Abstract
Metastatic breast cancer (MBC) patients often experience pain which can trigger pain behaviors, such as distorted ambulation. Psychological variables, such as individuals’ attitudes toward pain, play a role in pain intervention. In this study, we used the cognitive-behavioral model of pain to examine the influence of patients’ attitudes toward pain (as measured by the Survey of Pain Attitudes or SOPA) on their pain behaviors (as measured by the Pain Behaviors Checklist). Two hundred-one MBC patients completed surveys at treatment initiation and again three and six months later. Linear Mixed Model with repeated measures analyses showed that SOPA-solicitude, SOPA-emotions, SOPA-cure, SOPA-disability, and SOPA-medication pain attitudes were consistently significantly associated with pain behaviors at each assessment time point. Additionally, the belief that a medical cure for pain exists buffered the positive association between pain severity and pain behaviors. Our findings support and extend the cognitive-behavioral model of pain and suggest that it may be useful to target pain attitudes in pain management interventions for MBC patients.
Keywords: cancer, pain, pain attitudes, pain behaviors, cognitive-behavioral model of pain
Introduction
Pain is one of the most commonly experienced and inadequately treated side-effects of cancer and its treatment (Fairchild, 2010). It is a major concern for women with metastatic breast cancer (MBC) (Butler et al., 2003) with 56–68% of MBC patients reporting severe pain compared to 33–52% of patients with non-metastatic breast cancer (McGuire & Sheidler, 1992). One reason why MBC patients report higher levels of pain relative to other cancer patients is that bone is the most common site of breast cancer metastasis (Coleman & Rubens, 1987). The high levels of pain experienced by MBC patients make pain management interventions a critical component of improving their cancer care and quality of life.
One way to determine how well patients with pain are functioning is to assess the pain behaviors they display (Jensen, Turner, Romano, & Lawler, 1994) such as distorted ambulation and facial/audible expressions of pain (Badr & Milbury, 2011; Kerns et al., 1991). Because pain behaviors are often associated with functional disability and poor quality of life (Ahles et al., 1990; Ahles et al., 1983), it is important to target these behaviors in pain management interventions. Unfortunately, inadequate pain management is common in routine clinical practice, often because of under-treatment or misguided concerns about medication addiction (Portenoy & Lesage, 1999). Moreover, 25% of cancer patients do not use analgesics to relieve their pain because they are either unable to pay for them, fear addiction or dependence, or their health care provider did not prescribe or recommend them (Simone et al., 2012). As a result, other methods of managing pain are needed.
One option for managing pain is to teach patients to modify their attitudes toward pain. Indeed, pain attitudes have been found to play a central role in predicting pain behaviors (Jensen et al., 1999) and refer to individuals’ perceptions of the treatment and curability of their pain as well as what their pain indicates (e.g., disability) (Tait & Chibnall, 1997). Some pain attitudes reveal general beliefs such as the belief that medication is an effective treatment for pain (Tait & Chibnall, 1997). Others refer to either negative or positive beliefs about pain. The distinction between pain attitudes as positive or negative comes from pain attitudes’ orientation toward pain as either uncontrollable (negative) or manageable (positive) (Tait & Chibnall, 1997) as well as their associations with psychological distress and effective pain management (Jensen & Karoly, 1992). For instance, beliefs that pain indicates disability, that engaging in activities will be harmful when one feels pain, or that emotions exacerbate pain all represent negative attitudes because they reflect the belief that pain is damaging and uncontrollable (Tait & Chibnall, 1997). Some of these negative pain attitudes (such as belief that pain indicates disability) have been negatively associated with psychological functioning and activity levels among pain patients (Jensen & Karoly, 1992). In contrast, the belief in a medical cure for pain and the belief that pain can be controlled represent positive attitudes because they reflect the belief that pain can be effectively managed (Tait & Chibnall, 1997). Positive attitudes, such as belief in a medical cure for pain, have been positively associated with utilization of professional services for pain management (Jensen & Karoly, 1992). Additionally, belief that pain can be controlled has been positively associated with psychological functioning (Jensen & Karoly, 1991). Similar to past research on the association between positive and negative attitudes and psychological and physical functioning, positive and negative pain attitudes may also predict the degree to which individuals engage in pain behaviors. Namely, attitudes that would be expected to be associated with more pain behaviors could be viewed as negative and attitudes that would be expected to be associated with fewer pain behaviors could be viewed as positive. Examining both positive and negative pain attitudes as predictors of pain behaviors simultaneously would help researchers to understand which pain attitudes are more strongly associated with pain behaviors. This, in turn, would allow for the development of more targeted pain interventions that focus on pain attitudes with the strongest associations with pain behaviors.
The hypothesis that pain attitudes predict pain behaviors comes from the cognitive-behavioral model of pain (Turk et al., 1987). This model was developed from research demonstrating that attitudes about one’s pain predict patient reports of pain severity even after controlling for the effects of physical pathology (Sharp, 2001). Strong empirical evidence demonstrates that pain-related attitudes influence one’s ability to cope with pain and, as a result, the impact of pain on patients’ lives (Sharp, 2001). Research supporting the cognitive-behavioral model of pain has demonstrated that attitudes toward pain are associated with both physical disability (Jensen et al., 1994) and the number of pain behaviors displayed by chronic pain patients (Jensen et al., 1987; Jensen et al., 1999). However, little is known about the relationship between positive pain attitudes (e.g., belief in a medical cure for pain) and pain behaviors.
Despite extensive research on the relationship between pain attitudes and pain behaviors in chronic pain populations (Jensen et al., 1999, 1994; Strong et al., 1992; Tait & Chibnall, 1997), studies have yet to examine whether pain attitudes buffer or exacerbate the relationship between pain severity and pain behaviors. Knowing which pain attitudes exacerbate or buffer this relationship could help clinicians improve pain management programs for patients with MBC. Just as different pain attitudes should differentially predict pain behaviors, they should also differentially affect the relationship between pain and pain behaviors. For example, negative attitudes regarding pain-related disability and harm might intensify or exacerbate the association between pain severity and pain behaviors whereas positive attitudes such as the belief in a medical cure for pain might attenuate or buffer this association.
Given this and the present review of the literature, this study sought to examine associations between pain, pain attitudes, and pain behaviors across three time points among MBC patients. The following hypotheses were tested: (1) negative pain attitudes (e.g., belief that pain indicates disability, engaging in activities when one feels pain is harmful) are positively associated with pain behaviors (across time), (2) positive pain attitudes (e.g., belief in a medical cure for pain) are negatively associated with pain behaviors (across time), (3) negative pain attitudes exacerbate the association between pain severity and pain behaviors, and (4) positive pain attitudes buffer the relationship between pain severity and pain behaviors. In addition to examining the association between pain behaviors and each pain attitude individually, we also sought to explore which pain attitudes were most strongly associated with pain behaviors.
Methods
Study Design
Data were taken from a larger study of pain and spousal relationships that was approved by The University of Texas MD Anderson Cancer Center’s Institutional Review Board. Details regarding the larger study and the recruitment process are presented elsewhere (Badr et al., 2010; Badr & Milbury, 2011). Briefly, during routine clinical visits, female MBC patients were identified through medical chart review and approached to participate in the study. Patients were eligible if they: (1) were beginning treatment for MBC; (2) had a physician-rated Eastern Cooperative Oncology Group (ECOG) (Oken et al., 1982) performance status score ≤ 2 (i.e., ambulatory and capable of all self-care but unable to perform any work activities); (3) rated their average pain as ≥ 1 on the Brief Pain Inventory (BPI) (Cleeland & Syrjala, 1992, in which 0=no pain and 10=worst pain imaginable); (4) could speak and understand English; and (5) had a male partner (spouse or significant other) with whom they had lived for the past year.
Patients and their partners completed questionnaires and returned them in individually sealed postage-paid envelopes at the initiation of patients’ treatment for MBC (baseline) and 3 and 6 months later. They received gift cards worth $10 upon the return of each completed survey. Because the focus of this study was on associations between pain severity, pain attitudes, and pain behaviors and because these items were only assessed for patients, only patient data was used.
Assessment Measures
The assessment battery comprised frequently used instruments with established reliability and validity. Individual scales are described below.
Pain severity
Patients completed the four-item pain severity subscale of the BPI (Cleeland & Syrjala, 1992). The BPI asks patients to rate their current pain (right now), as well as their worst, least, and average pain over the last seven days on an 11-point Likert-type scale (0 = no pain to 10 = pain as bad as you can imagine). As outlined by Cleeland and Syrjala (1992), an overall pain-severity score was created by averaging responses to these four items. The BPI has been shown to be a reliable and valid assessment of pain among cancer patients (Cleeland, 1991). Data from this scale were internally consistent (Cronbach’s α = .91).
Pain behaviors
To assess pain behaviors, patients completed the 17-item Pain Behavior Checklist (PBCL; Kerns et al., 1991). This self-report measure was developed by Kerns et al. (1991) as a proxy measure for observed pain behaviors and has been shown to have significant positive correlations with third-party observations of pain behaviors such as guarding, bracing, and grimacing. The PBCL assesses the extent to which patients engaged in four categories of pain behaviors (distorted ambulation, facial/audible expression of pain, seeking help, and affective distress) during the previous week on a 7-point Likert-type scale (0 = never to 6 = very often). Patients indicated how many of these pain behaviors they engaged in and how often. A total pain behaviors score was calculated by summing responses to the different subscales. Data from this scale were internally consistent (Cronbach’s α = .88).
Pain attitudes
Patients completed the 30-item brief version of the Survey of Pain Attitudes (SOPA; Tait & Chibnall, 1997) which assesses patients’ attitudes toward their pain across seven dimensions using a 5-point Likert-type scale (0 = very untrue to 4 = very true). The seven dimensions measured were: (1) solicitude [belief that it is the responsibility of others to assist the patient with his or her pain experience (five items)], (2) emotions [the degree to which emotions, such as stress, influence how much pain a patient feels (four items)], (3) medical cure [belief that there is a medical cure for one’s pain (five items)], (4) control [belief that patients can control the degree of pain they feel (five items)], (5) harm [belief that pain signifies damage and that exercise and activity could make this damage worse (four items)], (6) disability [the belief that the patient is disabled due to the pain (four items)], and (7) medication [belief that medications are the best treatment strategy for pain (three items)]. Subscale scores were created by averaging item responses for each dimension. Data from each subscale were internally consistent: solicitude (Cronbach’s α = .75), emotions (Cronbach’s α = .84), cure (Cronbach’s α = .71), control (Cronbach’s α = .71), harm (Cronbach’s α = .75), disability (Cronbach’s α = .75), and medication (Cronbach’s α = .64).
Demographic/medical variables
Patients provided demographic information including age, gender, race, ethnicity, marital status, and occupational status. They were also asked questions about time since cancer diagnosis, disease stage at diagnosis, and treatment.
Data Analysis Plan
To examine the change in pain behaviors across time within individuals, we utilized a linear mixed model with repeated measures. One advantage of mixed modeling is that it can accommodate missing data points, which are common in longitudinal datasets, and thus maximize the utility of existing data (Krueger & Tian, 2004). To rule out potential confounding variables, we examined patient demographic (i.e., age, income, education) and medical variables (i.e., time since diagnosis, stage at diagnosis) as predictors of pain behaviors as well. Race and ethnicity were not included as control variables because this was a predominately (92%) White, non-Hispanic sample.
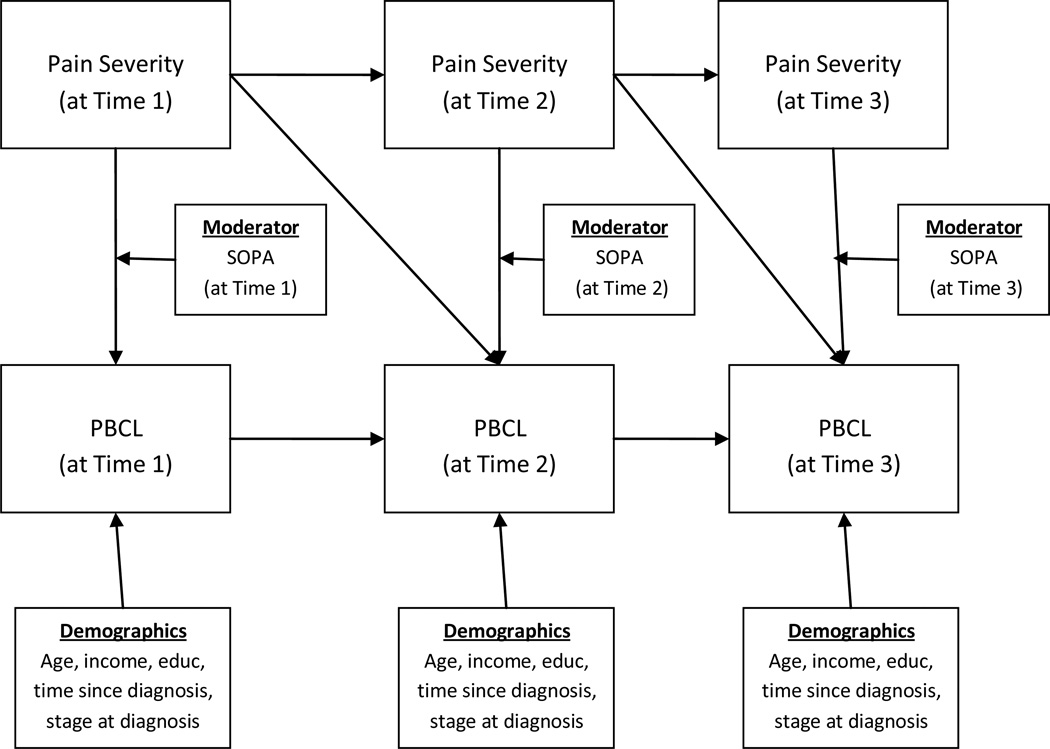
To test our four main hypotheses, we ran a series of seven linear mixed models with repeated measures to examine whether: (1) pain attitudes (SOPA) were associated with self-reported pain behaviors (PBCL) and (2) each pain attitude (SOPA) subscale moderated the association between pain severity (BPI) and pain behaviors (PBCL). These mixed models were run to examine the associations between each individual pain attitude subscale and pain behaviors. In an initial base mixed model, we entered time, pain severity, and demographic and medical control variables. In the seven linear mixed models with repeated measures, specific pain attitudes (SOPA) as well as an interaction term (pain severity × a specific pain attitude) were added to the models to determine their associations with patient pain behaviors. Figure 1 provides a conceptual model of the linear mixed models with repeated measures that were conducted.
Figure 1.
Conceptual model depicting the linear mixed model with repeated measures analyses that were conducted.
To test our exploratory aim of examining the association between all pain attitudes and pain behaviors, we ran a separate linear mixed model with repeated measures. In this model, all seven of the pain attitude subscales as well as their interactions with pain severity (pain severity × specific pain attitude) were entered into the linear mixed model, along with pain severity.
Results
Recruitment and Sample Characteristics
Three hundred sixty-seven female MBC patients and their male partners were approached by research staff. Of these, 26 patients (6.5%) were ineligible (7 did not live with their partner; 11 had no pain; 2 did not speak English; and 6 could not provide informed consent). Of the 341 patients remaining, 50 (14.6%) declined participation (4 felt too distressed to participate and 46 were not interested), resulting in 291 patients who consented to participate. Patient participants were compared to those who declined based on available data for: age, ECOG performance status, race, average pain (BPI) at time of recruitment, and primary metastatic site. The only significant difference was in reported pain severity [t(351) = −8.49, p=.001]. Specifically, patients who agreed to participate reported more severe pain (M=4.34, SD=3.02) than did those who declined to participate in the study (M=1.44, SD=1.34).
Of the 291 patients who consented to participate, 10 had partners who were unreachable which precluded the patient from participating further. Of the remaining 281 patients who consented to participate, 75 (27%) did not return their baseline surveys and were therefore classified as passive refusers. African American, Hispanic, and Asian patients had a greater likelihood of passive refusal than did white patients [χ2 (3, 273) = 5.79, p=.02]. After accounting for the 75 passive refusers, 206 couples remained. Among those were 15 couples in which only the patient (n=10) or the partner (n=5) returned the survey. Given that the focus of the present study is on patients only, the final baseline sample comprised 201 patients.
Ten patients who completed the baseline survey died or were referred to hospice before the 3-month follow-up surveys were mailed, and only patients whose partners had completed a baseline survey were sent follow-up surveys. As such, only 181 surveys were mailed out at the 3-month follow-up. Of those, 122 surveys were returned (67% of the 181 mailed out). Prior to the 6-month survey being mailed, 8 additional patients died and 22 either dropped out of the study or were lost due to follow-up. This resulted in a mail-out of 151 6-month surveys. Of the 151 surveys mailed out, 108 were returned by patients (72% of the 151 mailed out; 54% of the original 201). Six of the 6-month surveys were not returned because the patient had died. Comparisons were made between patients who completed the study and those who did not. There were no significant differences based on age, ECOG performance status, race/ethnicity, pain severity, or pain behaviors. Demographic and medical characteristics of the baseline sample are presented in Table 1.
Table 1.
Demographic and Medical Characteristics of Baseline MBC Patient Sample
| Patients (N = 201) | |
|---|---|
| White (%) | 185 (92.0) |
| Age (mean ± SD) (range) yrs | 52.20±10.5 (23–78) |
| College ≥2 yrs (%) | 141 (70.1) |
| Employment status (%) | |
| Full-time | 50 (24.9) |
| Part-time | 21 (10.40 |
| Unemployed | 63 (31.3) |
| Retired | 52 (25.9) |
| Unknown | 15 (7.5) |
| Married (%) | 199 (99.0) |
| Years of marriage (range) | 25.57 ± 13.02 (Range = 1–58 yrs) |
| Stage at time of initial cancer diagnosis: (%) | |
| I | 24 (11.9) |
| II | 51 (25.4) |
| III | 41 (20.4) |
| IV | 51 (25.4) |
| Unknown | 34 (16.9) |
| Years since diagnosis (range) | 5.43 ± 5.20 (5 wks–25.6 yrs) |
| Primary metastatic site (%) | |
| Bone | 113 (56.2) |
| Lung | 42 (20.9) |
| Liver | 38 (18.9) |
| Brain | 8 (4.0) |
| Treatment (%) | |
| Chemotherapy | 171 (85.1) |
| Hormonal therapy | 22 (10.9) |
| Palliative radiation | 8 (4.0) |
SD: standard deviation.
Descriptive Results
Patients reported an average pain level of 2.23 (SD=2.00) on the 0-to-10 overall BPI severity scale. Eleven percent of patients rated their pain as higher than the mid-point (i.e., “5”) of the BPI scale. On average, patients reported a 25.09 (SD=18.18) on the PBCL (possible range: 0 to 102). Table 2 shows the univariate correlations between the major study variables as well as the univariate descriptive statistics (means, standard deviations, and ranges) for each measure.
Table 2.
Descriptive Results for MBC Patients on Major Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Mean+SD | Range | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Pain severity (BPI) | --- | 2.23 ± 2.00 | 0 – 9.75 | ||||||||
| 2. Pain behaviors (PBCL) | .62** | --- | 25.09 ± 18.18 | 0 – 93.0 | |||||||
| 3. SOPA-Solicitude | .23** | .34** | --- | 1.52 ± .94 | 0 – 4.00 | ||||||
| 4. SOPA-Emotions | .09 | .24** | .51** | --- | 1.82 ± 1.14 | 0 – 4.00 | |||||
| 5. SOPA-Cure | −.23** | −.31** | −.19* | −.09 | --- | 2.70 ± .89 | 0 – 4.00 | ||||
| 6. SOPA-Control | −.15** | −.18** | .06 | .30** | .27** | --- | 2.13 ± .92 | 0 – 4.00 | |||
| 7. SOPA-Harm | .29** | .32** | .05 | −.14** | −.18** | −.48** | --- | 1.93 ± 1.01 | 0 – 4.00 | ||
| 8. SOPA-Disability | .43** | .57** | .33** | .22** | −.34** | −.31** | .45** | --- | 1.88 ± 1.08 | 0 – 4.00 | |
| 9. SOPA-Medication | .33** | .41** | .28** | .26** | −.30** | −.12* | .29** | .44** | --- | 2.27 ± 1.06 | 0 – 4.00 |
Note:
p < .05;
p < .01
BPI = Brie Pain Inventory Severity subscale; PBCL = Pain Behaviors Checklist Total Score; SOPA = Survey of Pain Attitudes
All measures represent univariate measures (across all three time points)
Base Linear Mixed Model with Repeated Measures
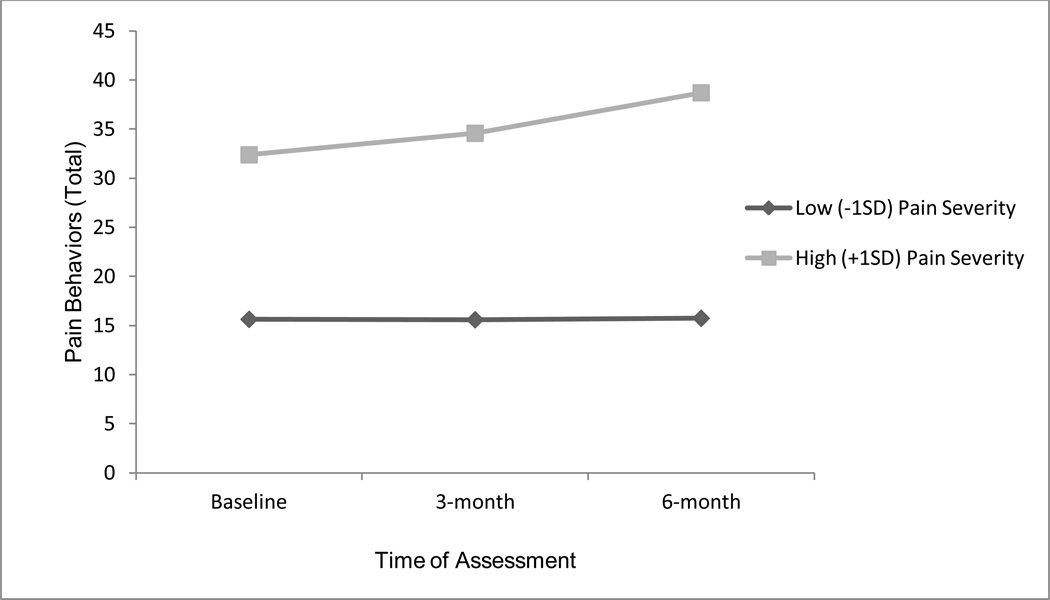
Although time was not a significant predictor of pain behaviors,1 there was a significant time × pain severity interaction (F = 4.00, p = .02). Follow-up tests examining this interaction are reported in Table 3. Namely, individuals with low levels of pain severity (−1SD) did not show a significant change in pain behaviors across time but individuals with high levels of pain severity (+1SD) did show a significant change in pain behaviors across time (see Figure 2). Because there was a significant interaction between time and pain severity, time, pain severity, and the time × pain severity interaction were included in all further analyses.
Table 3.
Differences of least squares means for the significant time × pain severity interaction
| Level of BPI severity |
Time point 1 | Mean† | Time point 2 | Mean† | Mean difference | t value |
|---|---|---|---|---|---|---|
| −1SD | Baseline | 15.62 | 3 month | 15.58 | 0.04 | .03 |
| Baseline | 15.62 | 6 month | 15.73 | −.12 | −.08 | |
| 3 month | 15.58 | 6month | 15.73 | −.16 | −.10 | |
| +1SD | Baseline | 32.38 | 3 month | 34.58 | −2.20 | −1.34 |
| Baseline | 32.38 | 6 month | 38.70 | −6.32 | −3.45** | |
| 3 month | 34.58 | 6month | 38.70 | −4.12 | −2.08* | |
Note:
p < .05;
p < .01;
p < .001;
BPI = brief pain inventory;
Mean of Pain behaviors checklist (PBCL) at a specific level of BPI severity and at a certain time point
Figure 2.
Results of linear mixed model with repeated measures regressing pain behaviors (PBCL) on to time with pain (BPI-severity) as a moderator.
To rule out potential confounding variables, we examined patient demographic (i.e., age, income, education) and medical variables (i.e., time since diagnosis, stage at diagnosis) as predictors of pain behaviors (with time, pain severity, and the time × pain severity interaction included in the model).
Individual Pain Attitude Linear Mixed Models with Repeated Measures
As noted earlier, because none of the demographic or medical variables were significant predictors of pain behaviors, they were dropped from all analyses in order to increase power. As such, only time, pain severity, pain attitudes, the time × pain severity interaction, and the pain severity × pain attitude interaction were included as predictors. We examined all two-way and three-way interactions between time, pain severity, and pain attitudes, but there were no significant two-way interactions between time and pain attitudes or any three-way interactions between time, pain severity, and pain attitudes. As such, these two-way and three-way interactions were not included in the final models. The results of each linear mixed model with repeated measures are discussed below and presented in Table 4.
Table 4.
Linear mixed model with repeated measures analyses predicting pain behaviors from time, pain severity, the time × pain severity interaction, pain attitudes, and the pain severity × pain attitudes interaction
| Variable | F | B | SE | t | Effect size (r) |
|---|---|---|---|---|---|
| Base Model | |||||
| Time | .01 | -- | -- | -- | -- |
| Pain severity | 128.31*** | 5.75 | .56 | 10.29*** | .58 |
| Time × pain severity | 4.00* | -- | -- | -- | -- |
| SOPA-Solicitude | |||||
| Time | .22 | -- | -- | ||
| Pain severity | 28.80*** | 5.31 | .99 | 5.35*** | .38 |
| Time × pain severity | 2.48 | -- | -- | -- | |
| SOPA-solicitude | 7.47** | 2.94 | 1.08 | 2.73** | .21 |
| Pain × SOPA-solicitude | .22 | .19 | .40 | .47 | -- |
| SOPA-Emotions | |||||
| Time | .42 | -- | -- | -- | -- |
| Pain severity | 37.66*** | 4.75 | .90 | 5.28*** | .38 |
| Time × pain severity | .92 | -- | -- | -- | -- |
| SOPA-emotions | 4.00* | 1.74 | .87 | 2.00* | .15 |
| Pain × SOPA-emotions | 2.04 | .42 | .29 | 1.43 | -- |
| SOPA-Cure | |||||
| Time | .17 | -- | -- | -- | -- |
| Pain severity | 47.98*** | 8.20 | 1.05 | 7.82*** | .52 |
| Time × pain severity | 1.36 | -- | -- | -- | -- |
| SOPA-cure | 1.02 | 1.20 | 1.19 | 1.01 | -- |
| Pain × SOPA-cure | 5.88* | −.98 | .41 | −2.43* | .18 |
| SOPA-Control | |||||
| Time | .09 | -- | -- | -- | -- |
| Pain severity | 53.45*** | 6.45 | .83 | 7.80*** | .52 |
| Time × pain severity | 3.56* | -- | -- | -- | -- |
| SOPA-control | .07 | −.24 | .91 | −.27 | -- |
| Pain × SOPA-control | .63 | −.26 | .33 | −.80 | -- |
| SOPA-Harm | |||||
| Time | .14 | -- | -- | -- | -- |
| Pain severity | 27.43*** | 5.44 | 1.08 | 5.02*** | .36 |
| Time × pain severity | 2.27 | -- | -- | -- | -- |
| SOPA-harm | 2.55 | 1.66 | 1.04 | 1.60 | -- |
| Pain × SOPA-harm | .03 | .06 | .34 | .18 | -- |
| SOPA-Disability | |||||
| Time | .12 | -- | -- | -- | -- |
| Pain severity | 27.31*** | 5.55 | .99 | 5.58*** | .40 |
| Time × pain severity | 3.24* | -- | -- | -- | -- |
| SOPA-disability | 29.29*** | 5.05 | .93 | 5.41*** | .39 |
| Pain × SOPA-disability | .03 | −.05 | .31 | −.16 | -- |
| SOPA-Medication | |||||
| Time | .02 | -- | -- | -- | -- |
| Pain severity | 18.06*** | 5.12 | 1.13 | 4.54*** | .33 |
| Time × pain severity | 3.48* | -- | -- | -- | -- |
| SOPA-medication | 5.36* | 2.25 | .97 | 2.31* | .17 |
| Pain × SOPA-medication | .66 | .29 | .36 | .81 | -- |
Note:
p < .05;
p < .01;
p < .001;
B =raw coefficient, effect size
BPI = Brief Pain Inventory Severity subscale; SOPA = Survey of Pain Attitudes;
F values provided for all variables; B estimates and t values provided for all continuous predictor variables
SOPA-solicitude
As Table 4 shows, the interaction between SOPA-solicitude and pain severity was not significant, but there was a significant main effect for SOPA-solicitude (B = 2.94, p < .01). Namely, SOPA-solicitude attitudes were significantly associated with more self-reported pain behaviors.
SOPA-emotions
As Table 4 shows, the interaction between SOPA-emotions and pain severity was not significant, but there was a significant main effect for SOPA-emotions (B = 1.74, p < .05). Namely, SOPA-emotions attitudes were significantly associated with more self-reported pain behaviors.
SOPA-cure
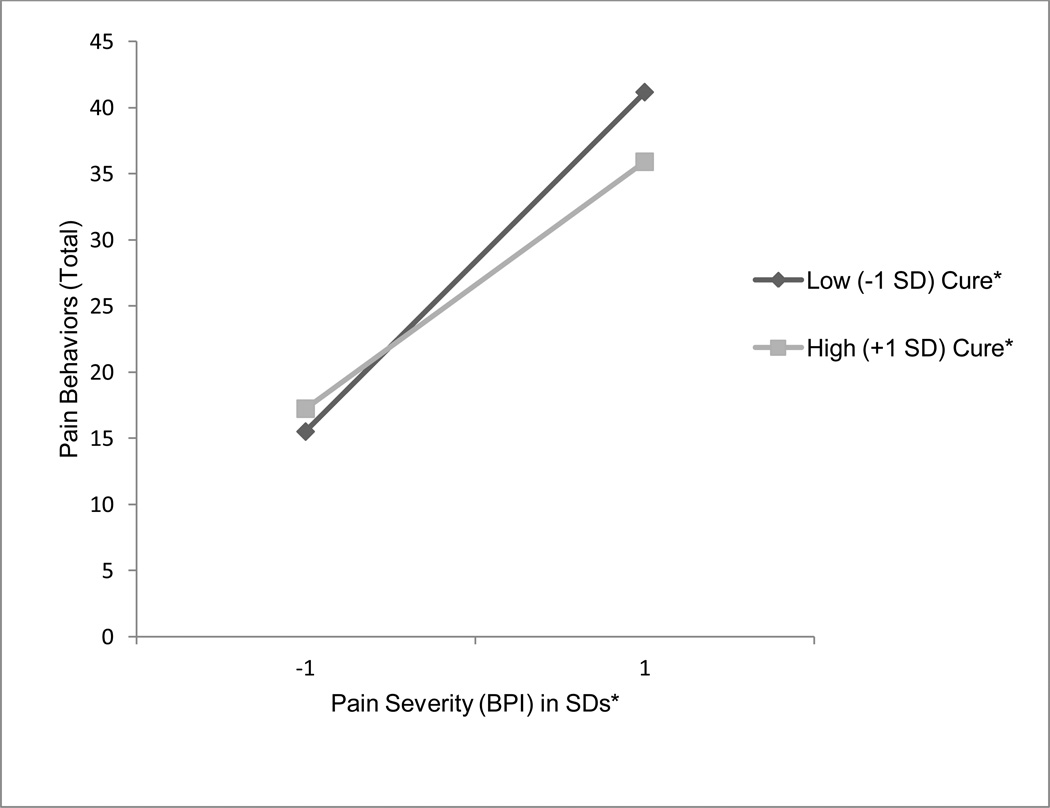
As Table 4 shows, although there was no main effect of SOPA-cure on pain behaviors, the pain × SOPA-cure interaction was significantly associated with pain behaviors (B = −.98, p < .01). Tests of the simple slopes indicated that there was a significant positive association between pain severity and pain behaviors for patients with low levels (−1SD) of SOPA-cure attitudes [t(167)= 3.60, p = .0004]. The relationship between pain severity and pain behaviors was only marginally significant for patients with high levels (+1SD) of SOPA-cure attitudes [t(167)= 1.87, p = .06]. Thus, higher levels of pain severity were associated with higher levels of pain behaviors, but only for those with low levels of SOPA-cure attitudes (see Figure 3).
Figure 3.
Results of linear mixed model with repeated measures regressing pain behaviors (PBCL) on to pain (BPI-severity) with SOPA-cure as a moderator.
SOPA-disability
As Table 4 shows, the interaction between SOPA-disability and pain severity was not significant, but there was a significant main effect for SOPA-disability (B = 5.05, p < .001). Namely, SOPA-disability attitudes were significantly associated with more self-reported pain behaviors.
SOPA-medication
As Table 4 shows, the interaction between SOPA-medication and pain severity was not significant, but there was a significant main effect for SOPA-medication (B = 2.25, p < .05). Namely, SOPA-medication attitudes were significantly associated with more self-reported pain behaviors.
There were no significant main effects or interaction effects for SOPA-control or SOPA-harm. See Table 4 for results of these analyses.
Linear Mixed Model with Repeated Measures for All Pain Attitudes
After examining each pain attitude individually, we entered all the pain attitudes that were significantly associated with pain behaviors into the same linear mixed model with repeated measures (SOPA-solicitude, SOPA-emotions, SOPA-cure, SOPA-disability, and SOPA-medication as a cure for pain). Additionally, we included time, pain severity (BPI), and the time × pain severity interaction described earlier. When attitudes were examined simultaneously, only SOPA-cure (B = 3.30, p < .01) and SOPA-disability (B = 4.80, p < .001) significantly predicted pain behaviors (see Table 5). The main effect of SOPA-cure was qualified, however, by a significant interaction between SOPA-cure and pain severity (B = −1.21, p < .05) as seen earlier in the individual models. Tests of the simple slopes indicated that, similar to earlier analyses, the relationship between pain severity and pain behaviors was significant (and positive) for those with lower SOPA-cure scores (t = 2.35, p = .02) but was not significant for those with higher SOPA-cure scores (t = 1.10, p = .27).
Table 5.
Linear mixed model with repeated measures analysis predicting pain behaviors from time, pain severity, the time × pain severity interaction, pain attitudes, and the pain severity × pain attitudes interactions
| F | B | SE | t | |
|---|---|---|---|---|
| Time | .31 | -- | -- | -- |
| Pain severity (BPI) | 19.99*** | 7.65 | 1.65 | 4.65*** |
| Time × pain severity | .52 | -- | -- | -- |
| SOPA-Solicitude | 2.76 | 1.94 | 1.17 | 1.66 |
| SOPA-Emotions | 1.28 | 1.08 | .96 | 1.13 |
| SOPA-Cure | 7.27** | 3.30 | 1.22 | 2.70** |
| SOPA-Disability | 19.82*** | 4.80 | 1.08 | 4.45*** |
| SOPA-Medication | .87 | .94 | 1.01 | .93 |
| Pain × SOPA-solicitude | 1.30 | −.46 | .41 | −1.14 |
| Pain × SOPA-emotions | 1.71 | .35 | .27 | 1.31 |
| Pain × SOPA-cure | 10.26** | −1.21 | .38 | −3.20* |
| Pain × SOPA-disability | .30 | −.19 | .35 | −.54 |
| Pain × SOPA-medication | .55 | .27 | .36 | .74 |
Note.
B = raw coefficient, SE = standard error;
p < .05;
p < .01
p < .001
BPI = Brief Pain Inventory Severity subscale; SOPA = Survey of Pain Attitudes
F statistics provided for all variables, B estimates and t values provided for continuous variables
Discussion
The study results support our hypothesis that pain attitudes are associated with pain behaviors. Specifically, the following pain attitudes were positively associated with pain behaviors: SOPA-solicitude, SOPA-emotions, SOPA-disability, and SOPA-medication. When examining all pain attitudes simultaneously, only SOPA-cure and SOPA-disability were significantly associated with pain behaviors. The main effect of SOPA-cure, however, was qualified by its significant interaction with pain severity. These results mirror and extend previous findings that have examined the relationship between pain attitudes and pain behaviors (Jensen et al., 1999; Tait & Chibnall, 1997). Similar to the results of past research, the attitudes that patients are disabled because of pain (SOPA-disability) and that medication is the best treatment for pain (SOPA-medication) had the strongest relationships with pain behaviors (Tait & Chibnall, 1997) when examined individually. SOPA-disability, however, showed a much stronger association with pain behaviors among MBC patients (r = .57) than among chronic pain patients (r = .27) (Jensen et al., 1999). These two correlations are significantly different (z = 3.19, p < .01), and this difference may be attributable to the differing role of behavioral responses to pain among MBC patients. The association between disability and pain behaviors may be stronger among cancer patients than among chronic pain patients because cancer patients are more concerned about the damage or disease progression that pain may indicate (Raphael et al., 2010).
In addition to examining the relationship between pain attitudes and pain behaviors, we also examined whether pain attitudes would moderate the positive association between pain severity and pain behaviors. Results partially support our hypotheses that negative pain attitudes exacerbate the association between pain severity and pain behaviors and that positive pain attitudes buffer the effects of pain severity on pain behaviors. Specifically, we found that a positive pain attitude -- belief in a medical cure for pain -- buffered the association between pain severity and pain behaviors among patients. Namely, the positive relationship between pain severity and pain behaviors only existed among individuals who were low in SOPA-cure. For those high in SOPA-cure, the relationship between pain severity and pain behaviors was not significant. This seemingly indicates that increasing individuals’ beliefs in a medical cure for pain could help to buffer against the negative effects of pain severity on pain behaviors. As such, SOPA-cure could be targeted as a form of pain management for patients whose disease status indicates that cure of pain is a possible outcome.
When examining all pain attitudes simultaneously, only SOPA-disability and SOPA-cure were significantly associated with pain behaviors. This main effect of SOPA-cure, however, was qualified by a significant interaction between pain severity and SOPA-cure. Namely, SOPA-cure served as a buffer against the negative effects of pain severity on pain behaviors. These findings indicate that both negative (i.e., SOPA-disability) and positive (i.e., SOPA-cure) pain attitudes could have the ability to shape the number of pain behaviors in which individuals engage. This extends previous research, which has focused primarily on the association of negative pain attitudes such as SOPA-disability, SOPA-harm, and SOPA-solicitude with pain behaviors (Jensen et al., 1999), by expanding to the effects of positive pain attitudes on pain behaviors. Whereas previous research has focused on targeting negative cognitions about one’s pain (i.e., SOPA-disability) to understand patients adjustment to pain (Jensen et al., 1999), the present study presents evidence that it is also important to target positive cognitions surrounding one’s pain (i.e., SOPA-cure) in pain management. Focusing on reducing negative cognitions as well as increasing positive cognitions, such as SOPA-cure for one’s pain, may lead to the greatest benefit and reduction of pain behaviors among MBC patients.
Finally, it should be noted that, in the present study, pain severity moderated the effects of time on pain behaviors. Namely, pain behaviors did not change significantly over time for individuals with low levels of pain (−1SD) but did change significantly for individuals with high levels of pain (+1SD). This, coupled with the significant interaction between pain severity and SOPA-cure indicate that there may be a stronger need for intervention among patients with high levels of pain severity than for those with low levels of pain severity. Whereas pain behaviors remain relatively stable (and low) over time for individuals with low levels of pain severity, pain behaviors show a steady increase over time for those with high levels of pain severity. As such, patients with high levels of pain severity may benefit most from interventions targeting pain attitudes as a mode to improving pain management.
Together, our findings support the cognitive-behavioral model of pain (Turk et al., 1987), which posits that pain attitudes predict pain behaviors. Namely, pain attitudes consistently predicted pain behaviors across time. The findings from this study suggest that pain attitudes or cognitions related to pain should be targeted in future pain management interventions for women with MBC. Targeting cognitions about pain, specifically pain attitudes, is in line with research demonstrating that cognitive-behavioral therapy is one of the most successful forms of therapy for treating pain. A meta-analysis of 33 papers across 25 trials found that cognitive-behavioral therapy has significant effect sizes on all domains of pain (median across domains =.50; Morley et al., 1999). When cognitive-behavioral therapy was compared to alternative active treatments, cognitive-behavioral treatments produced significantly greater changes in the domains of pain experience and reduced behavioral expressions of pain. Cognitive-behavioral therapy has also been shown to reduce reported pain in breast cancer patients specifically (Tatrow & Montgomery, 2006). Our findings demonstrate that cognitions associated with pain attitudes, as well as psychological issues related to one’s pain (SOPA-emotions), could be targeted as a mode to reduce behavioral expressions of pain and possibly the experience of pain among patients with MBC.
Interventions designed to decrease MBC patients’ pain behaviors could employ a two-pronged approach in which interventions focus on decreasing negative attitudes and on increasing positive attitudes (i.e., SOPA-cure). Thus, the practical application of these findings in the design of clinical interventions for pain management would likely be to target multiple pain attitudes at the same time. For example, our findings suggest that psycho-educational interventions that seek to correct patient’s negative or misguided beliefs about pain while at the same time reinforcing their positive beliefs about pain management may be effective in reducing pain behaviors. Simultaneously targeting positive and negative pain attitudes in this way, as opposed to focusing on a specific pain attitude, may also bolster the overall effectiveness of the intervention. It is important to note, however, that one ought to consider a patient’s disease status before recommending endorsement of SOPA-cure. For instance, if curing a patient’s pain is not an achievable goal, it might best to encourage patients to focus on palliating their pain and achieving reasonable management of pain rather than focusing on absolute cure of the pain.
Other interventions could be used to teach cancer patients how to solicit support more effectively and how to develop healthy attitudes about pain. For instance, targeting the erroneous belief that movement and exercise are harmful to individuals who have cancer could lead to a decrease in pain behaviors and an increase in exercise. Some exercises, such as yoga, have been shown to reduce both pain and fatigue levels among MBC patients (Carson et al., 2007). As a result, a pain management program designed to change the maladaptive beliefs about pain that lead cancer patients to avoid exercise could benefit these individuals by increasing their activity levels and ultimately decreasing their pain. Given the strong association between SOPA-disability and pain behaviors, this pain attitude ought to be specifically targeted as perhaps the most effective pain attitude with which to intervene.
Despite its strengths, this study had some limitations. Although one of the strengths of this study is its longitudinal design, the results are still correlational and thus preclude any conclusions regarding the direction of causality. It may be that the amount of pain experienced influences pain attitudes or that the pain behaviors themselves shape pain attitudes. Additionally, pain behaviors did change over time, but this was dependent on the level of pain severity. As such, pain behaviors did not uniformly change across time. One explanation for this lack of uniform change across the sample is that the average level of reported pain in our sample was lower than the pain levels reported in other published studies (Turk et al., 1998). Data on use of pain medication were not collected, but it is likely that these patients’ pain was well-managed because they were being treated at a major cancer center with a well-integrated palliative care program. Despite reporting lower levels of pain, however, the patients in our study reported a higher number of pain behaviors than did patients in other chronic pain samples (Romano et al., 2000). Further investigation is needed to determine why pain behaviors consistently rose over time for individuals with high levels of pain severity but not for those with low levels of pain severity. Possible explanations could be the amount of distress individuals feel or the length of time they have been suffering from metastatic disease. By examining how long it has been since diagnosis of metastatic disease (as opposed to just breast cancer), we may be better able to assess why individuals with higher levels of pain show significant increases in pain behaviors over time.
While promising, the relationships explored in this study need to be examined in a broader cultural context. Because this study was fairly homogenous with regard to race and ethnicity, it had insufficient power to examine cultural differences. Additionally, because all patients were women, we could not examine gender differences. Moreover, patients in the present study were all partnered women which may be a uniquely different population than non-partnered women. Although there is no published data to suggest that pain attitudes are differentially associated with pain behaviors among men and non-partnered women, this is a possibility and should be investigated in future studies. Additionally, our study may have been underpowered to examine all significant interactions between pain attitudes and pain severity. However, with the exception of the interaction between SOPA-emotions and pain severity, most interactions between pain severity and pain attitudes had very small effect sizes, indicating this was likely not the case.
Our study had a considerable number of passive refusals. A possible reason for this may be that patients were just beginning treatment for MBC, and filling out a lengthy survey may have been viewed as too taxing or not a priority. In addition, these individuals may have been experiencing psychological distress, making them unable or unwilling to fill out the survey. Because these data were not collected at recruitment, we cannot determine whether passive refusers were more or less distressed than were study participants or whether they felt overwhelmed by filling out a survey at the start of treatment.
Finally, although we refer to positive and negative pain attitudes in the present study, more research is needed to indicate whether these particular attitudes represent adaptive and maladaptive coping with pain. However, previous studies as well as the present study seemingly indicate they do. For instance, past research shows positive attitudes (i.e., SOPA-cure) have been positively associated with utilizing professional medical services to cope with pain (Jensen & Karoly, 1992) and psychological functioning (i.e., SOPA-control; Jensen & Karoly, 1991) whereas negative attitudes (i.e., SOPA-disability) have been associated with poorer physical and psychological functioning (Jensen & Karoly, 1991). Confirming and extending past research, the present study demonstrates that the “negative” pain attitudes were associated with increases in pain behaviors and “positive” pain attitudes buffered the relationship between pain severity and pain behaviors.
In conclusion, our study findings demonstrate that pain attitudes play an important role in patient pain behaviors in the context of cancer. More specifically, we found that: (1) pain attitudes are significantly associated with pain behaviors and (2) attitudes about a medical cure for pain moderate the relationship between pain severity and pain behaviors. More research is needed to investigate how cognitive-behavioral interventions designed at targeting pain attitudes can be integrated into conventional pharmacological and medical pain interventions in the context of MBC. Such integrated treatment approaches are likely to improve pain management and may even reduce the negative moods that are often associated with negative pain attitudes.
Acknowledgments
Dr. Shen’s work on this project was supported by a cancer prevention fellowship from the National Cancer Institute (5R25CA081137). Dr. Redd’s work was supported through a Research and Mentoring in Psychosocial Oncology award from the National Cancer Institute (5K05CA108955). Dr. Badr’s work was supported by a multi-disciplinary award from the U. S. Army Medical Research and Materiel Command W81XWH-0401-0425 and K07CA124668
Footnotes
In a base repeated measures mixed model, in which time was included as the sole predictor of PBCL, time was not a significant predictor of PBCL (F = .86, p = .43).
References
- Ahles TA, Blanchard EB, Ruckdeschel JC. The multidimensional nature of cancer-related pain. Pain. 1983;17(3):277–288. doi: 10.1016/0304-3959(83)90100-8. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Coombs DW, Jensen L, Stukel T, Maurer LH, Keefe FJ. Development of a behavioral observation technique for the assessment of pain behaviors in cancer patients. Behavior Therapy. 1990;21(4):449–460. [Google Scholar]
- Badr H, Carmack CL, Kashy DA, Cristofanilli M, Revenson TA. Dyadic coping in metastatic breast cancer. Health Psychology. 2010;29(2):169–180. doi: 10.1037/a0018165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr H, Milbury K. Associations between depression, pain behaviors, and partner responses to pain in metastatic breast cancer. PAIN. 2011;152(11):2596–2604. doi: 10.1016/j.pain.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Butler LD, Koopman C, Cordova M, Garlan R, DiMiceli S, Spiegel D. Psychological distress and pain significantly increase before death in metastatic breast cancer patients. Psychosomatic Medicine. 2003;65:416–426. doi: 10.1097/01.psy.0000041472.77692.c6. [DOI] [PubMed] [Google Scholar]
- Carson JW, Carson KM, Porter LS, Keefe FJ, Shaw H, Miller JM. Yoga for women with metastatic breast cancer: Results from a pilot study. Journal of Pain and Symptom Management. 2007;33(3):331–341. doi: 10.1016/j.jpainsymman.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Cleeland CS. Pain assessment in cancer. In: Osoba D, editor. Effect of cancer on quality of life. Boca Raton, FL: CRC Press, Inc.; 1991. pp. 293–305. Retrieved from http://scholar.google.com/scholar?q=cleeland+pain+assessment+in+cancer+1991&btnG=&hl=en&as_sdt=0%2C33. [Google Scholar]
- Cleeland Charles S, Syrjala K. How to assess cancer pain. In: Turk D, Melzack R, editors. Handbook of Pain Assessment. New York: Guilford Press; 1992. pp. 362–387. [Google Scholar]
- Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. British Journal of Cancer. 1987;55(1):61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild A. Under-treatment of cancer pain. Current Opinion in Supportive and Palliative Care. 2010;4(1):11–15. doi: 10.1097/SPC.0b013e328336289c. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Karoly P. Control beliefs, coping efforts, and adjustment to chronic pain. Journal of Consulting and Clinical Psychology. 1991;59(3):431–438. doi: 10.1037//0022-006x.59.3.431. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Karoly P. Pain-specific beliefs, perceived symptom severity, and adjustment to chronic pain. The Clinical Journal of Pain. 1992;8(2):123–130. doi: 10.1097/00002508-199206000-00010. [DOI] [PubMed] [Google Scholar]
- Jensen Mark P, Romano JM, Turner JA, Good AB, Wald LH. Patient beliefs predict patient functioning: further support for a cognitive-behavioural model of chronic pain. Pain. 1999;81(1–2):95–104. doi: 10.1016/s0304-3959(99)00005-6. [DOI] [PubMed] [Google Scholar]
- Jensen Mark P, Karoly P, Huger R. The development and preliminary validation of an instrument to assess patients’ attitudes toward pain. Journal of Psychosomatic Research. 1987;31(3):393–400. doi: 10.1016/0022-3999(87)90060-2. [DOI] [PubMed] [Google Scholar]
- Jensen Mark P, Turner JA, Romano JM, Lawler BK. Relationship of pain-specific beliefs to chronic pain adjustment. Pain. 1994;57(3):301–309. doi: 10.1016/0304-3959(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Kerns RD, Haythornthwaite J, Rosenberg R, Southwick S, Giller EL, Jacob MC. The pain behavior check list (PBCL): Factor structure and psychometric properties. Journal of Behavioral Medicine. 1991;14(2):155–167. doi: 10.1007/BF00846177. [DOI] [PubMed] [Google Scholar]
- Krueger C, Tian L. A comparison of the general linear mixed model and repeated measures ANOVA using a dataset with multiple missing data points. Biological Research For Nursing. 2004;6(2):151–157. doi: 10.1177/1099800404267682. [DOI] [PubMed] [Google Scholar]
- McGuire DB, Sheidler VR. Pain. In: Groenwald SL, Frogge MH, Goodman M, Yarbro CH, editors. Manifestations of cancer and cancer treatment. Boston: Jones and Bartlett Publishers; 1992. pp. 985–1041. [Google Scholar]
- Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80(1–2):1–13. doi: 10.1016/s0304-3959(98)00255-3. [DOI] [PubMed] [Google Scholar]
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American journal of clinical oncology. 1982;5(6):649–655. [PubMed] [Google Scholar]
- Portenoy RK, Lesage P. Management of cancer pain. The Lancet. 1999;353(9165):1695–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- Raphael J, Ahmedzai S, Hester J, Urch C, Barrie J, Williams J, Farquhar-Smith P, et al. Cancer Pain: Part 1: Pathophysiology; Oncological, Pharmacological, and Psychological Treatments: A Perspective from the British Pain Society Endorsed by the UK Association of Palliative Medicine and the Royal College of General Practitioners. Pain Medicine. 2010;11(5):742–764. doi: 10.1111/j.1526-4637.2010.00840.x. [DOI] [PubMed] [Google Scholar]
- Romano JM, Jensen MP, Turner JA, Good AB, Hops H. Chronic pain patient-partner interactions: Further support for a behavioral model of chronic pain. Behavior Therapy. 2000;31(3):415–440. [Google Scholar]
- Sharp TJ. Chronic pain: a reformulation of the cognitive-behavioural model. Behaviour Research and Therapy. 2001;39(7):787–800. doi: 10.1016/s0005-7967(00)00061-9. [DOI] [PubMed] [Google Scholar]
- Simone CB, Vapiwala N, Hampshire MK, Metz JM. Cancer patient attitudes toward analgesic usage and pain intervention. The Clinical Journal of Pain. 2012;28(2):157–162. doi: 10.1097/AJP.0b013e318223be30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong J, Ashton R, Chant D. The measurement of attitudes towards and beliefs about pain. Pain. 1992;48(2):227–236. doi: 10.1016/0304-3959(92)90062-G. [DOI] [PubMed] [Google Scholar]
- Tait CR, Chibnall JT. Development of a brief version of the Survey of Pain Attitudes. Pain. 1997;70(2–3):229–235. doi: 10.1016/s0304-3959(97)03330-7. [DOI] [PubMed] [Google Scholar]
- Tatrow K, Montgomery G. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: A meta-analysis. Journal of Behavioral Medicine. 2006;29(1):17–27. doi: 10.1007/s10865-005-9036-1. [DOI] [PubMed] [Google Scholar]
- Turk DC, Sist TC, Okifuji A, Miner MF, Florio G, Harrison P, Massey J, et al. Adaptation to metastatic cancer pain, regional/local cancer pain and non-cancer pain: role of psychological and behavioral factors. Pain. 1998;74(2–3):247–256. doi: 10.1016/s0304-3959(97)00187-5. [DOI] [PubMed] [Google Scholar]
- Turk Dennis C, Meichenbaum D, Genest M. Pain and behavioral medicine: A cognitive-behavioral perspective. Guilford Press; 1987. [Google Scholar]