Abstract
Isolation rearing is a neurodevelopmental manipulation that produces neurochemical, structural, and behavioral alterations in rodents that have consistencies with schizophrenia. Symptoms induced by isolation rearing that mirror clinically relevant aspects of schizophrenia, such as cognitive deficits, open up the possibility of testing putative therapeutics in isolation-reared animals prior to clinical development. We investigated what effect isolation rearing would have on cognitive flexibility, a cognitive function characteristically disrupted in schizophrenia. For this purpose, we assessed cognitive flexibility using between- and within-session probabilistic reversal-learning tasks based on clinical tests. Isolation-reared rats required more sessions, though not more task trials, to acquire criterion performance in the reversal phase of the task and were slower to adjust their task strategy after reward contingencies were switched. Isolation-reared rats also completed fewer trials and exhibited lower levels of overall activity in the probabilistic reversal-learning task compared to socially reared rats. This finding contrasted with the elevated levels of unconditioned investigatory activity and reduced levels of locomotor habituation that isolation-reared rats displayed in the behavioral pattern monitor. Finally, isolation-reared rats also exhibited sensorimotor gating deficits, reflected by decreased prepulse inhibition of the startle response, consistent with previous studies. We conclude that isolation rearing constitutes a valuable, noninvasive manipulation for modeling schizophrenia-like cognitive deficits and assessing putative therapeutics.
Keywords: isolation rearing, probabilistic learning, reversal learning, prepulse inhibition, behavioral pattern monitor, schizophrenia, habituation, investigatory behavior, startle response, rats
Introduction
Isolation rearing is a neurodevelopmental manipulation developed to mimic psychosocial deprivation. Social isolation of rodents after weaning produces permanent neurochemical, structural, and behavioral alterations (Bianchi et al., 2006; Fone & Porkess, 2008; C. A. Jones, Brown, Auer, & Fone, 2011; King, Seeman, Marsden, & Fone, 2009; Powell, 2010; Schubert, Porkess, Dashdorj, Fone, & Auer, 2009). At least some of these effects, such as disruptions in sensorimotor gating, are developmentally specific, in that they result only when animals are isolated as juveniles; social isolation of rodents after they have reached adulthood does not produce these alterations (Cilia, Reavill, Hagan, & Jones, 2001; Wilkinson et al., 1994).
Early life stressors have been associated with neuropsychiatric vulnerability and an increased risk of several mental illnesses, including schizophrenia (Agid et al., 1999; Lim, Chong, & Keefe, 2009). Moreover, social isolation and impaired social functioning are nonspecific symptoms of schizophrenia that have been observed to predate the onset of psychotic symptoms in both retrospective and prospective studies (Addington, Penn, Woods, Addington, & Perkins, 2008; Hafner, Loffler, Maurer, Hambrecht, & an der Heiden, 1999; Moller & Husby, 2000) and that can predict conversion to psychosis in high-risk patients (Cannon et al., 2008). Notably, several of the abnormalities produced by isolation rearing strongly resemble findings in human schizophrenia (Fone & Porkess, 2008; Geyer, Wilkinson, Humby, & Robbins, 1993; Powell, 2010). For example, prepulse inhibition (PPI) of the startle response, an operational measure of sensorimotor gating, is robustly decreased in patients with schizophrenia (D. Braff et al., 1978; 2001; 1992), possibly reflecting a pervasive information processing deficit (D. L. Braff & Geyer, 1990). Isolation rearing reliably decreases PPI in rats (Cilia, Hatcher, Reavill, & Jones, 2005, 2001; Geyer et al., 1993; Varty & Geyer, 1998; Varty & Higgins, 1995; Wilkinson et al., 1994). Strikingly, these PPI deficits are absent in juvenile isolation-reared rats but emerge after puberty (Bakshi & Geyer, 1999), resembling the time course of schizophrenia (Weinberger, 1987). Impairments in cognitive performance have also been observed in isolation-reared animals (Bianchi et al., 2006; Gresack et al., 2010; Hellemans, Benge, & Olmstead, 2004; C. A. Jones et al., 2011; Valzelli, 1973), matching the cognitive deficits seen in schizophrenia (Elvevåg & Goldberg, 2000; Mortimer, 1997; Nelson et al., 1990; Nuechterlein et al., 2004; Sharma & Antonova, 2003; Tyson, Laws, Flowers, Tyson, & Mortimer, 2006). Several of the behavioral alterations induced by isolation rearing have been found to be reversible with antipsychotic treatment (Bakshi, Swerdlow, Braff, & Geyer, 1998; Cilia et al., 2001; Geyer et al., 1993; Li, Wu, & Li, 2007; Varty & Higgins, 1995; Wilkinson et al., 1994), further supporting the utility of this manipulation as an inducing condition for models of schizophrenia symptoms.
Schizophrenia patients frequently exhibit cognitive inflexibility (i.e., the inability to alter behavior in reaction to changing situational demands) (Goldberg, Weinberger, Berman, Pliskin, & Podd, 1987; Leeson et al., 2009; Morice, 1990; Murray et al., 2008), a characteristic deficit of executive functioning that contributes to the difficulties with problem solving encountered by many schizophrenia sufferers (Hatashita-Wong, Smith, Silverstein, Hull, & Willson, 2002; Nuechterlein et al., 2004). A distinguishing symptom of this deficit is perseveration in outdated behavioral strategies that are no longer rewarded. Cognitive flexibility can be assayed in humans and experimental animals using reversal-learning tasks (Boulougouris, Dalley, & Robbins, 2007; Fellows & Farah, 2003). These tasks require the subject to first learn a reward contingency, and then to detect that the reward contingency has been switched to its exact opposite, i.e., that the response previously associated with reward is now associated with non-reward and vice versa. Several studies have reported impairments in reversal-learning tasks in isolation-reared animals (G. H. Jones, Marsden, & Robbins, 1991; Krech, Rosenzweig, & Bennett, 1962; Li et al., 2007; Schrijver, Pallier, Brown, & Wurbel, 2004); but see (Wongwitdecha & Marsden, 1996).
Animal behavioral tasks frequently use reward contingencies that predict reward or non-reward with 100% certainty. Such all-or-nothing contingencies may not suitably reflect real-life problem-solving situations, which usually involve maximizing the probability of reward under circumstances where a given action does not guarantee a given outcome 100% of the time. Probabilistic learning tasks, in contrast, require the subject to learn to choose the response that has the highest probability of resulting in a reward based on probabilistic contingencies which predict a certain outcome in some, but not all, cases. The subject’s performance is therefore guided by punishing and rewarding feedback that is “degraded” (i.e., misleading on a subset of trials) and may more accurately represent real-life problem solving situations. Schizophrenia patients exhibit significant impairments in a probabilistic reversal-learning task (Waltz & Gold, 2007), suggesting that deficits in feedback processing and reinforcement learning may contribute to the cognitive inflexibility observed in this population. The probabilistic reversal-learning paradigm was selected by Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) – a translational research initiative sponsored by the National Institute of Mental Health – to assess reinforcement learning in schizophrenia (Ragland et al., 2009). Measuring such learning across species was supported by the development of a rat probabilistic reversal-learning task sensitive to serotonergic manipulation (Bari et al., 2010).
In this study, we examined the performance of isolation-reared rats in two versions of a probabilistic reversal-learning task based on that described by Bari et al. (2010). Rats were trained to respond preferentially in a location that offered a high probability of a food reward. In a minority of trials, however, responding in this “target” location resulted in a punitive timeout and no reward. Likewise, in a certain percentage of trials, responding in the “non-target” location resulted in reward delivery, even though responding in this location resulted in a timeout and no reward during the majority of trials. These target/non-target contingencies were then switched upon attainment of a set criterion; thus, probabilistic learning and reversal learning were measured in the same task.
In order to confirm the effectiveness of our isolation rearing protocol, we also tested these rats in an acoustic startle/PPI paradigm. Additionally, rats were tested in the behavioral pattern monitor (BPM), a test used in rats (Geyer, Russo, & Masten, 1986), mice (Risbrough et al., 2006), and humans (Perry et al., 2009; Young, Minassian, Paulus, Geyer, & Perry, 2007) to profile the amount and pattern of unconditioned exploratory activity. Isolation rearing has been reported to decrease locomotor habituation and increase rearing and exploratory holepoking in the BPM or similar locomotor activity tests (Lapiz, Mateo, Parker, & Marsden, 2000; Paulus, Bakshi, & Geyer, 1998; Powell, Swerdlow, Pitcher, & Geyer, 2002; Sahakian, Robbins, Morgan, & Iversen, 1975; Varty, Paulus, Braff, & Geyer, 2000). Such reduced habituation is consistent with findings in patients with schizophrenia (Perry et al, 2009). A generalized increase in locomotion was also found by some (Hall, Huang, Fong, Pert, & Linnoila, 1998; G. H. Jones, Robbins, & Marsden, 1989; Lapiz et al., 2000; Powell et al., 2002; Sahakian et al., 1975), but not all studies (Paulus et al., 1998; Varty et al., 2000) and may be strain-dependent. Increases in unconditioned locomotor activity have been widely used as a model to develop antipsychotic treatments for schizophrenia (van den Buuse, 2010). We hypothesized that isolation-reared rats would exhibit: 1) impaired reversal learning in the probabilistic task; 2) impaired PPI; and 3) reduced locomotor habituation in a novel environment, thus mimicking the cognitive and behavioral changes observed in patients with schizophrenia.
Materials and Methods
Animals
Twelve timed-pregnant Long Evans dams were shipped to our facility at gestation day 18. On postnatal day 3, litters were culled to 10 with an equal number of males and females kept in each litter for a total of 118 rats (one litter had 8 pups). Rats were weaned at 24 days postnatal and a total of 56 male Long-Evans rats (Charles River Laboratories, Wilmington, MA) were used in this study. 29 of the pups were single-housed (isolation-reared rats or isolates), while the 27 remaining pups were housed in groups of three (socially reared rats or socials). One of the socials died during the experiment, and its cagemates remained housed as a pair; as a result 26 socials were tested in the study. Behavioral testing commenced at 8 weeks post-weaning. Rats were allowed to reach a body weight of at least 300 g before initiation of food restriction, which was calibrated to keep rats at 90% of their free-feeding weight. Water was available ad libitum at all times except during testing. Rats were housed on a 12 h:12 h reversed light-dark cycle (lights off at 7:00 am); all behavioral testing was conducted during the animals’ dark cycle. Animals were treated in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council’s Guide for Care and Use of Laboratory Animals. All experiments were approved by the Animal Care and Use Committee of the University of California San Diego.
Study Design
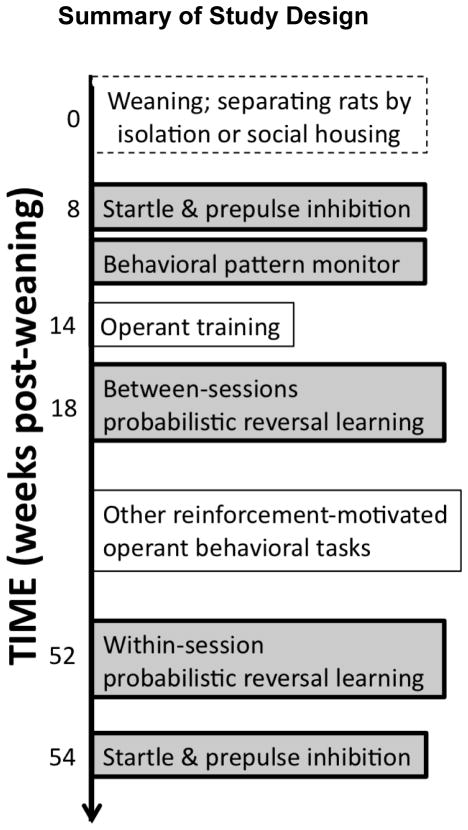
After 8 weeks of isolation or social rearing, rats were tested in an acoustic startle/PPI paradigm, followed by testing in the BPM. Food restriction was then initiated. After rats’ weight had stabilized at ~90% of free-feeding weight, they were trained to respond for a food reward in the operant testing chamber. Rats were first tested in a between-sessions probabilistic learning task. To further explore whether the performance differences observed in isolates in this task reflected a true learning deficit, we then tested the rats in a within-session version of the probabilistic learning task. To minimize overtraining in the probabilistic learning task and ensure a significant learning component for the within-session probabilistic learning task, rats were also trained and tested in several other reinforcement-motivated operant behavioral tasks for several months between the between-sessions probabilistic learning task and the within-session probabilistic learning task. After completion of all other behavioral testing, 54 weeks post-weaning, rats were re-tested in the acoustic startle/PPI paradigm. See Figure 1 for an overview of the study design.
Figure 1. Summary of Study Design.
Rats were initially weaned and either isolated or grouped into triads for the rest of the study. Tasks presented in this article are highlighted in grey.
Startle Apparatus
Startle and PPI were assessed in startle chambers (SR-LAB system, San Diego Instruments, San Diego, CA). Each chamber contained a Plexiglas cylinder (9 cm in diameter) into which the rat was placed. The jump or flinch responses of the rat were detected by a piezoelectric accelerometer mounted at the bottom of the cylinder. A loudspeaker (Radio Shack Supertweeter) located 24 cm above the cylinder provided the broadband background noise and acoustic stimuli. Each apparatus was housed within a ventilated, sound-attenuating startle chamber (39 × 38 × 58 cm). Presentations of the acoustic stimuli were controlled by the SR-LAB software and interface system, which also rectified, digitized using a 12-bit (0–4095) digitizer, and recorded responses from the accelerometer. Calibrations were performed monthly to maintain accurate acoustic stimuli and mechanical vibration measures.
Startle/PPI Procedures
Rats were allowed to acclimate to the testing room for 60 min before being placed in the startle chambers. The experimental session consisted of a 5-min acclimatization period to a 65-dB background noise (continuous throughout the session), followed by a 15-min PPI test session. Five trial types were presented: a 40-ms, 120-dB startle pulse (P120); three 20-ms prepulse + P120 pulse combinations with different prepulse intensities (3 dB, 6 dB, or 12 dB above background); and no-stimulus trials, which consisted of accelerometer recordings obtained in the absence of any stimulus and served to assess baseline motor activity. The interstimulus interval between prepulse and pulse was 100 ms. The first four trial types were presented in a pseudorandom order (12 presentations of the P120 trial, 12 presentations of each prepulse + pulse trial). The intertrial interval varied between 7 and 23 s, with an average duration of 15 s. No-stimulus trials were interspersed between each of these trials. In addition, six P120 trials were presented at both the beginning (Block 1: to assess startle reactivity before appreciable habituation) and the end of the acoustic test session (Block 2: to assess habituation across the session by comparison with Block 1). Mean startle magnitude for each trial type presentation was determined by averaging 100 one-millisecond readings taken from the onset of the P120 stimulus.
The following measures were calculated for the startle/PPI test:
%PPI: the percent decrease in startle magnitude in the prepulse + pulse trials compared to the P120 trials, calculated using the formula ([1 − (startle magnitude on prepulse + pulse trials/startle magnitude on P120 trials)] × 100), such that a 0% value indicated no difference between the responses to prepulse + pulse trials and P120 trials (i.e., no PPI)
Startle reactivity: mean startle response to the P120 trials during the PPI test session
Startle habituation: the percent decrease in startle response to the P120 trials in Block 2 compared to Block 1, calculated using the formula ([1 − (mean startle for Block 2/mean startle for Block 1)] × 100)
%PPI was calculated separately for each prepulse intensity along with mean %PPI across all prepulse intensities.
Behavioral Pattern Monitor (BPM) Apparatus
Unconditioned exploratory locomotor activity was assessed in behavioral pattern monitors (BPM), 30.5×61.0-cm black Plexiglas chambers each enclosed within a ventilated sound-attenuated cabinet. Each chamber was outfitted with a 4×8 array of photobeams 2 cm above the floor used to detect the rat’s position in an x–y coordinate system with a resolution of 3.8 cm. Ten holes (2.5 cm in diameter; three in each long wall, one in the rear short wall and three along the center of the floor) containing photobeams were used to detect investigatory holepokes. Metal touchplates located along the walls 15.2 cm above the floor of the chamber were used to detect rearing. The status of all photobeams was monitored and recorded with 55-ms temporal resolution by an IBM PC-compatible computer. BPM chambers were lit by 7.5-W red lights.
BPM Procedure
Rats were allowed to acclimate to the testing room for 60 min. They were then gently placed into the BPM chambers and allowed to explore the chambers for 60 min.
The following measures were calculated for the BPM test:
Crossings: number of transitions among eight equal 15.25 × 15.25 cm sectors, a measure of horizontal locomotion in the BPM
Holepokes: number of nosepokes into the holes in the BPM wall and floor
Rears: number of times the rat reared up against the wall of the BPM
Crossings were assessed separately for the first and second 30 min period of the session. In addition, locomotor habituation was assessed by calculating the percent decrease in locomotion in the second 30 min period compared to the first 30 min period, using the formula ([1 − (crossings for second period/crossings for first period)] × 100).
Probabilistic Learning Apparatus
Training and testing were conducted in 9-hole operant testing chambers enclosed in ventilated sound-attenuating cabinets (Med Associates Inc., St. Albans, VT and Lafayette Instrument Company, Lafayette, IN). Each testing chamber contained a curved rear wall with nine contiguous apertures. Metal inserts could be used to cover the apertures in the testing chamber. An infrared beam located at the entrance of each aperture detected nosepoke responses, and an LED stimulus light was located at the rear of each aperture. Liquid reinforcement in the form of strawberry milkshake (Nesquik® plus non-fat milk, 40 μl) could be delivered into a magazine located in the opposite wall via peristaltic pump; an infrared beam detected head entries into the magazine. A house light was located in the middle of the chamber ceiling. The control of stimuli and recording of responses were managed by a SmartCtrl Package 8-In/16-Out with additional interfacing by MED-PC for Windows (Med Associates Inc., St. Albans, VT) using custom programming (R.F. Sharp & J.W. Young).
Operant Response Training
Rats were trained to retrieve the liquid reward from the magazine for two consecutive days in 10 min sessions, during which a 40 μl increment of strawberry milkshake was delivered noncontingently into the magazine every 15 s (stage 1). Delivery of the reward was accompanied by illumination of the magazine light. Head entries into the magazine to consume the reward led to extinction of the magazine light until the delivery of the next reward.
Consequently, the rats were trained to nosepoke for the reward using a fixed ratio 1 schedule of reinforcement (stage 2). Rats were trained in daily 30-min sessions. Metal inserts covered eight of the nine apertures in the testing chamber, leaving open only the central aperture (aperture 5). All trials were initiated by a head entry into the food magazine. An initial noncontingent liquid reward was delivered into the magazine at the start of each session to facilitate initiation of the first trial. Upon removal of the head from the magazine, a 4 s intertrial interval (ITI) began, after which the central aperture was illuminated. A nosepoke response into the aperture resulted in extinction of the aperture light and delivery of a reward into the food magazine.
Between-Sessions Probabilistic Learning Task
During the initial learning phase, rats were trained on the initial reward contingency using daily 30-minute sessions. Metal inserts covered seven of the nine apertures in the testing chamber, leaving open only apertures 3 and 7. Trials were initiated by a head entry into the food magazine. Upon removal of the head from the magazine, a 2 s ITI began, after which both of the uncovered apertures were illuminated. A nosepoke in the target aperture resulted in “reward” (i.e., extinction of the aperture light and delivery of a reward into the food magazine) 80% of the time and in “punishment” (i.e., extinction of the aperture light, no reward delivery, and illumination of the house light for a 4 s timeout) 20% of the time, following a randomized schedule. Nosepokes into the non-target aperture resulted in reward 20% of the time and punishment 80% of the time. Target and non-target locations were counterbalanced so that the target aperture was located on the right for half the animals and on the left for the other half. Nosepokes in either aperture made during the ITI, before illumination of the apertures (“premature responses”), were punished by a timeout and no reward. Continued nosepokes made into the same aperture after a rewarded response before retrieval of the reward (“reward-perseverative responses”), as well as continued responses after a punished response made into the same aperture (“punished-perseverative responses”) or the other aperture (“timeout responses”) were recorded, but had no programmed consequences. Animals were tested daily until they reached criterion performance, i.e., until >90% of their responses occurred in the target location on two consecutive days while performing at least 50 trials per session. This criterion performance was considered as reflective of having learned the target location.
Once a rat had reached criterion performance, it was switched to the reversal-learning phase. For each individual rat, this phase was started as soon as this rat had reached criterion performance, consistent with human reversal-learning tasks. Beginning with the next session after reaching criterion performance, reward contingencies were reversed, so that the previous location of the target aperture was now the non-target aperture and vice versa. For example, if the left aperture had served as the target location in the initial learning phase and the right aperture as the non-target location, the right aperture became the target location and the left aperture the non-target location in the reversal-learning phase. Daily 30-min sessions continued with these reversed contingencies until the rat once again reached criterion performance (>90% of responses into the new target location, ≥50 trials per session), which was considered indicative of successful reversal learning.
Once a rat reached criterion performance in the reversal phase, its reward contingencies were switched again on the next day. (In the above example, the target location, which had been on the left during the initial learning phase and on the right during the reversal phase, would now be again located on the left.) If the animal then reached criterion performance again under these re-reversed conditions, reward contingencies were again switched starting on the next day, and so on.
Rats were tested in the between-sessions probabilistic learning task for 15 days. During this time, reward contingencies were switched back and forth for each given animal as often as it managed to reach criterion performance. Unless otherwise stated, the analysis of results focuses on the animals’ performance of the initial learning phase and the first reversal-learning phase that directly followed the initial learning phase. Not all rats managed to reach criterion performance in this first reversal phase, or even in the initial learning phase, during the 15 days of testing (see Results).
Within-Session Probabilistic Learning Task
This task version was based on a similar task developed by Bari et al. (2010). Briefly, the task matched the between-sessions probabilistic learning task in terms of apertures uncovered, trial initiation, initial response contingencies, and parameters recorded. In contrast to the between-sessions task, however, sessions lasted one hour, and criterion performance was now defined as the performance of eight consecutive nosepoke responses (rewarded or unrewarded) into the target location. As soon as this criterion was reached, the reward contingency switched (former target location became non-target location and vice versa). This switch was not signaled to the rat in any way. If rats succeeded in again performing eight consecutive responses into the new target location, reward contingencies switched again, and continued to switch every time the rat reached criterion performance until the end of the session or completion of a maximal number of trials, whichever came first. The maximum trial number was set at 240 during the first session. For all subsequent sessions, it was increased to 600 to ensure that all rats performed the task for the full one-hour duration. Rats were tested in five sessions of this task on five separate days to assess improvements in reversal learning. Unlike the task employed by Bari et al. (but like the between-sessions task used earlier in this study), the illumination of the apertures did not end after a set time, but continued until the rat performed a nosepoke. This ensured that the focus of the task was strictly on the animal’s ability to learn the target location, without any component of attention to a limited-duration stimulus.
Probabilistic Learning Measures
The following measures were calculated to assess task performance:
Measures assessed in the between-sessions task only
Sessions to initial acquisition: number of sessions required to reach criterion performance under the initial task contingencies
Sessions to reversal: number of sessions required to reach criterion performance under the reversed task contingencies.
Measures assessed in both versions of the probabilistic learning task
Overall performance
Number of reversals: number of times rat reached criterion performance and was switched to reversed task contingencies during the 15 days of the experiment (between-sessions task) or during the session (within-session task).
Trials per session: number of trials completed per session.
Trials to initial acquisition: number of trials required to reach criterion performance under the initial task contingencies.
Trials to first reversal: number of trials required to reach criterion performance after task contingencies were reversed for the first time.
Strategy formation
Target win-stay ratio: proportion of target responses following a reward from responding in the target location: [# target responses after rewarded target response/# total responses after rewarded target response]
Non-target win-stay ratio: proportion of non-target responses following a reward from responding in the non-target location: [# non-target responses after rewarded non-target response/# total responses after rewarded non-target response]
Target lose-shift ratio: proportion of non-target responses following a punished response into the target location: [# non-target responses after punished target response/# total responses after punished target response]
Non-target lose-shift ratio: proportion of target responses following a punished response into the non-target location: [# target responses after punished non-target response/# total responses after punished non-target response]
% premature responses: number of premature responses per session divided by the number of trials per session.
% reward-perseverative responses: number of reward-perseverative responses per session divided by the number of trials per session.
% punished-perseverative responses: number of punished-perseverative responses per session divided by the number of trials per session.
% timeout responses: number of timeout responses per session divided by the number of timeout periods per session.
Statistical Analyses
Data were analyzed using mixed-factor two-way ANOVA, with Rearing as the between-subjects factor and the following within-subjects factors specific to the various tests:
PPI: Prepulse Intensity
BPM (crossings): Time Block
Between-sessions probabilistic learning task (sessions to initial acquisition vs. sessions to first reversal; trials to initial acquisition vs. trials to first reversal): Task Phase
Between-sessions probabilistic learning task (win-stay ratios, lose-shift ratios): Day
Within-session probabilistic learning task (all measures): Day
In addition, trials to initial acquisition vs. trials to first reversal in the within-session probabilistic learning task were analyzed using three-way ANOVA with the factors Rearing, Day, and Task Phase. When statistically significant effects were found in the ANOVA, post hoc comparisons among means were conducted using Bonferroni tests. All remaining measures were analyzed using two-tailed t-tests; Welch’s correction was used where variances were significantly different among groups. The level of significance was set at 0.05. Statistical trends (0.05 < p < 0.1) are discussed in the Results. Data were analyzed using GraphPad Prism® (GraphPad, San Diego, CA), BMDP (Statistical Solutions Inc., Saugus, MA) or SPSS Version 19 (IBM Corporation, Armonk, NY, USA).
Results
One isolate and one social were excluded from the probabilistic learning tasks because they never developed sufficient levels of operant responding. One additional isolate was excluded due to profoundly elevated levels of non-task-related nosepoking. Between the between-sessions and the within-session probabilistic learning task, one isolate had to be euthanized due to suspicion of parvovirus infection. As a result, final group sizes were 26 socials and 28 isolates (initial PPI and BPM tests), 25 socials and 27 isolates (between-sessions probabilistic learning task), 25 socials and 26 isolates (within-session probabilistic learning task), or 26 socials and 27 isolates (final PPI test).
PPI
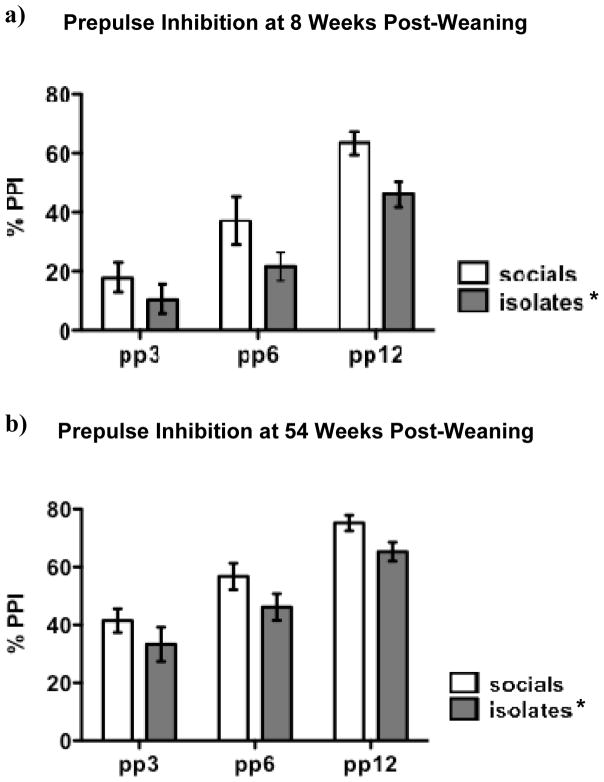
Isolates exhibited consistently lower %PPI than socials (see Figure 2), reflected by a significant main effect of Rearing irrespective of age [8 weeks post-weaning: F(1,104) = 4.8, p < 0.05; 54 weeks post-weaning: F(1,102) = 4.9, p < 0.05]. A significant main effect of Prepulse Intensity in %PPI was also found [8 weeks post-weaning: F(2,104) = 61.3, p < 0.0001; 54 weeks post-weaning: F(2,102) = 37.7, p < 0.0001], with no Rearing x Prepulse Intensity interaction on %PPI. Isolates and socials did not differ significantly in terms of absolute startle or startle habituation, although there was a trend towards less habituation in the isolates during the re-test at 54 weeks post-weaning [t(51) = 1.8, p = 0.072]. A similar reduction in habituation in isolates was also seen at 8 weeks post-weaning, but did not reach significance or trend level [t(40) = 1.7 with Welch’s correction, p = 0.102; see Table 1].
Figure 2. Effects of Isolation Rearing on Prepulse Inhibition of the Startle.
Response Isolation-reared rats (isolates) had decreased levels of prepulse inhibition (PPI) compared to socially reared rats (socials). pp3, pp6, pp12: prepulse intensity of 3, 6, or 12 dB over background. Values at 8 weeks (a) and 54 weeks (b) post-weaning are expressed as mean ± SEM. Asterisks (*p < 0.05) denote significant differences compared to socials; asterisks next to the group name reflect an overall main effect of Rearing rather than a significant difference at any individual prepulse intensity.
Table 1. Effects of Isolation Rearing on Acoustic Startle.
Absolute startle and startle habituation did not differ significantly between isolates and socials. However, there was a trend towards less habituation in isolates compared to socials during at 54 weeks post-weaning (p = 0.072)
| socially reared | isolation-reared | ||
|---|---|---|---|
| startle | 8 weeks post-weaning | 269.69 ± 41.58 | 313.71 ± 45.54 |
| 54 weeks post-weaning | 364.31 ± 66.66 | 335.51 ± 42.85 | |
| startle habituation | 8 weeks post-weaning | 66.19% ± 7.87% | 51.34% ± 4.48% |
| 54 weeks post-weaning | 68.13% ± 5.58% | 53.87% ± 5.59% | |
Values are expressed as mean ± SEM
BPM
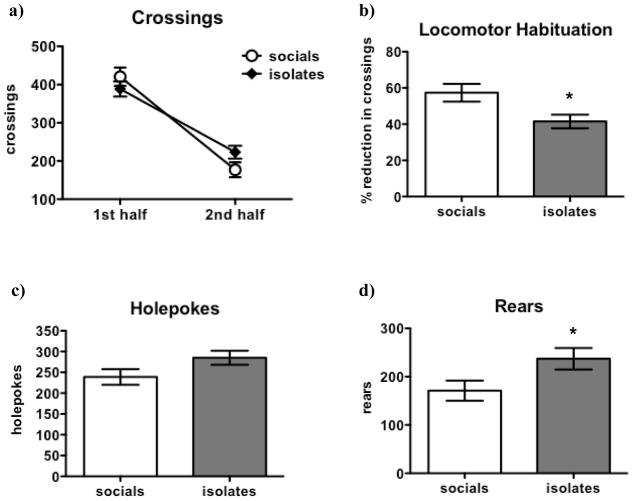
Crossings decreased in the second half of the session [F(1,52) = 282.5, p < 0.0001], as would be expected given habituation to the BPM environment. No main effect of Rearing on crossings was observed, but a Rearing x Time Block interaction [F(1,52) = 10.3, p < 0.01; see Figure 3a] was found. Isolates exhibited less locomotor habituation in the BPM compared to socials [t(52) = 2.6, p < 0.05; see Figure 3b]. There was a trend towards more holepokes in isolates [t(52) = 1.8, p = 0.073; see Figure 3c], and isolates performed more rears than socials [t(52) = 2.2, p < 0.05; see Figure 3d].
Figure 3. Effects of Isolation Rearing on Locomotion and Exploratory Behavior in the Behavioral Pattern.
Monitor Crossings (a) decreased from the first to the second half of the test in both isolates and socials. However, a significant Rearing x Session Period interaction reflected the fact that crossings decreased less in isolates over time than in socials. This finding was confirmed by the fact that locomotor habituation (b) was significantly lower in isolates compared to socials. There was a trend (p = 0.073) towards more holepokes (c) in isolates, and isolates also performed more rears (d) than socials. Values are expressed as mean ± SEM. Asterisks (*p < 0.05) denote significant differences compared to socials.
Between-Sessions Probabilistic Learning Task
Overall performance
Rats required both more sessions [F(1,46) = 58.2, p < 0.0001] and more trials [F(1,46) = 51.7, p < 0.0001] to reach criterion performance after reward contingencies were reversed (see Figure 4a, b). No rats reached the accuracy criterion for task performance (>90% of responses into the target location) without also reaching the trial criterion (≥50 trials per session). Failures to reach criterion were therefore driven predominantly by lack of selectivity for the target location, not low levels of responding.
Figure 4. Effects of Task Phase on Performance in the Between-Sessions Probabilistic Learning Task.
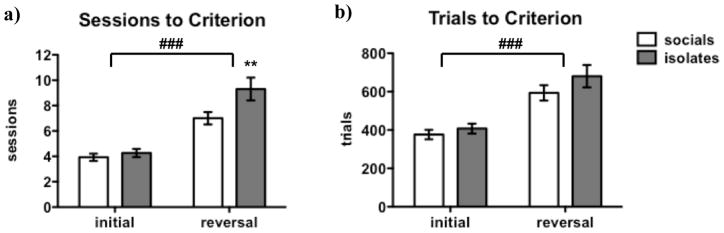
Rats required more sessions (a) and more trials (b) to reach criterion performance in the reversal phase compared to the initial learning phase. Initial: initial learning phase; reversal: reversal-learning phase. Values are expressed as mean ± SEM. Pound signs (###p < 0.0001) denote significant differences between initial learning phase and reversal phase; asterisks (**p < 0.01) denote significant differences compared to socials. Rats that did not reach criterion during the reversal-learning phase are excluded from the analysis due to ANOVA requirements.
No main effect of Rearing and no Rearing x Task Phase interaction were found for trials to initial acquisition/first reversal. There was, however, a significant main effect of Rearing on the number of sessions required to reach initial acquisition/first reversal [F(1,46) = 5.6, p < 0.05]. While there was no Rearing x Task Phase interaction on sessions to initial acquisition/first reversal, post hoc testing detected significant differences between isolates and socials only during the reversal phase (p < 0.01), with no significant differences during the initial learning phase.
It should be noted, however, that not all rats reached criterion performance after the first reversal of reward contingencies. The comparisons of initial vs. reversal acquisition therefore did not include the initial learning phase performance of rats that did not reach criterion during the reversal phase. In order to compare the performance of all rats during the initial learning phase, we also performed separate t-tests comparing performance during either the initial learning phase or the reversal-learning phase alone. The following analyses, depicted in Figure 5, include the rats that did not reach criterion during the reversal-learning phase.
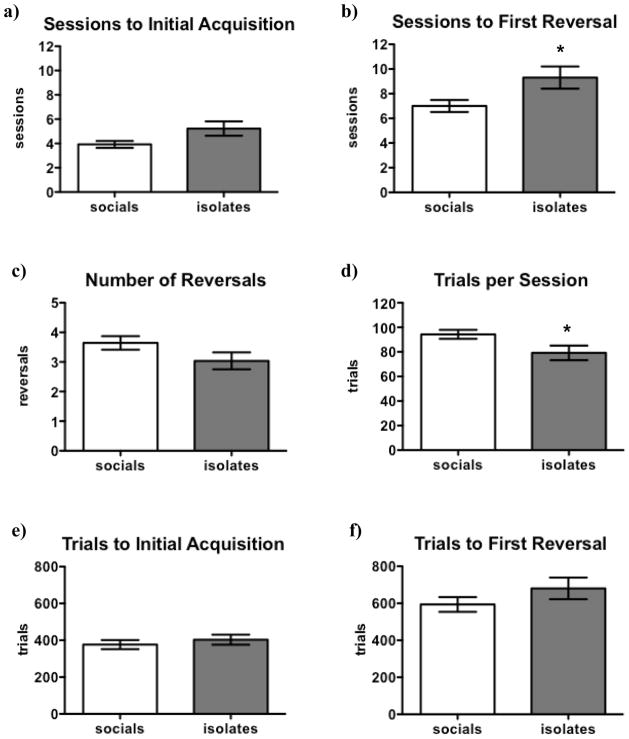
Figure 5. Effects of Isolation Rearing on Performance in the Between-Sessions Probabilistic Learning Task.
There was a strong trend (p = 0.054) for isolates to require more sessions to reach criterion performance in the initial learning phase (a), and isolates required significantly more sessions to reach criterion in the reversal phase (b). Isolates did not differ significantly from socials in terms of reversals completed during the duration of the experiment (c). Isolates performed fewer trials per session (d) compared to socials. There were no significant differences regarding trials to initial acquisition (e) and trials to first reversal (f) between isolates and socials. Values are expressed as mean ± SEM. Asterisks (*p < 0.05) denote significant differences compared to socials. The depicted analyses include rats that did not reach criterion during the reversal-learning phase.
Isolates strongly tended to take longer to attain initial criterion performance compared to socials [t(37) = 2.0 with Welch’s correction, p = 0.054; see Figure 5a]. Isolates also required significantly more sessions to reach criterion performance under reversed task conditions [t(34) = 2.3 with Welch’s correction, p < 0.05; see Figure 5b]. Indeed, four isolates never reached criterion performance under the reversed task conditions during the 15 days of the experiment; while all socials did. Inspection of the data also indicated that isolates on average tended to complete slightly fewer reversals during the 15 days of the experiment (see Figure 5c), although the difference between the groups did not reach statistical significance [t(50) = 1.6, p = 0.109].
Isolates also performed significantly fewer trials per session than socials [t(43) = 2.2, p < 0.05; see Figure 5d]. Therefore, when summing trials across days, isolates did not require more trials than socials to attain initial learning [t(50) = 0.7, p > 0.1; see Figure 5e] or reversal [t(46) = 1.3, p > 0.1; see Figure 5f].
Strategy formation
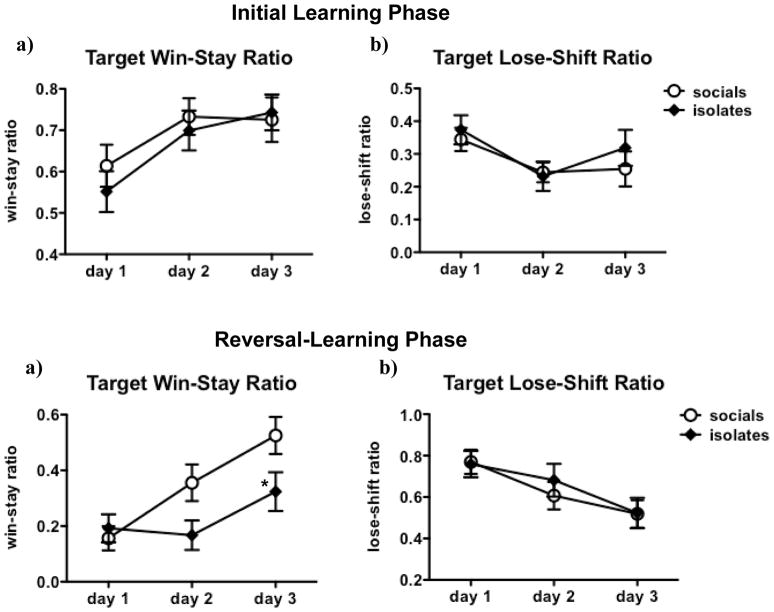
We analyzed win-stay and lose-shift ratios for the first three days of both the initial learning phase and the reversal-learning phase. We focused on these initial sessions because significant numbers of rats reached criterion performance within these three sessions. During the initial learning phase, rats increased their target win-stay ratio [F(2,50) = 7.8, p < 0.001] and decreased their target lose-shift ratio [F(2,50) = 4.1, p < 0.05] across days, indicating that they became more likely over time to continue responding at the target location after receiving a reward from that location, and less likely to shift away from the target location after a punishment (see Figure 6a, b). Non-target shifting or staying did not change over days (see Table 2). Rearing did not affect shifting and staying for either the target or the non-target location during the initial learning phase, and there was no Rearing x Day interaction.
Figure 6. Effects of Isolation Rearing on Target Win-Stay and Lose-Shift Ratios in the Between-Sessions Probabilistic Learning Task.
Rats increased their target win-stay ratio and decreased their target lose-shift ratio during the first three days of the initial learning phase (a, b) and again during the first three days of the reversal phase (c, d). Isolates exhibited less increase in their target win-stay ratio and less decrease in their non-target win-stay ratio compared to socials. Values are expressed as mean ± SEM. Asterisks (*p < 0.05, **p < 0.01) denote significant differences compared to socials.
Table 2. Effects of Isolation Rearing on Non-Target Win-Stay and Lose-Shift Ratios in the Between-Sessions Probabilistic Learning Task.
During the first three days of the initial learning phase, there were no significant differences across days or between isolates and socials regarding non-target win-stay and lose-shift ratios. In contrast, during the first three days of the reversal-learning phase, rats decreased their non-target win-stay ratio and increased their non-target lose-shift ratio over time, and isolates exhibited less decrease in their non-target win-stay ratio compared to socials
| socially reared | isolation-reared | ||
|---|---|---|---|
| initial learning phase | |||
| non-target win-stay ratio | day 1 | 0.33 ± 0.06 | 0.33 ± 0.05 |
| day 2 | 0.20 ± 0.04 | 0.33 ± 0.07 | |
| day 3 | 0.31 ± 0.07 | 0.27 ± 0.06 | |
| non-target lose-shift ratio | day 1 | 0.61 ± 0.04 | 0.59 ± 0.04 |
| day 2 | 0.67 ± 0.04 | 0.64 ± 0.06 | |
| day 3 | 0.68 ± 0.05 | 0.63 ± 0.04 | |
| reversal-learning phase | |||
| non-target win-stay ratio | day 1 | 0.88 ± 0.03 | 0.83 ± 0.04 |
| day 2 | 0.68 ± 0.06 | 0.77 ± 0.05 | |
| day 3 | 0.47 ± 0.07 | 0.70 ± 0.06 ** | |
| non-target lose-shift ratio | day 1 | 0.20 ± 0.03 | 0.17 ± 0.03 |
| day 2 | 0.28 ± 0.04 | 0.21 ± 0.03 | |
| day 3 | 0.43 ± 0.05 | 0.32 ± 0.05 | |
Values are expressed as mean ± SEM. Asterisks (**p < 0.01) denote significant differences compared to socials
After reward contingencies had been reversed, rats increased their tendency to continue responding at the new target location after receiving a reward from that location [F(2,49) = 17.8, p < 0.0001] and decreased their tendency to shift away from this new target location after a punishment [F(2,48) = 11.1, p < 0.0001] across days (see Figure 6c, d). Additionally, they decreased their tendency to continue responding at the new non-target location after receiving a reward from that location [F(2,49) = 22.8, p < 0.0001] and increased their tendency to shift away from this new non-target location after a punishment [F(2,49) = 28.7, p < 0.0001] over time (see Table 2). Rearing interacted significantly with Day on both target win-stay ratio [F(2,49) = 4.9, p < 0.01] and non-target win-stay ratio [F(2,49) = 6.5, p < 0.01] during the first reversal phase. Post hoc testing revealed that isolates exhibited lower target win-stay ratios (p < 0.05) and higher non-target win-stay ratios (p < 0.01) than socials on Day 3 of the reversal-learning phase. In other words, when reward contingencies were reversed, isolates were less successful in increasing their tendency to continue responding in the new target location after rewarded target responses (see Figure 6c), and in decreasing their tendency to continue responding in the new non-target location after rewarded non-target responses (see Table 2).
The groups did not differ significantly regarding their mean target latency; while target latencies were shorter in socials, this difference did not reach significance or trend level [t(50) = 1.4, p = 0.159]. There was a trend towards longer non-target latencies in isolates [t(32) = 1.7 with Welch’s correction, p = 0.098]. Isolates exhibited lower % premature responses than socials [t(50) = 2.4, p < 0.05], but did not differ in terms of % reward-perseverative responses, % punished-perseverative responses, or % timeout responses (see Table 3).
Table 3. Effects of Isolation Rearing on Activity in the Between-Sessions Probabilistic Reversal-Learning Task.
Isolates and socials did not differ significantly in terms of mean target latency, but there was a trend towards longer non-target latencies in isolates (p = 0.098). Premature responding rates were lower in isolates than in socials, but there were no differences in % reward-perseverative responses, % punished-perseverative responses, or % timeout responses
| socially reared | isolation-reared | |
|---|---|---|
| target latency (s) | 6.49 ± 2.14 | 11.18 ± 2.53 |
| non-target latency (s) | 7.00 ± 2.18 | 17.69 ± 6.00 |
| reward latency (s) | 1.17 ± 0.06 | 1.43 ± 0.27 |
| % premature responses | 5.90% ± 0.82% | 3.40% ± 0.72% * |
| % reward-perseverative responses | 2.07% ± 0.20% | 1.76% ± 0.19% |
| % punished-perseverative responses | 12.11% ± 1.06% | 11.32% ± 1.07% |
| % timeout responses | 22.60% ± 1.86% | 20.94% ± 2.34% |
Values are expressed as mean ± SEM. Asterisks (*p < 0.05) denote significant differences compared to socials
Within-Session Probabilistic Learning Task
Overall performance
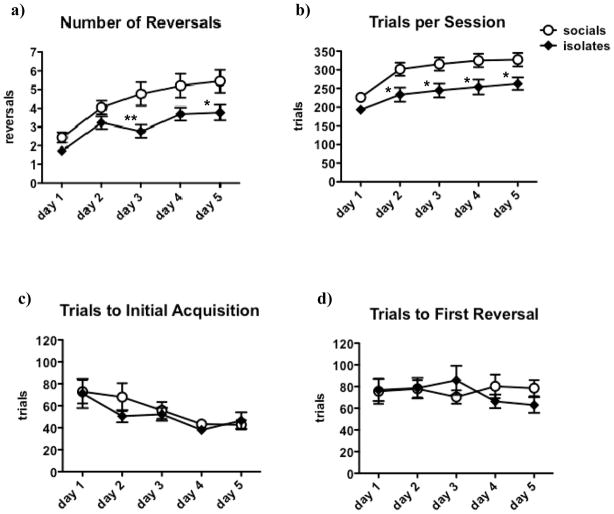
Isolates completed fewer reversals per session [F(1,196) = 8.1, p < 0.01] as well as fewer trials per session [F(1,196) = 8.1, p < 0.01] compared to socials (see Figure 7a, b). Post-hoc testing showed that the difference in reversals per session reached significance on Day 3 (p < 0.01) and Day 5 (p < 0.05), though a similar pattern could be observed on the other days of the task (see Figure 7a). For trials per session, post-hoc testing showed significant differences between isolate and socials on Days 2–5 (p < 0.05; see Figure 7b).
Figure 7. Effects of isolation rearing on performance in the within-session probabilistic learning task.
Isolates completed fewer reversals per session (a) and performed fewer trials per session (b) compared to socials. Over time, all rats completed more reversals per session and performed more trials per session. Values are expressed as mean ± SEM. Asterisks (*p < 0.05, **p < 0.01) denote significant differences compared to socials.
The number of reversals per session [F(4,196) = 19.0, p < 0.0001] and trials per session [F(4,196) = 38.6, p < 0.0001] increased robustly over time. The large increase in trials per session after Day 1 was likely due to the change in task conditions, which increased the maximum number of trials rats could complete per session. The main effect of Day on trials per session remained, however, even when analyzing only Days 2–4 [F(3,147) = 6.3, p < 0.001]. There was no Rearing x Day interaction on either reversal per session or trials per session.
Comparing across days, rats required fewer trials to reach initial learning over time [F(1,196) = 5.8, p < 0.001], but the number of trials to first reversal did not change significantly. Rearing did not affect trials to initial learning or first reversal, nor did Rearing interact with Day on either measure (see Figure 7c, d).
Three-way ANOVA comparing trials to criterion in both task phases (i.e., both initial learning phase and reversal-learning phase) for both groups revealed that rats required more trials to reach criterion in the first reversal phase compared to the initial learning phase of the task [F(1,26) = 15.8, p < 0.001]. Three-way ANOVA analysis also confirmed a main effect of Day on trials to criterion [F(4,104) = 4.3, p < 0.01], likely driven by the decrease in trials to initial learning across days.
Strategy formation
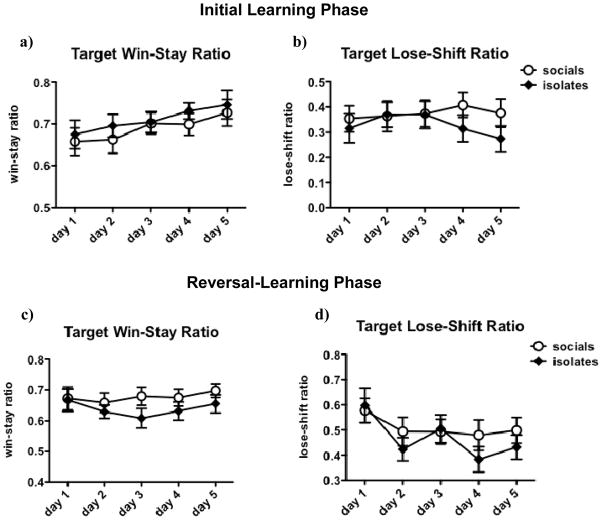
Target win-stay ratios increased steadily across days [F(4,196) = 2.6, p < 0.05; see Figure 8a]. While there was a main effect of Day on non-target win-stay ratio [F(4,196) = 3.8, p < 0.01], there was no clear pattern explaining this finding (see Table 4). While on days 1–3, rats appeared to more efficiently disregard rewards from non-target locations, this effect was apparently reversed on days 4 and 5. Target and non-target lose-shift ratios were unaffected by Day (Figure 8b, Table 4). Rearing did not affect staying or shifting during the initial learning phase, and no Rearing x Day interactions were observed. There was a trend towards a main effect of Rearing on non-target lose-shift ratio [F(1,196) = 3.0, p = 0.091], but inspection of the data reveals no clear pattern of difference between isolates and socials on this measure (see Table 4).
Figure 8. Effects of Isolation Rearing on Target Win-Stay and Lose-Shift Ratios in the Within-Session Probabilistic Learning Task.
During the initial learning phase, rats increased their target win-stay ratio over time (a); there were no systematic changes over time in target lose-shift ratio (b). During the reversal-learning phase, there was a trend (p = 0.089) towards lower target win-stay ratios (c) in isolates compared to socials. All rats decreased their target lose-shift ratio (d) over time during the reversal-learning phase. Values are expressed as mean ± SEM.
Table 4. Effects of Isolation Rearing on Non-Target Win-Stay and Lose-Shift Ratios in the Within-Session Probabilistic Learning Task.
During the initial learning phase, there was a significant main effect of Day on non-target win-stay ratio and a trend (p = 0.091) towards a main effect of Rearing on non-target lose-shift ratio, but no clear patterns explaining these findings could be identified. No other effects on non-target win-stay and lose-shift ratios were seen during the initial learning phase. Likewise, no effects of Day or Rearing on non-target win-stay and lose-shift ratios and no interactions were found during the reversal-learning phase
| socially reared | isolation-reared | ||
|---|---|---|---|
| initial learning phase | |||
| non-target win-stay ratio | day 1 | 0.42 ± 0.05 | 0.32 ± 0.03 |
| day 2 | 0.35 ± 0.05 | 0.38 ± 0.06 | |
| day 3 | 0.24 ± 0.06 | 0.18 ± 0.04 | |
| day 4 | 0.40 ± 0.06 | 0.39 ± 0.07 | |
| day 5 | 0.41 ± 0.07 | 0.36 ± 0.05 | |
| non-target lose-shift ratio | day 1 | 0.59 ± 0.04 | 0.64 ± 0.03 |
| day 2 | 0.65 ± 0.03 | 0.64 ± 0.04 | |
| day 3 | 0.62 ± 0.04 | 0.68 ± 0.03 | |
| day 4 | 0.59 ± 0.03 | 0.69 ± 0.04 | |
| day 5 | 0.68 ± 0.04 | 0.69 ± 0.04 | |
| reversal-learning phase | |||
| non-target win-stay ratio | day 1 | 0.62 ± 0.05 | 0.52 ± 0.04 |
| day 2 | 0.60 ± 0.06 | 0.65 ± 0.05 | |
| day 3 | 0.67 ± 0.05 | 0.58 ± 0.05 | |
| day 4 | 0.48 ± 0.05 | 0.56 ± 0.05 | |
| day 5 | 0.51 ± 0.07 | 0.62 ± 0.06 | |
| non-target lose-shift ratio | day 1 | 0.48 ± 0.03 | 0.51 ± 0.03 |
| day 2 | 0.52 ± 0.04 | 0.60 ± 0.04 | |
| day 3 | 0.52 ± 0.04 | 0.59 ± 0.04 | |
| day 4 | 0.54 ± 0.03 | 0.53 ± 0.03 | |
| day 5 | 0.55 ± 0.03 | 0.52 ± 0.03 | |
Values are expressed as mean ± SEM
During first reversal-learning phase, isolates tended to have lower target win-stay ratios [F(1,184) = 3.01, p = 0.089; see Figure 8c]. Target lose-shift ratio decreased slightly across days [F(4,184) = 4.8, p < 0.01; see Figure 8d]. No other main effects of Day and Rearing on staying and shifting and no Rearing x Day interactions were found (see Figure 8c, d, Table 4).
Isolates had longer target latencies [F(1,196) = 4.38, p < 0.05] and non-target latencies [F(1,196) = 6.29, p < 0.05] compared to socials. Post-hoc testing detected a significant difference in non-target latencies on Day 4. No main effects of Day and no Rearing x Day interaction on target and non-target latency were seen. Reward latencies decreased over days [F(4,196) = 30.02, p < 0.0001]; there was no main effect of Rearing and no Rearing x Day interaction on reward latency (see Table 5).
Table 5. Effects of Isolation Rearing on Activity in the Within-Session Probabilistic Reversal-Learning Task.
Isolates had longer target and non-target latencies compared to socials. Reward latencies decreased across days, but did not differ between isolates and socials. Premature, reward-perseverative, and punished-perseverative responses, but not timeout responses, decreased over time. Isolates exhibited lower rates of premature responding and a trend (p = 0.074) toward lower timeout responding compared to socials
| socially reared | isolation-reared | ||
|---|---|---|---|
| target latency# (s) | day 1 | 2.53 ± 0.36 | 3.77 ± 0.55 |
| day 2 | 2.58 ± 0.54 | 4.51 ± 1.18 | |
| day 3 | 2.63 ± 0.70 | 4.25 ± 0.71 | |
| day 4 | 2.07 ± 0.38 | 3.83 ± 0.77 | |
| day 5 | 2.03 ± 0.36 | 3.15 ± 0.38 | |
| non-target latency# (s) | day 1 | 2.93 ± 0.41 | 5.45 ± 0.99 |
| day 2 | 2.72 ± 0.50 | 5.50 ± 1.18 | |
| day 3 | 2.68 ± 0.63 | 4.83 ± 0.89 | |
| day 4 | 2.39 ± 0.47 | 5.74 ± 1.63 | |
| day 5 | 2.91 ± 0.69 | 4.09 ± 0.72 * | |
| reward latency (s) | day 1 | 1.16 ± 0.04 | 1.15 ± 0.05 |
| day 2 | 0.93 ± 0.06 | 1.02 ± 0.05 | |
| day 3 | 0.90 ± 0.06 | 1.01 ± 0.07 | |
| day 4 | 0.86 ± 0.06 | 0.91 ± 0.04 | |
| day 5 | 0.86 ± 0.06 | 0.92 ± 0.05 | |
| % premature responses# | day 1 | 0.28% ± 0.13% | 0.02% ± 0.02% |
| day 2 | 0.25% ± 0.10% | 0.03% ± 0.03% | |
| day 3 | 0.50% ± 0.17% | 0.07% ± 0.05% | |
| day 4 | 0.97% ± 0.33% | 0.12% ± 0.07% | |
| day 5 | 0.98% ± 0.38% | 0.15% ± 0.06% ** | |
| % reward-perseverative responses | day 1 | 2.39% ± 0.55% | 1.24% ± 0.32% ** |
| day 2 | 1.63% ± 0.45% | 1.79% ± 0.40% | |
| day 3 | 1.56% ± 0.35% | 1.67% ± 0.34% | |
| day 4 | 1.47% ± 0.37% | 0.97% ± 0.22% | |
| day 5 | 1.11% ± 0.19% | 1.03% ± 0.19% | |
| % punished-perseverative responses | day 1 | 5.12% ± 0.81% | 5.54% ± 0.75% |
| day 2 | 3.37% ± 0.49% | 4.02% ± 0.64% | |
| day 3 | 3.71% ± 0.52% | 4.87% ± 0.59% | |
| day 4 | 4.13% ± 0.53% | 4.72% ± 0.58% | |
| day 5 | 3.59% ± 0.48% | 4.16% ± 0.62% | |
| % timeout responses | day 1 | 2.86% ± 0.55% | 1.47% ± 0.32% |
| day 2 | 1.74% ± 0.42% | 1.28% ± 0.38% | |
| day 3 | 2.75% ± 0.46% | 1.85% ± 0.39% | |
| day 4 | 2.17% ± 0.54% | 1.43% ± 0.32% | |
| day 5 | 1.94% ± 0.57% | 1.47% ± 0.43% |
Values are expressed as mean ± SEM. Pound signs (#p < 0.05) denote a significant main effect of Rearing; asterisks (*p < 0.05, **p < 0.01) denote significant differences compared to socials
Isolates exhibited lower % premature responses than socials [F(1,196) = 9.8, p < 0.01]. While % premature responses increased over time [F(4,196) = 4.3, p < 0.01], there was a strong trend toward an interaction of Day with Rearing [F(4,196) = 2.4, p = 0.055]. Post hoc testing confirmed that the levels of premature responding were lower in isolates than in socials, a difference that reached significance on Days 4 and 5 (p < 0.01). Reward-perseverative responses [F(4,196) = 3.4, p < 0.05] and punished-perseverative responses [F(4,196) = 5.43, p < 0.001] decreased slightly over time; no main effect of Rearing and no Rearing x Day interaction were found on these measures. Finally, isolates tended to exhibit lower % timeout responses compared to socials [F(1,196) = 3.32, p = 0.074]. No main effect of Day and no Rearing x Day interaction on % timeout responding were observed (see Table 5).
Discussion
Consistent with previous studies (Cilia et al., 2005; 2001; Geyer et al., 1993; Varty & Geyer, 1998; Varty & Higgins, 1995; Wilkinson et al., 1994), isolates had decreased PPI compared to social controls (see Figure 2). These PPI deficits were robustly detected both at the beginning and the end of our study, confirming that the behavioral effects of isolation rearing persisted throughout the duration of the study. Isolates also exhibited increased investigatory behavior as measured by increased holepokes and rears in the BPM (see Figure 3c, d). Importantly, while the overall locomotor activity of isolates in the BPM did not differ from that of socials, isolates exhibited less locomotor habituation (see Figure 3a, b), consistent with findings in patients with schizophrenia (Perry et al., 2009). These BPM results were also consistent with other studies of isolates (Lapiz et al., 2000; Paulus et al., 1998; Powell et al., 2002; Sahakian et al., 1975; Varty et al., 2000).
The present data extend the behavioral phenotype of isolates to deficits in cognitive flexibility, using a cross-species relevant test of reinforcement learning (Ragland et al., 2009). Conceptual validation of the cognitive flexibility required for this probabilistic reversal-learning task is supported by the finding that all rats required more trials to reach criterion during the reversal phase compared to the initial learning phase in both versions of the task. Likewise, in the between-sessions version of the task, sessions to criterion were also higher in the reversal-learning phase (see Figure 4). This pattern reflects the increased difficulty of task performance after the reversal of reward contingencies, because in addition to learning the new reward contingency, the animal must also inhibit the previously learned strategy and suppress the pre-potent previously rewarded responses, a process that is likely to engage executive processes (Gilmour et al., 2012).
In the between-sessions probabilistic learning task, isolates required more sessions to reach criterion performance than socials (see Figure 5a, b). This difference reached significance only during the reversal-learning phase of the task, possibly highlighting a selective effect of isolation rearing on reversal learning vs. simple acquisition, although there was a strong trend towards a group difference during the initial learning phase also, with no Rearing x Task Phase interaction. Isolates also exhibited a non-significant tendency towards completing fewer reversals over the duration of the experiment compared to socials (see Figure 5c). Finally, four isolates never reached criterion performance under reversal phase conditions within the 15 days of testing, while all socials completed at least the first reversal phase during this period.
Although the above findings support deficient learning in isolates under probabilistic learning conditions, these animals also completed significantly fewer trials per session (see Figure 5d). No difference between isolates and socials was observed in the total number of trials required to reach criterion in either phase (see Figure 5e, f). Given that isolates reached criterion performance within the same number of trials as socials, the difference in sessions to acquisition and first reversal in the between-sessions probabilistic learning task may not reflect a true learning deficit, and may instead simply be driven by a lower trial completion rate of isolates in the probabilistic reversal-learning task.
To clarify the role of trials per session in the effects of isolation rearing on probabilistic reversal-learning performance, we tested the same group of rats in a version of the probabilistic reversal-learning task that assessed reversal learning within a single testing session. Isolates reached criterion less often and completed significantly fewer reversals per session compared to socials (see Figure 7a). Again, however, this difference could be explained by fewer trials completed per session by isolates (see Figure 7b). Isolates did not differ from socials in terms of the number of trials to initial acquisition or reversal (see Figure 7c, d).
To further explore the effect of isolation rearing on rule acquisition and reversal learning under probabilistic conditions, we analyzed the response strategies of our rats in the task by calculating their win-shift and lose-stay ratios after target vs. non-target responses. This analysis allows the assessment of whether subjects use model-free or model-based choice strategies to perform a task. Model-free strategies entail a nonselective bias towards choosing previously rewarded locations or shifting away from punished locations. In the present task, a model-free strategy would result in a preference for responding in a previously rewarded location (i.e., making a win-stay choice) or shifting from a previously punished location (i.e., making a lose-shift choice) regardless of whether this location constitutes the target or non-target site. In contrast, model-based strategies reflect the subject forming a model of which location represents the higher probability of reward and selectively favoring positive feedback and discounting negative feedback from this target location (Otto, Gershman, Markman, & Daw, in press; Wunderlich, Smittenaar, & Dolan, 2012).
Over the course of the first three sessions of the initial learning phase of the between-sessions probabilistic learning task, rats selectively increased their win-stay and decreased their lose-shift ratios after responses in the target location only (see Figure 6a, b), indicating the development of a model-based strategy. Moreover, in the first days after reward contingencies were switched, the rats selectively increased their target win-stay and non-target lose-shift ratios while decreasing their non-target win-stay and target lose-shift ratios (see Figure 6c, d, Table 2).
Repeated challenge with the within-session probabilistic learning task led to further selective increases in target win-stay ratio (during the initial learning phase of each session) and decreases in target lose-shift ratio (during the first reversal phase of each session) across days (see Figure 8). Hence, with increased repetitions of the task, rats learned to more quickly favor positive feedback and discount negative feedback associated with an apparent target location. With increased repetitions of the task, rats also managed to complete more reversals per session, i.e., reach criterion performance more often during the time allotted (see Figure 7a) consistent with earlier reports (Bari et al., 2010), supporting the idea that they were acquiring a higher-order strategy optimizing their performance in the task. The development of such higher-order strategies in response to “serial reversal learning”, i.e. exposure to multiple contingency reversals, has been postulated in standard (non-probabilistic) reversal-learning tasks (Rygula, Walker, Clarke, Robbins, & Roberts, 2010; Warren, 1966).
Isolates did not differ from socials in terms of win-stay and lose-shift ratios during the initial learning phase of the between-sessions probabilistic reversal-learning task (see Figure 6a, b). Once reward contingencies were switched, however, isolates were significantly slower than socials to increase their win-stay ratios after responses in the new target location (see Figure 6c). At the same time, isolates were slower to decrease their win-stay ratio after responses in the new non-target location (see Table 2). A similar pattern was seen in the within-session probabilistic reversal-learning task, with no differences between isolates and socials during the initial learning phase (see Figure 8a, b), but a trend towards slower increases in target win-stay ratios during the reversal-learning phase (see Figure 8c). In other words, isolates were less able to shift their focus towards newly significant positive feedback and discount formerly significant positive feedback that had become insignificant. This inability, in combination with the normal win-stay and lose-shift ratios seen in isolates during the initial acquisition phase, suggests that isolates were able to develop an intact model-based choice strategy, but unable to readily update this model to respond to changing environmental demands. Thus, despite possible interpretative difficulties of overall performance due to reduced trials completed, isolates did exhibit decision-making deficiencies in the probabilistic reversal-learning tasks in response to changing contingencies. Such a deficit reflects a specific impairment in cognitive flexibility, a cognitive modality that is characteristically impaired in schizophrenia (Goldberg et al., 1987; Leeson et al., 2009; Morice, 1990; Murray et al., 2008). This finding is consistent with the results seen in other reversal-learning tasks, where isolates showed no deficit in the initial acquisition of a rotating T-maze task (Li et al., 2007), a simple visual discrimination task (G. H. Jones et al., 1991; Krech et al., 1962), or an odor/digging medium discrimination task (Schrijver et al., 2004), but exhibited impairment in the reversal-learning phase of these tasks. Selective deficits in cognitive flexibility in isolates were also detected using a related assay, the attentional set-shifting task (McLean et al., 2010; Schrijver & Wurbel, 2001). Wongwitdecha and Marsden (1996), however, reported improved reversal learning of isolates in a water maze task. Task differences such as using rewarding vs. aversive motivators (see below) and/or differences in handling and housing during the isolation procedure may explain these divergent findings. Similarly, subtle differences in task requirements between the between-sessions and the within-session probabilistic learning tasks may explain why, in our study, the difference between isolates and socials in reversal-phase target win-stay ratios reached significance in the former, but not the latter task. A possible explanation is the more stringent performance criterion in the between-sessions task, which required >90% of responses into the target location out of at least 50 trials per session in two consecutive sessions (i.e., at least 45 accurate target responses on two consecutive days), vs. only eight consecutive target responses in the within-session probabilistic learning task. Moreover, in the between-sessions design, 24 h pass between consecutive assessments of criterion performance, which adds a long-term memory component that is largely absent from the within-session design. In previous studies, isolates exhibited memory deficits in the novel object recognition task that were apparent after long (3.5 – 24 h), but not shorter delays (Bianchi et al., 2006; Lukasz et al., 2013; McLean et al., 2010). The memory demands of the between-sessions probabilistic learning tasks may therefore make this task particularly challenging for isolates, contributing to more robust deficits.
Notably, in our study, the lose-shift ratios of isolates did not differ from those of socials during the reversal phase. In other words, isolates were just as successful as socials in decreasing their lose-shift ratios after responses into the new target location (see Figures 6b and 8b) and increasing their lose-shift ratios after responses into the new non-target location (see Table 2) during the reversal phase. The reversal-learning deficit in isolates was therefore driven predominantly by impaired processing of positive feedback, while negative feedback processing appeared largely unaltered. Such dissociation of positive/negative reward feedback learning may explain why isolates were not impaired in the aversely motivated water maze task (Wongwitdecha & Marsden, 1996), but were impaired in these reward-based tasks. Importantly, studies of reinforcement learning in schizophrenia patients have found the same pattern of deficient positive feedback processing, but normal processing of negative feedback (Waltz, Frank, Robinson, & Gold, 2007). This is particularly relevant because social isolation also plays a role in psychiatric illnesses other than schizophrenia, such as major depression (Barnett & Gotlib, 1988; Becker & Kleinman, 1991). Likewise, probabilistic reversal learning is disrupted in a number of different psychiatric disorders, such as depression (Murphy, Michael, Robbins, & Sahakian, 2003) and bipolar disorder (Roiser et al., 2009). In these other psychiatric conditions, however, poor reinforcement learning is mediated by differing aspects, such as hypersensitivity to reward in patients with bipolar disorder (Meyer, Johnson, & Winters, 2001), or hypersensitivity to punishment in depressed subjects (Santesso et al., 2008; Taylor Tavares et al., 2008). Isolation rearing may therefore most closely mirror the deficient reinforcement learning of people with schizophrenia, rather than that seen in other disorders.
It is not clear why isolates completed fewer trials per session in the probabilistic reversal-learning tasks. While there was no significant difference in target and non-target latencies between isolates and socials in the between-sessions version of the task, isolates tended to have longer response latencies (see Table 3). Such long latencies of isolates were significant in the within-session version of the task (see Table 5). Longer response latencies may have prevented isolates from completing as many trials during the session as socials. Moreover, although reward latencies of isolates did not differ from those of socials in either version of the task (see Tables 3 and 5), new trials were only initiated upon removal of the head from the magazine. Thus, isolates may have taken more time to consume the reward, delaying their initiation of the next trial. In addition, isolates may have taken longer than socials to perform head entries into the magazine after the end of the timeout after a punished response. Given the lack of effect of isolation rearing on overall locomotor activity in the BPM (see Figure 3a) and on reward latencies (see Tables 3 and 5), the lower trial completion rate in isolates was unlikely to be attributable to locomotor impairment, sedation, or decreased motivation, a conclusion further corroborated by their normal breakpoints when responding in a progressive ratio schedule (Amitai et al., unpublished data).
The lower response rates of isolates in the probabilistic reversal-learning task were also observed as reduced levels of premature responding in both versions of the task (see Tables 3 and 5). In the within-session task, isolates also tended to perform fewer timeout responses (see Table 5). These decreased rates of behavior across various types of responding – target, non-target, premature, and timeout responses – in isolates are in striking contrast to the increased levels of unconditioned locomotor activity in the BPM, which include increased holepokes and rears, along with less locomotor habituation to a novel environment (see Figure 3). Isolation rearing may therefore produce a pattern of elevated unconditioned activity, but decreased activity in a goal-oriented task. This notion is supported by findings of increased locomotion, holepokes, and rearing in the BPM or open-field test in isolation-reared rats (Hall et al., 1998; G. H. Jones et al., 1989; Lapiz et al., 2000; Paulus et al., 1998; Powell et al., 2002; Sahakian et al., 1975; Varty et al., 2000), along with fewer total trials and premature responses with longer choice latencies in a gambling task (Zeeb, Wong, & Winstanley, 2012) and longer response latencies in an ethanol self-administration task (McCool & Chappell, 2009) in these animals. Isolates also tended to make fewer premature responses during ITI manipulations in the five-choice serial reaction time task (Dalley, Theobald, Pereira, Li, & Robbins, 2002), although increased perseverative responses during presentation of an auditory distractor were also observed.
These contrasting effects of isolation rearing may be due to differential changes in neurotransmission in cortical vs. subcortical brain structures of isolates. While the effects of isolation rearing on neurotransmitter levels and function remains to be fully elucidated, some studies have reported increased basal dopamine levels (Hall, 1998; Robbins, Jones, & Wilkinson, 1996), increased dopamine turnover (Blanc et al., 1980; Heidbreder et al., 2000), and/or psychostimulant- (Howes, Dalley, Morrison, Robbins, & Everitt, 2000; G. H. Jones, Hernandez, Kendall, Marsden, & Robbins, 1992) or footshock-induced (Fulford & Marsden, 1998) dopamine hyper-responsivity in the nucleus accumbens, which plays an essential role in exploratory locomotion (Mogenson & Nielsen, 1984; Mogenson & Wu, 1991; Svensson & Ahlenius, 1983). On the other hand, dopamine turnover in the prefrontal cortex was found to be decreased (Hall, 1998; Heidbreder et al., 2000; Robbins et al., 1996). These findings are not surprising, since cortical and subcortical dopamine projections often show reciprocal changes in activity. Given the critical involvement of the prefrontal cortex in higher-level cognition and executive function, this pattern of neurochemical changes may explain why unconditioned exploratory activity is increased in isolates, while responding in cognitive tasks, such as the probabilistic reversal-learning task, is reduced. Indeed, an increase in non-goal-specific behaviors such as exploration of the testing chamber may have contributed to a less efficient performance of the task (leading, for example, to longer pauses before initiation of a new trial after the timeout after a punished response) and thus fewer trials completed per session.
In summary, isolation rearing produced robust sensorimotor gating deficits and changes in exploratory locomotor behavior. Cognitive deficits in isolation-reared rats were more subtle, and were driven by poor processing of positive feedback reinforcement learning. Additionally, isolates exhibited a general response suppression in the cognitive tasks that contrasted with the elevated levels of unconditioned exploratory locomotion seen in these animals. The isolation rearing model recreates many of the behavioral and cognitive deficits characteristic of schizophrenia and thus may prove suitable for testing putative therapeutics for treating dysfunction in schizophrenia.
Acknowledgments
Supported by NIH grants R21 MH091571 and R01 MH091407. Mark A. Geyer holds equity interest in San Diego Instruments. The authors would like to thank Dr. Neal Swerdlow, Dr. Adam Halberstadt, and Ms. Mahalah Buell for their assistance.
Footnotes
No other authors have any potential conflict of interest to declare.
References
- Addington J, Penn D, Woods SW, Addington D, Perkins DO. Social functioning in individuals at clinical high risk for psychosis. Schizophr Res. 2008;99(1–3):119–124. doi: 10.1016/j.schres.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4(2):163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA. Ontogeny of isolation rearing-induced deficits in sensorimotor gating in rats. Physiol Behav. 1999;67(3):385–392. doi: 10.1016/s0031-9384(99)00082-7. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Swerdlow NR, Braff DL, Geyer MA. Reversal of isolation rearing-induced deficits in prepulse inhibition by Seroquel and olanzapine. Biol Psychiatry. 1998;43(6):436–445. doi: 10.1016/s0006-3223(97)00246-1. [DOI] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, et al. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35(6):1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett PA, Gotlib IH. Psychosocial functioning and depression: distinguishing among antecedents, concomitants, and consequences. Psychol Bull. 1988;104(1):97–126. doi: 10.1037/0033-2909.104.1.97. [DOI] [PubMed] [Google Scholar]
- Becker J, Kleinman A, editors. Psychosocial aspects of depression. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. [Google Scholar]
- Bianchi M, Fone KF, Azmi N, Heidbreder CA, Hagan JJ, Marsden CA. Isolation rearing induces recognition memory deficits accompanied by cytoskeletal alterations in rat hippocampus. Eur J Neurosci. 2006;24(10):2894–2902. doi: 10.1111/j.1460-9568.2006.05170.x. [DOI] [PubMed] [Google Scholar]
- Blanc G, Herve D, Simon H, Lisoprawski A, Glowinski J, Tassin JP. Response to stress of mesocortico-frontal dopaminergic neurones in rats after long-term isolation. Nature. 1980;284(5753):265–267. doi: 10.1038/284265a0. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179(2):219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15(4):339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47(2):181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156(2–3):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49(3):206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia J, Hatcher PD, Reavill C, Jones DN. Long-term evaluation of isolation-rearing induced prepulse inhibition deficits in rats: an update. Psychopharmacology (Berl) 2005;180(1):57–62. doi: 10.1007/s00213-004-2139-5. [DOI] [PubMed] [Google Scholar]
- Cilia J, Reavill C, Hagan JJ, Jones DN. Long-term evaluation of isolation-rearing induced prepulse inhibition deficits in rats. Psychopharmacology (Berl) 2001;156(2–3):327–337. doi: 10.1007/s002130100786. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Pereira EA, Li PM, Robbins TW. Specific abnormalities in serotonin release in the prefrontal cortex of isolation-reared rats measured during behavioural performance of a task assessing visuospatial attention and impulsivity. Psychopharmacology (Berl) 2002;164(3):329–340. doi: 10.1007/s00213-002-1215-y. [DOI] [PubMed] [Google Scholar]
- Elvevåg B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14(1):1–2. [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126(Pt 8):1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32(6):1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Fulford AJ, Marsden CA. Effect of isolation-rearing on conditioned dopamine release in vivo in the nucleus accumbens of the rat. J Neurochem. 1998;70(1):384–390. doi: 10.1046/j.1471-4159.1998.70010384.x. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25(1):277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry. 1993;34(6):361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- Gilmour G, Arguello A, Bari A, Brown VJ, Carter C, Floresco SB, et al. Measuring the construct of executive control in schizophrenia: Defining and validating translational animal paradigms for discovery research. Neurosci Biobehav Rev. 2012 doi: 10.1016/j.neubiorev.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR, Berman KF, Pliskin NH, Podd MH. Further evidence for dementia of the prefrontal type in schizophrenia? A controlled study of teaching the Wisconsin Card Sorting Test. Arch Gen Psychiatry. 1987;44(11):1008–1014. doi: 10.1001/archpsyc.1987.01800230088014. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Risbrough VB, Scott CN, Coste S, Stenzel-Poore M, Geyer MA, et al. Isolation rearing-induced deficits in contextual fear learning do not require CRF(2) receptors. Behav Brain Res. 2010;209(1):80–84. doi: 10.1016/j.bbr.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner H, Loffler W, Maurer K, Hambrecht M, an der Heiden W. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatr Scand. 1999;100(2):105–118. doi: 10.1111/j.1600-0447.1999.tb10831.x. [DOI] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12(1–2):129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on locomotion, anxiety and responses to ethanol in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998;139(3):203–209. doi: 10.1007/s002130050705. [DOI] [PubMed] [Google Scholar]
- Hatashita-Wong M, Smith TE, Silverstein SM, Hull JW, Willson DF. Cognitive functioning and social problem-solving skills in schizophrenia. Cogn Neuropsychiatry. 2002;7(2):81–95. doi: 10.1080/13546800143000168. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, et al. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100(4):749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res. 2004;150(2):103–115. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl) 2000;151(1):55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Jones CA, Brown AM, Auer DP, Fone KC. The mGluR2/3 agonist LY379268 reverses post-weaning social isolation-induced recognition memory deficits in the rat. Psychopharmacology (Berl) 2011;214(1):269–283. doi: 10.1007/s00213-010-1931-7. [DOI] [PubMed] [Google Scholar]
- Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW. Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav. 1992;43(1):17–35. doi: 10.1016/0091-3057(92)90635-s. [DOI] [PubMed] [Google Scholar]
- Jones GH, Marsden CA, Robbins TW. Behavioural rigidity and rule-learning deficits following isolation-rearing in the rat: neurochemical correlates. Behav Brain Res. 1991;43(1):35–50. doi: 10.1016/s0166-4328(05)80050-6. [DOI] [PubMed] [Google Scholar]
- Jones GH, Robbins TW, Marsden CA. Isolation-rearing retards the acquisition of schedule-induced polydipsia in rats. Physiol Behav. 1989;45(1):71–77. doi: 10.1016/0031-9384(89)90167-4. [DOI] [PubMed] [Google Scholar]
- King MV, Seeman P, Marsden CA, Fone KC. Increased dopamine D2High receptors in rats reared in social isolation. Synapse. 2009;63(6):476–483. doi: 10.1002/syn.20624. [DOI] [PubMed] [Google Scholar]
- Krech D, Rosenzweig MR, Bennett EL. Relations between chemistry and problem-solving among rats raised in enriched and impoverished environments. J Comp Physiol Psychol. 1962;55:801–807. doi: 10.1037/h0044220. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Mateo Y, Parker T, Marsden C. Effects of noradrenaline depletion in the brain on response on novelty in isolation-reared rats. Psychopharmacology (Berl) 2000;152(3):312–320. doi: 10.1007/s002130000534. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66(6):586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wu X, Li L. Chronic administration of clozapine alleviates reversal-learning impairment in isolation-reared rats. Behav Pharmacol. 2007;18(2):135–145. doi: 10.1097/FBP.0b013e3280d3ee83. [DOI] [PubMed] [Google Scholar]
- Lim C, Chong SA, Keefe RS. Psychosocial factors in the neurobiology of schizophrenia: a selective review. Ann Acad Med Singapore. 2009;38(5):402–406. [PubMed] [Google Scholar]
- Lukasz B, O’Sullivan NC, Loscher JS, Pickering M, Regan CM, Murphy KJ. Peripubertal viral-like challenge and social isolation mediate overlapping but distinct effects on behaviour and brain interferon regulatory factor 7 expression in the adult Wistar rat. Brain Behav Immun. 2013;27(1):71–79. doi: 10.1016/j.bbi.2012.09.011. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33(2):273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean S, Grayson B, Harris M, Protheroe C, Woolley M, Neill J. Isolation rearing impairs novel object recognition and attentional set shifting performance in female rats. J Psychopharmacol. 2010;24(1):57–63. doi: 10.1177/0269881108093842. [DOI] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Winters R. Responsiveness to Threat and Incentive in Bipolar Disorder: Relations of the BIS/BAS Scales With Symptoms. J Psychopathol Behav Assess. 2001;23(3):133–143. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Nielsen M. Neuropharmacological evidence to suggest that the nucleus accumbens and subpallidal region contribute to exploratory locomotion. Behav Neural Biol. 1984;42(1):52–60. doi: 10.1016/s0163-1047(84)90424-2. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Wu M. Quinpirole to the accumbens reduces exploratory and amphetamine-elicited locomotion. Brain Res Bull. 1991;27(5):743–746. doi: 10.1016/0361-9230(91)90057-q. [DOI] [PubMed] [Google Scholar]
- Moller P, Husby R. The initial prodrome in schizophrenia: searching for naturalistic core dimensions of experience and behavior. Schizophr Bull. 2000;26(1):217–232. doi: 10.1093/oxfordjournals.schbul.a033442. [DOI] [PubMed] [Google Scholar]
- Morice R. Cognitive inflexibility and pre-frontal dysfunction in schizophrenia and mania. Br J Psychiatry. 1990;157:50–54. doi: 10.1192/bjp.157.1.50. [DOI] [PubMed] [Google Scholar]
- Mortimer AM. Cognitive function in schizophrenia: do neuroleptics make a difference? Pharmacol Biochem Behav. 1997;56(4):789–795. doi: 10.1016/s0091-3057(96)00425-x. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Michael A, Robbins TW, Sahakian BJ. Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychol Med. 2003;33(3):455–467. doi: 10.1017/s0033291702007018. [DOI] [PubMed] [Google Scholar]
- Murray GK, Cheng F, Clark L, Barnett JH, Blackwell AD, Fletcher PC, et al. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008;34(5):848–855. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE, Pantelis C, Carruthers K, Speller J, Baxendale S, Barnes TRE. Cognitive functioning and symptomatology in chronic schizophrenia. Psychol Med. 1990;20:357–365. doi: 10.1017/s0033291700017670. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72(1):29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Otto AR, Gershman SJ, Markman AB, Daw ND. The curse of planning: dissecting multiple reinforcement learning systems by taxing the central executive. Psychol Sci. doi: 10.1177/0956797612463080. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Bakshi VP, Geyer MA. Isolation rearing affects sequential organization of motor behavior in post-pubertal but not pre-pubertal Lister and Sprague-Dawley rats. Behav Brain Res. 1998;94(2):271–280. doi: 10.1016/s0166-4328(97)00158-7. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, et al. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66(10):1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB. Models of neurodevelopmental abnormalities in schizophrenia. Current Topics in Behavioral Neurosciences. 2010;4:435–481. doi: 10.1007/7854_2010_57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Swerdlow NR, Pitcher LK, Geyer MA. Isolation rearing-induced deficits in prepulse inhibition and locomotor habituation are not potentiated by water deprivation. Physiol Behav. 2002;77(1):55–64. doi: 10.1016/s0031-9384(02)00817-x. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Cools R, Frank M, Pizzagalli DA, Preston A, Ranganath C, et al. CNTRICS final task selection: long-term memory. Schizophr Bull. 2009;35(1):197–212. doi: 10.1093/schbul/sbn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31(11):2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Jones GH, Wilkinson LS. Behavioural and neurochemical effects of early social deprivation in the rat. J Psychopharmacol. 1996;10(1):39–47. doi: 10.1177/026988119601000107. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Cannon DM, Gandhi SK, Taylor Tavares J, Erickson K, Wood S, et al. Hot and cold cognition in unmedicated depressed subjects with bipolar disorder. Bipolar Disord. 2009;11(2):178–189. doi: 10.1111/j.1399-5618.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Walker SC, Clarke HF, Robbins TW, Roberts AC. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci. 2010;30(43):14552–14559. doi: 10.1523/JNEUROSCI.2631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakian BJ, Robbins TW, Morgan MJ, Iversen SD. The effects of psychomotor stimulants on stereotypy and locomotor activity in socially-deprived and control rats. Brain Res. 1975;84(2):195–205. doi: 10.1016/0006-8993(75)90975-0. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Steele KT, Bogdan R, Holmes AJ, Deveney CM, Meites TM, et al. Enhanced negative feedback responses in remitted depression. Neuroreport. 2008;19(10):1045–1048. doi: 10.1097/WNR.0b013e3283036e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijver NC, Pallier PN, Brown VJ, Wurbel H. Double dissociation of social and environmental stimulation on spatial learning and reversal learning in rats. Behav Brain Res. 2004;152(2):307–314. doi: 10.1016/j.bbr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Schrijver NC, Wurbel H. Early social deprivation disrupts attentional, but not affective, shifts in rats. Behav Neurosci. 2001;115(2):437–442. [PubMed] [Google Scholar]
- Schubert MI, Porkess MV, Dashdorj N, Fone KC, Auer DP. Effects of social isolation rearing on the limbic brain: a combined behavioral and magnetic resonance imaging volumetry study in rats. Neuroscience. 2009;159(1):21–30. doi: 10.1016/j.neuroscience.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Sharma T, Antonova L. Cognitive function in schizophrenia: deficits, functional consequences, and future treatment. Psychiatr Clin North Am. 2003;26(1):25–40. doi: 10.1016/s0193-953x(02)00084-9. [DOI] [PubMed] [Google Scholar]
- Svensson L, Ahlenius S. Suppression of exploratory locomotor activity in the rat by the local application of 3-PPP enantiomers into the nucleus accumbens. Eur J Pharmacol. 1983;88(4):393–397. doi: 10.1016/0014-2999(83)90592-7. [DOI] [PubMed] [Google Scholar]
- Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008;42(3):1118–1126. doi: 10.1016/j.neuroimage.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson PJ, Laws KR, Flowers KA, Tyson A, Mortimer AM. Cognitive function and social abilities in patients with schizophrenia: relationship with atypical antipsychotics. Psychiatry Clin Neurosci. 2006;60(4):473–479. doi: 10.1111/j.1440-1819.2006.01534.x. [DOI] [PubMed] [Google Scholar]
- Valzelli L. The “isolation syndrome” in mice. Psychopharmacologia. 1973;31(4):305–320. doi: 10.1007/BF00421275. [DOI] [PubMed] [Google Scholar]
- van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010;36(2):246–270. doi: 10.1093/schbul/sbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varty GB, Geyer MA. Effects of isolation rearing on startle reactivity, habituation, and prepulse inhibition in male Lewis, Sprague-Dawley, and Fischer F344 rats. Behav Neurosci. 1998;112(6):1450–1457. doi: 10.1037//0735-7044.112.6.1450. [DOI] [PubMed] [Google Scholar]
- Varty GB, Higgins GA. Examination of drug-induced and isolation-induced disruptions of prepulse inhibition as models to screen antipsychotic drugs. Psychopharmacology (Berl) 1995;122(1):15–26. doi: 10.1007/BF02246437. [DOI] [PubMed] [Google Scholar]
- Varty GB, Paulus MP, Braff DL, Geyer MA. Environmental enrichment and isolation rearing in the rat: effects on locomotor behavior and startle response plasticity. Biol Psychiatry. 2000;47(10):864–873. doi: 10.1016/s0006-3223(99)00269-3. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62(7):756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93(1–3):296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JM. Reversal learning and the formation of learning sets by cats and rhesus monkeys. J Comp Physiol Psychol. 1966;61(3):421–428. doi: 10.1037/h0023269. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Killcross SS, Humby T, Hall FS, Geyer MA, Robbins TW. Social isolation in the rat produces developmentally specific deficits in prepulse inhibition of the acoustic startle response without disrupting latent inhibition. Neuropsychopharmacology. 1994;10(1):61–72. doi: 10.1038/npp.1994.8. [DOI] [PubMed] [Google Scholar]
- Wongwitdecha N, Marsden CA. Effects of social isolation rearing on learning in the Morris water maze. Brain Res. 1996;715(1–2):119–124. doi: 10.1016/0006-8993(95)01578-7. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Smittenaar P, Dolan RJ. Dopamine enhances model-based over model-free choice behavior. Neuron. 2012;75(3):418–424. doi: 10.1016/j.neuron.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: rodent and human studies in the Behavioral Pattern Monitor. Neurosci Biobehav Rev. 2007;31(6):882–896. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Wong AC, Winstanley CA. Differential effects of environmental enrichment, social-housing, and isolation-rearing on a rat gambling task: Dissociations between impulsive action and risky decision-making. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2822-x. [DOI] [PubMed] [Google Scholar]