5. Conceptual priming and recognition and the hippocampus
We also ran parallel ROI- and voxel-based analyses on the hippocampus to quantify its role during implicit and explicit memory retrieval. First, mean parameter estimates were extracted for primed, unprimed, and baseline trials from left and right hippocampal ROIs and used in a two-way repeated measures ANOVA (Condition×Hemisphere) to assess conceptual priming. This revealed no significant main effects of Condition (F[2,40] = 1.47, p > .05) or Hemisphere (F[1,20] = 3.03, p > .05), and no interaction (F[2,40] = 1.48, p > .05), indicating that the hippocampus was insensitive to conceptual priming . Furthermore, an anatomically-constrained voxel-based analysis of the hippocampus revealed no suprathreshold clusters exhibiting less activity for primed relative to unprimed and baseline words. The reverse contrast also did not yield any suprathreshold clusters. Additionally, behavioral priming and the pattern of fMRI results in PRC and hippocampus did not differ when excluding one participant who reported the intentional (i.e., explicit) retrieval of previously studied targets during the free association priming task.
Next, we evaluated whether the hippocampus was sensitivity to familiarity-based recognition. A bidirectional linear trend contrast of the mean parameter estimates for ‘1’ to ‘5’ confidence responses in the hippocampal ROIs indicated that activity in left and right hippocampus did not decrease as a function of recognition confidence (Fs[1,19] < 1). Similarly, a voxel-based analysis of the hippocampus did not yield any suprathreshold clusters exhibiting decreasing - or increasing - activity from ‘1’ to ‘5’ responses.
Lastly, we examined the role of the hippocampus during recollection-based recognition. Here we assessed whether there was any difference in activity for ‘6’ responses (i.e., recollected) relative to the average of all other responses (i.e., 1-5) in the hippocampal ROIs. This analysis indicated that there was significantly more activity for ‘6’ responses relative to 1-5 responses in both left (t[19] = 3.14, p = .005) and right (t[19] = 2.42, p < .05) hippocampus. Furthermore, the voxel-based analysis of recollection-related activity in the hippocampus revealed a significant cluster in left hippocampus (MNI: x = -30, y = -15, z = -18; CS = 60; Z = 4.46), with no suprathreshold clusters exhibiting the opposite pattern of activity.
4. Discussion
In the current study, we tested the hypothesis that the PRC plays a critical role in conceptual implicit memory retrieval, and further assessed whether this region overlapped with regions within the PRC supporting familiarity-based recognition. To test this, we examined PRC activity reductions as it related to conceptual implicit memory as measured by free association priming, and familiarity as measured by recognition memory confidence. The ROI results indicated that decreased activity in left and right PRC was associated with successful conceptual priming and that the magnitude of this neural priming effect was significantly correlated with the magnitude of behavioral priming across participants. The voxel-based analysis yielded converging results, and additionally indicated that conceptual priming was related to activity reductions in an anterior portion of the PRC. Finally, activity in an overlapping region in anterior PRC decreased across recognition confidence, in line with previous data linking the PRC to familiarity-based responses.
Previous work has observed that making repeated semantic judgments elicits activity reductions in the PRC (Heusser et al., 2013; O'Kane et al., 2005; Voss et al., 2009; Voss et al., 2012) and that the magnitude of these neural priming effects are related to speeded reaction time measures of behavioral priming across participants (Heusser et al., 2013; Voss et al., 2009). These previous studies, however, did not distinguish between conceptual and perceptual priming, or between conceptual and response priming, the latter of which can especially elicit repetition-related deactivations in cortical regions associated with conceptual processing (Schacter et al., 2004). The free association priming paradigm utilized in the current study allowed us to address these concerns by isolating conceptual implicit retrieval in the absence of any perceptual or response repetition. Furthermore, this task allowed us to assess whether the PRC was simply sensitive to conceptual repetition (i.e., deactivations to a related cue, regardless of whether or not the paired target was generated), or if it indexed successful conceptual implicit retrieval (i.e., deactivations to related cues only when the target was successfully primed). Our finding of activity reductions in the PRC, but not the hippocampus, for conceptually primed trials relative to both unprimed and baseline trials offers strong evidence of PRC involvement during a conceptual implicit memory retrieval task without any response or perceptual repetition. Additionally, our results accord with evidence that damage to the PRC, but not the hippocampus, impairs conceptual implicit memory, and evidence that PRC activity at encoding predicts subsequent conceptual priming (Wang et al, 2010).
Many studies have shown that the PRC is sensitive to short-term semantic priming or fluency manipulations (Dew & Cabeza, 2013; Nobre & McCarthy, 1995), involved in on-line semantic processing or integration tasks (Kivisaari, Tyler, Monsch, & Taylor, 2012; McCarthy, Nobre, Bentin, & Spencer, 1995; Meyer et al., 2005; Moss, Rodd, Stamatakis, Bright, & Tyler, 2005; Tyler et al., 2004), and recruited during tasks that require crossmodal integration (Taylor, Moss, Stamatakis, & Tyler, 2006). Furthermore, there is also evidence that PRC is responsive to meaningful objects and familiar faces (i.e., those with preexisting conceptual representations), relative to novel objects and faces, during perceptual discriminations (Barense, Henson, & Graham, 2011). This has led some researchers to propose that PRC may be critical for semantic memory (Davies, Graham, Xuereb, Williams, & Hodges, 2004; Davies, Halliday, Xuereb, Kril, & Hodges, 2009), possibly by differentiating between objects with overlapping conceptual features (Bright, Moss, Stamatakis, & Tyler, 2005; Tyler et al., 2013). Thus, the present results implicating PRC in the facilitated retrieval of previously studied concepts could be indicative of a more general role for this region in conceptual processing.
The current study also investigated how PRC activity varied as a function of recognition memory confidence. More specifically, we sought to replicate previous results indicating that the PRC is sensitive to familiarity-based recognition (Daselaar, Fleck, & Cabeza, 2006; Daselaar, Fleck, Dobbins et al., 2006; Gonsalves et al., 2005; Montaldi et al., 2006; but see Voss et al., 2012; Yonelinas et al., 2005), and to clarify conflicting findings indicating that the PRC is not involved in either recognition memory retrieval or conceptual repetition priming (Donaldson et al., 2001; Voss et al., 2008). The ROI-based analysis did not reveal a significant pattern of decreasing PRC activity with increasing memory confidence from ‘1’ to ‘5’, but a targeted contrast indicated that a region in right anterior PRC did exhibit this pattern, and a conjunction analysis indicated that this PRC region overlapped with the region found to be involved in conceptual priming. Thus, the results from the current study suggest that while PRC activity does not appear to be particularly sensitive to familiarity strength when averaged across the entire PRC cortex, there is a region within the PRC (Figure 6c) that exhibits activity reductions at retrieval during both explicit familiarity-based recognition and conceptual implicit memory.
It is important to note that the demands of the recognition and priming tasks were quite different. In the free association priming task, participants were presented with conceptually-related and baseline cues and were asked to respond overtly with the first word that came to mind. In contrast, test probes in the recognition task were studied and unstudied words and participants were asked to respond on a button box using a 6-point scale. In spite of these differences, the finding of overlapping priming- and familiarity-related deactivations in PRC supports the idea that there may be a common neural mechanism across these two forms of mnemonic retrieval (e.g., Dew & Cabeza, 2011; Henke, 2010; Wang & Yonelinas, 2012a; Yonelinas, 2002). Whether these effects reflect the operation of a common process that supports performance on both measures, however, or the influence of conceptual priming during familiarity-based recognition judgments (Paller, Voss, & Boehm, 2007; Voss, Lucas, & Paller, 2012), remains an important topic for future research.
In addition to indexing familiarity-based recognition, we also observed greater activity for high confidence ‘6’ responses relative to all other responses in the PRC ROIs, with the voxel-based analysis implicating a posterior portion of the PRC as a neural correlate of recollection. Although this finding conflicts with previous claims that the PRC is not involved in recollection (e.g., Bowles et al., 2007; Brown & Aggleton, 2001; Diana et al., 2007; Eichenbaum et al., 2007), it is consistent with prior studies that have reported PRC activations at time of retrieval when ‘item’ information is recollected (Diana, Yonelinas, & Ranganath, 2010; Hannula, Libby, Yonelinas, & Ranganath, 2013; Staresina, Fell, Do Lam, Axmacher, & Henson, 2012; Wang, Yonelinas, & Ranganath, 2013). That is, if the PRC supports the processing of item information, then test probes that lead to the successful retrieval of associated item information (i.e., recollection) may also lead to an increase in PRC activity. Our results accord with this possibility; ROI- and voxel-based analyses of the hippocampus revealed the same recollection-related pattern of activity (i.e., increased activity for ‘6’ responses relative to ‘1-5’ responses), but no patterns of activity related to familiarity-based recognition or conceptual priming.
The fact that we observed increases in activity when assessing recollection and activity reductions when assessing familiarity indicates that this non-monotonic pattern of PRC activity associated with recognition memory retrieval cannot be simply accounted for by a memory-strength response. Rather, our results indicate that there may be two distinct retrieval processes that contribute to recognition memory within the PRC, a familiaritylike process related to activity reductions, and a distinct recollection-like process related to increased activity (e.g., Staresina et al., 2012). This finding also demonstrates that activity reductions associated with conceptual implicit retrieval was not driven by recollective processes, because recollection was associated with increased activity in both hippocampus and PRC, whereas both priming and familiarity were associated with activity reductions in the PRC, but not the hippocampus. Additionally, we note that the pattern of behavioral and fMRI results did not change when excluding participants who reported using intentional retrieval strategies, further indicating that our results were not likely to be contaminated by conscious recall or recollection. The present results illustrate the utility of assessing and comparing activity during explicit and implicit tasks in order to better understand the role of PRC in different retrieval processes.
A growing body of work has implicated the PRC in a variety of mnemonic, perceptual, and associative tasks (Murray & Richmond, 2001). The role that PRC may play in a wide range of cognitive tasks is of particular relevance given the variety and severity of neurological diseases that differentially affect PRC structure and function, including Alzheimer's disease, herpes encephalitis, semantic dementia, and temporal lobe epilepsy (Ranganath & Ritchey, 2012). Thus, our work specifying the relationship between conceptual priming and PRC will hopefully help better characterize mnemonic deficits associated with diseases that damage this region and in general add to our understanding of the PRC's complex role in human cognition.
Supplementary Material
Highlights.
We examined the role of perirhinal cortex (PRC) in conceptual implicit memory retrieval.
Free association priming was related to activity reductions in PRC.
These reductions correlated with the magnitude of behavioral priming across participants.
An overlapping PRC region exhibited decreasing activity as a function of familiarity.
Results implicate PRC in both conceptual priming and familiarity-based recognition.
Acknowledgments
This study was supported by grants R01 MH083734 and F31 MH096346 from the NIMH. The authors thank Dr. Jon Simons for helpful feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;15:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Barense MD, Henson RN, Graham KS. Perception and conception: Temporal lobe activity during complex discriminations of familiar and novel faces and objects. Journal of Cognitive Neuroscience. 2011;23:3052–3067. doi: 10.1162/jocn_a_00010. [DOI] [PubMed] [Google Scholar]
- Bowles B, Crupi C, Mirsattari SM, Pigott SE, Parrent AG, Pruessner JC, Yonelinas AP, Kohler S. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proceedings of the National Academy of Sciences. 2007;104:16382–16387. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright P, Moss HE, Stamatakis EA, Tyler LK. The anatomy of object processing: the role of anteromedial temporal cortex. The Quarterly Journal of Experimental Psychology: B. 2005;58:361–377. doi: 10.1080/02724990544000013. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–62. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brozinsky CJ, Yonelinas AP, Kroll NE, Ranganath C. Lag-sensitive repetition suppression effects in the anterior parahippocampal gyrus. Hippocampus. 2005;15(5):557–61. doi: 10.1002/hipo.20087. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Memory, perception, and the ventral visual-perirhinal-hippocampal stream: thinking outside of the boxes. Hippocampus. 2007;17:898–908. doi: 10.1002/hipo.20320. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. Journal of Neurophysiology. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cerebral Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies RR, Graham KS, Xuereb JH, Williams GB, Hodges JR. The human perirhinal cortex and semantic memory. European Journal of Neuroscience. 2004;20:2441–2446. doi: 10.1111/j.1460-9568.2004.03710.x. [DOI] [PubMed] [Google Scholar]
- Davies RR, Halliday GM, Xuereb JH, Kril JJ, Hodges JR. The neural basis of semantic memory: evidence from semantic dementia. Neurobiology of Aging. 2009;30:2043–2052. doi: 10.1016/j.neurobiolaging.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Dew ITZ, Cabeza R. The porous boundaries between explicit and implicit memory: Behavioral and neural evidence. Annals of the New York Academy of Sciences. 2011;1224:174–190. doi: 10.1111/j.1749-6632.2010.05946.x. [DOI] [PubMed] [Google Scholar]
- Dew ITZ, Cabeza R. A broader view of perirhinal function: From recognition memory to fluency-based decisions. The Journal of Neuroscience. doi: 10.1523/JNEUROSCI.1413-13.2013. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. Journal of Cognitive Neuroscience. 2010;22:1808–1818. doi: 10.1162/jocn.2009.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007 Sep;11(9):379–86. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Buckner RL. Dissociating memory retrieval processes using fMRI: evidence that priming does not support recognition memory. Neuron. 2001;31:1047–1059. doi: 10.1016/s0896-6273(01)00429-9. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human hippocampus: Functional anatomy, vascularization and serial sections with MRI. 3. New York: Springer; 2005. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G, Tendolkar I. The rhinal cortex: ‘Gatekeeper’ of the declarative memory system. Trends in Cognitive Sciences. 2006;10:358–362. doi: 10.1016/j.tics.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods, Experimental Design, and Scientific Inference. Oxford: Oxford University Press; 1990. [Google Scholar]
- Franko E, Insausti AM, Artacho-Perula E, Insausti R, Chavoix C. Identification of the human medial temporal lobe regions on magnetic resonance images. Human Brain Mapping. 2012 doi: 10.1002/hbm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee AC. Going beyond LTM in the MTL: A synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Greene CM, Soto D. Neural repetition effects in the medial temporal lobe complex are modulated by previous encoding experience. PLoS One. 2012;7:e40870. doi: 10.1371/journal.pone.0040870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Libby LA, Yonelinas AP, Ranganath C. Medial temporal lobe contributions to cued retrieval of items and contexts. Neuropsychologia. 2013 doi: 10.1016/j.neuropsychologia.2013.02.011. doi:0.1016/j.neuropsychologia.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K. A model for memory systems based on processing modes rather than consciousness. Nature Reviews Neuroscience. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- Henson RN, Cansino S, Herron JE, Robb WG, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Josephs O, Dolan RJ. Functional magnetic resonance imaging of proactive interference during spoken cued recall. Neuroimage. 2002;17:543–558. [PubMed] [Google Scholar]
- Heusser AC, Awipi T, Davachi L. The ups and downs of repetition: Modulation of the perirhinal cortex by conceptual repetition predicts priming and long-term memory. Neuropsychologia. 2013 doi: 10.1016/j.neuropsychologia.2013.04.018.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner AJ, Henson RN. Priming, response learning and repetition suppression. Neuropsychologia. 2008;46:1979–1991. doi: 10.1016/j.neuropsychologia.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. American Journal of Neuroradiology. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Kivisaari SL, Tyler LK, Monsch AU, Taylor KI. Medial perirhinal cortex disambiguates confusable objects. Brain. 2012;135:3757–3769. doi: 10.1093/brain/aws277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior-medial temporal lobe: I. Intracranial distribution and neural generators. The Journal of Neuroscience. 1995;15:1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer P, Mecklinger A, Grunwald T, Fell J, Elger CE, Friederici AD. Language processing within the human medial temporal lobe. Hippocampus. 2005;15:451–459. doi: 10.1002/hipo.20070. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Moss HE, Rodd JM, Stamatakis EA, Bright P, Tyler LK. Anteromedial temporal cortex supports finegrained differentiation among objects. Cerebral Cortex. 2005;15:616–627. doi: 10.1093/cercor/bhh163. [DOI] [PubMed] [Google Scholar]
- Murray EA, Richmond BJ. Role of perirhinal cortex in object perception, memory, and associations. Current Opinion in Neurobiology. 2001;11:188–193. doi: 10.1016/s0959-4388(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida free association, rhyme, and word fragment norms. Behavior Research Methods, Instruments, & Computers. 2004;36:402–407. doi: 10.3758/bf03195588. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G. Language-related field potentials in the anterior-medial temporal lobe: II. Effects of word type and semantic priming. The Journal of Neuroscience. 1995;15:1090–1098. doi: 10.1523/JNEUROSCI.15-02-01090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kane G, Insler RZ, Wagner AD. Conceptual and perceptual novelty effects in human medial temporal cortex. Hippocampus. 2005;15:326–332. doi: 10.1002/hipo.20053. [DOI] [PubMed] [Google Scholar]
- Okada K, Vilberg KL, Rugg MD. Comparison of the neural correlates of retrieval success in tests of cued recall and recognition memory. Human Brain Mapping. 2012;33:523–533. doi: 10.1002/hbm.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Voss JL, Boehm SG. Validating neural correlates of familiarity. Trends in Cognitive Sciences. 2007;11:243–250. doi: 10.1016/j.tics.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Implicit memory in normal human subjects. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Vol. 8. Amsterdam: Elsevier; 1993. pp. 63–131. [Google Scholar]
- Schacter DL, Dobbins IG, Schnyer DM. Specificity of priming: A cognitive neuroscience perspective. Nature Reviews Neuroscience. 2004;5:853–862. doi: 10.1038/nrn1534. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Implicit memory: History and current status. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:501–518. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Fell J, Do Lam AT, Axmacher N, Henson RN. Memory signals are temporally dissociated in and across human hippocampus and perirhinal cortex. Nature Neuroscience. 2012;15:1167–1173. doi: 10.1038/nn.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KI, Moss HE, Stamatakis EA, Tyler LK. Binding crossmodal object features in perirhinal cortex. Proceedings of the National Academy of Sciences. 2006;103:8239–8244. doi: 10.1073/pnas.0509704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Chiu S, Zhuang J, Randall B, Devereux BJ, Wright P, Clarke A, Taylor KI. Objects and Categories: Feature Statistics and Object Processing in the Ventral Stream. Journal of Cognitive Neuroscience. 2013;25:1723–1735. doi: 10.1162/jocn_a_00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Bright P, Acres K, Abdallah S, Rodd JM, Moss HE. Processing objects at different levels of specificity. Journal of Cognitive Neuroscience. 2004;16:351–362. doi: 10.1162/089892904322926692. [DOI] [PubMed] [Google Scholar]
- Wais PE. fMRI signals associated with memory strength in the medial temporal lobes: A meta-analysis. Neuropsychologia. 2008;46:3185–3196. doi: 10.1016/j.neuropsychologia.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Wang WC, Lazzara MM, Ranganath C, Knight RT, Yonelinas AP. The medial temporal lobe supports conceptual implicit memory. Neuron. 2010;68:835–842. doi: 10.1016/j.neuron.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Yonelinas AP. Familiarity and conceptual implicit memory: Individual differences and neural correlates. Cognitive Neuroscience. 2012a;3:213–214. doi: 10.1080/17588928.2012.689968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Yonelinas AP. Familiarity is related to conceptual implicit memory: An examination of individual differences. Psychonomic Bulletin & Review. 2012b;19:1154–1164. doi: 10.3758/s13423-012-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Yonelinas AP, Ranganath C. Dissociable neural correlates of item and context retrieval in the medial temporal lobes. Behavioral Brain Research. 2013;254:102–107. doi: 10.1016/j.bbr.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Federmeier KD, Paller KA. The potato chip really does look like Elvis! Neural hallmarks of conceptual processing associated with finding novel shapes subjectively meaningful. Cerebral Cortex. 2012;22:2354–2364. doi: 10.1093/cercor/bhr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Hauner KK, Paller KA. Establishing a relationship between activity reduction in human perirhinal cortex and priming. Hippocampus. 2009;19:773–778. doi: 10.1002/hipo.20608. [DOI] [PubMed] [Google Scholar]
- Voss JL, Lucas HD, Paller KA. More than a feeling: Pervasive influences of memory processing without awareness of remembering. Cognitive Neuroscience. 2012;3:193–207. doi: 10.1080/17588928.2012.674935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Reber PJ, Mesulam MM, Parrish TB, Paller KA. Familiarity and conceptual priming engage distinct cortical networks. Cerebral Cortex. 2008;18:1712–1719. doi: 10.1093/cercor/bhm200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver operating characteristics in recognition memory: Evidence for a dual process model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The contribution of recollection and familiarity to recognition and source memory: An analysis of receiver operating characteristics and a formal model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:1415–1434. doi: 10.1037//0278-7393.25.6.1415. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Consciousness, control and confidence: The three Cs of recognition memory. Journal of Experimental Psychology: General. 2001;130:361–379. doi: 10.1037//0096-3445.130.3.361. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. The Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


